Abstract
The storage and transport of cells is a fundamental technology which underpins cell biology, biomaterials research, and emerging cell-based therapies. Inspired by antifreeze and ice-binding proteins in extremophiles, macromolecular (polymer) cryoprotectants are emerging as exciting biomaterials to enable the reduction and/or replacement of conventional cryoprotective agents such as DMSO. Here, we critically study post-thaw cellular outcomes upon addition of macromolecular cryoprotectants to provide unambiguous evidence that post-thaw culturing time and a mixture of assays are essential to claim a positive outcome. In particular, we observe that only measuring the viability of recovered cells gives false positives, even with non-cryoprotective polymers. Several systems gave apparently high viability but very low total cell recovery, which could be reported as a success but in practical applications would not be useful. Post-thaw culture time is also shown to be crucial to enable apoptosis to set in. Using this approach we demonstrate that polyampholytes (a rapidly emerging class of cryoprotectants) improve post-thaw outcomes across both measures, compared to poly(ethylene glycol), which can give false positives when only viability and short post-thaw time scales are considered. This work will help guide the discovery of new macromolecular cryoprotectants and ensure materials which only give positive results under limited outcomes can be quickly identified and removed.
Introduction
The banking of cells underpins all cell biology and biomaterials research, removing the need for continuous culture (which results in phenotype drift,1 as well as consuming large amounts of resources) and enables successful delivery of emerging cell-based therapies.2,3 Conventional cryoprotectants (CPAs), which protect the cells from cold-associated stress during freezing, include DMSO (the most common), glycerol, trehalose, and sucrose.4 While DMSO is still the gold standard cryoprotectant, it is desirable to reduce or remove DMSO due to toxicity issues,5 epigenetic changes,6 and DMSO sensitivity with certain cells (e.g., RAW 264.7).7 To address this, there has been a resurgence of interest in the discovery of molecules and materials which can modulate the damage during cryopreservation,8−12 initially inspired by how extremophiles survive subzero temperatures.13,14 These organisms produce antifreeze proteins (AFP) and antifreeze glycoproteins (AFGP),15,16 which demonstrate potent ice recrystallization inhibition (IRI) activity, a key cause of cell death during thawing in vitro.17,18 Biomaterials that mimic the IRI properties of AFPs,19,20 such as poly(vinyl alcohol) (PVA), have been shown to improve post-thaw cell recoveries.21−23 Other IRI active examples include polyproline,24,25 small molecules,26 and graphene oxide.27 Polyampholytes (polymers containing a mix of both positive and negative charges) have emerged as a new class of macromolecular cryoprotectant, which (while having some IRI activity)28 appear to work by an alternative mechanism which might include membrane stabilization.11,29,30 The first polyampholyte used in cryopreservation was reported by Matsumura et al. using a carboxylated ε-poly-l-lysine derivative for DMSO-free cryopreservation.11 Polyampholytes have been used to successfully cryopreserve stem cells,31 cell monolayers,32 and mouse oocytes.33 Structure–property relationships for these materials are still missing however.34
One particular challenge in this emerging biomaterials field is that there is no standardized test for assessing a cryoprotectant for cell recovery, and there are many cell lines (or primary cells) which survive freezing differently. Therefore, it is currently hard to compare how potent two macromolecular cryoprotectants are. It is clear, however, that there is a mismatch between the two common methods for measuring cryoprotective outcome: the viability of the cells recovered (the ratio of live cells to total cells post-thaw, this is most commonly reported)35−37 and the total number of cells recovered (the ratio of total live cells post-thaw to total cells initially frozen), with the former tending to give higher values than the latter. Furthermore, the post-thaw interval differs between studies, from analyzing cells immediately, to up to 48 h post-thaw. These two factors are especially crucial when assessing new macromolecular cryoprotectants which may function by different mechanisms (compared to conventional CPAs) and result in unanticipated stresses (or protection).9 For example, Stöver and co-workers reported polyampholytes for DMSO-free cryopreservation;38 cell viabilities immediately post-thaw were similar to that of 10% DMSO, but the cells did not adhere well, and post-thaw growth curves suggested the polymer did not produce viable cells unless additional DMSO was added. Matsumura used vitrification (using 6.5 M ethylene glycol) for mesenchymal stromal (stem) cell cryopreservation with added polyampholytes.39 Near 100% cell viability could be achieved, but post-thaw growth rates were suppressed relative to controls (but superior to conventional vitrification). Crucially, the number of cells at zero hours (post-thaw) was greater than after 1 day culture. Similarly, Sharp et al. observed lower cell densities after 24 h compared to immediately post-thaw.40 Yang and co-workers measured cell survival over time (after cryopreservation) and found it peaked at 1–2 h post-thaw but decreased after 24 h incubation,41 highlighting that immediate post-thaw measurements lead to significant overestimation of cryoprotectant activity. Mercado et al. showed that adding an amphiphilic polymer to SAOS-2 cells along with 200 mM trehalose gave a cryoprotective benefit but found significant differences between the two assessment methods (trypan blue and MTS assay) when the cells were analyzed immediately post-thaw.42 These studies further highlight that immediate post-thaw values can fail to predict long-term cryoprotective outcomes; clearly, the primary aim of cryopreservation must be to obtain sufficient numbers of viable cells suitable for experiments or therapy, and new cryoprotective biomaterials should be designed to achieve this.
Considering the above, it is clear that the potential for false positives in this emerging field of macromolecular cryoprotectants is significant and that single measurements (especially viability) can give the impression of exceptional cryopreservation performance when in reality few cells are recovered. Therefore, the aim of this manuscript is to critically evaluate the post-thaw culture conditions and assessment methods on the outcome of cellular cryopreservation upon addition of several polymers. It is shown that short culture times post-thaw lead to severe overestimation of cryoprotectant function and that the use of viability measurements alone also gives significant false positives. We hope this will help guide the development of new materials for this important biotechnological process.
Experimental Section
Materials
Advanced Dulbecco’s Modified Eagle’s Medium, Ham’s F-12K media, and penicillin/streptomycin/amphotericin B were obtained from Gibco. Poly(ethylene glycol) (Mn 8 kDa) was obtained from MP Biomedicals. Dimethylaminoethanol, dimethyl sulfoxide, fetal bovine serum, Dulbecco’s phosphate-buffered saline, poly(ethylene glycol)methyl ether (Mn 20 kDa), and poly(methyl vinyl ether-alt-maleic anhydride) (Mn 80 kDa) were obtained from Sigma-Aldrich. A live/dead viability/cytotoxicity kit and CellEvent Caspase-3/7 Green Detection Reagent were obtained from Thermo Fisher. All solvents were purchased from VWR or Sigma-Aldrich, and reagents were used without further purification unless indicated.
Methods
Polyampholyte Synthesis
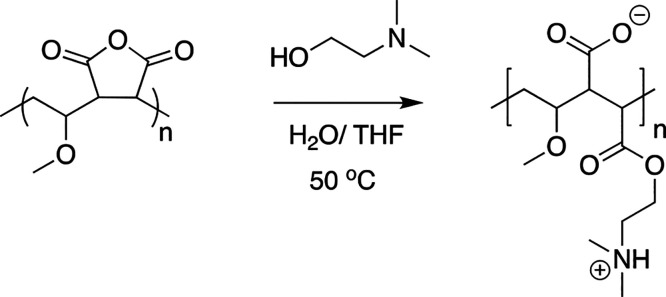
Polyampholyte was synthesized as previously described.32 Briefly, poly(methyl vinyl ether-alt-maleic anhydride), average Mn 80 kDa (1 g), was dissolved in tetrahydrofuran (50 mL) and heated to 50 °C with stirring, Scheme 1. Once dissolved, dimethylaminoethanol (2 g) was added in excess, forming a pink waxy solid, which was allowed to stir for 30 min. Water (50 mL) was added, and the reaction was left to stir overnight followed by purification in dialysis tubing (Spectra Por, 12–14 kDa MWCO) for 48 h. The resulting solution was freeze dried to produce a white solid.
Scheme 1. Synthesis of Poly(methyl vinyl ether-alt-maleic anhydride) Polyampholyte.
Cell Culture
Human caucasian lung carcinoma A549 cells (ECACC 86012804) and human colon adenocarcinoma SW480 cells (ECACC 87092801) were obtained from the European Collection of Authenticated Cell Cultures and cultured in Ham’s F-12K media (Gibco) and Advanced Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco), supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich), 100 units·mL–1 penicillin, 100 μg·mL–1 streptomycin, and 250 ng·mL–1 amphotericin B (PSA). Cells were maintained in a humidified atmosphere at 37 °C, 5% CO2, and subcultured every 3–4 days or when 90% confluent.
Cryopreservation of Cell Suspensions
Polymer cryoprotectants were prepared at a 2× final concentration in culture media containing 20% FBS and 5% DMSO and allowed to dissolve before sterile filtering through a 0.2 μm membrane. Cells were removed from culture by treatment with 0.25% trypsin plus 1 mM ethylenediaminetetraacetic acid (EDTA) in balanced salt solution for 5 min at 37 °C before being neutralized with complete cell culture media and centrifuged at 180 × g for 5 min. Supernatant was removed, a sample of cells was diluted 1:1 with 0.4% trypan blue, and the number of viable cells was determined by counting with a hemocytometer. The cell density was adjusted to obtain a cell suspension containing 2 × 105 cells·mL–1, and a second cell count was performed to obtain an accurate prefreeze value. A 500 μL amount of cell suspension was added to 500 μL of cryoprotectant solution in a cryovial and mixed 3 times. Final polymer solutions consisted of 10% FBS, 2.5% DMSO, and 20 mg·mL–1 polymer. Triplicate samples were prepared for each freezing condition. The cryovials were transferred to a CoolCell freezing box and frozen at 1 °C·min–1 in a −80 °C freezer. After 2 h at −80 °C the cryovials were transferred to liquid nitrogen storage for 24 h. To thaw, cryovials were removed from liquid nitrogen and suspended in a water bath heated to 37 °C. The contents of each vial were added to 9 mL of complete media and centrifuged at 180 × g for 5 min to pellet cells. The supernatant was discarded, and the cell pellet was resuspended in 500 μL of complete cell media and then transferred to individual wells of a 24-well plate. Plates were maintained in a humidified atmosphere at 37 °C, 5% CO2 for either 6 or 24 h.
For samples analyzed immediately post-thaw (0 h time point), cryovials were thawed as described above. After centrifugation, the supernatant was removed and cells were resuspended in 400 μL of complete cell media. A sample of the cell suspension was used for the trypan blue exclusion assay. For samples analyzed 6 or 24 h post-thaw, the supernatant in the well was collected and cells were washed once with 250 μL of PBS. The PBS wash was combined with the well supernatant, centrifuged at 180 × g for 5 min, and then resuspended in 100 μL of complete media. The cells were counted using a hemocytometer to determine the number of nonattached cells post-thaw. Following PBS wash, the cells in the well plate were treated with 0.25% trypsin plus 1 mM ethylenediaminetetraacetic acid (EDTA) in balanced salt solution for 5 min at 37 °C, 5% CO2, neutralized with complete cell media, and centrifuged at 180 × g for 5 min. The supernatant was removed, and cells were resuspended in 400 μL of complete media.
Trypan Blue Exclusion Assay
For all time points, a sample of cells was mixed 1:1 in 0.4% trypan blue and counted using a hemocytometer. Cell recovery was calculated as the ratio of unstained cells to the number of cells initially frozen. Cell viability was calculated as the ratio of unstained cells to the sum of the stained plus unstained cells.
Live/Dead Viability Assay
Cells were frozen with cryoprotectant solutions and thawed as described and plated into 24-well plates. After either 6 or 24 h post-thaw, cell media was removed and cells were washed with 300 μL of sterile phosphate-buffered saline. A live/dead solution was prepared in sterile PBS containing 2 μM calcein-AM and 4 μM ethidium homodimer-1 and vortexed to mix. A 250 μL amount of live/dead solution was added to each well, and the plate was incubated at room temperature protected from light for 30 min. After 30 min, phase contrast and fluorescence images were captured for two different areas of each well at 530 and 645 nm on a CKX41 microscope with pE-300-W LED illumination and a XC30 camera. Four wells were analyzed for each condition. Image analysis was performed using ImageJ software, version 1.52. Cell viability was calculated as the ratio of cells stained with calcein-AM (green fluorescence) to the sum of cells stained with calcein-AM (green fluorescence) and ethidium homodimer-1 (red fluorescence).
Apoptosis Assay
Cells were frozen with cryoprotectant solutions and thawed as described above. The number of viable cells for each sample was determined by the trypan blue exclusion assay using a hemocytometer, and the cell density was adjusted to 1 × 105 cells·mL–1. A 100 μL amount of each sample was added to individual wells of a 96-well plate (1 × 104 cells·well–1). CellEvent Caspase-3/7 Green Detection Reagent was prepared in complete cell media at 2× final concentration (8 μM, final concentration 4 μM), and 100 μL was added to all wells. Plates were maintained in a humidified atmosphere at 37 °C, 5% CO2 until ready for analysis. At 6 and 24 h post-thaw, phase contrast and fluorescence images (Ex/Em 502/530 nm) were captured on a CKX41 microscope with pE-300-W LED illumination and a XC30 camera. Image analysis was performed using ImageJ software, version 1.52.
Results and Discussion
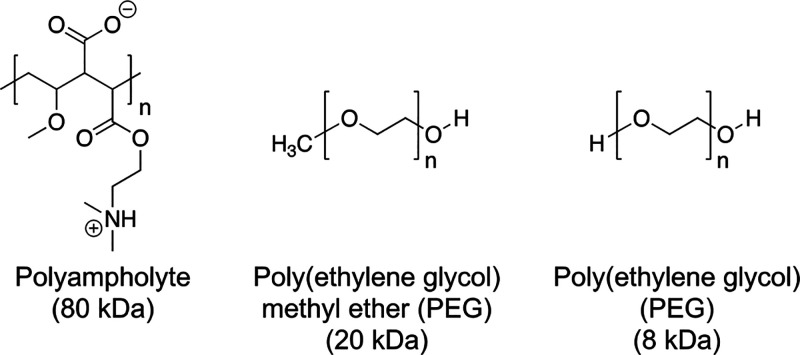
Two model cell lines were employed in this work, A549 and SW480. These were chosen as we found them useful for screening for cryopreservation outcomes and are easily available (unlike, e.g., primary cells). They have doubling rates of 24–48 h,43 allowing the study of proliferation (and onset of apoptosis) within a reasonable time frame in the laboratory. With these a panel of polymers was selected based on their reported cryoprotective, or lack of, properties. A polyampholyte comprising a poly(methyl vinyl ether-alt-maleic anhydride) backbone functionalized with dimethylaminoethanol (Figure 1), which has been shown to increase suspension and monolayer cell cryopreservation, was synthesized according to previous procedures.32 This polymer is only weakly IRI active, with its mechanism of action suggested to be due to membrane stabilization.32 Poly(ethylene glycol) (PEG) (8 and 22 kDa) was chosen as it is a negative control in ice growth assays19,20,44 and cryoprotective assays, but in our initial work (explored below) it was capable of showing false positive results.
Figure 1.
Polymers used in this study.
To highlight the need for considering both viability (eq 1) and total recovery values (eq 2), control experiments with variable DMSO levels were conducted. A549 cells were cryopreserved with either 10% (v/v) or 2.5% (v/v) DMSO and then thawed and plated. After 24 h incubation, cell viability (live/dead assay) and total cell recovery (trypan blue exclusion assay) were assessed, Figure 2. If viability of the recovered population is considered, 10% DMSO gave 94% viability and 2.5% DMSO gave 80%, suggesting both are reasonable cryoprotectants. However, viability measurements (ratio of live to total cells recovered) do not consider the number of cells lost during the freezing process, leading to overestimates of success. From the micrographs in Figure 2 A–C it is clear that far fewer cells are recovered when frozen in 2.5% DMSO (14%) compared to 10% DMSO (55%). This effect was also apparent when 20 mg·mL–1 PEG (8 kDa) was added as a cryoprotectant, which showed comparable viability to 10% DMSO (90%) yet yielded much lower cell recoveries (36%).
| 1 |
| 2 |
| 3 |
Figure 2.
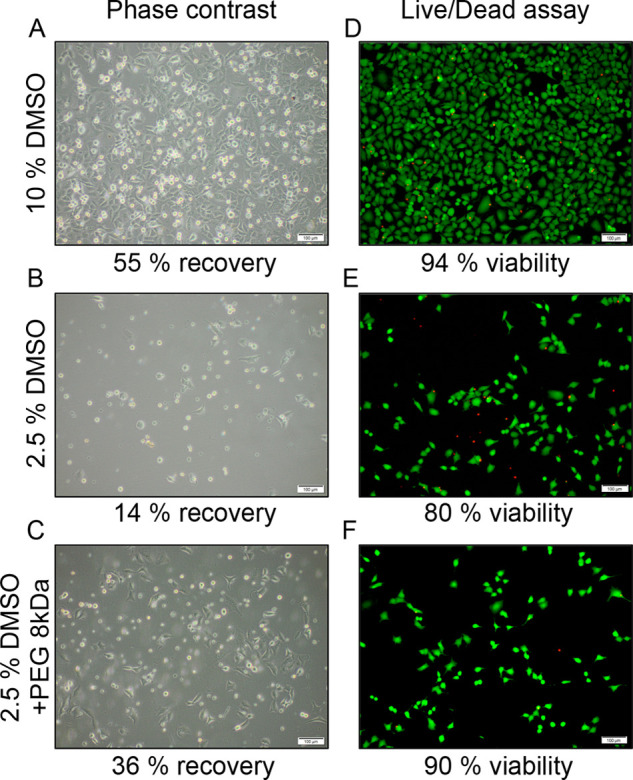
Post-thaw outcomes of cryopreserved A549 cells. (A–C) Phase contrast images 24 h post-thaw, after cryopreservation in the indicated conditions. Recoveries calculated by trypan blue exclusion assay. (D–F) Fluorescence microscopy images of A549 cells 24 h post-thaw, cryopreserved in the indicated conditions. Cells stained with calcein-AM (green fluorescence) and ethidium homodimer-1 (red fluorescence). Cell viability calculated from live/dead assay. Scale bar 100 μm.
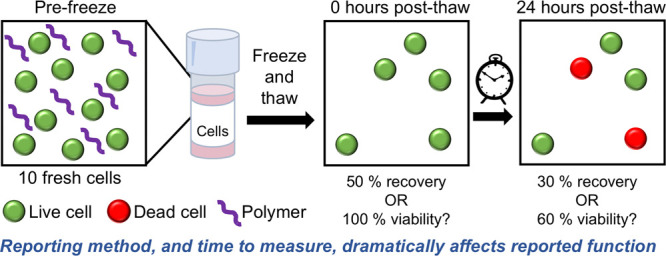
To exemplify this, Figure 3 shows a (simplified) schematic of how different post-thaw outcomes can be interpreted. If 10 cells were cryopreserved (Figure 3A), we show two scenarios. One scenario is where 8 intact cells are recovered (Figure 3B), with 6 live and 2 dead. This could be reported as 60% recovery or 75% viability; clearly different numbers. In the second scenario (Figure 3C), only 2 cells are recovered (20% recovery), but as both of them are viable, this is reported as 100% viability. The latter is clearly a “worse” outcome as fewer cells survived but based on common reporting methods would be seen as a positive result. This simplistic model highlights the challenges of making comparisons in this fast-emerging field and the need to ensure results are comparable and a “positive” result is true.
Figure 3.
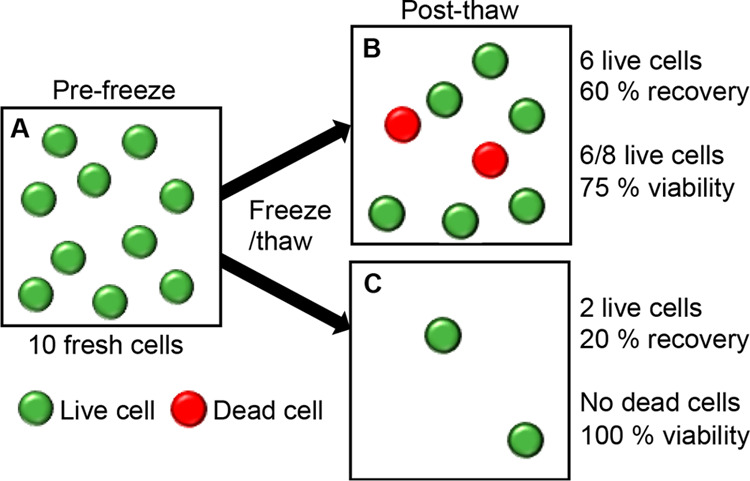
Schematic showing a comparison between reporting the total cell recovery and the cell viability of frozen and thawed cells using the live/dead assay. (A) Number of cells initially frozen; (B and C) hypothetical cryoprotective outcomes post-thaw.
To enable a systematic evaluation of how the post-thaw culture conditions and assay systems can impact the reported results and to test if the recently reported polyampholytes do provide a robust cryopreservation enhancement,32 a detailed post-thaw analysis was undertaken. For each cell line, the impact of adding 20 mg·mL–1 of the indicated polymer with 2.5% DMSO during cryopreservation was tested. This concentration was chosen to be non-optimal to allow benefits of polymers to be evaluated. This reproduces common conditions used for the discovery of macromolecular cryoprotectants.32,34 Both cell recovery and cell viability were measured using the trypan blue exclusion assay, where cells with compromised membranes take up the cell-impermeable dye, while cells with intact membranes remain unstained. This allowed measurement immediately post-thaw, as the live/dead assay cannot be used accurately at zero hours post-thaw since the cells have not yet adhered to their culture surface. Crucially, cells were analyzed at 0, 6, and 24 h time points to understand how these parameters changed over the post-thaw interval. High viability after 6 h, which then decreases at 24 h, is not useful for any procedure or process involving cells and is a key mechanism for introduction of false positives in the discovery of cryoprotectants.
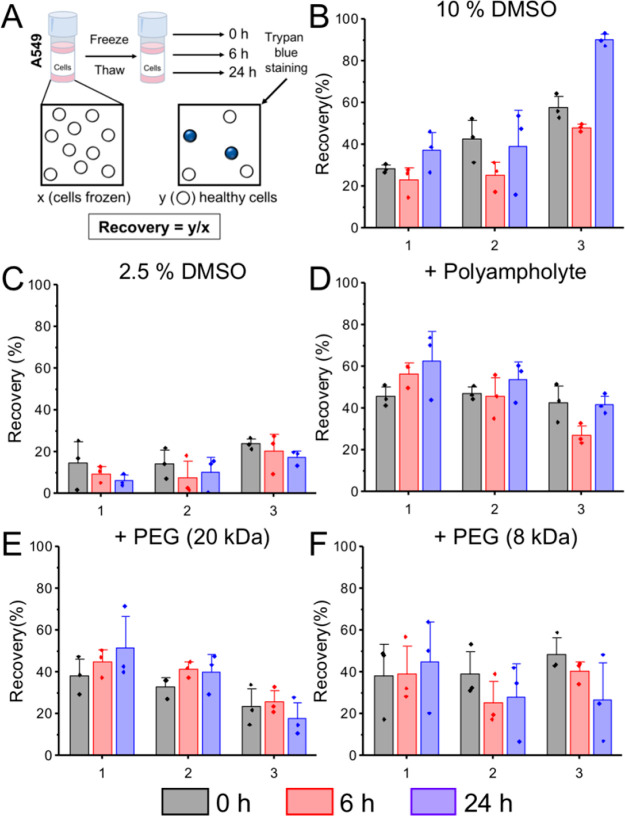
In the following sections, data is presented per biological repeat (with technical triplicates) rather than averaged to ensure trends are not smoothed out (averaged data is included in the Supporting Information, Figures S1–S4). Figure 4 shows the post-thaw total cell recovery for A549 cells under the indicated conditions. Using 2.5% DMSO as a cryoprotectant led to apparent cell recoveries between 16% and 23%, which decreased after 6 and 24 h in culture. In general, addition of any polymer showed some overall increase, especially using immediate post-thaw measurements. Similar recoveries were observed between 0 and 24 h for the polyampholyte and 20 kDa PEG (both with 2.5% DMSO), suggesting the recovered cells were capable of attaching and proliferating.
Figure 4.
Post-thaw total cell recovery of A549 cells. (A) Schematic for calculating percentage recovery. (B–F) Recovery calculated by trypan blue exclusion. Each plot shows biological repeats, with 3 technical replicates. Error bars are ± SEM. A549 cells were frozen in (B) 10% DMSO, (C) 2.5% DMSO, (D) 20 mg·mL–1 polyampholyte + 2.5% DMSO, (E) 20 mg·mL–1 poly(ethylene glycol) (20 kDa) + 2.5% DMSO, and (F) 20 mg·mL–1 poly(ethylene glycol) (8 kDa) + 2.5% DMSO.
However, only the polyampholyte cryopreserved cells showed increases in cell number over time for each biological repeat. Importantly, the recovery of cells frozen with 8 kDa PEG generally decreased from 0 to 6 and 24 h, similar to 2.5% DMSO alone. This highlights that recording cell recovery at 0 h post-thaw overestimates the cryoprotective ability of the polymers and promotes false positives. If recovery data was only shown at 0 h this could be interpreted as PEG is a potent cryoprotectant. Recovery after 24 h clearly shows that the cells die over time and hence are not well cryopreserved.
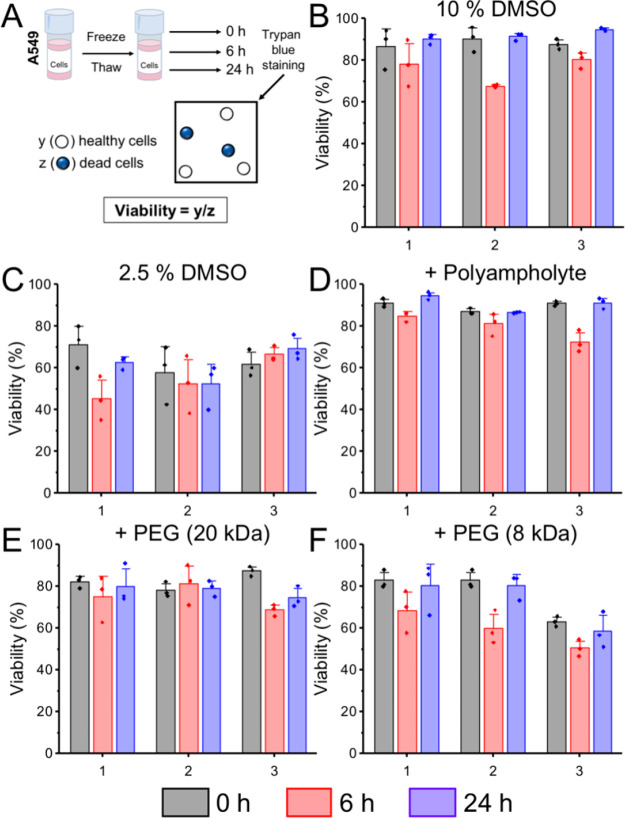
Next, the viability of the cells was measured using the trypan blue exclusion assay (eq 3, fraction of recovered cells which were viable), Figure 5. In all cases, the viability values were high and failed to capture subtle differences between the different polymer cryoprotectants. For each condition, a U-shaped trend was observed across the three time points, where viability decreased between 0 and 6 h and then increased again by 24 h post-thaw.
Figure 5.
Post-thaw cell viability of A549 cells. (A) Schematic for calculating percentage viability. (B–F) Viability data calculated by trypan blue exclusion. Each plot shows biological repeats, with 3 technical replicates. Error bars are ± SEM Error bars show ± SEM. A549 cells were frozen in (B) 10% DMSO, (C) 2.5% DMSO, (D) 20 mg·mL–1 polyampholyte + 2.5% DMSO, (E) 20 mg·mL–1 poly(ethylene glycol) (20 kDa) + 2.5% DMSO, and (F) 20 mg·mL–1 poly(ethylene glycol) (8 kDa) + 2.5% DMSO.
The viability of non-frozen control cells did not show this trend and remained above 95% at all time points (Figure S5). This U-shaped response suggests a delayed-onset cryoinjury, where cells initially appear healthy but then a large degree of cell death occurs after 6 h in culture. A comparison of this versus the recovery data from Figure 4 highlights how the common methods, when used alone, can give very different outcomes for the same system.
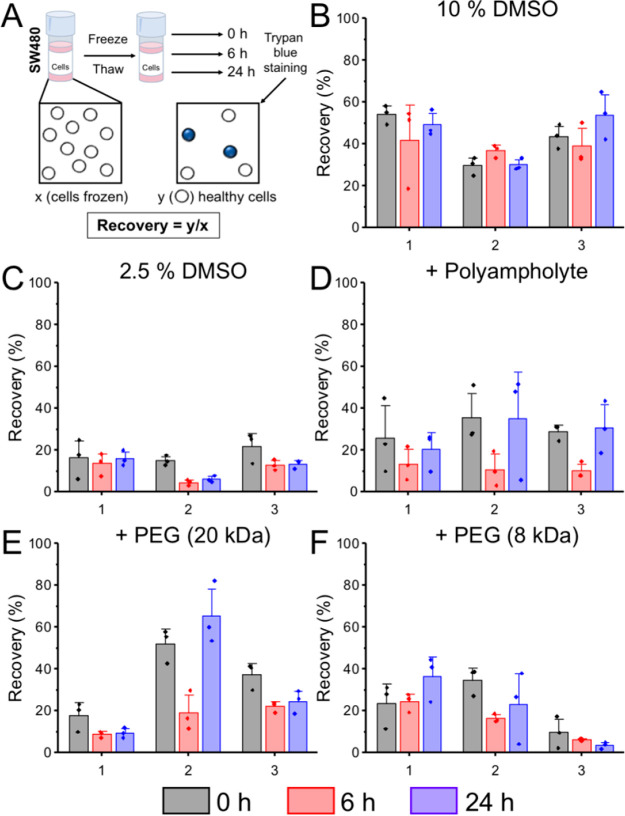
The same conditions were investigated in another cell line to validate the results. Figure 6 shows the post-thaw total cell recovery for SW480 cells. In general, cell recoveries were lower in the SW480 line compared to the A549s, as cryopreservation is cell line dependent. Cryopreservation with 2.5% DMSO alone led to cell recoveries of approximately 20% at 0 h, which decreased further after 6 and 24 h. Similar to the A549s, adding polymers provided some improvement to total cell recovery. Cryopreservation with the polyampholyte + 2.5% DMSO led to comparable recovery values at 0 and 24 h with a dip after 6 h, suggesting some cell death occurred, followed by proliferation. As seen with A549s, total cell recovery immediately post-thaw dramatically overestimated the degree of cryoprotection compared to 24 h when PEG (8 and 20 kDa) was added as a cryoprotectant.
Figure 6.
Post-thaw total cell recovery of SW480 cells. (A) Schematic for calculating percentage recovery. (B–F) Recovery calculated by trypan blue exclusion. Each plot shows biological repeats, with 3 technical replicates. Error bars are ± SEM. SW480 cells were frozen in (B) 10% DMSO, (C) 2.5% DMSO, (D) 20 mg·mL–1 polyampholyte + 2.5% DMSO, (E) 20 mg·mL–1 poly(ethylene glycol) (20 kDa) + 2.5% DMSO, and (F) 20 mg·mL–1 poly(ethylene glycol) (8 kDa) + 2.5% DMSO.
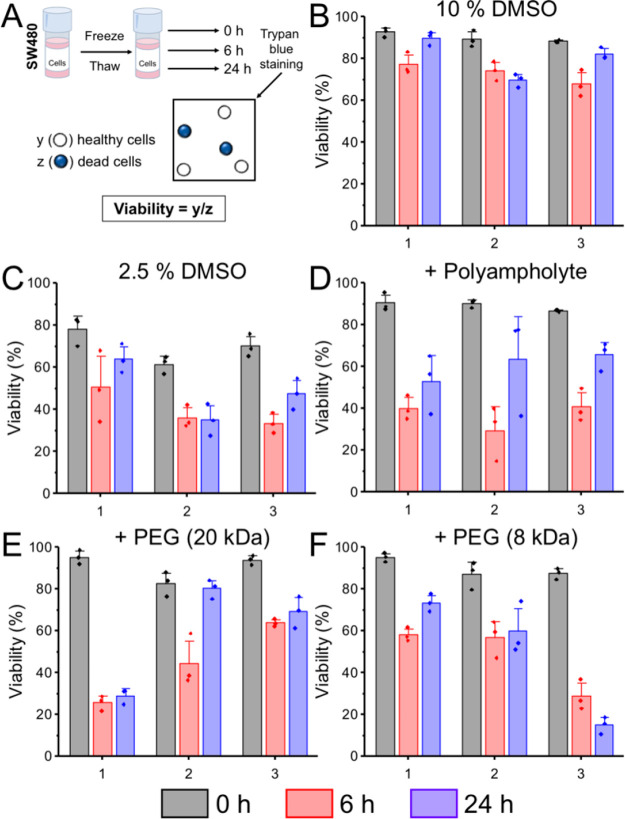
Cell viabilities were also recorded for SW480 cells using the trypan blue assay at 0, 6, and 24 h post-thaw. Viabilities of SW480 cells immediately post-thaw appeared high but dropped substantially between 0 and 6 h, and in some cases, the cells did not recover at all after 24 h in culture, Figure 7. For example, at 0 h post-thaw, the viability of SW480 cells cryopreserved with PEG (8 kDa) + 2.5% DMSO was 90−95%, but in once instance, this dropped to less than 60% after 6 h and, in one instance to 16% after 24 h. This provides further evidence that assessing cryopreservation immediately post-thaw overlooks delayed cryoinjury and leads to false-positive outcomes. Similar results have been described by other groups; Stöver reported DMSO-free cryopreservation of 3T3 cells with polyampholytes that showed >80% cell viabilities immediately post-thaw.38 However, after plating the cells and culturing for 1–3 days, they observed slower growth rates and fewer cells than expected, which could only be rescued by addition of 2% DMSO into the cryoprotective media. This finding would have been overlooked if only short-term culture conditions had been included. Mercado et al. reported the outcome of freezing osteosarcoma cells using an amphipathic polymer alongside 200 mM trehalose.42 While the number of viable cells (assessed by trypan blue) was higher in the presence of the polymer, cryosurvival by MTS assay was much lower. This could be due to low numbers of total recovered cells (overlooked by viability), which was highlighted only by using two different methods to assess cryosurvival.
Figure 7.
Post-thaw cell viability of SW480 cells. (A) Schematic for calculating percentage viability. (B–F) Viability data calculated by trypan blue exclusion. Each plot shows biological repeats, with 3 technical replicates. Error bars are ± SEM. SW480 cells were frozen in (B) 10% DMSO, (C) 2.5% DMSO, (D) 20 mg·mL–1 polyampholyte + 2.5% DMSO, (E) 20 mg·mL–1 poly(ethylene glycol) (20 kDa) + 2.5% DMSO, and (F) 20 mg·mL–1 poly(ethylene glycol) (8 kDa) + 2.5% DMSO.
Delayed onset apoptosis (programmed cell death) is the most likely cause of the U-shaped post-thaw trend, as the apoptotic cycle (which can take anywhere between 2 and 48 h depending on multiple factors)45 has not had time to initiate and complete immediately post-thaw. Using viability or counting at this point fails to identify apoptotic or pre-apoptotic cells.46 Cryopreservation-induced apoptosis47 has been observed in embryonic stromal (stem) cells,48 hepatocytes,49 and sperm.50 It has previously been reported that a loss of cell viability post-thaw has been attributed to apoptosis.51,46
To evaluate apoptosis after cryopreservation, the activity of caspase-3 and caspase-7, which are highly activated during apoptosis,52 was measured at 6 and 24 h post-thaw, in both cell lines, using the aforementioned cryoprotectants. To avoid bias, the thawed cells were counted and plated at the same density (1 × 105 cells·mL–1). From the micrographs (Figures S6–9), caspase-3 and -7 activity was present in all frozen samples (green fluorescence) at both 6 and 24 h post-thaw, strongly suggesting that apoptosis had been initiated. In addition, positively stained cells also displayed morphological shrinkage, a further trait of apoptosis.53 Importantly, substantial caspase-3 or -7 activity was not observed in non-frozen control cells, suggesting that treatment with trypsin, centrifugation, and replating was not the cause of apoptosis in the cryopreserved samples. This finding is supported by guidelines by Galluzzi et al., who advocate using multiple, complementary assays to confirm cellular death, including assessment of long-term survival to detect delayed cell death, such as apoptosis.54
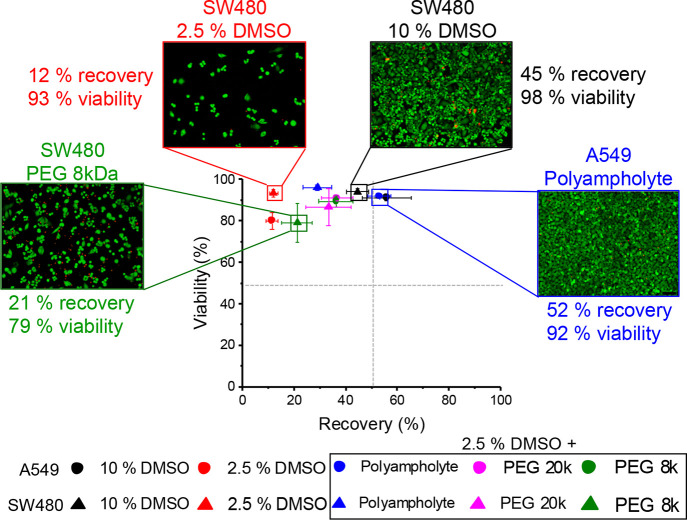
The data above provides conclusive evidence that short (or nonexistent) culture times post-thaw and only considering cell viability overestimates the potency of macromolecular cryoprotectants and can give the impression that a new material has cryoprotective function. To summarize this, we collated the averaged data and created a scatter plot, Figure 8. The bottom right quadrant (high recovery and no viability) is not possible to achieve (as recovery does not include non-viable cells). Most of the conditions tested here fell into the top left quadrant of high cell viability but low recoveries. Interestingly, only the polyampholytes enabled rescue of 2.5% (v/v) DMSO cryopreservation into the top-right quadrant of both high viability and recovery. Example images of different conditions using live/dead staining 24 h post-thaw are shown on the scatter plot. This shows, for example, how addition of 8 kDa PEG to 2.5% DMSO gives a minor increase in recovery but no benefit to viability, whereas 10% DMSO (current standard) performs well by both measures. The polyampholyte supplemented with 2.5% DMSO is the only macromolecular system which gives benefits in both measures and shows it matches or even outperforms compared to 10% DMSO cryopreservation. This highlights the necessity of performing cell counting and monitoring post-thaw outcomes to remove false positives from spreading in this emerging field.
Figure 8.
Scatter plot of total cell recovery against viability. Recovery (x axis) from trypan blue data and viability (y axis) from live/dead assay using calcein-AM and ethidium homodimer-1 24 h post-thaw. Circles are A549 cells; triangles are SW480 cells. All polymer samples contained 20 mg·mL–1 of the polymer and 2.5% DMSO. Each point is the mean of 8–9 repeats, and all error bars show ± SEM.
Conclusions
Here, we present how measuring cryopreservation outcomes can generate false positives when only cell viability is considered. We highlight that measuring cell viability alone leads to overestimation of the activity of some macromolecular materials. We demonstrated that using net recovery of viable cells provides a much more robust measure of cryoprotective outcome. Further, analysis of both cell viability and recovery over a 24 h period demonstrated that post-thaw culture time is essential to allow apoptosis to progress, which is crucial to account for delayed cryoinjury and confirm that remaining cells are healthy (which in a biomedical context is essential). Using PEG as a negative control, we showed that standard viability measurements can suggest significant increases in post-thaw outcomes, but when compared to the total number of cells, it is clear that PEG enhances cryopreservation in only a few cases. Combining the results in a 2-D analysis, it was observed that polyampholytes do indeed enhance post-thaw outcomes across all measures and that PEG can give false positives with high viability values yet low net cell recoveries. These results will help guide the discovery of macromolecular cryoprotectants, remove the large potential for false positives, and help provide critical comparisons of new and emerging biomaterials.
Acknowledgments
M.I.G. thanks the ERC for StG (638661) and PoC (789182) and the Royal Society for an Industry Fellowship (191037). We thank the Wellcome Trust-Warwick Quantitative Biomedicine Programme for a seed grant (Institutional Strategic Support Fund 105627/Z/14/Z) and MRC for partial support of K.M. (MC_PC_17203).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biomac.0c00591.
Additional methods, average cell recovery and viability, nonfrozen cell viability, and apoptosis (PDF)
The authors declare the following competing financial interest(s): MIG is a named inventor on a patent application involving material used in this article.
Notes
Background data is available at wrap.warwick.ac.uk.
Supplementary Material
References
- Geraghty R. J.; Capes-Davis A.; Davis J. M.; Downward J.; Freshney R. I.; Knezevic I.; Lovell-Badge R.; Masters J. R.; Meredith J.; Stacey G. N.; Thraves P.; Vias M. Guidelines for the Use of Cell Lines in Biomedical Research. Br. J. Cancer 2014, 111 (6), 1021–1046. 10.1038/bjc.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender E. Cell Based-Therapy: Cells on Trial. Nature 2016, 540 (7634), S106–S108. 10.1038/540S106a. [DOI] [PubMed] [Google Scholar]
- Fischbach M. A.; Bluestone J. A.; Lim W. A. Cell-Based Therapeutics: The next Pillar of Medicine. Sci. Transl. Med. 2013, 5 (179), 179ps7. 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. D.; Wang S.; Fuller B. J. Cryoprotectants: A Review of the Actions and Applications of Cryoprotective Solutes That Modulate Cell Recovery from Ultra-Low Temperatures. Cryobiology 2017, 76, 74–91. 10.1016/j.cryobiol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Syme R.; Bewick M.; Stewart D.; Porter K.; Chadderton T.; Glück S. The Role of Depletion of Dimethyl Sulfoxide before Autografting: On Hematologic Recovery, Side Effects, and Toxicity. Biol. Blood Marrow Transplant. 2004, 10 (2), 135–141. 10.1016/j.bbmt.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Verheijen M.; Lienhard M.; Schrooders Y.; Clayton O.; Nudischer R.; Boerno S.; Timmermann B.; Selevsek N.; Schlapbach R.; Gmuender H.; Gotta S.; Geraedts J.; Herwig R.; Kleinjans J.; Caiment F. DMSO Induces Drastic Changes in Human Cellular Processes and Epigenetic Landscape in Vitro. Sci. Rep. 2019, 9 (1), 4641–4653. 10.1038/s41598-019-40660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm M.; Saaby L.; Moesby L.; Hansen E. W. Considerations Regarding Use of Solvents in in Vitro Cell Based Assays. Cytotechnology 2013, 65 (5), 887–894. 10.1007/s10616-012-9530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockbank K.; Taylor M. Tissue Preservation. Adv. biopreservation 2006, 5 (3), 157–196. 10.1201/9781420004229.ch8. [DOI] [Google Scholar]
- Stubbs C.; Bailey T. L.; Murray K.; Gibson M. I. Polyampholytes as Emerging Macromolecular Cryoprotectants. Biomacromolecules 2020, 21 (1), 7–17. 10.1021/acs.biomac.9b01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S.; Matsusita M.; Morita T.; Kamachi H.; Tsukiyama S.; Furukawa Y.; Koshida S.; Tachibana Y.; Nishimura S.-I.; Todo S. Effects of Synthetic Antifreeze Glycoprotein Analogue on Islet Cell Survival and Function during Cryopreservation. Cryobiology 2006, 52 (1), 90–98. 10.1016/j.cryobiol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Hyon S. H. Polyampholytes as Low Toxic Efficient Cryoprotective Agents with Antifreeze Protein Properties. Biomaterials 2009, 30 (27), 4842–4849. 10.1016/j.biomaterials.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Capicciotti C. J.; Malay D.; Ben R. N.. Ice Recrystallization Inhibitors: From Biological Antifreeze to Small Molecules. Recent Developments in the Study of Recrystallization; InTech, 2013; pp 177–224. [Google Scholar]
- Rothschild L. J.; Mancinelli R. L. Life in Extreme Environments. Nature 2001, 409, 1092–1101. 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- Pikuta E. V.; Hoover R. B.; Tang J. Microbial Extremophiles at the Limits of Life. Crit. Rev. Microbiol. 2007, 33 (3), 183–209. 10.1080/10408410701451948. [DOI] [PubMed] [Google Scholar]
- Voets I. K. From Ice-Binding Proteins to Bio-Inspired Antifreeze Materials. Soft Matter 2017, 13 (28), 4808–4823. 10.1039/C6SM02867E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. L. Ice-Binding Proteins: A Remarkable Diversity of Structures for Stopping and Starting Ice Growth. Trends Biochem. Sci. 2014, 39 (11), 548–555. 10.1016/j.tibs.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Mazur P. Freezing of Living Cells: Mechanisms and Implications. Am. J. Physiol. Physiol. 1984, 247 (3), C125–C142. 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- Capicciotti C. J.; Kurach J. D. R.; Turner T. R.; Mancini R. S.; Acker J. P.; Ben R. N. Small Molecule Ice Recrystallization Inhibitors Enable Freezing of Human Red Blood Cells with Reduced Glycerol Concentrations. Sci. Rep. 2015, 5, 9692. 10.1038/srep09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs C. I.; Stubbs C.; Graham B.; Fayter A. E. R.; Hasan M.; Gibson M. I. Mimicking the Ice Recrystallization Activity of Biological Antifreezes. When Is a New Polymer “Active”?. Macromol. Biosci. 2019, 1900082, 1900082. 10.1002/mabi.201900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs C. I.; Bailey T. L.; Graham B.; Stubbs C.; Fayter A.; Gibson M. I. Polymer Mimics of Biomacromolecular Antifreezes. Nat. Commun. 2017, 8 (1), 1546. 10.1038/s41467-017-01421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller R. C.; Pessin J. E.; Vatish M.; Mitchell D. A.; Gibson M. I. Enhanced Non-Vitreous Cryopreservation of Immortalized and Primary Cells by Ice-Growth Inhibiting Polymers. Biomater. Sci. 2016, 4 (7), 1079–1084. 10.1039/C6BM00129G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller R. C.; Vatish M.; Mitchell D. A.; Gibson M. I. Synthetic Polymers Enable Non-Vitreous Cellular Cryopreservation by Reducing Ice Crystal Growth during Thawing. Nat. Commun. 2014, 5, 1–7. 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E.; Fayter A. E. R.; Deller R. C.; Hasan M.; Gutierrez-Marcos J.; Gibson M. I. Ice-Recrystallization Inhibiting Polymers Protect Proteins against Freeze-Stress and Enable Glycerol-Free Cryostorage. Mater. Horiz. 2019, 6 (2), 364–368. 10.1039/C8MH00727F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.; Bailey T. L.; Healey J. R. J.; Marcellini M.; Deville S.; Gibson M. I. Polyproline as a Minimal Antifreeze Protein Mimic That Enhances the Cryopreservation of Cell Monolayers. Angew. Chem., Int. Ed. 2017, 56 (50), 15941–15944. 10.1002/anie.201706703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q.; Zhao L.; Liu Z.; Liu T.; Qu J.; Zhang X.; Li R.; Yan L.; Yan J.; Jin S.; Wang J.; Qiao J. Bioinspired l -Proline Oligomers for the Cryopreservation of Oocytes via Controlling Ice Growth. ACS Appl. Mater. Interfaces 2020, 12 (16), 18352–18362. 10.1021/acsami.0c02719. [DOI] [PubMed] [Google Scholar]
- Capicciotti C. J.; Leclère M.; Perras F. A.; Bryce D. L.; Paulin H.; Harden J.; Liu Y.; Ben R. N. Potent Inhibition of Ice Recrystallization by Low Molecular Weight Carbohydrate-Based Surfactants and Hydrogelators. Chem. Sci. 2012, 3 (5), 1408–1416. 10.1039/c2sc00885h. [DOI] [Google Scholar]
- Geng H.; Liu X.; Shi G.; Bai G.; Ma J.; Chen J.; Wu Z.; Song Y.; Fang H.; Wang J. Graphene Oxide Restricts Growth and Recrystallization of Ice Crystals. Angew. Chem., Int. Ed. 2017, 56 (4), 997–1001. 10.1002/anie.201609230. [DOI] [PubMed] [Google Scholar]
- Stubbs C.; Lipecki J.; Gibson M. I. Regioregular Alternating Polyampholytes Have Enhanced Biomimetic Ice Recrystallization Activity Compared to Random Copolymers and the Role of Side Chain versus Main Chain Hydrophobicity. Biomacromolecules 2017, 18 (1), 295–302. 10.1021/acs.biomac.6b01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R.; Hayashi F.; Nagashima T.; Matsumura K. Toward a Molecular Understanding of the Mechanism of Cryopreservation by Polyampholytes: Cell Membrane Interactions and Hydrophobicity. Biomacromolecules 2016, 17 (5), 1882–1893. 10.1021/acs.biomac.6b00343. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Hayashi F.; Nagashima T.; Hyon S. H. Long-Term Cryopreservation of Human Mesenchymal Stem Cells Using Carboxylated Poly-l-Lysine without the Addition of Proteins or Dimethyl Sulfoxide. J. Biomater. Sci., Polym. Ed. 2013, 24 (12), 1484–1497. 10.1080/09205063.2013.771318. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Bae J. Y.; Kim H. H.; Hyon S. H. Effective Vitrification of Human Induced Pluripotent Stem Cells Using Carboxylated ε-Poly-l-Lysine. Cryobiology 2011, 63 (2), 76–83. 10.1016/j.cryobiol.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Bailey T. L.; Stubbs C.; Murray K.; Tomás R. M. F.; Otten L.; Gibson M. I. Synthetically Scalable Poly(Ampholyte) Which Dramatically Enhances Cellular Cryopreservation. Biomacromolecules 2019, 20 (8), 3104–3114. 10.1021/acs.biomac.9b00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H.; Kohaya N.; Kamoshita M.; Fujiwara K.; Matsumura K.; Hyon S. H.; Ito J.; Kashiwazaki N. Efficient Production of Live Offspring from Mouse Oocytes Vitrified with a Novel Cryoprotective Agent, Carboxylated ε-Poly-L-Lysine. PLoS One 2013, 8 (12), e83613. 10.1371/journal.pone.0083613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs C.; Murray K. A.; Ishibe T.; Mathers R. T.; Gibson M. I. Combinatorial Biomaterials Discovery Strategy to Identify New Macromolecular Cryoprotectants. ACS Macro Lett. 2020, 9, 290–294. 10.1021/acsmacrolett.0c00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M.; Rajan R.; Hyon S. H.; Matsumura K. Hydrogelation of Dextran-Based Polyampholytes with Cryoprotective Properties via Click Chemistry. Biomater. Sci. 2014, 2 (3), 308–317. 10.1039/C3BM60261C. [DOI] [PubMed] [Google Scholar]
- Rajan R.; Jain M.; Matsumura K. Cryoprotective Properties of Completely Synthetic Polyampholytes via Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization and the Effects of Hydrophobicity. J. Biomater. Sci., Polym. Ed. 2013, 24 (15), 1767–1780. 10.1080/09205063.2013.801703. [DOI] [PubMed] [Google Scholar]
- Rajan R.; Kazuaki M. Preparation of Novel Synthetic Cryoprotectants. Cryobiol. Cryotechnol. 2014, 60 (2), 99–103. [Google Scholar]
- Zhao J.; Johnson M. A.; Fisher R.; Burke N. A. D.; Stöver H. D. H. Synthetic Polyampholytes as Macromolecular Cryoprotective Agents. Langmuir 2019, 35 (5), 1807–1817. 10.1021/acs.langmuir.8b01602. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Kawamoto K.; Takeuchi M.; Yoshimura S.; Tanaka D.; Hyon S.-H. H. Cryopreservation of a Two-Dimensional Monolayer Using a Slow Vitrification Method with Polyampholyte to Inhibit Ice Crystal Formation. ACS Biomater. Sci. Eng. 2016, 2 (6), 1023–1029. 10.1021/acsbiomaterials.6b00150. [DOI] [PubMed] [Google Scholar]
- Sharp D. M. C.; Picken A.; Morris T. J.; Hewitt C. J.; Coopman K.; Slater N. K. H. Amphipathic Polymer-Mediated Uptake of Trehalose for Dimethyl Sulfoxide-Free Human Cell Cryopreservation. Cryobiology 2013, 67 (3), 305–311. 10.1016/j.cryobiol.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Cai N.; Zhai H.; Zhang J.; Zhu Y.; Zhang L. Natural Zwitterionic Betaine Enables Cells to Survive Ultrarapid Cryopreservation. Sci. Rep. 2016, 6 (1), 37458. 10.1038/srep37458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado S. A.; Slater N. K. H. Increased Cryosurvival of Osteosarcoma Cells Using an Amphipathic PH-Responsive Polymer for Trehalose Uptake. Cryobiology 2016, 73 (2), 175–180. 10.1016/j.cryobiol.2016.08.002. [DOI] [PubMed] [Google Scholar]
- ATCC . A549 (ATCC® CCL-185TM); American Type Culture Collections; https://www.lgcstandards-atcc.org/products/all/CCL-185.aspx?geo_country=gb (accessed Feb 18, 2020). [Google Scholar]
- Congdon T.; Notman R.; Gibson M. I. Antifreeze (Glyco)Protein Mimetic Behavior of Poly(Vinyl Alcohol): Detailed Structure Ice Recrystallization Inhibition Activity Study. Biomacromolecules 2013, 14 (5), 1578–1586. 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- Xiang J.; Wan C.; Guo R.; Guo D. Is Hydrogen Peroxide a Suitable Apoptosis Inducer for All Cell Types?. BioMed Res. Int. 2016, 2016, 1–6. 10.1155/2016/7343965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baust J. M.; Vogel M. J.; Van Buskirk R.; Baust J. G. A Molecular Basis of Cryopreservation Failure and Its Modulation to Improve Cell Survival. Cell Transplant. 2001, 10 (7), 561–571. 10.3727/000000001783986413. [DOI] [PubMed] [Google Scholar]
- Matsushita T.; Yagi T.; Hardin J. A.; Cragun J. D.; Crow F. W.; Bergen H. R.; Gores G. J.; Nyberg S. L. Apoptotic Cell Death and Function of Cryopreserved Porcine Hepatocytes in a Bioartificial Liver. Cell Transplant. 2003, 12 (2), 109–121. 10.3727/000000003108746696. [DOI] [PubMed] [Google Scholar]
- Xu X.; Cowley S.; Flaim C. J.; James W.; Seymour L.; Cui Z. The Roles of Apoptotic Pathways in the Low Recovery Rate after Cryopreservation of Dissociated Human Embryonic Stem Cells. Biotechnol. Prog. 2010, 26 (3), 827–837. 10.1002/btpr.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T.; Hardin J. A.; Valenzuela Y. M.; Miyoshi H.; Gores G. J.; Nyberg S. L. Caspase Inhibition Reduces Apoptotic Death of Cryopreserved Porcine Hepatocytes. Hepatology 2001, 33 (6), 1432–1440. 10.1053/jhep.2001.24560. [DOI] [PubMed] [Google Scholar]
- Paasch U.; Sharma R. K.; Gupta A. K.; Grunewald S.; Mascha E. J.; Thomas A. J.; Glander H.-J.; Agarwal A. Cryopreservation and Thawing Is Associated with Varying Extent of Activation of Apoptotic Machinery in Subsets of Ejaculated Human Spermatozoa1. Biol. Reprod. 2004, 71 (6), 1828–1837. 10.1095/biolreprod.103.025627. [DOI] [PubMed] [Google Scholar]
- Heng B. C.; Ye C. P.; Liu H.; Toh W. S.; Rufaihah A. J.; Yang Z.; Bay B. H.; Ge Z.; Ouyang H. W.; Lee E. H.; Cao T. Loss of Viability during Freeze-Thaw of Intact and Adherent Human Embryonic Stem Cells with Conventional Slow-Cooling Protocols Is Predominantly Due to Apoptosis Rather than Cellular Necrosis. J. Biomed. Sci. 2006, 13 (3), 433–445. 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- Riedl S. J.; Shi Y. Molecular Mechanisms of Caspase Regulation during Apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5 (11), 897–907. 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Kerr J F R; Wyllie A H; Currie A R Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Human Disease. Br. J. Cancer 1972, 26 (4), 239–257. 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L; Aaronson S A; Abrams J; Alnemri E S; Andrews D W; Baehrecke E H; Bazan N G; Blagosklonny M V; Blomgren K; Borner C; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Cell Death in Higher Eukaryotes. Cell Death Differ. 2009, 16 (8), 1093–1107. 10.1038/cdd.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.