Abstract
Riparian spiders are being used increasingly to track spatial patterns of contaminants in and fluxing from aquatic ecosystems. However, our understanding of the circumstances under which spiders are effective sentinels of aquatic pollution is limited. The present study tests the hypothesis that riparian spiders may be effectively used to track spatial patterns of sediment pollution by polychlorinated biphenyls (PCBs) in aquatic ecosystems with high habitat heterogeneity. The spatial pattern of ΣPCB concentrations in 2 common families of riparian spiders sampled in 2011 to 2013 generally tracked spatial variation in sediment ΣPCBs across all sites within the Manistique River Great Lakes Area of Concern (AOC), a rivermouth ecosystem located on the south shore of the Upper Peninsula, Manistique (MI, USA) that includes harbor, river, backwater, and lake habitats. Sediment ΣPCB concentrations normalized for total organic carbon explained 41% of the variation in lipid-normalized spider ΣPCB concentrations across 11 sites. Furthermore, 2 common riparian spider taxa (Araneidae and Tetragnathidae) were highly correlated (r2 > 0.78) and had similar mean ΣPCB concentrations when averaged across all years. The results indicate that riparian spiders may be useful sentinels of relative PCB availability to aquatic and riparian food webs in heterogeneous aquatic ecosystems like rivermouths where habitat and contaminant variability may make the use of aquatic taxa less effective. Furthermore, the present approach appears robust to heterogeneity in shoreline development and riparian vegetation that support different families of large web-building spiders. Environ Toxicol Chem 2017;36:1278–1286. Published 2016 Wiley Periodicals, Inc. on behalf of SETAC. This article is a US government work and, as such, is in the public domain in the United States of America.
INTRODUCTION
Understanding patterns and drivers of contaminant uptake by biological organisms is an important goal of biomonitoring and a requisite starting point for evaluating remedy effectiveness in impacted aquatic environments 1, 2. So-called sentinel organisms (also called biomonitors) that accumulate pollutants in their tissues without large toxic effects can be used to track spatial patterns of contaminant bioavailability 3–5 if they meet certain requirements. Potential sentinel organisms should be ubiquitous, abundant, and easily sampled, and they should also predictably accumulate contaminants of interest 3, 5. However, naturally occurring aquatic taxa that meet these requirements can be rare, especially in ecosystems that encompass a diverse array of habitats like large rivermouths and estuaries 6 where it can be difficult to find taxa that are abundant throughout. In such cases, riparian consumers of aquatic prey may prove useful alternatives to aquatic sentinel organisms because their distributions may be less likely to be constrained by variation in aquatic habitat 7–9, while individuals may reasonably represent local aquatic conditions (e.g., some spiders on webs 2). Furthermore, using riparian taxa to monitor aquatic pollution can provide the additional benefit of indicating potential impacts of aquatic contaminants on terrestrial ecosystems (e.g., spiders 2, 6, 10, 11, nestling birds 12, 13, and turtle eggs 14), which can occur even at trace concentrations 15.
Rivermouth ecosystems, defined as the mixing zone that occurs at the confluence of riverine and lentic ecosystems 6, encompass a mosaic of habitats including upstream river valleys, depositional receiving basins, associated wetlands, and nearshore lake regions that are important for regional biota, fish production, recreation, storm protection, sediment regulation, and aesthetics 6. Despite, or perhaps because of, their ecological and economic importance, these systems are also often hotspots of contaminant bioaccumulation 6. For example, in the Great Lakes region of North America, cities and industrial development are concentrated near rivermouths, which also receive polluted water and sediment from upstream. As a result, most of the International Joint Commission–designated Areas of Concern (AOCs) in the Great Lakes region are in rivermouths 16, 17. The AOCs are locations that have experienced environmental degradation, primarily in the form of contamination of water, sediments, and associated ecosystems by toxic chemicals such as metals and polychlorinated biphenyls (PCBs) 17. Polychlorinated biphenyls are bioaccumulative, persistent organic contaminants that can magnify up the food chain and have cellular, reproductive, and population-level impacts on higher order predators 9, 13, 18–21. Thus, understanding contaminant uptake into biota in rivermouth ecosystems is important for their effective management, but identifying relevant sentinels is challenging because of high aquatic habitat heterogeneity and contaminant patchiness. Challenges related to this heterogeneity can include 1) mismatches between the size of the contaminated area and home ranges of other aquatic species, such as resident fishes; 2) difficulties in finding aquatic species that occur across different habitat types; and 3) difficulties in sampling aquatic organisms in deep water (e.g., >5 m deep).
Sentinels have a long history of being used to monitor uptake of aquatic contaminants in polluted ecosystems. Bivalves, especially mussels, are some of the most commonly used aquatic taxa in biomonitoring (e.g., Mussel Watch programs 22) because of their global distribution and their tendency to accumulate a suite of contaminants from the environment 23–25. Fish and some non-mollusk aquatic invertebrates are also commonly used as sentinels 5, 26, 27. Despite the importance of these groups for monitoring contaminant uptake in biota, each one has potential drawbacks when used to estimate spatial patterns of contaminant concentrations across habitat types. For example, mussels can grow on hard and soft substrates, but are not widely distributed in some habitats (e.g., tidal flats and soft sediments experiencing high disturbance rates 25, 28). Macroinvertebrate distribution is also often reflective of habitat type 29, 30, although some are widely distributed (e.g., chironomid midges 31, 32) and can track environmental concentrations of some contaminants 33, 34. Fish, on the other hand, are less restricted by substrate and habitat type, but their mobility can make pinpointing sources of contaminant uptake difficult (unless they are caged, which presents other limitations 35).
Riparian consumers can provide useful and complementary information regarding aquatic contaminant uptake and availability to biota in aquatic and nearby terrestrial ecosystems 14, 19, 36–38. Riparian orb-weaving spiders in particular have been increasingly used in this capacity because they can consume mostly aquatic prey (45–100% of the diet for horizontal web-builders in small streams and rivers 39–41), are relatively sedentary, are easily sampled, may represent an exposure pathway for higher order predators, and are found in riparian areas adjacent to multiple aquatic habitats on natural and man-made riparian structures 11, 38, 42. Specifically, concentrations of some metals, nonmetal elements, and organic contaminants in riparian spiders that rely heavily on aquatic insect prey have been found to reflect aquatic pollution levels in or contaminant flux from streams, rivers, and ponds 2, 10, 43, 44. Riparian sentinels may be particularly effective at tracking some mainly softer metals (Hg, Se, Cd, As) and relatively hydrophobic organic contaminants (log octanol–water coefficient [KOW] of ∼6–8) such as PCBs because these chemicals biomagnify in consumers and are retained in the bodies of adult insect prey through metamorphosis 10, 43, 45–47.
The Manistique River and Harbor (MI, USA) AOC (hereafter referred to as the Manistique River AOC) is a US Environmental Protection Agency (USEPA) listed rivermouth ecosystem adjacent to Lake Michigan. The site, contaminated with high levels of PCBs from industrial sources, includes approximately 2.8 km of the Manistique River and Harbor as well as adjacent backwater habitats. In the present study we tested the hypothesis that riparian spiders may be used to track spatial patterns of sediment pollution by PCBs throughout the Manistique River AOC to determine whether spiders may be employed as sentinels of aquatic contamination and contaminant flux to terrestrial food webs in aquatic ecosystems with high habitat heterogeneity (i.e., rivermouth ecosystems). Specifically, we compared PCB contamination of sediments from 11 sites within and adjacent to the AOC across 4 habitat types (river channel, backwater, harbor, and lake shore) with tissue concentrations in 2 families of riparian spider over 3 yr (2011–2013).
METHODS
Study area background
The Manistique area has a long history of industrial pollution dating back to the mid-19th century when sawmills deposited an estimated 4.5 × 106 t of sawdust and woodchips into waters around Manistique 48. In 1959, Manistique Papers began de-inking wastepaper at the site, leaching PCBs into the sediment and waters surrounding the mill 49. Despite a number of remediation actions (e.g., dredging 49), areas of high sediment PCB concentrations are still patchily distributed throughout the AOC. Concentrations of PCBs in fish tissues are also higher than those at nearby reference sites 50. Because of ongoing PCB contamination, 2 beneficial use impairments remain in effect as of 2016: restriction on fish consumption and restriction on dredging 17.
Study area background
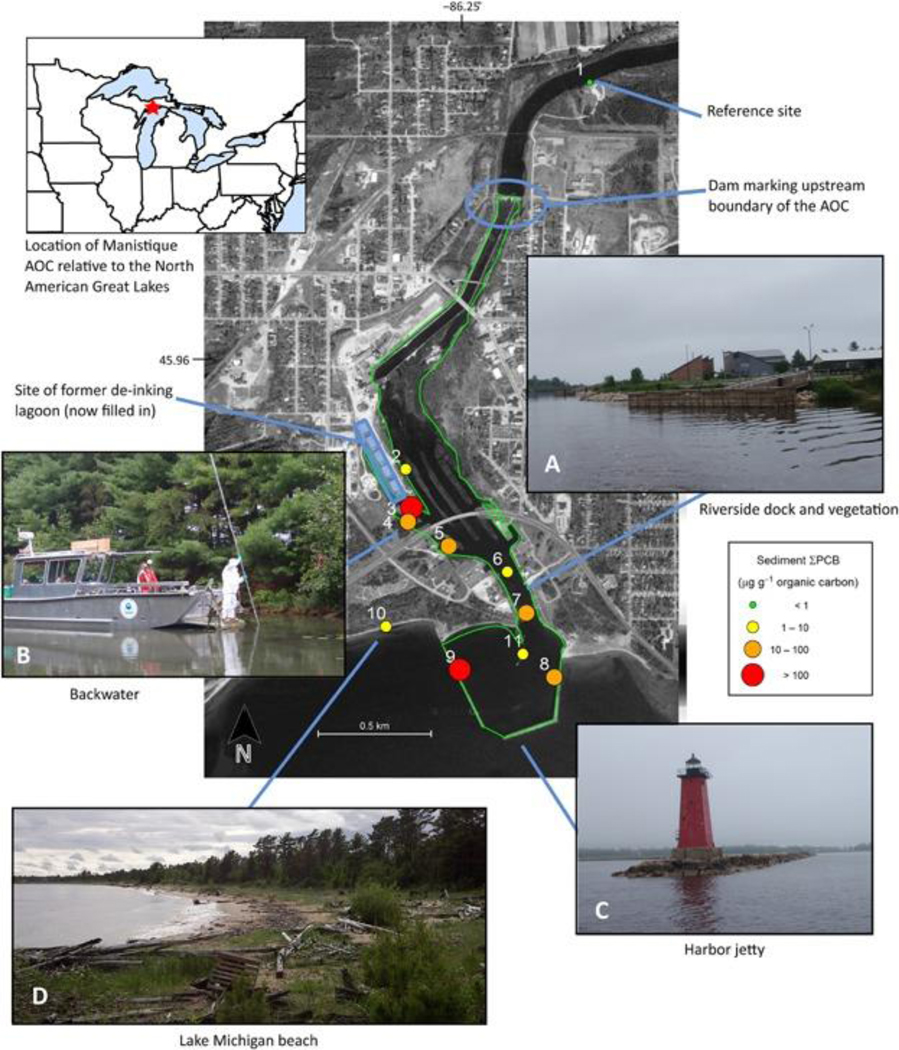
To test the effectiveness of riparian spiders as sentinels of AOC-wide sediment pollution, we sampled surface sediment and riparian spiders from 11 sites within and around the Manistique River AOC during the early fall of 2011 and summer of 2012 and 2013 (20–23 September 2011, 12–18 July 2012, and 23–28 July 2013; Figure 1 and Supplemental Data, Table S2). Sites were selected to characterize the wide range of physical habitat conditions, water chemistry, and PCB contamination levels present in the AOC. Sites included river sites (sites 2, 6, and 7) along the mainstem, backwater sites (sites 3, 4, and 5) known to have ongoing high and patchy sediment PCB concentrations 50, and harbor sites (sites 8 and 9) on the eastern and western edges of the harbor and on 1 of the jetties at the river mouth (site 11). One lake site (site 10) was located on the shore of Lake Michigan just west of the harbor outside the AOC boundaries in an area that historically received sawdust from the river, and a reference site (site 1) was located just outside of the AOC in the Manistique River approximately 2.8 km upstream of the mouth above a dam. The backwater sites were the most distinct biogeochemically (Supplemental Data, Table S3 and Figure S1). Riparian habitats at the sites ranged from a mixture of vegetation and developed structures such as docks (river sites) to mature trees and dense vegetation (backwater sites), large boulders making up the constructed harbor walls (harbor sites), and sandy beach/dune grass (lake site; Figure 1).
Figure 1.
Manistique River and Harbor Area of Concern (AOC). Map background shows an overview of the study area at the mouth of the Manistique River, with the locations of sampling sites and other features of interest; sampling sites are shown as colored symbols representing the annual site mean total organic carbon-normalized total polychlorinated biphenyl (ΣPCB) concentration in sediments averaged over 3 yr of sampling (2011–2013), that is, the grand means. The green outline indicates the designated AOC boundary. Photos show examples of the major riparian habitat types present in or near the AOC that were sampled in the present study: river (A), backwater (B), harbor (C), and lake shore (D). Map data: Google, Digital Globe, Landsat, USDA Farm Service Agency; software: Kahle and Wickham 70. Photo credit: D. Walters.
Sediment and spider sampling
To sample sediments and spiders at fairly narrow riverine sites (from ∼20–80 m wide), we established 1 transect running perpendicular to the river flow across the width of the channel and including the shoreline at each site (Supplemental Data, Figure S2). Transects were haphazardly placed within each habitat type, except for backwater sites, where transects were located in the middle of the site and 1 jetty where the entire length was sampled. We collected 4 sediment samples along each transect at 20%, 40%, 60%, and 80% of the transect width to capture environmental PCB exposure of potential insect prey to near-shore web-building spiders. We collected web-building spiders from 50-m shoreline zones upstream and downstream of the point where each transect intersected the right and left shorelines (n = 4 zones per site). At harbor and lake sites, which are relatively vast compared with riverine sites, the sampling design was similar except sediment transects ran for 200 m parallel to the shore. These transects were placed as close to the shoreline as possible to safely sample via boat (i.e., ∼10–100 m depending on site bathymetry), and this is the area of aquatic habitat that we expected would supply the majority of aquatic insects reaching shoreline spiders. Sediment samples from the reference site were collected only in 2011, and spider samples only in 2011 and 2012, because of high water and unsafe conditions in 2012 and 2013.
We collected surface sediments using a core sampler (Watermark Core Sampler, Forestry Suppliers) to at least 30-cm depth (core tube diameter = 6.8 cm). Only the top 15 cm of the core sample was retained, and a subsample was collected in a 250-mL USEPA-certified clean, amber glass jar with a Teflon-lined cap. Riparian spiders were sampled by hand at night using headlamps. Sampling was generally limited to habitats overhanging the water surface and up to 2 m inland from the shoreline; the exception was at the lake site (site 10) where sampling included an approximately 30-m swath of dune grass extending inland from the high water line because 1) this swath of habitat was relatively homogeneous and did not provide an obvious transition from water to shoreline vegetation that is typically seen along temperate rivers (e.g., forested riparian vegetation); 2) spider biomass was relatively low in beach grass, so we had to expand our sampling zone to collect sufficient spider biomass for chemical analysis; and 3) we observed aquatic insects (e.g., midges) throughout the beach grass habitats, giving us confidence that the spiders living within this habitat could be exposed to sediment contamination via consumption of adult aquatic insects, similar to those at river sites. Two families of orb-weaving spiders, Tetragnathidae and Araneidae, were collected within each zone at each site.
Tetragnathids found in riparian zones specialize in the consumption of aquatic insects that they catch in weak, horizontal orb webs 51–53. Araneids are generally distributed from riparian to upland habitats and build stronger, vertical orb webs in which they catch both aquatic and terrestrial insects 51, 54. We collected 4 replicate spider samples (i.e., ∼20 individual spiders from each of 4 zones) of each taxon at each site; variable spider abundance reduced this number in some years/sites (Supplemental Data, Figure S3). Sediment and spider samples were immediately placed on ice, transferred to a – 20 °C freezer at the end of each sampling day, and then shipped overnight for analysis.
Chemical analysis of sediments and tissues
Sediment and tissue (spider) samples were extracted and analyzed for PCB congeners, which were summed to give a measure of total PCB (∑PCB). Briefly, analysis was by gas chromatography/mass spectrometry, following USEPA methods 1668A 55 and SW846 8270D 56. Logistical constraints required moving to a new laboratory for chemical analysis after 2011. High-resolution mass spectrometry (ALS Environmental) was used to analyze 2011 samples, while low-resolution mass spectrometry (Battelle Memorial Institute) was used to analyze 2012 to 2013 samples, resulting in some differences in detection limits and in numbers of congeners analyzed between 2011 versus 2012 to 2013 (Supplemental Data, Table S4). Nonetheless, concentrations measured in 2011 and 2012 to 2013, respectively, are generally comparable 57, 58. Lipids (as total extractable organics) in tissue samples were measured using the gravimetric method and reported as percentage of lipids on a wet weight basis. Low mass of tetragnathid samples in 2011 prevented lipid content analysis of these samples. Total organic carbon (TOC) content in sediment samples was analyzed according to USEPA SW846 method 9060A 59 and reported as percentage of carbon on a dry weight basis. Details of sample extraction, analysis, and quality control procedures are provided in the Supplemental Data. All ΣPCB concentrations are reported to 2 significant digits to reflect the relative precision of this analysis (12% average relative percentage difference in ΣPCB concentration between laboratory duplicate samples, n = 15 pairs), but raw data were used for statistical analyses.
Data analysis
The ΣPCB concentration was calculated as the sum of the concentrations of all measured congeners above the detection limit. This approach to censored data (i.e., substituting a concentration of 0 for nondetects) is commonly used by studies summing concentrations across a large number of analytes 60–62. We investigated several lines of evidence confirming that this treatment of nondetects was appropriate for our dataset (Supplemental Data). Although for a majority of samples, the influence of nondetect results was negligible, low biomass (<1 g) of some spider samples resulted in relatively high detection limits and fewer detections for these samples with the low-resolution mass spectrometry analysis used in 2012 to 2013 (Supplemental Data, Table S5). However, after controlling for site means, no evidence was uncovered to indicate that the ΣPCB of 2012 to 2013 spider samples was related to sample biomass (F1,108 = 1.08, p = 0.30; see Supplemental Data), suggesting that any influence of sample biomass on measured ΣPCB was weak relative to the importance of variation among sites. Linear regression analysis was used to investigate relationships among sediment and spider ΣPCB concentrations across sites; ΣPCB concentrations were log-transformed prior to regression analysis to improve normality and homogeneity of variance. All analyses were conducted using R statistical computing software 63. The significance level was set at a < 0.05. Normalizations (TOC-normalized and lipid-normalized ΣPCB concentrations for sediment and tissue samples, respectively) were applied to reduce variation in PCB concentrations that are due to variation in TOC and lipid content among samples rather than to environmental exposures themselves, and were always calculated for individual samples prior to calculating site means. Nondetects for ΣPCB in an individual sample were included as 0s in calculation of site means; nondetects for TOC were assigned a value of ½ the method detection limit (i.e., 0.01% organic carbon).
RESULTS
PCB contamination in sediments
Averaged across 3 yr of sampling, grand mean ΣPCB concentrations measured in sediments at each site during the present study spanned 4 orders of magnitude, ranging from 0.87 ng g−1 dry weight at the reference site to 8200 ng g−1 at site 5 (Table 1). Within each sample year, annual site mean sediment ΣPCB concentrations at backwater sites 3 and 5 were consistently 1 to 3 orders of magnitude higher than at other sites (site 3: grand mean = 7300, annual range = 4900–11 000 ng g−1 dry wt; site 5: grand mean = 8200, annual range = 5800–9600 ng g−1 dry wt), with the exceptions of harbor site 9 and backwater site 4, which were within the same order of magnitude (Table 1 and Supplemental Data, Table S6 and Figure S3). The reference site had the lowest mean ΣPCB sediment concentration (0.87 ng g−1 dry wt), and low ΣPCB concentrations (typically < 20 ng g−1 dry wt) were also found at lake site 10 and river sites 2 and 6 (Table 1 and Supplemental Data, Table S6 and Figure S3). Within sites, the concentrations of individual sediment samples often differed by as much as 1 to 3 orders of magnitude (sites 3–9; Supplemental Data, Table S5).
Table 1.
Grand means ± standard deviation, (n) at each sampling site of total polychlorinated biphenyl (ΣPCB) concentration, % total organic carbon (TOC), and ΣPCB concentration normalized to TOC content in sediment samples from Manistique River Area of Concern across 2011, 2012, and 2013a
| Sediment | |||
|---|---|---|---|
| Site | ΣPCB (ng g−1 dry wt) | % TOC | ΣPCBTOC (ng g−1 organic carbon) |
| 1 | 0.87, (1) | 0.72, (1) | 200, (1) |
| 2 | 10 ± 5.6, (3) | 0.21 ± 0.12, (3) | 6300 ± 4800, (3) |
| 3 | 7300 ± 3300, (3) | 8.0 ± 1.7, (3) | 140 000 ± 100 000, (3) |
| 4 | 1100 ± 350, (3) | 6.9 ± 1.2, (3) | 17 000 ± 6600, (3) |
| 5 | 8200 ± 2100, (3) | 12 ± 1.2, (3) | 75 000 ± 23 000, (3) |
| 6 | 27 ± 36, (3) | 0.65 ± 0.69, (3) | 3900 ± 1800, (3) |
| 7 | 150 ± 220, (3) | 0.11 ± 0.018, (3) | 78 000 ± 93 000, (3) |
| 8 | 130 ± 100, (3) | 0.35 ± 0.15, (3) | 35 000 ± 37 000, (3) |
| 9 | 2300 ± 2200, (3) | 2.9 ± 1.0, (3) | 100 000 ± 120 000, (3) |
| 10 | 8.2 ± 6.0, (3) | 0.26 ± 0.075, (3) | 2500 ± 1100, (3) |
| 11 | 39 ± 16, (2) | 0.56 ± 0.38, (2) | 6200 ± 200, (2) |
Reported to 2 significant digits.
Mean percentage of TOC in sediment was consistently highest (>5%) at the 3 backwater sites where plant detritus and sawdust/wood chips inputs dominate. Harbor site 9 was also high (>2% organic carbon); elsewhere, TOC was almost always <1% (Table 1 and Supplemental Data, Table S6). Spatial patterns in in TOC-normalized ΣPCB concentrations in sediment were generally similar to those for dry weight concentrations, with the highest ΣPCBTOC levels in backwater and harbor sites, although normalization decreased the ΣPCB sediment concentrations of backwater site 5 (high TOC) and increased them for harbor sites 7 and 8 (low TOC) as well as 9 (moderate TOC) relative to the other sites (Table 1 and Figure 1). The TOC-normalized ΣPCB concentration at the reference site (200 ng g−1 organic carbon in 2011) was almost 1 order of magnitude less than the next lowest site (1500 ng g−1 organic carbon at site 10 in 2011).
PCB contamination in riparian spiders
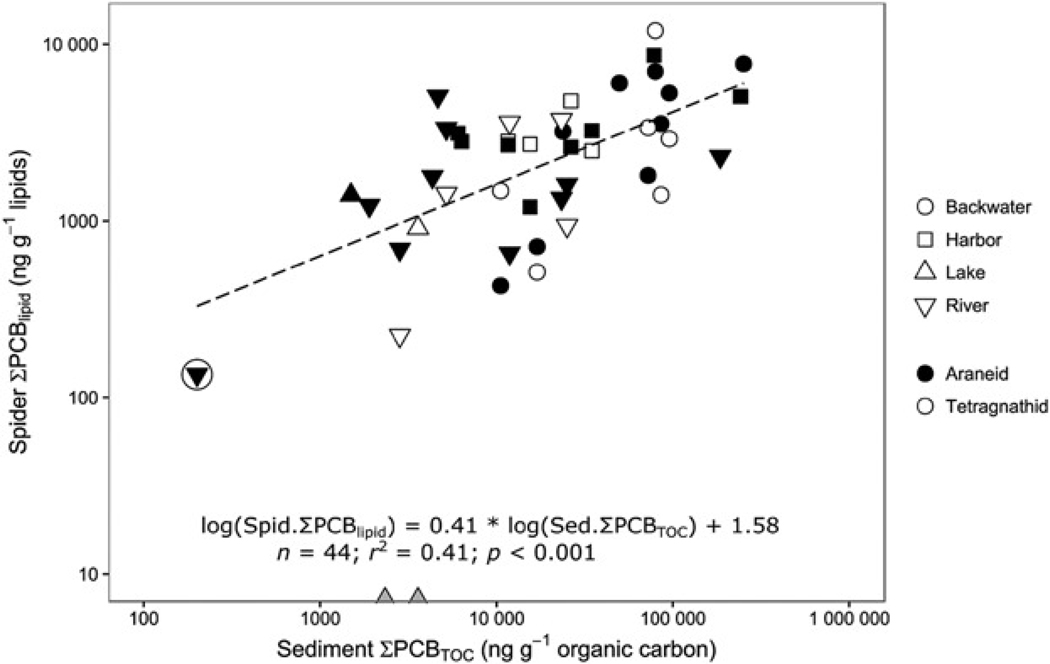
Grand mean ΣPCB concentrations measured in riparian spiders at each site ranged from 3.0 ng g−1 in araneids at the reference site (site 1) to 270 ng g−1 wet weight in tetragnathids at backwater site 5 (Table 2 and Supplemental Data, Tables S7 and S8, and Figure S3). Within sites, measured concentrations of individual samples were generally within 1 order of magnitude, with the exceptions of site 4 (range = 4.2–200 ng g−1) and site 8 (range = 37–550 ng g−1; Supplemental Data, Table S5; sites 1, 2, 4, and 6 all had at least 1 nondetected value for spider samples). Within each sample year, the variation in annual mean ΣPCB concentrations among sites was lower for spider samples (mean coefficient of variation [CV] = 0.82) than for sediment samples (mean CV = 1.8), and patterns in relative concentration among sites were less consistent from year to year (Supplemental Data, Tables S7 and S8, and Figures S3 and S4); nonetheless, the spatial pattern of ΣPCB concentrations in spiders generally tracked spatial variation in sediment ΣPCB levels (Figure 2). Across all years, spider ΣPCB concentrations were significantly and positively related to sediment ΣPCB concentrations. The TOC-normalized sediment ΣPCB concentrations explained 41% of the variation in lipid-normalized spider ΣPCB concentrations (r 2 = 0.41, p < 0.001, Figure 2; for spider wet wt: r 2 = 0.36, p < 0.001, log(Spid.ΣPCBww) = 0.34 × log(Sed.ΣPCBTOC) + 0.50; not shown). Mean ΣPCB concentrations in both spider taxa tended to be relatively high (>100 ng g−1 wet wt) at backwater sites 5 and 3; averaged across the 3 yr of sampling, site 5 had the highest spider concentrations relative to all other sites (Table 2). However, spider ΣPCB concentrations at harbor sites 8 and 9 were on average as high as those found at the highly contaminated backwater sites (Supplemental Data, Figure S4). Concentrations at river sites (sites 2, 6, and 7) were typically lower than those in backwater and harbor sites, and the lowest concentrations were found at the reference site (site 1) and the lake site (site 10), which were located outside of the AOC borders (Table 2).
Table 2.
Grand means ± standard deviation, (n) at each sampling site of total polychlorinated biphenyl (ΣPCB) concentration, % lipids, and ΣPCB concentration normalized to lipid content in riparian spider samples from Manistique River Area of Concern across 2011, 2012, and 2013a
| Araneid | Tetragnathid | |||||
|---|---|---|---|---|---|---|
| Site | ΣPCB (ng g−1 wet wt) | % Lipid | ΣPCBlipid (ng g−1 lipid) | ΣPCB (ng g−1 wet wt) | % Lipid | ΣPCBlipid (ng g−1 lipid) |
| 1 | 3.0 ± 4.3, (2) | 2.9 ± 2.2, (2) | 68 ± 96, (2) | 7.5 ± 11, (2) | 3.8, (1) | 0, (1) |
| 2 | 46 ± 7.5, (3) | 5.5 ± 2.7, (3) | 1000 ± 640, (3) | 49 ± 38, (3) | 3.4 ± 1.7, (2) | 1900 ± 2400, (2) |
| 3 | 110 ± 63, (3) | 3.3 ± 0.84, (3) | 4400 ± 3100, (3) | 120 ± 72, (3) | 3.5 ± 0.71, (2) | 2400 ± 1400, (2) |
| 4 | 55 ± 50, (3) | 5.4 ± 3.0, (3) | 1500 ± 1500, (3) | 42 ± 33, (3) | 2.6 ± 0.87, (2) | 1000 ± 680, (2) |
| 5 | 250 ± 63, (3) | 4.8 ± 1.8, (3) | 6100 ± 860, (3) | 270 ± 230, (3) | 7.9 ± 2.7, (2) | 7400 ± 6400, (2) |
| 6 | 120 ± 41, (3) | 4.9 ± 1.6, (3) | 3200 ± 1900, (3) | 93 ± 62, (2) | 4.0, (1) | 1400, (1) |
| 7 | 83 ± 24, (3) | 5.1 ± 2.3, (3) | 1800 ± 500, (3) | 70 ± 28, (3) | 4.0 ± 1.5, (2) | 2300 ± 2000, (2) |
| 8 | 190 ± 160, (3) | 5.3 ± 1.6, (3) | 4200 ± 4000, (3) | 170 ± 40, (3) | 6.1 ± 0.59, (2) | 2800 ± 72, (2) |
| 9 | 200 ± 31, (3) | 5.8 ± 1.2, (3) | 3600 ± 1300, (3) | 200 ± 96, (3) | 5.8 ± 0.58, (2) | 3600 ± 1600, (2) |
| 10 | 29 ± 41, (2) | 4.5 ± 0.59, (2) | 700 ± 990, (2) | 33 ± 31, (3) | 6.6 ± 3.4, (2) | 460 ± 640, (2) |
| 11 | 160 ± 59, (2) | 5.5 ± 1.7, (2) | 3000 ± 230, (2) | — | — | — |
Reported to 2 significant digits; lipids not measured for tetragnathids in 2011.
Figure 2.
Relationship between annual site mean total organic carbon (TOC)-normalized total polychlorinated biphenyl (ΣPCB) concentrations in sediments and lipid-normalized ΣPCB concentrations in spiders, across all sampling sites and across all years (2011–2013). The plot shows raw data values (with log-scaled axes), but the regression equation describes the relationship for log-transformed data. An extra circle around a point symbol indicates data from the reference site (site 1). Annual site mean concentrations of zero (i.e., where no PCBs were detected in any sample from the site) are excluded from the analysis; these data points are shown as gray triangles resting on the axis. Spid. = spider; Sed. = sediment.
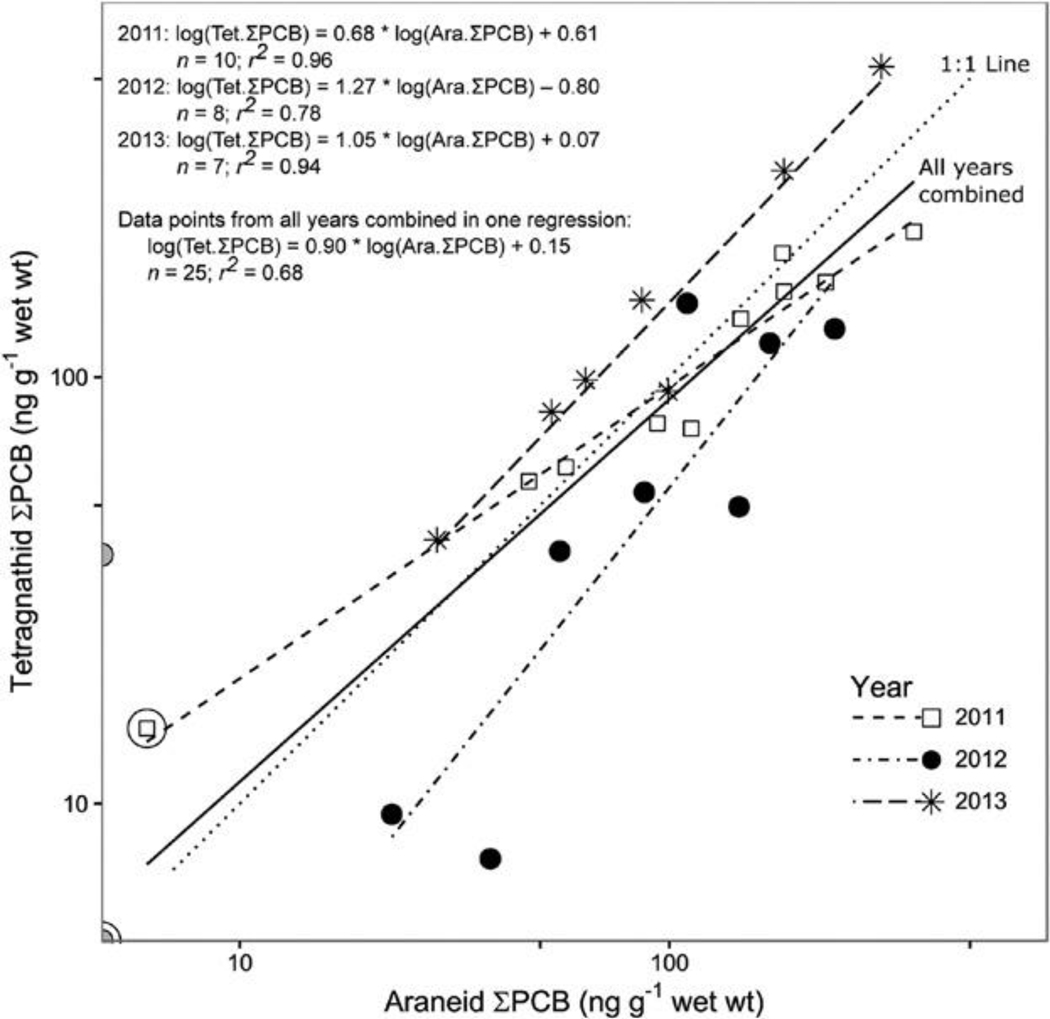
Annual site mean ΣPCB concentrations in araneids and in tetragnathids were highly correlated among sites within each year of sampling (r2 = 0.96, 0.78, and 0.94 in 2011, 2012, and 2013, respectively; all correlations were significant at p < 0.01; Figure 3). With data points from all years combined, the regression line (r2 = 0.67; p << 0.001) closely approaches 1:1 (95% confidence interval for regression slope [0.63, 1.2]; Figure 3).
Figure 3.
Relationships between annual site mean total polychlorinated biphenyl (ΣPCB) concentrations in 2 major riparian spider taxa across sites at Manistique River Area of Concern in 2011 to 2013. Note that although the plot shows raw data values on log-scaled axes, the regression equations describe relationships for log-transformed data. An equivalent figure with untransformed axes is provided in the Supplemental Data (Figure S5). For all regressions, p < 0.005. An extra circle around a point symbol indicates data from the reference site (site 1). Annual site mean concentrations of zero (i.e., where no PCBs were detected in any sample from the site) are excluded from the analysis; these data points are shown as gray circles resting on the axes. Tet = tetragnathid; Ara = araneid.
The mean lipid concentration of spider samples was fairly consistent, ranging from 1.4% (araneids at site 1 in 2012) to 9.8% (tetragnathids at site 5 in 2012), with no strong patterns in lipid concentration among sites or between families (Table 2; see also Supplemental Data, Tables S7 and S8). Spatial patterns in lipid-normalized ΣPCB data were very similar to those for wet-weight concentrations, although normalization increased the ΣPCB concentrations at site 3 in araneids (low percentage of lipid) relative to other sites.
DISCUSSION
Riparian spiders are increasingly being used to track spatial patterns of contaminant uptake in aquatic and nearby terrestrial ecosystems (Supplemental Data, Table S1). However, our understanding of situations in which spiders may be used as effective sentinels of aquatic pollution is limited. In the present study we found that riparian spiders may be good sentinels for reflecting spatial patterns of sediment PCB contamination in heterogeneous aquatic ecosystems where it can be difficult to find representative aquatic taxa that are abundant across all habitat types. Specifically, we found that the spatial pattern of ΣPCB concentration in spiders generally tracked spatial variation in sediment ΣPCBs among sites at the Manistique River AOC, and that 2 common riparian spider taxa (Araneidae and Tetragnathidae) had similar mean ΣPCB concentrations when averaged across all years. Harbor sites (8 and 9) showed the least congruence between mean spider and sediment PCB concentrations: PCB concentrations in spiders were high relative to sediments compared with other sites. Higher adult aquatic insect production from large lentic systems 64 could result in higher PCB transfer from sediment to spiders at these sites.
In river systems draining to marine or lake environments, both human development and sediment contamination are often concentrated near estuaries and rivermouths 6. These dynamic ecological systems are typically characterized by a complex mosaic of habitat types that vary in biogeochemistry, geomorphology, and shoreline habitats, often within a relatively small spatial area. These features complicate the use of aquatic sentinels for assessing risks from contamination. However, despite considerable heterogeneity in aquatic and riparian habitats, we found that riparian spiders performed reasonably well in identifying locations within the Manistique River AOC where exposures to fish and wildlife are likely to be highest. The spatial patterns in spider concentrations consistently reflected both highly contaminated hot spots and relatively uncontaminated sites in the sediment. However, the explanatory power of sediment concentration levels relative to spider concentrations was lower (41%) than has been demonstrated previously in other systems. For example, in the Twelve Mile Creek arm of Lake Hartwell (SC, USA), sediment ΣPCB concentrations explained 52% and 89% of the variation in lipid-normalized ΣPCB concentrations in araneids and tetragnathids, respectively. (One species of araneid analyzed separately [Mecynogea lemniscata] had a lipid-normalized r2 = 0.69 1). Furthermore, methyl mercury (MeHg) flux from small ponds in small adult aquatic insects was also strongly correlated (r 2 = 0.74) with MeHg concentrations in tetragnathids 44.
For riparian consumers to be used as sentinels of aquatic contamination, several conditions must be met: 1) changes in contaminant concentrations in water or sediment must be reflected by changes in concentrations in larval insects; 2) concentrations in larval insects must be reflected in adult aquatic insects; and 3) adult aquatic insects must be reflected in riparian consumers 2. In the case of the present study, the weaker statistical relationship between sediments and spiders at the Manistique River AOC compared with a lake ecosystem (Lake Hartwell) may be partially explained by a potential disconnect between local insect and sediment PCB concentrations as a result of relatively high spatial heterogeneity in sediment contamination levels (patchiness) within sites and possible dispersal of adult insects from areas of highly disparate ΣPCB sediment concentrations for some sites (e.g., harbor). Sediment concentrations at Lake Hartwell are more gradual (fairly predictable, gradual change in sediment concentrations along a longitudinal gradient), whereas sediment concentrations at Manistique are patchy and vary by orders of magnitude over relatively small spatial scales (10s of meters). Furthermore, variation in riparian habitat composition among sites in our system may have affected bioaccumulation of contaminants in some riparian spiders by altering the availability/adequacy of web-building structures, which presumably allow more efficient capture and assimilation of aquatic insect prey 7.
The riparian spiders we sampled were ubiquitous, inhabiting variable shoreline habitats that ranged from tree limbs to jetty boulders. However, there were some differences among the spider taxa in terms of their distribution: tetragnathids were more likely to be found on riparian vegetation overhanging or near the water, whereas araneids were more likely to be found on man-made structures such as bridges and bulkheads. Thus, collection of both taxa was required to achieve good spatial coverage throughout the Manistique River AOC. The combined use of both taxa as interchangeable sentinels was supported by their comparable contaminant concentrations and spatial patterns: ΣPCB concentrations measured across sites in araneid and tetragnathid spiders were closely correlated to each other within each year, with a relationship approaching 1:1 for all years together. However, comparing contaminant accumulation data from different taxa can be problematic 5, and araneids and tetragnathids do not always show a 1:1 relationship in terms of PCB accumulation, although they are still well correlated: Walters et al. 2 found that ΣPCB concentrations in araneids at Lake Hartwell were consistently approximately twice as high as tetragnathids, perhaps because of dietary differences. This variability among study sites suggests that the relationship between ΣPCB concentrations of araneids and tetragnathids may need to be evaluated for each site to determine comparability of these taxa for monitoring aquatic contamination. Further investigation into the physiological and ecological processes determining the extent of bioaccumulation in these spiders is needed.
In general, factors that affect riparian spider diet and contaminant burden in adult aquatic insect prey can independently and interactively alter the transfer of aquatic contaminants to riparian spiders. For example, ponds with fish showed lower aquatic insect emergence and Hg concentrations in spiders 44, but Hg transfer to riparian spiders by aquatic insects can also be affected by aquatic habitat characteristics (e.g., dissolved organic carbon content 65 and habitat type 66). Contaminant transfer can also be modulated by insect metamorphosis 10, 46 and distance of spiders from the shoreline 67–69. Even among web-building riparian spiders, aquatic insects in the diet can vary with web structure and seasonal changes in insect availability (e.g., 52–90% of the diet was aquatic for horizontal orb-weavers but <1–10% of the diet was found for vertical web-builders 40; similar numbers are seen in Akamatsu and Toda 39), although presumably for spiders on man-made structures near productive aquatic systems the aquatic contributions would be higher 42. Thus, using riparian organisms as sentinels of aquatic pollution requires consideration of these conditions and others, including spatial heterogeneity of contaminants, when assessing whether riparian spiders are a good choice of sentinel of aquatic pollutants in a given system.
In terms of direct threats to wildlife, the PCB concentrations reported in the present study are relatively low compared with another highly contaminated area, the Lake Hartwell Superfund site 2. With the exception of 1 of our sites (530 ng/g for tetragnathids at site 5, in 2013), in most sites, mean spider ∑PCB concentrations in the Manistique River AOC (<370 ng/g) were equivalent to or less than the lowest site mean values reported in riparian araneid and tetragnathids at Lake Hartwell (349 ng/g and 320 ng/g, respectively 2). However, 3 spider samples from our study did show ΣPCB concentrations that exceeded a modeled wildlife value for the protection of avian spider predators (>529 ng g−1 wet wt, for 12-d-old chickadees 2).
Management implications
The present study at the Manistique River AOC provides an example of how riparian spiders may be used to affirm and inform management decisions in contaminated rivermouth systems. First, our results confirm that the boundaries of the AOC encompass the most contaminated sites: ΣPCB concentrations in spider samples collected outside the borders of the AOC were relatively lower than within AOC sites, matching the pattern seen in sediment samples. Second, within the AOC boundaries, contamination levels in spiders identify hot spot locations of contamination risk for targeted remediation actions (such as capping or dredging 58), which are often costly. Although these locations are often identified based on bulk sediment data, spider concentrations reflect PCBs bioavailibility to both aquatic and terrestrial biota and may be more easily/inexpensively sampled than sediment or some aquatic taxa. Finally, concentrations detected in riparian spiders during the present study could serve as baseline data for monitoring the effectiveness of any future remedial actions. If such actions are successful at reducing the bioavailability of contaminants to fish and wildlife, this should be reflected in lower tissue concentrations of riparian spiders.
Supplementary Material
Acknowledgment
We thank A. Mucha and S. Cieniawski (US Environmental Protection Agency [USEPA], Great Lakes National Program Office) for contributing to different aspects of the present study, including study design, project management, and additional contractor support. The present study was supported by a USEPA Great Lakes Restoration Initiative (GLRI) grant to D.M. Walters and by the USEPA Office of Research and Development.
Footnotes
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3658.
Disclaimer
The present study was subjected to US Geological Survey and USEPA review and approved for publication. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Data availability
Data are available on request. Contact D. Walters at waltersd@usgs.gov
Contributor Information
Johanna M. Kraus, Fort Collins Science Center, US Geological Survey, Fort Collins, Colorado, USA
Polly P. Gibson, Fort Collins Science Center, US Geological Survey, Fort Collins, Colorado, USA.
David M. Walters, Fort Collins Science Center, US Geological Survey, Fort Collins, Colorado, USA
Marc A. Mills, National Risk Management Laboratory, US Environmental Protection Agency, Cincinnati, Ohio, USA
References
- 1.Pennuto CM, Smith M. 2015. From midges to spiders: Mercury biotransport in riparian zones near the Buffalo River Area of Concern (AOC), USA. Bull Environ Contam Toxicol 95: 701–706. [DOI] [PubMed] [Google Scholar]
- 2.Walters DM, Mills MA, Fritz KM, Raikow DF. 2010. Spider-mediated flux of PCBs from contaminated sediments to terrestrial ecosystems and potential risks to arachnivorous birds. Environ Sci Technol 44: 2849–2856. [DOI] [PubMed] [Google Scholar]
- 3.Beeby A. 2001. What do sentinels stand for? Environ Pollut 112: 285–298. [DOI] [PubMed] [Google Scholar]
- 4.Gourlay-Francé C, Tusseau-Vuillemin M-H. 2013. Bioavailability of contaminants In Férard J-F, Blaise C, eds, Encyclopedia of Aquatic Ecotoxicology. Springer Netherlands, Dordrecht, Netherlands, pp 181–190. [Google Scholar]
- 5.Rainbow PS, Phillips DJH. 1993. Cosmopolitan biomonitors of trace metals. Mar Pollut Bull 26: 593–601. [Google Scholar]
- 6.Larson JH, Trebitz AS, Steinman AD, Wiley MJ, Mazur MC, Pebbles V, Braun HA, Seelbach PW. 2013. Great Lakes rivermouth ecosystems: Scientific synthesis and management implications. J Gt Lakes Res 39: 513–524. [Google Scholar]
- 7.Alberts JM, Sullivan SMP. 2016. Factors influencing aquatic-to-terrestrial contaminant transport to terrestrial arthropod consumers in a multiuse river system. Environ Pollut 213: 53–62. [DOI] [PubMed] [Google Scholar]
- 8.Iwata T. 2007. Linking stream habitats and spider distribution: Spatial variations in trophic transfer across a forest-stream boundary. Ecol Res 22: 619–628. [Google Scholar]
- 9.Walters DM, Fritz KM, Johnson BR, Lazorchak JM, McCormick FH. 2008. Influence of trophic position and spatial location on polychlorinated biphenyl (PCB) bioaccumulation in a stream food web. Environ Sci Technol 42: 2316–2322. [DOI] [PubMed] [Google Scholar]
- 10.Kraus JM, Schmidt TS, Walters DM, Wanty RB, Zuellig RE, Wolf RE. 2014. Cross-ecosystem impacts of stream pollution reduce resource and contaminant flux to riparian food webs. Ecol Appl 24: 235–243. [DOI] [PubMed] [Google Scholar]
- 11.Speir SL, Chumchal MM, Drenner RW, Cocke WG, Lewis ME, Whitt HJ. 2014. Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environ Toxicol Chem 33: 2506–2509. [DOI] [PubMed] [Google Scholar]
- 12.Burger J, Gochfeld M. 2004. Marine birds as sentinels of environmental pollution. EcoHealth 1: 263–274. [Google Scholar]
- 13.Custer CM, Custer TW, Dummer PM, Munney KL. 2003. Exposure and effects of chemical contaminants on tree swallows nesting along the Housatonic River, Berkshire County, Massachusetts, USA, 1998–2000. Environ Toxicol Chem 22: 1605–1621. [PubMed] [Google Scholar]
- 14.De Solla SR, Fernie KJ, Letcher RJ, Chu SG, Drouillard KG, Shahmiri S. 2007. Snapping turtles (Chelydra serpentina) as bioindicators in Canadian Areas of Concern in the Great Lakes Basin. 1. Polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in eggs. Environ Sci Technol 41: 7252–7259. [DOI] [PubMed] [Google Scholar]
- 15.Rowse LM, Rodewald AD, Sullivan SMP. 2014. Pathways and consequences of contaminant flux to Acadian flycatchers (Empidonax virescens) in urbanizing landscapes of Ohio, USA. Sci Total Environ 485–486: 461–467. [DOI] [PubMed] [Google Scholar]
- 16.Environment and Climate Change Canada. 2015. Great Lakes Areas of Concern. Gatineau, QC: [cited 2016 February 14]. Available from: http://www.ec.gc.ca/raps-pas/ [Google Scholar]
- 17.US Environmental Protection Agency. 2016. Great Lakes Areas of Concern. Chicago, IL: [cited 2016 February 14]. Available from: http://www.epa.gov/great-lakes-aocs [Google Scholar]
- 18.Folland WR, Newsted JL, Fitzgerald SD, Fuchsman PC, Bradley PW, Kern J, Kannan K, Remington RE, Zwiernik MJ. 2016. Growth and reproductive effects from dietary exposure to Aroclor 1268 in mink (Neovison vison), a surrogate model for marine mammals. Environ Toxicol Chem 35: 604–618. [DOI] [PubMed] [Google Scholar]
- 19.Kay DP, Blankenship AL, Coady KK, Neigh AM, Zwiernik MJ, Millsap SD, Strause K, Park C, Bradley P, Newsted JL, Jones PD, Giesy JP. 2005. Differential accumulation of polychlorinated biphenyl congeners in the aquatic food web at the Kalamazoo River Superfund Site, Michigan. Environ Sci Technol 39: 5964–5974. [DOI] [PubMed] [Google Scholar]
- 20.Salice CJ, Rowe CL, Eisenreich KM. 2014. Integrative demographic modeling reveals population level impacts of PCB toxicity to juvenile snapping turtles. Environ Pollut 184: 154–160. [DOI] [PubMed] [Google Scholar]
- 21.Yadetie F, Karlsen O, Eide M, Hogstrand C, Goksøyr A. 2014. Liver transcriptome analysis of Atlantic cod (Gadus morhua) exposed to PCB 153 indicates effects on cell cycle regulation and lipid metabolism. BMC Genomics 15: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg ED. 1975. The mussel watch—A first step in global marine monitoring. Mar Pollut Bull 6: 111. [Google Scholar]
- 23.Boening DW. 1999. An evaluation of bivalves as biomonitors of heavy metals pollution in marine waters. Environ Monit Assess 55: 459–470. [Google Scholar]
- 24.Jernelov A. 1996. The international mussel watch: A global assessment of environmental levels of chemical contaminants. Sci Total Environ 188(Suppl): S37–S44. [DOI] [PubMed] [Google Scholar]
- 25.Ji J, Choi HJ, Ahn I-Y. 2006. Evaluation of Manila clam Ruditapes philippinarum as a sentinel species for metal pollution monitoring in estuarine tidal flats of Korea: Effects of size, sex, and spawning on baseline accumulation. Mar Pollut Bull 52: 447–453. [DOI] [PubMed] [Google Scholar]
- 26.Albalat A, Potrykus J, Pempkowiak J, Porte C. 2002. Assessment of organotin pollution along the Polish coast (Baltic Sea) by using mussels and fish as sentinel organisms. Chemosphere 47: 165–171. [DOI] [PubMed] [Google Scholar]
- 27.Fialkowski W, Klonowska-Olejnik M, Smith BD, Rainbow PS. 2003. Mayfly larvae (Baetis rhodani and B. vernus) as biomonitors of trace metal pollution in streams of a catchment draining a zinc and lead mining area of Upper Silesia, Poland. Environ Pollut 121: 253–267. [DOI] [PubMed] [Google Scholar]
- 28.Bially A, Macisaac HJ. 2000. Fouling mussels (Dreissena spp.) colonize soft sediments in Lake Erie and facilitate benthic invertebrates. Freshw Biol 43: 85–97. [Google Scholar]
- 29.Buss DF, Baptista DF, Nessimian JL, Egler M. 2004. Substrate specificity, environmental degradation and disturbance structuring macroinvertebrate assemblages in neotropical streams. Hydrobiologia 518: 179–188. [Google Scholar]
- 30.Richards C, Haro R, Johnson L, Host G. 1997. Catchment and reach-scale properties as indicators of macroinvertebrate species traits. Fresh. Biol 37: 219–230. [Google Scholar]
- 31.Rosenberg DM. 1992. Freshwater biomonitoring and Chironomidae. Netherland J Aquat Ecol 26: 101–122. [Google Scholar]
- 32.Pinder LCV. 1995. Biology of the eggs and first-instar larvae In Armitage PD, Cranston PS, Pinder LCV, eds, The Chironomidae: The Biology and Ecology of Non-Biting Midges. Chapman & Hall, London, UK, pp 87–106. [Google Scholar]
- 33.Lazorchak JM, Griffith MB, Mills M, Schubauer-Berigan J, McCormick F, Brenner R, Zeller C. 2015. Proof of concept for the use of macroinvertebrates as indicators of polychlorinated biphenyls (PCB) contamination in Lake Hartwell. Environ Toxicol Chem 34: 1277–1282. [DOI] [PubMed] [Google Scholar]
- 34.Klemm DJ, Lewis PA, Fulk F, Lazorchak JM. 1990. Macroinvertebrate field and laboratory methods for evaluating the biological integrity of surface waters. EPA/600/4-90/030. US Environmental Protection Agency, Cincinnati, OH, USA. [Google Scholar]
- 35.Lotufo GR, Burton GA, Rosen G, Fleeger JW. 2013. Assessing biological effects In Reible DD, ed, Process, Assessment and Remediation of Contaminated Sediment. Springer, New York, NY, USA, pp 131–176. [Google Scholar]
- 36.Alberts JM, Sullivan SMP, Kautza A. 2013. Riparian swallows as integrators of landscape change in a multiuse river system: Implications for aquatic-to-terrestrial transfers of contaminants. Sci Total Environ 463–464: 42–50. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan SMP, Rodewald AD. 2012. In a state of flux: The energetic pathways that move contaminants from aquatic to terrestrial environments. Environ Toxicol Chem 31: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 38.Walters DM, Fritz KM, Otter RR. 2008. The dark side of subsidies: Adult stream insects export organic contaminants to riparian predators. Ecol Appl 18: 1835–1841. [DOI] [PubMed] [Google Scholar]
- 39.Akamatsu F, Toda H. 2011. Aquatic subsidies transport anthropogenic nitrogen to riparian spiders. Environ Pollut 159: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 40.Kato C, Iwata T, Nakano S, Kishi D. 2003. Dynamics of aquatic insect flux affects distribution of riparian web-building spiders. Oikos 103: 113–120. [Google Scholar]
- 41.Krell B, Röder N, Link M, Gergs R, Entling MH, Schäfer RB. 2015. Aquatic prey subsidies to riparian spiders in a stream with different land use types. Limnol Ecol Manag Inland Waters 51: 1–7. [Google Scholar]
- 42.Greene A, Coddington JA, Breisch NL, De Roche DM, Pagac BB Jr. 2010. An immense concentration of orb-weaving spiders with communal webbing in a man-made structural habitat. Am Entomol 56: 147. [Google Scholar]
- 43.Otter RR, Hayden M, Mathews T, Fortner A, Bailey FC. 2013. The use of tetragnathid spiders as bioindicators of metal exposure at a coal ash spill site. Environ Toxicol Chem 32: 2065–2068. [DOI] [PubMed] [Google Scholar]
- 44.Tweedy BN, Drenner RW, Chumchal MM, Kennedy JH. 2013. Effects of fish on emergent insect-mediated flux of methyl mercury across a gradient of contamination. Environ Sci Technol 47: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 45.Beck ML, Hopkins WA, Jackson BP. 2014. Variation in riparian consumer diet composition and differential bioaccumulation by prey influence the risk of exposure to elements from a recently remediated fly ash spill. Environ Toxicol Chem 33: 2595–2608. [DOI] [PubMed] [Google Scholar]
- 46.Kraus JM, Walters DM, Wesner JS, Stricker CA, Schmidt TS, Zuellig RE. 2014. Metamorphosis alters contaminants and chemical tracers in insects: Implications for food webs. Environ Sci Technol 48: 10957–10965. [DOI] [PubMed] [Google Scholar]
- 47.Mogren CL, Walton WE, Parker DR, Trumble JT. 2013. Trophic transfer of arsenic from an aquatic insect to terrestrial insect predators. PLOS One 8: e67817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macalady DL, Wissler SR. 1981. Sawdust and wood chip deposits in near-shore Lake Michigan waters near Manistique, Michigan. National Oceanic and Atmospheric Administration, Rockville, MD, USA. [Google Scholar]
- 49.US Environmental Protection Agency. 2012. Historical document review and information summary: Manistique River Area of Concern, Manistique, MI. Washington, DC. [Google Scholar]
- 50.Engineering EA, Science, and Technology. 2013. Conceptual site model for the Manistique River Area of Concern, Schoolcraft County, Michigan. EA Project 6254305.0004; Hunt Valley, MD, USA. [Google Scholar]
- 51.Ballinger A, Lake PS. 2006. Energy and nutrient fluxes from rivers and streams into terrestrial food webs. Mar Freshw Res 57: 15–28. [Google Scholar]
- 52.Gillespie RG. 1987. The mechanism of habitat selection in the long-jawed orb-weaving spider Tetragnatha elongata (Araneae, Tetragnathidae). J Arachnol 15: 81–90. [Google Scholar]
- 53.Williams DD, Ambrose LG, Browning LN. 1995. Trophic dynamics of two sympatric species of riparian spider (Araneae: Tetragnathidae). Can J Zool 73: 1545–1553. [Google Scholar]
- 54.Kato C, Iwata T, Wada E. 2004. Prey use by web-building spiders: Stable isotope analyses of trophic flow at a forest-stream ecotone. Ecol Res 19: 633–643. [Google Scholar]
- 55.US Environmental Protection Agency. 1999. Method 1668, Revision A: Chlorinated biphenyl congeners in water, soil, sediment, and tissue by HRGC/HRMS. Washington, DC: [cited 2015 November 2]. Available from: https://www.o2si.com/docs/epa-method-1668A.pdf [Google Scholar]
- 56.US Environmental Protection Agency. 1998. EPA Method 8270D (SW-846): Semivolatile organic compounds by gas chromatography/mass spectrometry (GC-MS). Washington, DC: [cited 2015 November 2]. Available from: http://www2.epa.gov/homeland-security-research/epa-method-8270d-sw-846-semivolatile-organic-compounds-gas [Google Scholar]
- 57.Gibson PP, Mills MA, Kraus JM, Walters DM. 2016. A modeling approach comparing ΣPCB concentrations between congener-specific analyses. Integr Environ Assess Manag DOI: 10.1002/ieam.1821 [DOI] [PubMed] [Google Scholar]
- 58.National Research Council. 2001. A Risk-Management Strategy for PCB-Contaminated Sediments. National Academies, Washington, DC. [Google Scholar]
- 59.US Environmental Protection Agency. 2004. EPA Method 9060A: Total organic carbon. Washington, DC: [cited 2015 December 16]. Available from: http://www3.epa.gov/epawaste/hazard/testmethods/sw846/pdfs/9060a.pdf [Google Scholar]
- 60.DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ. 2005. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res 98: 284–302. [DOI] [PubMed] [Google Scholar]
- 61.Ragland JM, Arendt MD, Kucklick JR, Keller JM. 2011. Persistent organic pollutants in blood plasma of satellite-tracked adult male loggerhead sea turtles (Caretta caretta). Environ Toxicol Chem 30: 1549–1556. [DOI] [PubMed] [Google Scholar]
- 62.Van Geest JL, Poirier DG, Solomon KR, Sibley PK. 2011. A comparison of the bioaccumulation potential of three freshwater organisms exposed to sediment-associated contaminants under laboratory conditions. Environ Toxicol Chem 30: 939–949. [DOI] [PubMed] [Google Scholar]
- 63.R Core Team. 2014. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria. [cited 2015 November 2]. Available from: http://www.R-project.org/ [Google Scholar]
- 64.Gratton C, Zanden MJV. 2009. Flux of aquatic insect productivity to land: Comparison of lentic and lotic ecosystems. Ecology 90: 2689–2699. [DOI] [PubMed] [Google Scholar]
- 65.Chaves-Ulloa R, Taylor BW, Broadley HJ, Cottingham KL, Baer NA, Weathers KC, Ewing HA, Chen CY. 2016. Dissolved organic carbon modulates mercury concentrations in insect subsidies from streams to terrestrial consumers. Ecol Appl 26: 1771–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gann GL, Powell CH, Chumchal MM, Drenner RW. 2015. Hg-contaminated terrestrial spiders pose a potential risk to songbirds at Caddo Lake (Texas/Louisiana, USA). Environ Toxicol Chem 34: 303–306. [DOI] [PubMed] [Google Scholar]
- 67.Akamatsu F, Toda H. 2011. Aquatic subsidies transport anthropogenic nitrogen to riparian spiders. Environ Pollut 159: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 68.Muehlbauer JD, Collins SF, Doyle MW, Tockner K. 2014. How wide is a stream? Spatial extent of the potential ‘stream signature’ in terrestrial food webs using meta-analysis. Ecology 95: 44–55. [DOI] [PubMed] [Google Scholar]
- 69.Raikow DF, Walters DM, Fritz KM, Mills MA. 2011. The distance that contaminated aquatic subsidies extend into lake riparian zones. Ecol Appl 21: 983–990. [DOI] [PubMed] [Google Scholar]
- 70.Kahle D, Wickham H. 2013. ggmap: Spatial visualization with ggplot2. R J 5: 144–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.