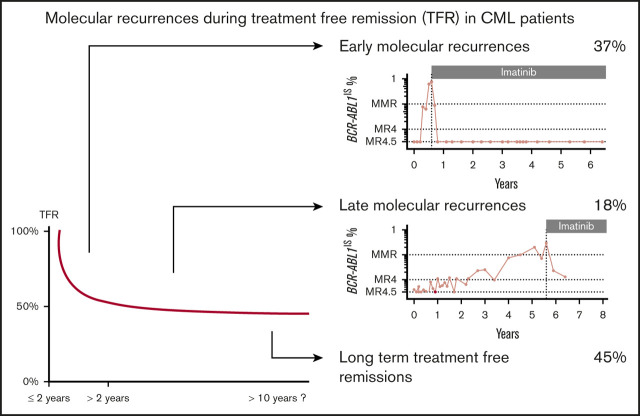
Key Points
Late molecular recurrences after 2 years in patients with CML experiencing TFR represent 14% of all molecular recurrences.
Patients with fluctuating minimal residual disease measurements during TFR are at high risk of late molecular recurrences.
Abstract
Treatment-free remission (TFR) is an opportunity for patients with chronic myeloid leukemia (CML). Reported cumulative incidence curves of molecular recurrence (MRec) arbor a 2-phase shape with mainly early events, but also some late events (late MRec [LMRec]). Having discontinued our first patient in 2004, we have access to a prolonged follow-up, enabling us to characterize these late events. Over 15 years, 128 patients from our institution were registered in the Stop Imatinib (STIM; A Study for Tyrosine Kinase Inhibitors Discontinuation [A-STIM]) trial. MRec was defined by the loss of major molecular response (BCR-ABL1IS >0.1%). At the first TFR attempt, patients had been taking a tyrosine kinase inhibitor for a median of 7.1 years and in BCR-ABL1IS ≤0.01% (MR4) for a median of 4 years. The median follow-up of patients in TFR was 6.5 years. The TFR rate was estimated to be 45.6% after 7 years. For 9/65 (14%) patients experiencing MRec, recurrence occurred after 2 years in TFR (median, 3.6 years). The residual rate of MRec after 2 years was estimated to be 18%. The probability of remaining in TFR was 65.4% for patients having experienced fluctuations of their minimal residual disease (MRD) (at least 2 consecutive measurements BCR-ABL1IS >0.0032% or loss of MR4), whereas it was 100% for those with stable MRD (P = .003). After 2 years in TFR, we observed an 18% residual rate of LMRec. These late events represent 14% of all MRec and occur in patients with fluctuating MRD measurements. A long-term molecular follow-up therefore remains mandatory for CML patients in TFR. The A-STIM study was registered at www.clinicaltrials.gov as #NCT02897245.
Visual Abstract
Introduction
Treatment-free remission (TFR) is becoming a realistic opportunity for patients with chronic myeloid leukemia (CML) treated with tyrosine kinase inhibitors (TKIs) and achieving a prolonged and continued deep molecular remission (DMR). Since the pioneer experience of the pilot Stop Imatinib (STIM)1 study, and following the report of the multicentric prospective STIM study,2,3 more and more patients have been offered to discontinue TKIs.4-8 Despite the absence of consensus regarding the entry criteria for a TFR attempt, recent studies have adopted the loss of major molecular response (MMR, BCR-ABL1IS ≤0.1%) as the criteria defining molecular recurrence (MRec) and triggering treatment resumption.8,9
In spite of different patient populations and heterogeneous criteria for MRec definition, all cumulative incidence curves of MRec arbor a similar 2-phase shape: (1) a majority of relapses within the first 12 to 18 months and (2) a “plateau” with few late events. However, the absence of long-term follow-up in most studies may limit capture of late events.
At our institution, the first discontinuation of imatinib in a STOP study was in June 2004. We took advantage of this prolonged follow-up to better characterize late MRec (late MRec [LMRec]) events by analyzing our cohort of patients registered in the A Study for Tyrosine Kinase Inhibitors Discontinuation (A-STIM) study (#NCT02897245).
Patients and methods
The A-STIM registry is an observational study of CML patients who discontinued TKIs intentionally after achieving a DMR (BCR-ABL1IS ≤0.01%). The registry was approved by the ethics committee “CPP ILE DE FRANCE XI” in October 2012 and registered as #NCT02897245 at www.clinicaltrials.gov. All patients gave their informed consent before participating in the study. All patients registered retrospectively or prospectively were aged 18 years or more, had a chronic phase CML, and had achieved a DMR when offered to intentionally discontinue TKI treatment. Patients could have attempted TFR more than once. Exclusion criteria included unintentional treatment discontinuation for whatever cause (adverse event, disease progression, pregnancy) and participation in a prospective STOP clinical trial. Patients with a previous history of resistance and those with mutations in the BCR-ABL1 tyrosine kinase domain were also excluded. Per study protocol, all patients had a homogeneous follow-up after TKI discontinuation: BCR-ABL1 transcripts were monitored monthly during the first 12 months, every 2 to 3 months during the second year, and every 3 to 6 months thereafter. BCR-ABL1 transcripts were quantified according to the laboratory recommendations of the European Treatment and Outcome Study for CML 2012 and 2015.10,11 From 2003 to 2012, the Standardized Europe against Cancer reverse transcription (RT) polymerase chain reaction (PCR) and quantitative RT-PCR protocols were used for quantification of BCR-ABL1 and ABL1 transcripts.12 All analytical series included BCR-ABL1 and ABL1 DNA standard dilutions titrating 105 to 10 copies (purchased from Ipsogen, Marseille, France). RNA samples were analyzed in duplicates for both targets. Analytical performance matched the following expectations: slopes for BCR-ABL1 and ABL1 standard curves ranging from −3.1 to −3.9 and positive amplification of the 10 copies dilution of BCR-ABL1 standard. The methods used to ensure the quality of individual RNA varied over time. From 2003 to 2005, a ΔCt of ≤3.3 between a given RNA sample and a reference value of 25.3 was retained to qualify RNA samples. This reference value of 25.3 corresponded to the median ABL1 Ct value obtained in our laboratory for nonleukemic samples and was considered to represent 18 000 ABL1 copies.13 After 2005, these criteria were replaced by the amount of ABL1 transcripts present in a given RNA sample. The minimal amount requested was fixed at 10 000 up to 2010 and was thereafter increased to 32 000 copies. Hence, we reasonably consider that our ability to score DMR has been at least compatible with the current MR4.0 status defined by Cross et al in 2003.10,11
The primary outcome measure was TFR without MRec. MRec was defined as the loss of MMR (ie, BCR-ABL1IS >0.1%) on 1 occasion and led to the resumption of TKI treatment. Patient characteristics were compared by nonparametric tests, either the Fisher's exact test for qualitative variables or the Kruskal-Wallis test for quantitative variables. The censored end points were estimated by the nonparametric Kaplan-Meier method in the absence of competing event (death).
Results
Patients characteristics
From June 2004 to December 2018, 128 consecutive patients were recorded in the A-STIM registry at our institution. During the same time period, 512 prevalent CML patients were followed-up for at least 3 years, resulting in an estimated 25% proportion of patients experiencing a first TFR attempt (TFR1). Median age at diagnosis was 48.2 years (range, 18.9-80.2), sex ratio (male/female) was 0.49, and Sokal score was low, intermediate, and high for 58%, 26%, and 16% of patients, respectively. Median treatment duration on TKI before TFR1 was 7.1 years. Thirty-eight patients (29.7%) were previously treated with interferon, 76 (61%) received imatinib (IM) alone, and 52 (39%) were on second-generation TKIs (2G-TKIs) at the time of discontinuation (14 as first-line therapy and 38 after a switch for suboptimal response or intolerance). All patients had achieved MR4 at the time of TKI discontinuation, and 84% of them had a stable MR4 (all consecutive BCR-ABL1 assessments ≤0.01% during the MR4 period). The median duration of MR4 was 4 years (range, 1.4-12.9). In 109 cases (85%), molecular response was deeper (BCR-ABL1IS ≤0.0032%, MR4.5), and 74 of them (67.8%) had a stable MR4.5. The median duration of MR4.5 was 2.9 years (range, 0.5-12.8) (Table 1).
Table 1.
Characteristics of the patients before the first TFR attempt
| Characteristics | Values |
|---|---|
| Demography | |
| Age, median (range), y, NS | 48.5 (18.9-80.2) |
| Sex ratio (M/F) | 0.49 |
| Disease characteristics | |
| Sokal score %, low/intermediate/high, NS | 58/26/16 |
| CML duration before TFR1, median (range), y, NS | 7.6 (3.3-28.5) |
| Therapy before TFR1 | |
| TKI duration, median (range), y, NS | 8.4 (3-18.8) |
| Ongoing TKI | |
| Imatinib, n (%) | 76 (61) |
| Including imatinib post-IFN, n | 25 |
| 2G TKI postimatinib, n (%) | 38 (28) |
| Including 2G TKI postimatinib and IFN, n | 12 |
| 2G TKI first line, n (%) | 14 (11) |
| Including 2G TKI first line plus IFN, n | 1 |
| Previous IFN (total) | 38 (29.7) |
| Response before TFR1 | |
| MR4, % | 100 |
| MR4 duration, median (range), y | 4 (1.4-12.9) |
| Stable MR4, % | 81 |
| MR4.5, % | 85 |
| MR4.5 duration, median (range), y | 2.9 (0.5-12.8) |
| Stable MR4.5, % | 67.2 |
F, female; IFN, interferon; M, male; MR4, BCR-ABL1IS ≤0.01%; MR4.5, BCR-ABL1IS ≤0.0032%; NS, values not significant.
First TFR attempt
Median follow-up in TFR1 was 6.5 years, with the longest duration of ongoing TFR1 being 14.9 years. TFR1 rates were 56.5% (95% confidence interval [CI], 47.4-64.6) at 1 year, 53% (95% CI, 43.9-61.3) at 3 years, 51.1% (95 CI, 42-59.5) at 5 years, and 45.6% (95% CI, 36.1-54.6) after 7 years (Figure 1A). Length of TKI treatment and duration of MR4 were associated with a higher TFR1 rate at 7 years in multivariate analysis. Sokal risk score and the specific TKI used (IM vs 2G-TKIs) did not influence TFR1 rate. A nonsignificant trend was observed in favor of patients previously exposed to interferon. Interestingly, the 14 patients treated first line with 2G-TKIs experienced a remarkable 68.2% TFR1 rate by 5 years, but this benefit was not statistically significant.
Figure 1.
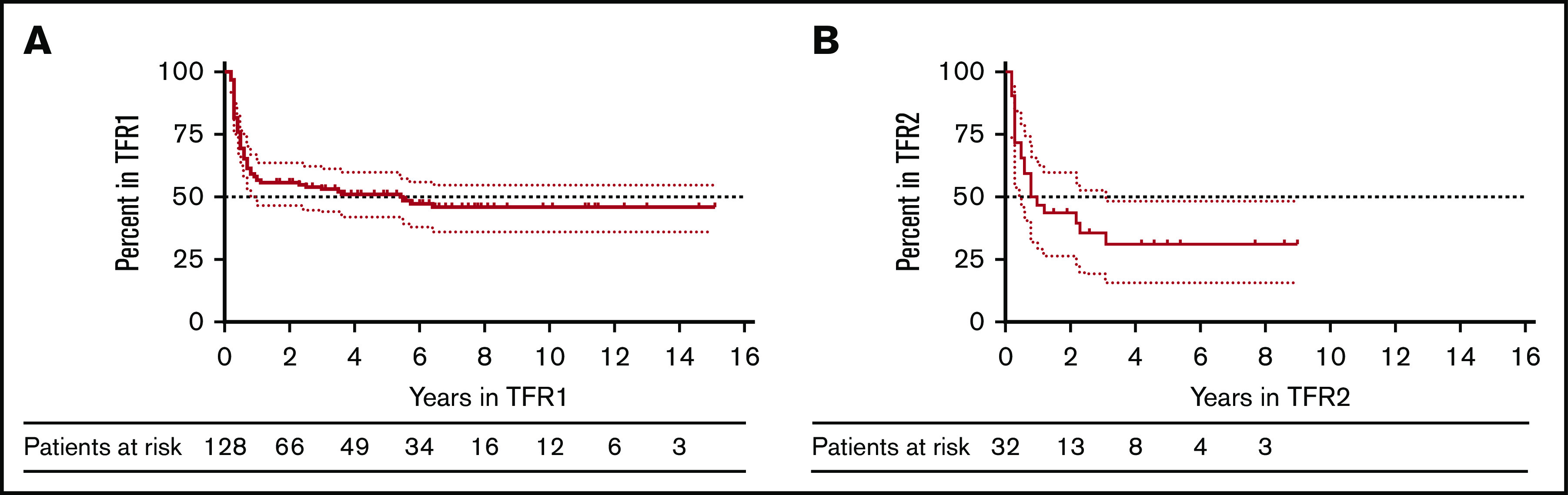
Molecular recurrence and treatment-free remission (TFR) after TKI discontinuation. (A) TFR after the first discontinuation attempt (TFR1) in 128 patients. (B) TFR after the second discontinuation attempt (TFR2) in 32 patients. The dotted lines indicate the upper and lower limits of the 95% CI for the estimated probability.
LMRec after TFR1
In accordance to the shape of our TFR1 curve showing a first plateau after 18 months in TFR1 (Figure 1A), we decided to define LMRec as MRec occurring after 2 years in TFR. Nine patients of 65 with an MRec (13.8%) experienced an LMRec at 2.3, 2.5, 3, 3.5, 3.6, 5.4, 5.5, 5.7, and 6.4 years (median time to LMRec, 3.6 years). We compared baseline characteristics (CML duration, Sokal score, gender, TKI duration, DMR duration, interferon exposure) in patients experiencing late (>2 years in TFR1) vs early (≤2 years in TFR1) MRec, and did not identify any significant difference between the 2 groups. To better characterize LMRec, we performed a landmark analysis after 2 years in TFR1 (median follow-up, 6.5 years). The probability of remaining in TFR1 on the long run for these 65 patients was 81.9% (95% CI, 67.6-90.3) (Figure 2A).
Figure 2.
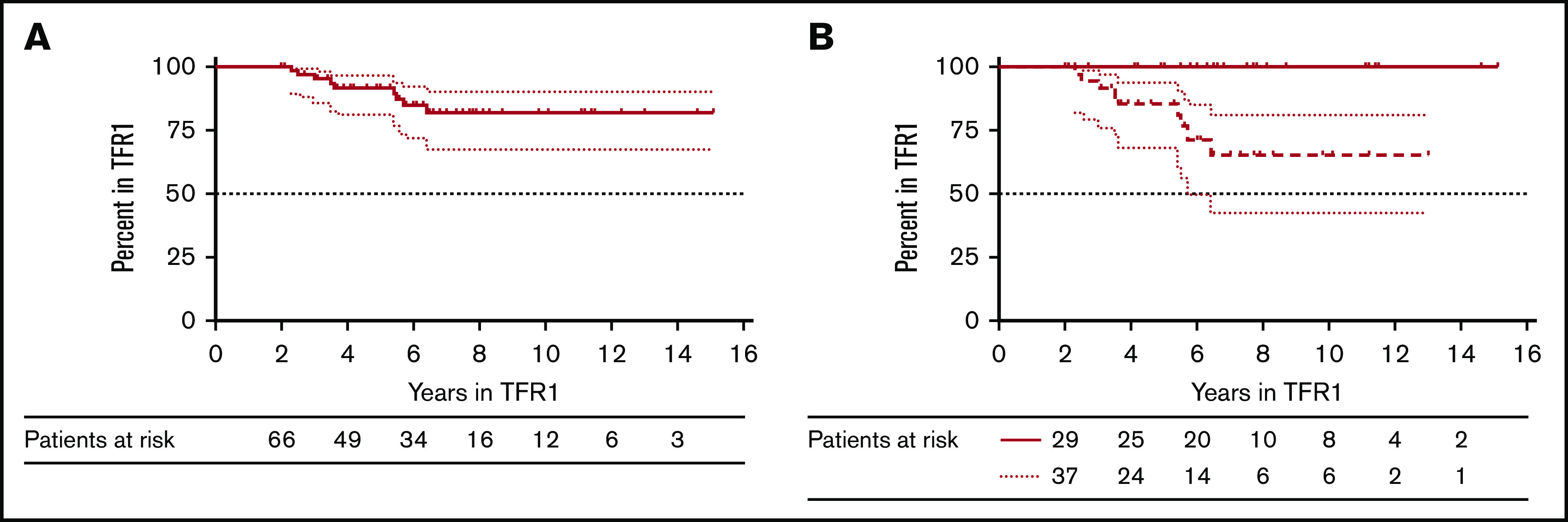
Landmark analysis at 2 years. (A) Treatment-free remission probabilities for the 66 patients in TFR1 for >2 years. (B) Treatment-free remission probabilities for the patients in TFR1 for >2 years and with a stable MR4.5 (BCR-ABL1 ≤0.0032%) (bold line) and treatment-free remission probabilities for the patients in TFR1 for >2 years and with an unstable molecular remission (dashed line).
We previously described TFR patients with fluctuating values of minimal residual disease (MRD) below the threshold of MMR. In accordance with our previous definition (at least 2 consecutive measurements >0.0032% and/or loss of MR4 on at least 1 occasion), these patients were categorized as cases with fluctuating molecular remission (FlucMR). All other patients were categorized as non-FlucMR. Using this definition, 55% of patients had FlucMR, whereas 45% did not. We then analyzed LMRec and found that the 9 late relapsing patients had unstable molecular remissions. The increase in MRD values was slow in patients experiencing LMRec as opposed to those experiencing early MRec (Figure 3). The resulting probabilities to remain in TFR1 on the long run for patients with FlucMR vs non-FlucMR patients were 100% and 65.4% (95% CI, 42.3-81), respectively (P = .003) (Figure 2B).
Figure 3.
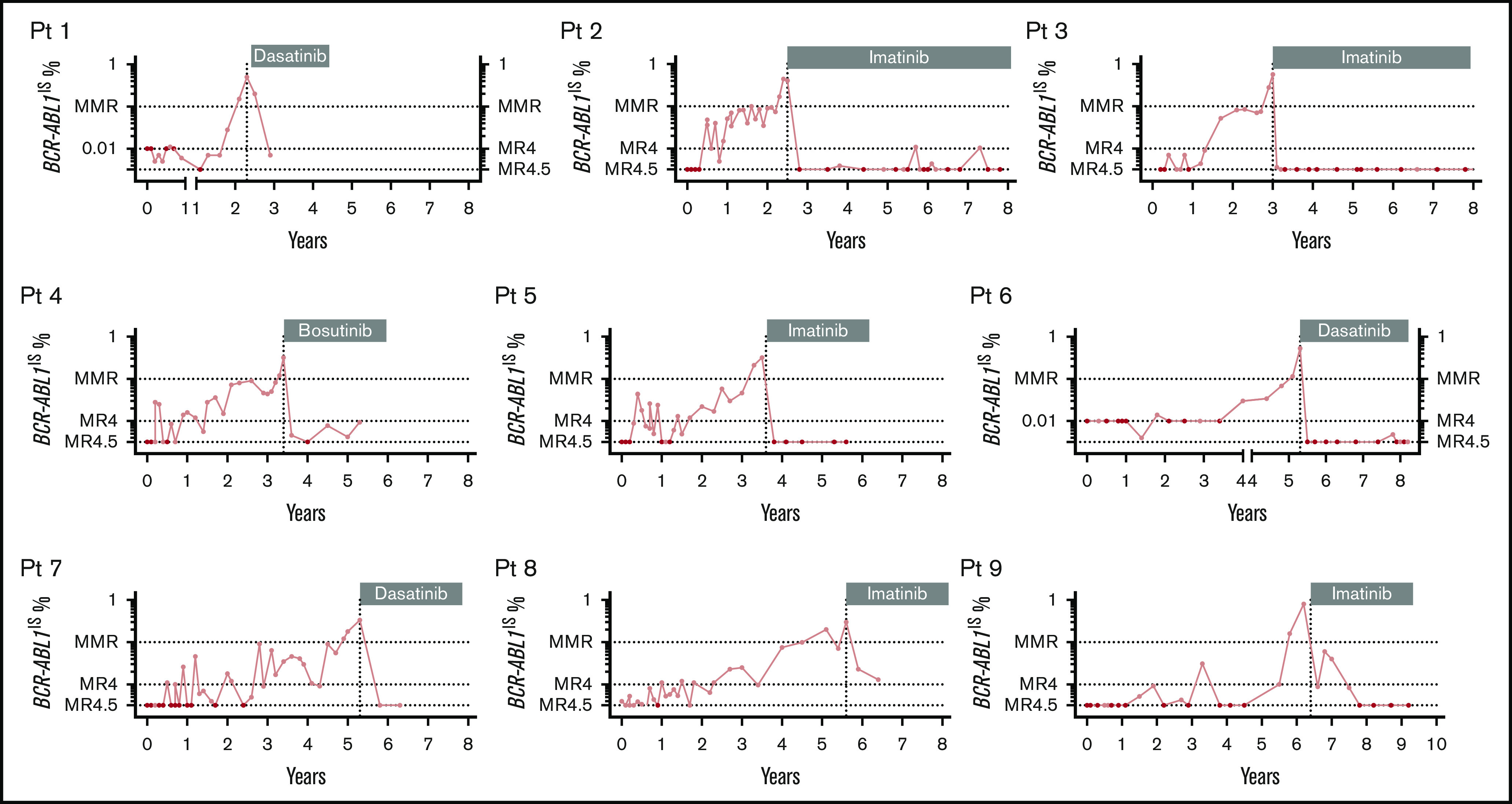
Molecular recurrence for the 9 patients experiencing a late MRec. Patient are classified based on the length of TFR1. Patients 1 and 6 started TFR 1 and 4 years before 2010, respectively. Sensitivity was fixed at 10 000 ABL1 copies to define PCR negativity in these early days (left x-segment and left y-axis). A sensitivity of 32 000 ABL1 copies to define PCR negativity was applied from 2010 (right x-segment and right y-axis). A sensitivity of 32 000 ABL1 copies was available for patients 2, 3, 4, 5, 7, 8, and 9. All patients experienced a slow kinetic of molecular recurrence. MR4, molecular response 4 log (BCR-ABL1IS ≤0.01%); MR4.5, molecular response 4.5 log (BCR-ABL1IS ≤0.0032%). Vertical dotted line: TKI resumption. Black circles indicate undetectable BCR-ABL1 transcripts.
Second TFR attempt and LMRec after the second TFR attempt
Of the 65 patients who restarted a TKI, 32 (49.2%) underwent a second TFR attempt (TFR2). The baseline characteristics of these patients were similar to those of the 33 patients who did not reattempt TFR (Table 2). The majority of patients attempting TFR2 had not changed TKI following failure of their first TFR attempt: 13/22 (59%) were still on IM, and 7/10 (70%) were on the same 2G-TKI. A smaller number of patients had changed TKI: 9 switched from IM to 2G-TKIs and 3 switched from 2G-TKIs to IM. Overall, 16 patients (50%) were on IM and 16 patients (50%) were on 2G-TKIs at the time of the second stop. The median duration of TKI treatment between TFR1 and TFR2 was 3.4 years (range, 1.4-6.8), and the total duration of TKI exposure before TFR2 was 9.4 years (range, 5-15.6). The median follow-up in TFR2 was 4.9 years, the longest ongoing TFR2 being 9 years. TFR2 rates were 46.8% (95% CI, 29.1-62.7) at 1 year, 35.8% (95% CI, 52.5-19.4) at 3 years, and 31.3% (95 CI, 15.6-48.4) at 5 years (Figure 1B). Total MR4 duration and longer TFR1 were both associated with an improved likelihood of successful TFR2 (supplemental Figure 1). There was a nonsignificant trend for a longer TFR2 in patients having switched from IM to 2G-TKIs (n = 9) after failure of TFR1 compared with those having resumed IM (n = 13). Median TFR2 duration was 0.8 years for IM-IM patients and was not reached for IM-2G-TKIs patients (supplemental Figure 2). Using the same criteria for LMRec (MRec after 2 years in TFR), the LMRec rate after the second stop was 14.3% (3/21 patients with MRec had an LMRec at 2.2; 2.3 and 3.1 years).
Table 2.
Characteristics of the patients before the second TFR attempt
| Patient characteristics | Patients accepting a TFR2, n = 32 | Patients not accepting a TFR2, n = 33 |
|---|---|---|
| Demography | ||
| Age, median (range), y, NS | 47.6 (18.9-77.5) | 51.3 (33.5-80.2) |
| Sex ratio (M/F) | 0.43 | 0.47 |
| Disease characteristics | ||
| Sokal score %, low/intermediate/high, NS | 44/28/28 | 55/30/15 |
| CML duration before TFR1, median, y, NS | 10.25 | 8.05 |
| Therapy before TFR1 | ||
| TKI duration, median (range), y, NS | 6 (2.2-12.5) | 7.9 (2.2-16) |
| Therapy before TFR2 | ||
| TKI duration post-TFR1, median (range), y | 3.4 (2.2-12.5) | |
| Total TKI duration (pre- and post-TFR1), median (range), y | 9.5 (5-15.6) | |
| TKI post-TFR1 | ||
| Imatinib, n (%) | 16 (50) | |
| 2G TKI, n (%) | 16 (50) | |
| Imatinib duration from end TFR1, median, y | 3.1 | |
| 2G TKI duration from end TFR1, median, y | 3.2 | |
| Response before TFR2 | ||
| MR4, % | 100 | |
| MR4 duration, median (range), y | 2.3 (0.8-5.8) | |
| Stable MR4, % | 100 | |
| MR4.5, % | 94 | |
| MR4.5 duration, median (range), y | 2.3 (0.6-5.6) | |
| Stable MR4.5, % | 66 |
NS, nonsignificant; TFR2, second attempt of treatment-free remission.
Discussion
Our prolonged follow-up of patients in TFR enabled us to demonstrate that 14% of the MRec occur after 2 years in TFR. The rate of these late events (LMRec) was the same after a first or a second stop attempt. We previously validated the loss of MMR as a trigger to restart TKIs after a TFR attempt,8 a criterion also used in the European Stop Tyrosine Kinase Inhibitor (EURO-SKI) study.9 This allowed us to follow patients in TFR with detectable long-term fluctuations of MRD below the MMR threshold. These FlucMR would have been missed in STOP studies using more stringent criteria to define MRec (ie, loss of MR5, MR4.5, or MR4).4-8 We found most low-level MRD positivity events to occur within the first 2 years of TFR, which probably explains why LMRec were not recorded in previous studies. Based on our definition of LMRec (molecular relapse after 2 years in TFR), no event was reported in the long-term follow-up of the STIM study (median TFR follow-up of 6.4 years).3 In contrast, based on the published TFR curve of the EURO-SKI study,9 in which molecular relapse was defined as loss of MMR, and despite a much shorter follow-up in TFR, we estimated roughly 10 LMRec events between months 24 and 36.
We were not able to identify any marker associated with LMRec, possibly because of the limited number of patients. We did however note that LMRec occurred in patients with FlucMR. TFR attempt eventually failed in approximately one-third of patients with FlucMR. Conversely, no late event was recorded in patients with a stable molecular remission. Practical consequences may be derived from these findings: molecular monitoring every 6 to 12 months is appropriate for stable molecular responders, whereas closer long-term assessments (every 3 months) are recommended for patients with FlucMR.
Our study has the limitation of being a monocentric study and did not provide answers to some of the burning questions in the field, such as the role of exposure to interferon, the added value of 2G-TKIs on successful TFR rate, and the benefit to restart with a 2G-TKI following TFR1 failure. Given our limited number of LMRec events, we were not able to show a benefit for patients receiving 2G-TKI, although we did observe a trend for a better TFR1 rate in patients treated first line with dasatinib or nilotinib. Previous studies have reported results supporting the notion that 2G-TKIs (mainly in second line) provide the same TFR curves as imatinib, and lead to similar or sometimes superior TFR outcomes.14-18 In the Nilotinib Treatment-free Remission Study in CML Patients (ENESTfreedom) study, 190 patients entered the TFR phase. The median duration of nilotinib treatment before stopping was 43.5 months. At 12 months after stopping nilotinib, 51.6% of patients remained in MMR or better.17 The recently published Dasatinib Discontinuation (DADI) study tested dasatinib first-line therapy in newly diagnosed CML patients. For the 58 patients who entered the maintenance phase and maintained a DMR for at least 1 year, the reported 6-month TFR rate was 55.2%.18 Large national or international studies are needed to reach the statistical power to provide reliable answers to these questions. There is no doubt that the long-term follow-up analysis of the EURO-SKI study will provide robust and mature data in the field.
We also report anecdotal (because of low numbers) LMRec in patients undergoing a TFR2 attempt, with a rate similar to the TFR1 setting. This suggests a similar mechanism underlying LMRec in the 2 situations. Our TFR2 rate of 31% at 5 years, and is in line with previous reports.19
The biology underlying LMRec is largely unknown. Our patients experiencing LMRec exhibited a very slow rise of the MRD values, suggesting that the cell of origin may be different compared with relapses occurring within the first 24 months. CML quiescent cells are insensitive to IM and to TKIs in general.20,21 We previously reported that patients in DMR still have detectable CML progenitors and/or CML long-term culture-initiating cells (LTC-IC).22 Using DNA PCR testing, Ross et al4 demonstrated that patients in TFR with undetectable BCR-ABL messenger RNA transcript still have a positive fusion DNA signal in their bone marrow. The low rate of LMRec may be partially explained by the time-dependent falling levels of residual leukemic cells in TFR patients.23 Some patients in TFR with FlucMR exhibited MRD curves reminiscent of those reported in CML patients after allogenic hematopoietic stem cell transplantation, suggesting an effector-target effect.24 Two studies reported that NK cells levels may predict a successful TFR attempt, but neither identified the immune(s) effector(s) involved.25,26 In a recent study,27 BCR-ABL1 positivity was assessed in 20 patients in TFR for >1 year after cell sorting into granulocytes, monocytes, B cells, T cells, and NK cells. The MRD signal was identified predominantly in the lymphoid compartment and was not found in granulocytes. The authors concluded that MRD in the blood of TFR patients need not imply the persistence of multipotent CML cells. This observation may explain why patients with FlucMR in the long term did not experience LMRec. However, our study demonstrates that at least one-third of these patients will experience LMRec, strongly suggesting the persistence of functional CML multipotent cells.
To conclude, we report the first TFR study focusing on patients with LMRec (>2 years). We established that LMRec represents approximately 14% of all MRec. LMRec are predominantly observed in TFR patients with FlucMR, justifying a close long-term MRD monitoring of these patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the French CML group (FiLMC) for support, Laure Morisset and the Centre Hospitalier de Versailles for administrative support, and Luigina Mollica for editorial assistance.
Footnotes
Presented in abstract form at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2019.
Requests for original data should be sent to the corresponding author.
Authorship
Contribution: P.R. and M.D. collected and analyzed the data; M.S. and J.M.C. performed the MRD monitoring; and P.R., C.L., M.D., J.M.C., and M.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe Rousselot, Hematology Department, Centre Hospitalier de Versailles, 177 rue de Versailles, 78157 Le Chesnay, France; e-mail: phrousselot@ch-versailles.fr.
References
- 1.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58-60. [DOI] [PubMed] [Google Scholar]
- 2.Mahon FX, Réa D, Guilhot J, et al. ; Intergroupe Français des Leucémies Myéloïdes Chroniques . Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029-1035. [DOI] [PubMed] [Google Scholar]
- 3.Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298-305. [DOI] [PubMed] [Google Scholar]
- 4.Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515-522. [DOI] [PubMed] [Google Scholar]
- 5.Lee SE, Choi SY, Bang JH, et al. Predictive factors for successful imatinib cessation in chronic myeloid leukemia patients treated with imatinib. Am J Hematol. 2013;88(6):449-454. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N, Kyo T, Maeda Y, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97(6):903-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahon FX, Nicolini FE, Noël MP, et al. Preliminary report of the STIM2 study: a multicenter stop imatinib trial for chronic phase chronic myeloid leukemia de novo patients on imatinib [abstract] Blood. 2013;122(21):654 Abstract 654 [Google Scholar]
- 8.Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424-430. [DOI] [PubMed] [Google Scholar]
- 9.Saussele S, Richter J, Guilhot J, et al. ; EURO-SKI investigators . Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747-757. [DOI] [PubMed] [Google Scholar]
- 10.Cross NC, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26(10):2172-2175. [DOI] [PubMed] [Google Scholar]
- 11.Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of “real-time” quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17(12):2318-2357. [DOI] [PubMed] [Google Scholar]
- 13.Beillard E, Pallisgaard N, van der Velden VH, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using “real-time” quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17(12):2474-2486. [DOI] [PubMed] [Google Scholar]
- 14.Mahon FX, Boquimpani C, Kim DW, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168(7):461-470. [DOI] [PubMed] [Google Scholar]
- 15.Shah NP, García-Gutiérrez V, Jiménez-Velasco A, et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. [published online ahead of print October 24, 2019]. Leuk Lymphoma. 2020;61(3):650-659. [DOI] [PubMed] [Google Scholar]
- 16.Rea D, Nicolini FE, Tulliez M, et al. ; France Intergroupe des Leucémies Myéloïdes Chroniques . Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846-854. [DOI] [PubMed] [Google Scholar]
- 17.Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura S, Imagawa J, Murai K, et al. ; DADI Trial Group . Treatment-free remission after first-line dasatinib discontinuation in patients with chronic myeloid leukaemia (first-line DADI trial): a single-arm, multicentre, phase 2 trial. [published online ahead of print January 21, 2020]. Lancet Haematol. 2020;7(3):e218-e225. [DOI] [PubMed] [Google Scholar]
- 19.Legros L, Nicolini FE, Etienne G, et al. ; French Intergroup for Chronic Myeloid Leukemias . Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123(22):4403-4410. [DOI] [PubMed] [Google Scholar]
- 20.Graham SM, Jørgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319-325. [DOI] [PubMed] [Google Scholar]
- 21.Holyoake TL, Vetrie D. The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood. 2017;129(12):1595-1606. [DOI] [PubMed] [Google Scholar]
- 22.Chomel JC, Bonnet ML, Sorel N, et al. Leukemic stem cell persistence in chronic myeloid leukemia patients in deep molecular response induced by tyrosine kinase inhibitors and the impact of therapy discontinuation. Oncotarget. 2016;7(23):35293-35301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross DM, Pagani IS, Shanmuganathan N, et al. ; Australasian Leukaemia and Lymphoma Group (ALLG) . Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia. 2018;32(12):2572-2579. [DOI] [PubMed] [Google Scholar]
- 24.Kaeda J, O’Shea D, Szydlo RM, et al. Serial measurement of BCR-ABL transcripts in the peripheral blood after allogeneic stem cell transplantation for chronic myeloid leukemia: an attempt to define patients who may not require further therapy. Blood. 2006;107(10):4171-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilander M, Olsson-Strömberg U, Schlums H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31(5):1108-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea D, Henry G, Khaznadar Z, et al. Natural killer-cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study. Haematologica. 2017;102(8):1368-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagani IS, Dang P, Saunders VA, et al. Lineage of measurable residual disease in patients with chronic myeloid leukemia in treatment-free remission. [published online ahead of print November 25, 2019]. Leukemia. 2020;34(4):1052-1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.