Key Points
Real-world data on caplacizumab confirm treatment efficiency in 60 patients with aTTP independent of timing and treatment modalities.
Use of caplacizumab resulted in a reduced need for PEX supporting frontline use of caplacizumab for all patients.
Abstract
Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare but life-threatening condition. In 2018, the nanobody caplacizumab was approved for the treatment of adults experiencing an acute episode of aTTP, in conjunction with plasma exchange (PEX) and immunosuppression for a minimum of 30 days after stopping daily PEX. We performed a retrospective, observational analysis on the use of caplacizumab in 60 patients from 29 medical centers in Germany during acute disease management. Caplacizumab led to a rapid normalization of the platelet count (median, 3 days; mean 3.78 days). One patient died after late treatment initiation due to aTTP-associated complications. In 2 patients with initial disease presentation and in 4 additional patients with laboratory signs of an exacerbation or relapse after the initial therapy, PEX-free treatment regimens could be established with overall favorable outcome. Caplacizumab is efficacious in the treatment of aTTP independent of timing and ancillary treatment modalities. Based on this real-world experience and published literature, we propose to administer caplacizumab immediately to all patients with an acute episode of aTTP. Treatment decisions regarding the use of PEX should be based on the severity of the clinical presentation and known risk factors. PEX might be dispensable in some patients.
Visual Abstract
Introduction
Acquired thrombotic thrombocytopenic purpura (aTTP) is caused by the formation of inhibitory and, less frequently, noninhibitory autoantibodies directed against a disintegrin and metalloprotease with thrombospondin type 1 motifs, member 13 (ADAMTS13). von Willebrand factor (VWF)–multimers form on endothelial cells where they activate and bind platelets upon endothelial injury and protect procoagulatory factor VIII from proteolytic degradation. In Germany, the incidence of aTTP is reported with 2.1 to 2.2 cases per 1 million adults per year.1,2 Acquired TTP presents as acute onset microangiopathic hemolytic anemia with profound thrombocytopenia. Additional complaints depend on the predominantly affected organ systems but may be absent. aTTP is a medical emergency requiring urgent plasma exchange (PEX) and immunosuppressive agents. If untreated, mortality may reach up to 90%.
Recently, the therapeutic options have been expanded by the approval of a novel anti-VWF humanized single-variable domain nanobody, caplacizumab, inhibiting VWF-platelet aggregation. In 2 double-blind controlled trials, TITAN and HERCULES, caplacizumab was compared against placebo in addition to PEX in 75 and 145 patients with aTTP.3,4 The TITAN trial reported an increased number of relapses in the intervention group after termination of caplacizumab after 30 days, which was attributed to unresolved autoimmune activity. Therefore, the HERCULES protocol included the option to extend caplacizumab treatment beyond 30 days after PEX in case of sustained autoimmune activity. The treatment extension was able to decrease relapse rates significantly. Administration of caplacizumab resulted in a reduced time to platelet count response in an analysis stratified by neurological involvement. It also reduced the recurrence rate compared with placebo (12% vs 38%; P < .001). A composite outcome event of thrombotic thrombocytopenic purpura (TTP)–related death, recurrence of TTP, or a thromboembolic event during the treatment period was 74% lower with caplacizumab than with placebo (12% vs 49%; P < .001). Consequently, the drug was rapidly approved and can be prescribed in Germany since October 2018.
Real-world evidence may help to generalize findings from well-controlled clinical trials with low risk of bias and confirm the effectiveness of novel treatment approaches under ordinary circumstances. Whether caplacizumab should be used as frontline therapy or only in cases of severe organ dysfunction, refractory disease, exacerbation, or relapse remains elusive. To provide a rationale for the treatment with a potentially lifesaving but also expensive drug, we analyzed a cohort of 60 patients with aTTP that received caplacizumab between June 2018 and December 2019.
Methods
Study design and participants
This study was conducted as a retrospective, observational study at 29 German medical centers. In total, 60 patients (∼90% of all patients treated in Germany) with aTTP were identified who received caplacizumab between June 2018 and December 2019. The PLASMIC (platelets, lysis, active cancer, stem cell or solid organ transplant, MCV, INR, and creatinine) score (developed as a clinical predictive tool of severe ADAMTS13 deficiency) was determined retrospectively as published previously.5,6 Day 0 was defined as the day of diagnosis as indicated by the treating physician, beginning of aTTP-specific therapy, or a reported ADAMTS13 activity measurement of less than 10%. It is important to note that the reported date of ADAMTS13 activity measurements may not reflect the time point when the results became available to the treating physician. Testing was not available on site at most centers and results may have been reported with a delay of several days. Retrospectively, aTTP had likely begun prior to the day of diagnosis in some patients but was not recognized or treated under the presumption of a different diagnosis.
Time to normalization of platelet count after the start of caplacizumab treatment was defined as the first day with a count ≥150 × 109/L. Clinical remission, exacerbation, refractory disease, and relapse were defined as published previously with the exception that reinitiation of PEX was not a prerequisite of exacerbation and relapse because some of these instances were managed with conservative treatment only.7 Treatment according to the HERCULES protocol was defined as caplacizumab started before or after the first PEX and continued for a minimum of 30 days and a maximum of 58 days after the last PEX treatment. Frontline use was defined as a treatment started within 72 hours after diagnosis. Presence of acute kidney injury according to Kidney Disease: Improving Global Outcome (KDIGO) was determined by comparing the highest with the lowest serum creatinine measurement during the follow-up. Lowest serum creatinine was assumed to be the baseline. Urinary output was not taken into consideration because this information was not available.
Statistical analysis
Given the exploratory nature of this study, no sample size calculation and no adjustments for multiple testing were performed. All results are given as median (range) or mean with 95% confidence interval (CI) when appropriate, except for Figure 1, where the standard errors of the means are reported. Statistical analysis was conducted using Prism version 8.0.1 (GraphPad Software, San Diego, CA). Figures and tables were designed and composed using Microsoft Office (Microsoft, Seattle, WA) and Adobe Illustrator (Adobe Systems Software Ireland Ltd, Dublin, Ireland).
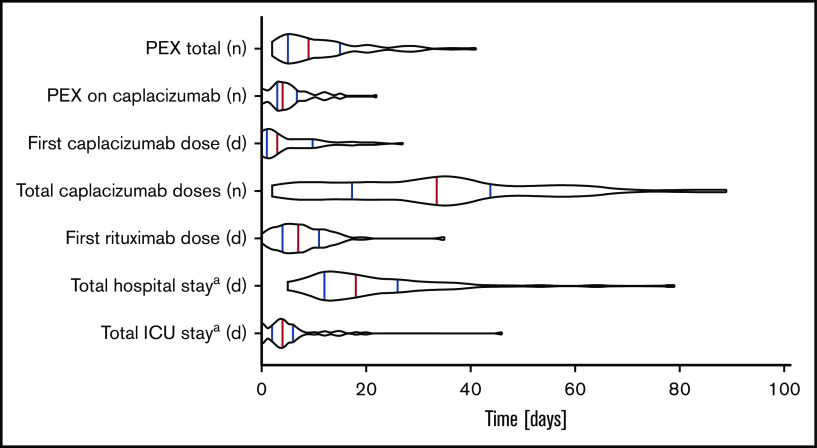
Figure 1.
Violin blots depict the distribution of main treatment and outcome parameters. Red lines indicate medians; blue lines indicate 75th and 25th interpercentile quartile. aTotal stay includes before, during, and after caplacizumab treatment.
Results
Individual patient characteristics, treatment modalities as well as individual courses comprising platelet counts, ADAMTS13 activity measurements, and relevant adverse events are shown in supplemental Figures 1 and 2.
Patient characteristics and initial disease presentation
Patient characteristics and initial disease presentation are shown in Figure 1 and Table 1. Sixty patients were followed up for a median of 108.5 days (range, 5-330 days) for a total of 7484 patient days. Female patients comprised 70.0% of the patient population (42 of 60 patients). Average age at onset was 45.7 years (range, 22-83 years). Fifty-nine patients were of white descent and one of African descent. Median body mass index (BMI) was 26.8 kg/m2 (range, 16.0-42.6 kg/m2). In 76.7% of the cases (46 of 60 patients), aTTP was considered a primary manifestation when presented with a disease relapse (23.3%; 14 of 60 patients). An initial ADAMTS13 activity of <10% was reported in all patients (median <0.3%; range, 0% to 8.0%). The median initial thrombocyte count was 13 × 109/L (range, 3-82 × 109/L). Eight patients (13.3%) presented with significant neurological symptoms as judged by a Glasgow coma scale (GCS) score of <13. All patients presented with a PLASMIC score of ≥4, indicating intermediate to high risk for thrombotic microangiopathy (TMA) related to ADAMTS13 deficiency. Thirty-six patients had a PLASMIC score of 7 (60.0%); 22 patients had a score of 5 or 6 (36.7%), and 1 patient had a score of 4 (1.7%). In only 32 subjects (53%), troponin was measured at least once. Twenty-seven of 32 patients (84%) with troponin measurements showed levels above the upper limit of normal suggesting concomitant myocardial damage. Three patients (5.0%) presented with a serum creatinine above 2.0 mg/dL (152.6 mmol/L). During the disease course, 44 (73%) patients sustained acute kidney injury KDIGO stage 1 to 3 of any cause: 31 patients reached KDIGO stage 1; 7 patients reached KDIGO stage 2; and 6 patients reached KDIGO stage 3.
Table 1.
Baseline patient characteristics and disease parameters of the cohort reported in this manuscript and the HERCULES trial
| Germany* (n = 60) | HERCULES cohort (N = 145)4 | Comparison of Germany vs HERCULES (Fisher’s exact test), P | |
|---|---|---|---|
| Age, mean (range), y | 45.7 (22-83) | 46 (18-79) | |
| Female sex, % (n/total) | 70 (42/60) | 69 (100/145) | >.9999 |
| White, % (n/total) | 98.3 (59/60) | 71.0 (97/137) | <.0001 |
| BMI, median (range), kg/m2 (n = 59) | 27 (16.0-42.6) | 30 (18-59) | |
| First occurrence of TTP, % (n/total) | 76.7 (46/60) | 57 (82/145) | .0072 |
| Hemoglobin, initial, median (range), g/dL | 8.2 (5.0-13.6) | Placebo arm (n = 63), 8.7 (6.4-15.1); verum arm (n = 62), 8.6 (5.9-15.9)† | |
| Hemoglobin, minimum, median (range), g/dL | 6.3 (4.5-10.9) | Not reported | |
| Platelets, initial, median (range), ×109/L | 13.0 (3-85) | 24 (3 – 133) | |
| Platelets, minimum, median (range), ×109/L | 10 (2-62) | Not reported | |
| LDH, initial, median (range), U/L | 985 (316-2588) | 422 (120-3343) | |
| LDH, maximum, median (range), U/L | 1088 (316-2918) | Not reported | |
| Troponin above ULN at any point, % (n/total) (n = 32) | 84.4 (27/32) | cTnI above ULN at baseline, 49.0 (71/145)‡ | |
| Creatinine, initial, median (range), µmol/L | 96.5 (52.2-470) | 80 (35-717) | |
| Creatinine, maximum, median (range), µmol/L | 120.4 (55.8-470) | Not reported | |
| ADAMTS13 activity below 10%, % (n/total) | 100 (60/60) | 85 (123/145) | .0004 |
| ADAMTS13 antibody above 12 IU/ml (ULN) at any point, % (n/total) | 91.7 (55/60) | Not reported | |
| ADAMTS13 antibody concentration, maximum, median (range), IU/ml | 75.5 (0.0-131.6) | Not reported | |
| Glasgow Coma Scale, % (n/total) | .1909 | ||
| <13 | 13.3 (8/60) | 8 (11/145) | |
| 13-15 | 83.3 (50/60) | 91 (132/145) | |
| Data missing | 3.3 (2/60) | 1 (2/145) | |
| PLASMIC score, % (n/total)5-7 | |||
| 0-4 | 1.7 (1/60) | Not reported | |
| 5-6 | 36.7 (22/60) | Not reported | |
| 7 | 60.0 (36/60) | Not reported | |
| Data missing | 1.7 (1/60) | Not reported |
Bold P values indicate statistical significance (P < .05).
cTnl, cardiac troponin; IULN, upper limit of normal.
n = 60 unless indicated otherwise due to missing data. Differences in the HERCULES cohort were tested using the Fisher’s exact test if sufficient data were available.
Source data from Sanofi are on file shared by the company.
Data source is the Orphan Maintenance Assessment Report (EMA/541816/2018). Please note the difference in the time point of troponin assessment (at any time point in the German cohort and at baseline in HERCULES).
Disease course and treatment modalities
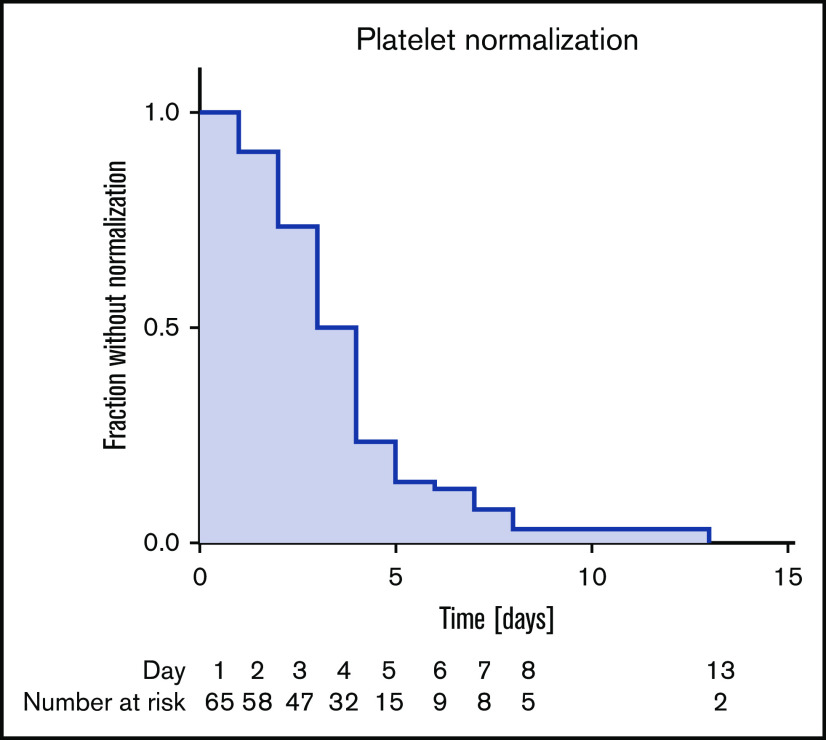
Disease course and treatment modalities are listed in Tables 2 and 3. Due to the severity of symptoms and the need for PEX, most patients (44 of 60 patients; 73.3%; 6 patients had data missing) were initially treated on intensive care units (ICU), accumulating 301 ICU days with a median of 4 days per patient. Patients were discharged from the hospital after 18 days (range, 5 to 79 days). Patients received a total of 1979 daily doses of caplacizumab with a median of 34.0 doses per patient (range, 2 to 89 doses). Only 8 (13.3%) patients were treated according to the HERCULES trial protocol. Caplacizumab treatment was started with a median of 3 days after diagnosis (range, 0 to 27 days). Caplacizumab was used as a frontline agent in 35 of 60 patients (58.3%); 25 of 60 patients (41.7%) received the drug to treat disease exacerbation or refractoriness or to prevent relapse. In an analysis of platelet counts and lactate dehydrogenase immediately before and after the start of the caplacizumab treatment, we could detect a robust tendency toward improvement as an indicator of waning TMA (Figure 2). Platelet counts normalized to above 150 × 109/L in a median of 3 days (range, 1 to 13 days) after commencing caplacizumab treatment (Figure 3).
Table 2.
Treatment modalities
| Germany (n = 60) | HERCULES cohort (N = 145) | Comparison of Germany vs HERCULES (Fisher’s exact test), P | |
|---|---|---|---|
| Rituximab treatment, % (n/total) | 80 (48/60) | 43 (63/145) | <.0001 |
| Rituximab, % (n/total) | |||
| Frontline (within 72 h) | 18.3 (11/60) | 17 (25/145) | .8398 |
| Delayed | 60.0 (36/60) | ||
| Data missing | 1.7 (1/60) | ||
| Day of first rituximab after disease onset, median (range), d | 7 (0-35) | Not reported | |
| Cumulative rituximab dose, median (range), mg | 2000 (562.5-5000) | Not reported | |
| PEX treatment, % (n/total) | 96.7 (58/60) | 100 (145/145) | .0846 |
| Days of PEX treatment (n = 58) | |||
| Median, no. (range) | 9 (2-41) | ||
| Mean, no. (95% CI) | 11.8 (9.5-14.2) | ||
| Days of PEX under caplacizumab treatment | |||
| Median, no. (range) | 4 (0-22) | Verum arm (n = 72), 5 (1-35) | |
| Mean, no. (95% CI) | 5.3 (4.2-6.4) | Verum arm (n = 72), 5.8 (4.8-6.8) | |
| Caplacizumab doses, median (range), no. | 34.0 (2-89) | ||
| Caplacizumab treatment duration, median (range), d | 34 (2-211) | 35 (1-65)* | |
| Caplacizumab as frontline (within 72 h), % (n/total) | 58.3 (35/60) | Not applicable | |
| Caplacizumab start after disease onset, median (range), d | 3 (0-27; 27) | Not applicable | |
| Treatment according to HERCULES protocol, % (n/total)† | 13.3 (8/60) | Not applicable | |
| Steroid treatment, % (n/total) | |||
| Any dose | 98.3 (59/60) | 97 (140/145) | .6734 |
| High dose‡ | 76.6 (46/60) | ||
| Non-high dose | 8.3 (5/60) | ||
| Data missing | 13.3 (8/60) | ||
| Steroid initial dose (n = 51), median (range), mg/kg BW§ | 1.40 (0.64-15.11) | Not reported | |
| Other treatments, % (n/total) | |||
| Mycophenolate | 5.9 (3/60) | 4 (6/145) | |
| Hydroxychloroquine | 1.7 (1/60) | 2 (3/145) | |
| Vincristine | 1.7 (1/60) | Not reported | |
| Ciclosporin | 3.3 (2/60) | 1 (2/145) | |
| Azathioprine | 5.0 (3/60) | Not reported | |
| Splenectomy | 0.0 (0/60) | 2 (3/145) | |
| IVIG | 0.0 (0/60) | 3 (4/145) | |
| Bortezomib | 0.0 (0/60) | 1 (2/145) | |
| Immunoadsorption | 0.0 (0/60) | 0.7 (1/145) |
N = 60 unless indicated otherwise.
BW, body weight; IVIG, IV immunoglobulin.
Data source is the Orphan Maintenance Assessment Report (EMA/541816/2018). Differences from the HERCULES cohort were tested using the Fisher’s exact test if sufficient data were available.
Defined as caplacizumab start before or after first PEX and caplacizumab continued for a minimum of 30 d after last PEX treatment.
High doses are defined as ≥1 mg/kg body weight of prednisone equivalent.
Milligram of prednisone or prednisone equivalence dose if another glucocorticoid has been used.
Table 3.
Outcome data
| Germany (n = 60) | |
|---|---|
| Mortality, % (n/total) | 1.7 (1/60) |
| Follow-up, median (range), d | 108.5 (5-330) |
| Clinical response, % (n/total)* | |
| Achieved | 93.3 (56/60) |
| Not achieved | 3.3 (2/60) |
| Insufficient follow-up | 3.3 (2/60) |
| Exacerbation, % (n/total) | 38.3 (23/60) |
| While on caplacizumab | 3.3 (2/60) |
| Refractory disease, % (n/total) | 31.7 (19/60) |
| Refractoriness, grade, % (n/total)† | |
| Severe | 68.4 (13/19) |
| Mild | 31.6 (6/19) |
| Relapse, % (n/total) | |
| Relapsing disease | 3.3 (2/60) |
| Insufficient follow-up | 13.3 (8/60) |
| Time to final platelet normalization after diagnosis, median (range), d | 12 (3-152) |
| Reached at end of follow-up, % (n/total) | 95.0 (57/60) |
| Not reached at end of follow-up, % (n/total) | 5.0 (3/60) |
| Time to final platelet normalization after caplacizumab start (n = 64)‡§, d | |
| Median (range) | 3.0 (1-13) |
| Mean (95% CI) | 3.78 (3.19-4.38) |
| Duration of hospital stay (n = 59), d | |
| Median (range) | 18 (5-79) |
| Mean (95% CI) | 21.6 (18.0-25.2) |
| Duration of ICU stay (n = 54), d | |
| Median (range) | 4 (0-46) |
| Mean (95% CI) | 5.8 (3.8-7.7) |
Clinical response as defined in Scully et al7: a sustained normalization of platelet counts >150 × 109/L and of LDH <1.5 upper limit of normal after cessation of PEX.
Refractoriness grade as defined in Scully et al7. Refractory TTP is defined as persistent thrombocytopenia <50 × 109/L and persistently raised LDH levels despite 5 PEX and steroid treatments. It is defined as severe if thrombocytopenia remains <30 × 109/L.
Platelets >150 × 109/L. In 3 patients, platelet count did not normalize due to ongoing sepsis or concomitant liver cirrhosis. These cases were excluded from the calculation (patients 10, 49, and 53). One patient (patient 18) was not computed because of aTTP-related death.
Instances (n = 64) rather than patient numbers are reported here reflecting the fact that, in some patients, caplacizumab treatment was stopped and restarted. Every time caplacizumab was restarted, the time to platelet normalization was measured again.
Figure 2.
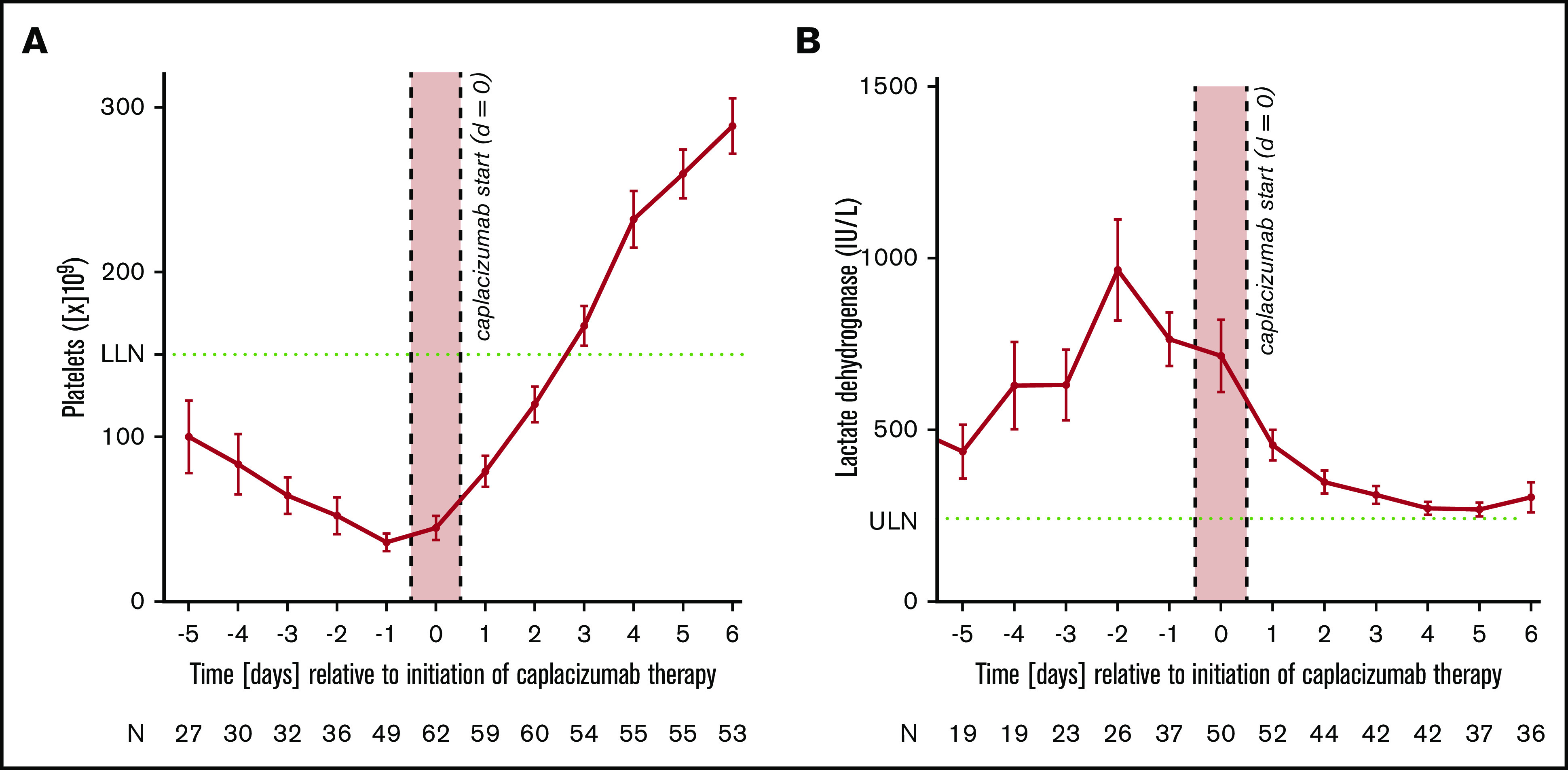
Average platelet counts and standard error of the mean in relation to the start of caplacizumab treatment. Average platelet counts (A) and lactate dehydrogenase (B) and standard error of the mean in relation to the start of caplacizumab treatment. The x-axis (time in days) denotes the time elapsed since the start of caplacizumab and not since the initial diagnosis (ie, day 0). Please note that there is no fixed correlation between the beginning of caplacizumab and the beginning of the treatment, because caplacizumab was not used as a frontline agent in a relevant number of instances. Green dotted lines indicate upper limit of normal (ULN) or lower limit of normal (LLN).
Figure 3.
Kaplan-Meier curves of patient fractions remaining without final normalization of platelet count (>150 × 109/L) after the start of caplacizumab treatment.
Fifty-eight patients (96.7%) received PEX, with a median of 9 PEX treatments (range, 2 to 41 treatments). In the majority of cases, PEX (38 of 60 patients; 63.3%) was continued until platelets normalized for at least 1 day. In 15 instances, PEX was stopped immediately when platelet count rose above 150 × 109/L. In 10 instances, PEX was stopped with stabilizing or rising platelets, albeit still below the lower limit of normal. Four patients were taken off PEX while on caplacizumab treatment with platelets still below 100 × 109/L. In one of these patients, PEX was already stopped after only 2 PEX procedures and 1 dose of caplacizumab when the platelet count had risen from 9 × 109/L to 42 × 109/L (patient 36). Fifty-nine patients (98.3%) received glucocorticoids with a median dose of 1.40 mg (range, 0.64 to 15.11 mg) per kg of body weight as the initial dose. One patient did not receive glucocorticoids for safety concerns due to an acute HIV infection. A total of 76.6% of patients (46 of 60 patients) received high-dose glucocorticoids, defined as a dose of >1.0 mg/kg of body weight. In 8 patients, the respective data were missing. Ten patients received additional immunosuppressants to treat aTTP. Azathioprine was used in 3 cases; mycophenolate was used in 3 cases, ciclosporin was used in 2 cases, and hydroxychloroquine and vincristine were used in 1 patient each. Forty-eight patients (80.0%) were treated with 1 to 6 doses of rituximab with a median cumulative dose of 2000 mg (range, 562.5 to 5000 mg). On average, patients received the first dose of rituximab on day 7 (range, day 0 to day 26). In 11 patients (18.3%), rituximab was used as a frontline agent.
The clinical courses and outcomes are summarized in Table 3. Clinical remission was achieved in 56 patients as defined in Scully et al.7 For 3 patients, insufficient follow-up was available (patients 10, 19, and 59) to assess clinical remission. One patient (patient 18) died of aTTP complications. This patient with known aTTP developed symptoms 2 weeks before admission. PEX treatment was commenced immediately after admission into the hospital but did not improve aTTP activity (supplemental Figure 1). On day 3, the patient developed abdominal symptoms and received the first dose of caplacizumab 4 hours prior to intestinal surgery. This resulted in an immediate thrombocyte response. Surgery revealed the presence of mesenteric ischemia and intestinal gangrene, and a resection was performed. Thrombotic occlusion of the vessels was detected in the pathological examination. Despite surgery and treatment with caplacizumab, the patient died the following day as a result of sepsis and multiorgan failure. One patient (patient 49) was ruled to have achieved clinical remission despite persistent thrombocytopenia as the platelet count stabilized at the previously established level related to concomitant liver cirrhosis. In 19 patients (31.7%), aTTP proved refractory to initial treatment without caplacizumab. Thirteen of these 19 instances were classified as severe, with platelets remaining below 30 × 109/L after 5 days of effective PEX. Twenty-three patients (38.3%) experienced any form of exacerbation. Four patients sustained >1 episode of exacerbation (1 patient with 3 episodes; 1 patient with 4 episodes). An exacerbation occurred in 2 patients (3.3%; patients 19 and 30) while on daily caplacizumab. Two patients were diagnosed with relapsing disease, 80 days and 43 days after the end of PEX respectively (3.3%; patients 33 and 54). All relapsing and refractory patients for whom such data were available showed suppressed ADAMTS13 activity levels <10% at the time point of refractoriness or relapse.
Caplacizumab treatment without PEX
In this study, we report 2 cases in which the treating physicians opted against standard of care and did not initiate PEX upon the initial presentations (patients 37 and 56). This was based on shared decision making with their patients. Patient 37 was admitted to an external hospital because of facial paresthesia, aphasia, and petechial bleeding. Magnetic resonance tomography ruled out stroke. Laboratory results revealed TMA (platelets, 17 × 109/L; hemoglobin, 7.5 g/dl; lactate dehydrogenase [LDH], 902 U/L; schistocytes, 4.6%; haptoglobin, not detectable; and creatinine, 63.7 µmol/L). The patient was transferred to one of the participating centers for treatment. The patient was informed about benefits and risks of PEX and decided against the procedure. Five and 6 hours after the first IV caplacizumab dose, the patient’s platelet count increased to 30 × 109/L and 35 × 109/L, respectively (supplemental Figure 1). The patient’s platelets normalized within 3 days. The diagnosis of aTTP was confirmed by nondetectable ADAMTS13 activity and the presence of autoantibodies. Patient 56 had a history of aTTP and presented with a mild relapse with nondetectable ADAMTS13 activity, platelets of 62 × 109/L, and LDH of 298 U/L. The patient received high-dose glucocorticoids and caplacizumab without PEX. The patient’s platelets normalized within 4 days.
In addition, we detected 3 instances of exacerbation or relapse after the initial caplacizumab treatment was stopped (patients 33, 42, and 43) and 1 instance of exacerbation or relapse by inadvertent omission of doses (patient 29), which could be successfully managed by reinitiating the caplacizumab treatment without additional PEX. In all cases, ADAMTS13 activities were still <10%. Recurrence of disease activity after the last dose of caplacizumab was detected at a median of 6 days (range, 4-14 days).
Safety events
Overall, safety events were reported only sporadically. One major bleeding complication in the form of recurrent vaginal hemorrhages 2 weeks after delivery was reported in 1 patient (patient 8); as a result, caplacizumab was initially paused and then stopped. Other minor bleeding complications comprised epistaxis and gingival bleeding without the need to stop caplacizumab. No patient suffered debilitating hemorrhage. In all cases, with the exception of patient 8 the need for red blood cell transfusions was deemed related to ongoing hemolysis rather than active bleeding. Six patients reported drug-related injection site reactions.
Discussion
Here, we summarize the clinical courses of the first patients treated with caplacizumab in Germany during June 2018 and December 2019. Overall, our experience confirms the positive results of the TITAN and HERCULES trials.3,4 Caplacizumab was effective in the treatment of aTTP and led to a rapid normalization of the platelet count (median, 3 days; mean, 3.78 days) in our cohort vs 2.69 and 3.0 days reported in HERCULES and TITAN.3,4 In HERCULES, these calculations were stratified by neurological involvement. Therefore, a direct comparison of values should not be considered.
The cohort reported herein shares many characteristics with the HERCULES cohort but differs significantly in several important aspects. In the German cohort, patients were predominantly of white descent and presented more often as first manifestation of aTTP rather than relapsing disease. All German patients were reported with an ADAMTS13 activity below 10% (median, <0.3%; range, <0.3% to 8.0%), whereas the HERCULES cohort included 7 patients with other forms of TMA because ADAMTS13 activity measurements were not part of the inclusion criteria. There appears to be a trend toward leaner (BMI, 26.8 kg/m2 vs 30 kg/m2) but more severely affected patients (median initial LDH, 985 IU/mL vs 422 IU/mL, and median initial platelet count, 13 × 109/L vs 24 × 109/L), raising the possibility that sicker patients may have been chosen for caplacizumab treatment. Markers of organ damage including myocardial markers, creatinine, and GCS score; however, did not differ widely. With regards to treatment modalities, more German patients than those in HERCULES received rituximab treatment at any time point with no significant differences in steroid-regimens in the acute setting and adjunct medications.
German patients accumulated almost twice as many days of PEX (mean, 11.8 vs 5.8 days), remained longer on ICU (mean, 5.8 vs 3.4 days), and were hospitalized for a mean of 21.6 days, whereas in the verum arm HERCULES patients averaged only 9.9 days of hospitalization.4 This most likely reflects that German physicians frequently used caplacizumab to treat refractoriness or exacerbation rather than using it as a frontline agent. Caplacizumab was used as a frontline agent in only 35 patients (58.3%). After the start of caplacizumab, platelets normalized within a median of 3 days (mean, 3.78), and only a median of 4 additional PEX were necessary. Of note, the decision on PEX modalities and cessation after thrombocyte recovery was highly variable and did not follow a stringent protocol as in the HERCULES trial. Contrary to the results in the 2 registrations trials without mortality during the study period, 1 patient in this cohort died after a delayed caplacizumab treatment initiation due to aTTP related complications.3,4
Taken together, these real-world data confirm the results from the phase II and III studies and generate evidence of the effectiveness of caplacizumab in the standard clinical care setting. They not only demonstrate a clear trend toward an earlier control of the disease with a reduced need for adjunct treatment but also argue in favor of a frontline use of caplacizumab for all patients. It remains impossible to predict individual treatment responses, clinical outcomes, and PEX-associated complications or to identify those patients who would have had a benign course without caplacizumab treatment. In the real-world setting reported in this study, where, in most cases, several PEX have already been applied at the time of starting caplacizumab, there is no need for a first IV dose prior to PEX. Instead, we recommend starting with a subcutaneous injection of caplacizumab after PEX. In case of any delay in administrating PEX, in patients with a known history of aTTP and/or high clinical suspicion of relapse, the IV route remains an important option.
In the HERCULES trial, all patients were required to continue on PEX for 2 days after the platelet count normalized.4 This may be dispensable when caplacizumab is administered concomitantly. Our data suggest that PEX can be stopped when platelets rise to values above 100 × 109/L to 150 × 109/L, perhaps even earlier. Two recent case reports have suggested that even treatment without PEX is feasible in selected patients albeit off label.8,9 In the German cohort, we report on 6 instances with acute aTTP disease activity treated without PEX. As a consequence, the necessity and duration of plasmapheresis to treat acute episodes of aTTP is a matter of debate. In certain cases, PEX might be dispensable, in particular, if the disease activity is detected early and clinical symptoms are mild. However, it remains unclear whether the reduction of PEX in aTTP is solely beneficial or may also have detrimental effects with regard to maintaining long-term remission. It may be hypothesized that reducing PEX reduces procedure-associated complications and prevents further immunization with ADAMTS13 antigens and an increase of the ADAMTS13 inhibitor concentration that is frequently observed.10 In contrast, less PEX may not be as effective in depleting tissue depots of ADAMTS13 antibodies and reduces the amount of supplemented ADAMTS13 protein.
Our retrospective observational study has several limitations. Patients were treated in 29 different centers with 1 to a maximum of 8 patients. Based on the published aTTP disease incidence in Germany of 2.1 cases per 1 million adults per year, we assume ∼200 to 250 aTTP cases during the observation period.2 Hence, the cohort reported herein comprises an estimated 25% to 30% of cases treated for aTTP during this time period. One might speculate whether there is a bias with respect to disease severity and the use of caplacizumab. Moreover, we do not have detailed information about the duration from disease onset and first clinical symptoms to the time point when the diagnosis of aTTP was made, as well as when and how often the patients contacted professional health care providers during this period. The treatment regimens were heterogenous and based on individual patient characteristics rather than study protocols. A similar heterogeneity has been observed in the TITAN and HERCULES trials as well as in a national survey and reflects the fact that only a few randomized trials have been performed in patients with aTTP.3,4,11
In conclusion, our data confirm the results reported in the TITAN and HERCULES trials in the German population in a real-world setting despite considerably heterogenous treatment regimens. This real-word evidence allows us to generalize the effectiveness of caplacizumab to treat acute episodes of aTTP beyond the internal validity of randomized controlled trials.12 Based on these data and the available literature, we propose to administer caplacizumab to all patients with an acute episode of aTTP and to administer PEX until thrombocytes rise above 100 × 109/L to 150 × 109/L. In selected patients, treatment without PEX seems to be an option. A randomized study is required to clarify this important topic.
Supplementary Material
The full-text version of this article contains a data supplement.
Footnotes
Results presented in this article have not been published previously except in abstract form (ASN Kidney Week 2019, Washington DC, 5-10 November 2019; Congress for Nephrology, DGfN 2019; Düsseldorf, Germany, 10-13 October 2019).
Send data sharing requests via e-mail to the corresponding authors, Paul T. Brinkkoetter (paul.brinkkoetter@uk-koeln.de) and Jan Menne (menne.jan@mh-hannover.de).
Authorship
Contribution: P.T.B. and J.M. conceptualized the study; L.A.V., J.K., P.T.B., and J.M. determined the methodology; L.A.V. and H.C. performed statistics and calculations; L.A.V., J.K., S.B., A.M., A.S., J. Gaedeke, M.T., W.J.J., F.Ö., W.M., S.M., M.G., F.B., T.H.W., H.F., M.H., M.B., J. Gerth, M. Bieringer, M. Bommer, S.Z., J.S., S.E., A. Gawlik, A. Gäckler, A.K., V.S., U.S., M.R., J.R., J.B., A.M., R.H., A.H., S.A.P., R.W., C.v.A., P.T.B., and J.M. were responsible for investigation; L.A.V., J.K., L.K., P.T.B., and J.M. wrote, reviewed, and edited the manuscript; and P.T.B. and J.M. initiated and supervised the study.
Conflict-of-interest disclosure: P.T.B. reports grants from the German Research Foundation (BR2955/8) during the conduct of the study and personal fees from Alexion, Astellas, Bayer, Sanofi Genzyme, Pfizer, and Vifor (speaker honoraria, advisory boards). L.A.V. reports grants from the Else-Kroener-Fresenius Stiftung (2015_A224) and speaker honoraria from Sanofi-Genzyme. S.B. receives grant support from the German Research Foundation (BR4917/3). J.M. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria, advisory boards). S.P. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria, advisory boards). R.W. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria, advisory boards, educational materials and review writing). W.J.J. received speaker honoraria from Alexion and Ablynx (advisory boards). W.M. received honoraria from Ablynx, Takeda, and Shire (speaker honoraria, advisory boards). M.T. reports research grants from the Sonnenfeld Stiftung, Charité3R, and Novartis and personal fees from Baxter, Cytosorbents, Novartis, and Takeda (speaker honoraria, advisory boards). M.R. reports personal fees from Alexion, Diamed, Roche, and Sanofi Genzyme (speaker honoraria, travel support). A. Gäckler reports personal fees from Alexion and Ablynx (speaker honoraria, advisory boards). F.B. received honoraria from Amgen, Sanofi, Akcea, Hexal, MSD, AstraZeneca, Alexion, and Astellas (speaker honoraria, advisory boards). U.S. reports study fees, travel support and consultancy fees from Alexion and study fees and consultancy fees Ablynx. M. Bommer has received honoraria from Ablynx, Alexion, Sanofi, and Amgen, as well as study fees from Ablynx and Amgen. C.v.A. reports personal fees from Sanofi Genzyme (advisory boards). The remaining authors declare no competing financial interests.
Correspondence: Paul T. Brinkkoetter, Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Kerpener Str 62, D-50937 Cologne, Germany; e-mail: paul.brinkkoetter@uk-koeln.de; and Jan Menne, Department of Nephrology and Hypertension, Medical School Hannover, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: menne.jan@mh-hannover.de.
References
- 1.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. [DOI] [PubMed] [Google Scholar]
- 2.Miesbach W, Menne J, Bommer M, et al. . Incidence of acquired thrombotic thrombocytopenic purpura in Germany: a hospital level study. Orphanet J Rare Dis. 2019;14(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyvandi F, Scully M, Kremer Hovinga JA, et al. ; TITAN Investigators . Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. [DOI] [PubMed] [Google Scholar]
- 4.Scully M, Cataland SR, Peyvandi F, et al. ; HERCULES Investigators . Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346. [DOI] [PubMed] [Google Scholar]
- 5.Bendapudi PK, Hurwitz S, Fry A, et al. . Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4(4):e157-e164. [DOI] [PubMed] [Google Scholar]
- 6.Li A, Khalighi PR, Wu Q, Garcia DA. External validation of the PLASMIC score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. J Thromb Haemost. 2018;16(1):164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully M, Cataland S, Coppo P, et al. ; International Working Group for Thrombotic Thrombocytopenic Purpura . Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312-322. [DOI] [PubMed] [Google Scholar]
- 8.Chander DP, Loch MM, Cataland SR, George JN. Caplacizumab therapy without plasma exchange for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;381(1):92-94. [DOI] [PubMed] [Google Scholar]
- 9.Sukumar S, George JN, Cataland SR. Shared decision making, thrombotic thrombocytopenic purpura, and caplacizumab [published online ahead of print 1 January 2020]. Am J Hematol. doi:10.1002/ajh.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isonishi A, Bennett CL, Plaimauer B, Scheiflinger F, Matsumoto M, Fujimura Y. Poor responder to plasma exchange therapy in acquired thrombotic thrombocytopenic purpura is associated with ADAMTS13 inhibitor boosting: visualization of an ADAMTS13 inhibitor complex and its proteolytic clearance from plasma. Transfusion. 2015;55(10):2321-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patriquin CJ, Clark WF, Pavenski K, Arnold DM, Rock G, Foley SR; Canadian Apheresis Group . How we treat thrombotic thrombocytopenic purpura: results of a Canadian TTP practice survey. J Clin Apher. 2017;32(4):246-256. [DOI] [PubMed] [Google Scholar]
- 12.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359(9302):248-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.