Abstract
Objective
tExtracorporeal membrane oxygenation (ECMO) is a life-saving therapy for severe, reversible cardiopulmonary failure, but data regarding its use in pregnancy and the postpartum period are limited. We sought to quantify survival of pregnant and postpartum women necessitating ECMO in a contemporary cohort at a single tertiary institution.
Study Design
All women of reproductive age (14–44 years), who underwent ECMO at our institution between January 1, 2008, and December 31, 2017, were identified using a query of hospital encounters for ECMO-related CPT codes. We manually reviewed all charts of women of reproductive age; women who were pregnant or <6 weeks postpartum at the time of ECMO initiation were included. Clinical characteristics and maternal and fetal outcomes are described.
Results
In this study, 54 women of reproductive age underwent ECMO for cardiopulmonary failure. Of those, 9 (17%) were pregnant or <6 weeks postpartum at the time of ECMO initiation: 4 antepartum, 1 intraoperative at the time of cesarean delivery, and 4 postpartum (including 2 in whom ECMO was initiated on postpartum day 0 or 1). Overall, maternal survival was 33%. The median maternal age was 24 years (range 19–39 years); most women were nonsmokers without underlying medical comorbidities. The most common indication for ECMO use in pregnant and postpartum women was acute respiratory distress syndrome, which was present in 7 cases (78%), including 5 cases that were due to infectious etiologies and 2 cases that were attributed to preeclampsia. The median number of days on ECMO was 6 (range 1–14). There were no cases of obstetric hemorrhage. Venovenous ECMO was utilized in all but 1 case, in which emergent attempted venoarterial ECMO was unsuccessful in resuscitating a postpartum patient with cardiac arrest and a massive pulmonary embolism. A total of 4 women were initiated on ECMO during pregnancy: their gestational ages at ECMO initiation were 21, 22, 29, and 30 weeks; maternal survival was 50%, and fetal mortality was 50%. A case of ECMO initiated during cesarean section at 29 weeks’ gestation resulted in both maternal and fetal survival. Among 4 mothers with ECMO initiation after childbirth, none survived. Finally, we found a tendency toward survival in those patients for whom ECMO was initiated soon after mechanical ventilation, earlier in the disease process. In contrast, in this study, 23 of 45 women of reproductive age (51%) who were not pregnant but underwent ECMO survived.
Conclusion
When ECMO was initiated during pregnancy or during childbirth, 60% of mothers and fetuses survived, supporting current use of ECMO as a salvage therapy in pregnant and intrapartum women. In this generally young and healthy population, ECMO has the potential to increase the survival rates of both mother and fetus and should be considered a salvage therapy for peripartum women with reversible forms of cardiorespiratory failure.
Key words: critical care, pregnancy, respiratory failure, hypoxemia, extracorporeal membrane oxygenation
Introduction
Extracorporeal life support was first introduced as a life-saving therapy in the 1970s. After its debut, randomized controlled trials initially showed benefit only in neonates, with survival approaching 80% in this population, compared with 10% to 30% of adults receiving therapy. However, technological improvements, including heparin coating of equipment in the years 1990 to 2000, led to improved outcomes in adults, and currently, survival rates in adults are 50% to 70%.1 , 2
AJOG MFM at a Glance.
Why was this study conducted?
There are limited data regarding the use of extracorporeal membrane oxygenation (ECMO) during pregnancy and the postpartum period; we sought to add to the current literature by presenting a 10-year case series from a single institution.
Key findings
ECMO was most commonly indicated for cases of persistent hypoxemia on maximum ventilator settings in the context of acute respiratory distress syndrome. Overall rates of maternal survival (33%) and fetal survival (60%) were low, although maternal survival was higher (60%) if ECMO was initiated during pregnancy or intrapartum.
What does this add to what is known?
Use of ECMO for pregnant and postpartum women with reversible forms of cardiorespiratory failure remains a feasible salvage therapy in some cases. Outcomes in this single-institution case series that is less subject to reporting bias support meta-analysis results of smaller case series. Consideration of ECMO in unique circumstances (eg, emergent intraoperative cannulation during cesarean delivery) in this generally young and healthy population has the potential for both maternal and fetal survival.
Modern extracorporeal membrane oxygenation (ECMO) is utilized in many tertiary care centers across the United States and other developed countries as a life-saving technology for severe, reversible cardiopulmonary failure. According to the Extracorporeal Life Support Organization (ELSO), the past decade has seen an increase in ECMO use in adults by more than 10-fold, and rates of use continue to rise.3 , 4 Use of ECMO in adults worldwide in 2019 exceeded 12,000 cases, an increase of 30% from the previous year.5
Despite its widespread use, there are limited data regarding the safety and efficacy of ECMO when used in pregnant and postpartum patients. We reviewed the literature and estimated that fewer than 150 cases of ECMO use during pregnancy and the postpartum period have been published. The largest case series we are aware of consists of 18 women (4 pregnant and 14 postpartum)6; many publications are single case reports.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Although the ELSO closely monitors outcomes and complication rates among individuals receiving ECMO, they do not track pregnancy or postpartum status.1 Therefore, the purpose of this study is to add to the literature by providing additional cases of ECMO use in obstetric patients to further support claims of safety and efficacy when used in pregnant and postpartum women.
Material and Methods
This was a 10-year case series (2008–2017) of pregnant and postpartum women undergoing ECMO at our tertiary care institution. Cases were identified using a query of all hospital encounters for ECMO-related CPT codes in adolescents and/or women aged 14 to 44 years. Individual chart review was then performed to confirm ECMO utilization for cardiopulmonary failure and to identify patients with a confirmed intrauterine pregnancy of at least 6 weeks’ gestation at the time of ECMO initiation and up to 6 weeks postpartum. Clinical characteristics and maternal and/or fetal outcomes were abstracted via individual chart review.
Our primary outcome of interest was maternal survival. The secondary outcome of interest was fetal survival if the mother was pregnant at the time of ECMO initiation. Additional characteristics of interest included indication for ECMO, type of ECMO circuit (venovenous or venoarterial), clinical parameters prompting providers to initiate mechanical ventilation and ECMO, length of time on ECMO, and time on mechanical ventilation before ECMO cannulation. Obstetric characteristics were also collected, including gestational age, mode and timing of delivery, and postpartum hemorrhage. Survival was also recorded for the women of reproductive age identified by our query who were not pregnant or who were postpartum women. Descriptive analyses are reported.
This study was reviewed and approved by the institutional review board at the University of North Carolina-Chapel Hill under a waiver of informed consent.
Results
In this study, a total of 54 women of reproductive age underwent ECMO for cardiopulmonary failure, and of these, 9 (17%) were pregnant or postpartum. These cases included 4 women who were pregnant at the time of ECMO initiation, 1 who had ECMO cannulation during birth (cesarean delivery), and 4 who were postpartum (Table 1 ). The median maternal age was 24 (range 19–39) years. Among the 9 women, 7 (78%) were nonsmokers, and 5 (56%) self-identified as non-Hispanic black. Only 2 patients had medical history notable for underlying medical comorbidities: one with type 1 diabetes mellitus and the other with severe asthma.
Table 1.
Description of 9 peripartum ECMO cases, including etiology of illness and perinatal course
| # | Peripartum status at time of ECMO initiation | Etiology of illness/indication for ECMO | Maternal survival | Fetal survival | Delivery indication and mode of delivery | Birth details | ECMO circuit | Time on MV before ECMO | Days on ECMO | Time on MV post ECMO |
|---|---|---|---|---|---|---|---|---|---|---|
| A | Pregnant, 21 wk | Urosepsis, aspiration pneumonia, ARDS | Yes | Yes | Nonreassuring fetal testing in the setting of diabetic ketoacidosis; cesarean delivery | Live birth, 4 wk after ECMO (26 wk) | V-V | <12 h (intubated outside the hospital) | 8 | 8 d |
| B | Pregnant, 22 wk | Malaria-induced ARDS | Yes | No (previable) | Preterm labor, spontaneous vaginal delivery | Spontaneous preterm delivery of 22-wk twin gestation | V-V | 14 h | 6 | <1 d |
| C | Pregnant, 29 wk | Status asthmaticus, sepsis | No | No | Not delivered secondary to maternal death | Intrauterine fetal demise noted on ECMO day 4 at 30 wk | V-V | 3 d | 6 | a |
| D | Pregnant, 30 wk | H1N1 influenza–induced ARDS | No | Yes | Preterm labor; forceps-assisted vaginal delivery | Live birth on ECMO day 3 at 30 wk | V-V | 6 d | 14 | 6 d |
| E | Intraoperative, during cesarean delivery, 29 wk | Preeclampsia with severe features, flash pulmonary edema, aspiration, ARDS | Yes | Yes | Fetal bradycardia owing to maternal respiratory failure; cesarean delivery | Live birth at 29 wk | V-V | 1.5 h | 4 | 1 d |
| F | Postpartum, day 0 | Septic abortion with septic shock, ARDS | No | No (previable) | Dilation and evacuation | Fetal demise at 15 wk | V-V | 16.5 h | 1 | a |
| G | Postpartum, day 1 | Cholecystitis with septic shock and multiorgan failure/ARDS and subsequent pneumonia | No | No (previable) | Passed spontaneously | Spontaneous abortion at 12 wk | V-V | 12 h | 9 | a |
| H | Postpartum, day 14 | Preeclampsia with pulmonary edema, ARDS (and subsequent pneumonia) | No | Yesb | Failed labor induction; cesarean delivery | Live birth at term | V-V | 11 d (MV initiated on postpartum day 3) | 12 (ECMO d/c’d 2/2 systemic bleeding) | 2 d |
| I | Postpartum, day 36 | Large lower extremity and inferior vena cava deep venous thrombus, pulmonary embolism, cardiac arrest | No | Yesb | Labor induction; cesarean delivery | Live birth at term | V-A | <1 h | 1 | a |
ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation; V-A, venoarterial; V-V, venovenous.
Webster et al. Extracorporeal membrane oxygenation in pregnant and postpartum women. AJOG MFM 2020.
Death occurred on ECMO
Delivery occurred before presumed onset of maternal illness.
The most common indication for ECMO use in pregnancy and the postpartum period was ARDS, which was present in 7 of 9 (78%) cases, wherein 5 were due to infectious etiologies and 2 were attributed to preeclampsia. Among the 9 women, 6 (67%) underwent ECMO cannulation within 24 hours of initiation of mechanical ventilation, with timing ranging from <1 hour to 11 days (Table 1). Major clinical parameters present at the time of decision for mechanical ventilation and ECMO initiation are listed in Table 2 . Venovenous ECMO was utilized in all but 1 case and was most often initiated owing to persistent hypoxemia despite maximal ventilatory settings or because of the significant risk of secondary lung injury from barotrauma, caused by high airway pressures required to maintain oxygenation. The single case of venoarterial ECMO was an unsuccessful resuscitation of a postpartum patient who had cardiac arrest with a massive pulmonary embolism. Women received ECMO for a median of 6 (range 1–14) days.
Table 2.
Factors contributing to decision to intubate and decision to initiate ECMO
| # | Peripartum status at time of ECMO initiation | Etiology/indication | Decision/parameters prompting providers to intubate | Decision/parameters prompting providers to initiate ECMO |
|---|---|---|---|---|
| A | Pregnant, 21 wk | Urosepsis, aspiration pneumonia, ARDS | Episode of hypoglycemia led to loss of consciousness and need for cardiopulmonary resuscitation, complicated by aspiration | Emergently initiated ECMO upon arrival from outside the hospital due to manual ventilation required to maintain SPO2 >90% |
| B | Pregnant, 22 wk | Malaria-induced ARDS | Worsening hypoxemia on continuous PAP support (pH 7.42, PO2 66, pCO2 27.6) | Persistent respiratory acidosis and hypoxemia (pH 7.24, pO2 64, pCO2 49) despite 100% FiO2, PEEP 18, VT 380, rate 30 on PRVC |
| C | Pregnant, 29 wk | Status asthmaticus, sepsis | Severe acidosis despite bilevel positive airway pressure (pH 7.08, pO2 83, pCO2 24.6) | Progressive hypoxemia (pH 7.34, pO2 67, pCO2 45) despite 100% FiO2, PEEP 5, VT 480, rate 24 on ACV |
| D | Pregnant, 30 wk | H1N1 influenza–induced ARDS | Significant increase in oxygen requirement, intubation done outside the hospital to secure airway before transport | Worsening subcutaneous emphysema, new left apical pneumothorax concerning for barotrauma, maximum ventilator settings with worsening hypoxemia (pH 7.38, pO2 73, pCO2 45.1) |
| E | Intraoperative, during cesarean delivery, 29 wk | Preeclampsia with severe features, flash pulmonary edema, ARDS | Intubated at start of cesarean delivery owing to loss of consciousness, persistent oxygen saturations of 60%, witnessed aspiration on induction | Persistent hypoxemia and hypercarbia, multiple modes of ventilation failed, manual ventilation required secondary to high peak airway pressures |
| F | Postpartum, day 0 | Septic abortion with septic shock, ARDS | Intubated at start of suction dilation and evacuation for septic abortion; developed florid pulmonary edema intraoperatively (aggressive fluid and blood product resuscitation) | Progressive hypoxia (pH 7.33, pO2 67, pCO2 40) despite FiO2 100%, PEEP 20, PIP 41 on pressure-controlled ventilation |
| G | Postpartum, day 1 | Cholecystitis with septic shock and multiorgan failure/ARDS and subsequent pneumonia | Worsening tachypnea on 4 L nasal cannula (pH 7.25, pO2 83, pCO2 37) with subsequent acute respiratory decline requiring intubation | Progressive hypoxemia (pH 7.26, pO2 48, pCO2 37) despite FiO2 100%, PEEP 15, PIP 50 on pressure-controlled ventilation |
| H | Postpartum, day 14 | Preeclampsia with pulmonary edema, ARDS (and subsequent pneumonia) | Presented with worsening shortness of breath on postoperative day 3 after cesarean delivery; found to be severely hypertensive (210/170 mm Hg) and hypoxic with pulmonary edema. Rapidly intubated for persistent hypoxia (SPO2 65% on room air, improved only to 80% on nonrebreather) | Following initial improvement on mechanical ventilation for 9 days (weaned to pressure support ventilation), respiratory status worsened over 48 h with progressive hypoxemia (pH 7.32, PO2 57, pCO2 47) despite aggressive ventilatory settings on high-frequency percussive ventilation with FiO2 95%, PEEP 8 |
| I | Postpartum, day 36 | Large lower extremity and inferior vena cava deep venous thrombus, pulmonary embolism, cardiac arrest | Acute decompensation intraoperatively during thrombectomy | Increasingly difficult to ventilate with increased airway pressures, cardiogenic shock, undergoing active cardiopulmonary resuscitation |
ACV, assist-control ventilation; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; PAP, positive airway pressure; PEEP, positive end expiratory pressure; PIP, peak inspiratory pressure; PRVC, pressure-regulated volume control.
Webster et al. Extracorporeal membrane oxygenation in pregnant and postpartum women. AJOG MFM 2020.
The timing and duration of ECMO relative to obstetric outcomes are shown in Figure 1 . Four women were pregnant at the time of ECMO initiation; the gestational ages of these 4 women at the time of ECMO cannulation were 21, 22, 29, and 30 weeks. One additional patient underwent ECMO cannulation intraoperatively during an emergent cesarean delivery at 29 weeks’ gestation. The remaining women were between 0 and 36 days postpartum at the time of ECMO initiation. Among the postpartum women, pregnancy durations ranged from 12 weeks to full term. In addition to the aforementioned cesarean delivery, there were 2 additional deliveries that used ECMO, both owing to preterm labor—one with spontaneous vaginal delivery of a previable twin gestation and the other with forceps-assisted vaginal delivery of a preterm infant. There were no cases of obstetric hemorrhage.
Figure 1.
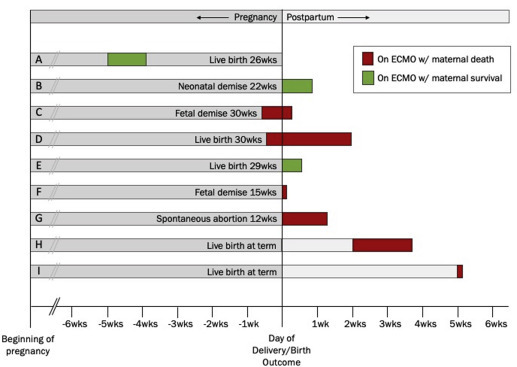
Timing and duration of ECMO relative to obstetrical outcomes for peripartum cases of ECMO at our tertiary care center (2008–2017)
Webster et al. Extracorporeal membrane oxygenation in pregnant and postpartum womens. AJOG MFM 2020.
Overall, 3 of 9 (33%) mothers survived. All 3 surviving patients were either pregnant or intrapartum at the time of ECMO cannulation, whereas none of the patients who initiated ECMO postpartum survived. In addition, in 5 of 9 women in whom ECMO was initiated while pregnant and/or intrapartum, maternal survival was 60%, and fetal survival was 60%; both mother and fetus survived in 2 pregnancies (40%).
Of the 3 surviving women, 1 woman had 1 subsequent pregnancy in our healthcare institution. She had an uncomplicated term delivery of an appropriate for gestational age neonate 21 months after her hospital discharge from her pregnancy requiring ECMO.
In addition, in this study, 23 of 45 (51%) women of reproductive age who were not pregnant or within 6 weeks postpartum who underwent ECMO at our institution survived.
Comment
In this 10-year study, 1 in 5 women of reproductive age undergoing ECMO at our institution were pregnant or <6 weeks postpartum. We found that if ECMO was initiated during pregnancy or intrapartum, 60% of mothers and fetuses survived, supporting the use of ECMO as a salvage therapy in pregnant and intrapartum women. Moreover, none of the pregnant women who had complicated pregnancies owing to severe maternal illness requiring ECMO during pregnancy or intrapartum delivered at term. Specifically, rates of spontaneous preterm labor and nonreassuring fetal status were high.
Despite a relative paucity of data in the obstetric population and the low maternal survival rate in this cohort, ECMO remains an attractive life-saving therapy for severe, reversible cardiopulmonary failure. Despite the full anticoagulation strategies required during ECMO, none of the antepartum or postpartum cases were complicated by obstetric hemorrhage. Although pregnant and postpartum women have unique risks for both infectious and noninfectious respiratory failure, most women in this study were young and lacked extensive medical comorbidities. Our case of emergent intrapartum ECMO initiation due to flash pulmonary edema and refractory hypoxemia and hypercarbia secondary to preeclampsia with severe features exemplifies a clinical scenario unique to pregnancy and illustrates a situation where tertiary care resources can be mobilized in nontraditional ways to meet acute patient needs. Furthermore, several women in our case series underwent ECMO initiation after only a short period of mechanical ventilation. There was a tendency toward survival in those patients for whom ECMO was initiated soon after mechanical ventilation, earlier in the disease process. Of the 3 patients who survived, all were cannulated for ECMO within 14 hours (range 1.5–14 hours) of intubation and mechanical ventilation, compared with nonsurvivors who were cannulated for ECMO later (range <1 hour–11 days).
Pregnancies complicated by ARDS, regardless of need for ECMO, are associated with a high rate of both maternal mortality (range 6.3%–76.2%) and perinatal mortality (range 8.3%–43.5%).20, 21, 22, 23, 24, 25, 26, 27, 28, 29 In a pooled analysis of 9 reports6 , 9 , 13, 14, 15, 16, 17, 18, 19 of 78 pregnant or postpartum women receiving ECMO (where each publication included at least 3 patients; range 3–18), the overall maternal mortality rate was 22.8% (95% confidence interval, 11.6%–35.9%), a rate lower than that of the general adult population that received ECMO.30 In addition, other single case reports of ECMO in pregnancy were published, the vast majority of which reported successful outcomes.7, 8, 9, 10, 11, 12 The wide range of outcomes in previous reports may reflect differences in patient-level factors and specific underlying etiologies of ARDS, in addition to publication biases. We posit that the differences we found in maternal outcomes in our cohort of women who received ECMO may reflect the unbiased reporting of all cases at our institution. In addition, other patient-specific and system-specific factors (eg, the underlying reason for ECMO, at what point in the disease process the individual was placed on ECMO, and other concomitant treatments) may explain the observed differences in outcomes.
Although pregnancy does not appear to influence the overall survival of women who develop ARDS, there are several key physiological differences in pregnancy that must be considered when caring for the critically ill pregnant patients.31 Reports regarding ventilatory management of ARDS in pregnancy suggest that pregnant women are more likely to develop severe ARDS requiring high ventilatory pressures. This is not unexpected, as the gravid uterus increases intraabdominal pressure, displaces the diaphragm upward, and reduces chest wall compliance.31 In addition to higher peak airway pressures required in pregnancy, accepted lung protective ventilatory strategies in ARDS, particularly permissive hypercapnia and associated respiratory acidosis, are at odds with fetal well-being and survival. It is possible that initiating ECMO early in the course of worsening ARDS may improve both maternal and fetal outcomes, compared with mechanical ventilation alone.
This study has several strengths. Our study is one of only a small number of case series6 , 9 , 13, 14, 15, 16, 17, 18, 19 to report on outcomes of a comprehensive case series of pregnant and postpartum women receiving ECMO and adds to the growing body of literature in this area. Our comprehensive chart review permitted evaluation of multiple medical and pregnancy-specific factors that may have influenced outcomes and also permitted us to describe in detail the specific conditions leading to deterioration of health of the patients resulting in both mechanical ventilation and ECMO. In addition, manual review of all records of women of reproductive age who received ECMO ensured that we were able to capture all pregnant and postpartum women, reducing the risk for a positive reporting bias. Nonetheless, these data should be interpreted with caution. Assessing outcomes in this population is limited by many factors, including the relative rarity of ECMO use and the difficulty in identifying and tracking pregnancy status in those undergoing ECMO. Furthermore, although this represents one of the largest case series to date on ECMO use in pregnancy and the postpartum period, numbers remain small.
Conclusion
In conclusion, ECMO is a key, potentially life-saving treatment for severe cardiopulmonary failure. Despite high maternal and perinatal mortality rates, therapy should not be withheld during pregnancy or the postpartum period. In this generally young and healthy population, ECMO has the potential to improve the survival rates of both mother and fetus and should be considered a salvage therapy for pregnant women with reversible forms of cardiorespiratory failure. We also noted a tendency toward survival in those patients whom ECMO was initiated soon after mechanical ventilation, earlier in the disease process. Obstetric clinicians should remain vigilant for the possibility of spontaneous preterm labor and nonreassuring fetal status. To better assess outcomes in this population, we implore the ELSO, which closely tracks ECMO cases in the United States, to consider tracking pregnancy and postpartum status.
Footnotes
The authors report no conflict of interest.
This study was supported by research grant numbers K24-ES031131 and R01-MD011609.
Cite this article as: Webster, CM, Smith, KA, Manuck, TA. Extracorporeal membrane oxygenation in pregnant and postpartum women: a ten-year case series. Am J Obstet Gynecol MFM 2020;2:100108.
References
- 1.Paden M.L., Rycus P.T., Thiagarajan R.R., ELSO Registry Update and outcomes in extracorporeal life support. Semin Perinatol. 2014;38:65–70. doi: 10.1053/j.semperi.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Lewandowski K. Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care. 2000;4:156–168. doi: 10.1186/cc689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Extracorporeal Life Support Organization ECLS Registry Report. International summary. January 2017. https://www.elso.org/Portals/0/Files/Reports/2017/International%20Summary%20January%202017.pdf Available at:
- 4.Extracorporeal Life Support Organization ECLS Registry Report. International summary. January 2018. https://www.elso.org/Portals/0/Files/Reports/2018/International%20Summary%20January%202018%20First%20Page.pdf Available at:
- 5.Extracorporeal Life Support Organization ECLS Registry Report. International summary. January 2019. https://www.elso.org/Portals/0/Files/Reports/2019/International%20Summary%20January%202019%20page%201.pdf Available at:
- 6.Agerstrand C., Abrams D., Biscotti M., et al. Extracorporeal membrane oxygenation for cardiopulmonary failure during pregnancy and postpartum. Ann Thorac Surg. 2016;102:774–779. doi: 10.1016/j.athoracsur.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Moore S.A., Dietl C.A., Coleman D.M. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016;151:1154–1160. doi: 10.1016/j.jtcvs.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Anselmi A., Ruggieri V.G., Letheulle J., et al. Extracorporeal membrane oxygenation in pregnancy. J Card Surg. 2015;30:781–786. doi: 10.1111/jocs.12605. [DOI] [PubMed] [Google Scholar]
- 9.Sharma N.S., Wille K.M., Bellot S.C., Diaz-Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J. 2015;61:110–114. doi: 10.1097/MAT.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa D., Jwa S.C., Seto T., et al. Successful treatment of severe acute respiratory distress syndrome due to group A streptococcus induced toxic shock syndrome in the third trimester of pregnancy-effectiveness of venoarterial extracorporeal membrane oxygenation: a case report. J Obstet Gynaecol Res. 2020;46:167–172. doi: 10.1111/jog.14138. [DOI] [PubMed] [Google Scholar]
- 11.Carlier L., Muller J., Debaveye Y., Verelst S., Rex S. Successful use of VV-ECMO in a pregnant patient with severe ARDS. Turk J Emerg Med. 2019;19:111–112. doi: 10.1016/j.tjem.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nnaoma C., Chika-Nwosuh O.Z., Isedeh A., Bustillo J., Al Twal A., Patel P. Venovenous extracorporeal membrane oxygenation in a gravid patient with acute respiratory distress syndrome: a case report. Am J Case Rep. 2019;20:705–708. doi: 10.12659/AJCR.914490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biderman P., Carmi U., Setton E., Fainblut M., Bachar O., Einav S. Maternal salvage with extracorporeal life support: lessons learned in a single center. Anesth Analg. 2017;125:1275–1280. doi: 10.1213/ANE.0000000000002262. [DOI] [PubMed] [Google Scholar]
- 14.Bouabdallaoui N., Demondion P., Leprince P., Lebreton G. Short-term mechanical circulatory support for cardiogenic shock in severe peripartum cardiomyopathy: la Pitié-Salpêtrière experience. Interact Cardiovasc Thorac Surg. 2017;25:52–56. doi: 10.1093/icvts/ivx106. [DOI] [PubMed] [Google Scholar]
- 15.Dubar G., Azria E., Tesnière A., et al. French experience of 2009 A/H1N1v influenza in pregnant women. PLoS One. 2010;5:e13112. doi: 10.1371/journal.pone.0013112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzgraefe B., Broomé M., Kalzén H., Konrad D., Palmér K., Frenckner B. Extracorporeal membrane oxygenation for pandemic H1N1 2009 respiratory failure. Minerva Anestesiol. 2010;76:1043–1051. [PubMed] [Google Scholar]
- 17.Hou X., Guo L., Zhan Q., et al. Extracorporeal membrane oxygenation for critically ill patients with 2009 influenza A (H1N1)-related acute respiratory distress syndrome: preliminary experience from a single center. Artif Organs. 2012;36:780–786. doi: 10.1111/j.1525-1594.2012.01468.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang K.Y., Li Y.P., Lin S.Y., Shih J.C., Chen Y.S., Lee C.N. Extracorporeal membrane oxygenation application in post-partum hemorrhage patients: is post-partum hemorrhage contraindicated? J Obstet Gynaecol Res. 2017;43:1649–1654. doi: 10.1111/jog.13426. [DOI] [PubMed] [Google Scholar]
- 19.Nair P., Davies A.R., Beca J., et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med. 2011;37:648–654. doi: 10.1007/s00134-011-2138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson M.W., Caruthers T.J., Whitty J.E., Gonik B. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol. 1998;91:108–111. doi: 10.1016/s0029-7844(97)00585-1. [DOI] [PubMed] [Google Scholar]
- 21.Catanzarite V., Willms D., Wong D., Landers C., Cousins L., Schrimmer D. Acute respiratory distress syndrome in pregnancy and the puerperium: causes, courses, and outcomes. Obstet Gynecol. 2001;97:760–764. doi: 10.1016/s0029-7844(00)01231-x. [DOI] [PubMed] [Google Scholar]
- 22.Munnur U., Karnad D.R., Bandi V.D., et al. Critically ill obstetric patients in an American and an Indian public hospital: comparison of case-mix, organ dysfunction, intensive care requirements, and outcomes. Intensive Care Med. 2005;31:1087–1094. doi: 10.1007/s00134-005-2710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfitscher L.C., Cecatti J.G., Pacagnella R.C., et al. Severe maternal morbidity due to respiratory disease and impact of 2009 H1N1 influenza A pandemic in Brazil: results from a national multicenter cross-sectional study. BMC Infect Dis. 2016;16:220. doi: 10.1186/s12879-016-1525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie J.K., Salibay C.J., Kang M., Glenn-Finer R.E., Murray E.L., Jamieson D.J. Pregnancy and severe influenza infection in the 2013–2014 influenza season. Obstet Gynecol. 2015;125:184–192. doi: 10.1097/AOG.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 25.Vasquez D.N., Das Neves A.V., Vidal L., et al. Characteristics, outcomes, and predictability of critically ill obstetric patients: a multicenter prospective cohort study. Crit Care Med. 2015;43:1887–1897. doi: 10.1097/CCM.0000000000001139. [DOI] [PubMed] [Google Scholar]
- 26.Lapinsky S.E., Rojas-Suarez J.A., Crozier T.M., et al. Mechanical ventilation in critically ill pregnant women: a case series. Int J Obstet Anesth. 2015;24:323–328. doi: 10.1016/j.ijoa.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Collop N.A., Sahn S.A. Critical illness in pregnancy. An analysis of 20 patients admitted to a medical intensive care unit. Chest. 1993;103:1548–1552. doi: 10.1378/chest.103.5.1548. [DOI] [PubMed] [Google Scholar]
- 28.Hung C.Y., Hu H.C., Chiu L.C., et al. Maternal and neonatal outcomes of respiratory failure during pregnancy. J Formos Med Assoc. 2018;117:413–420. doi: 10.1016/j.jfma.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A., Agarwal S., Kumar V., Nawal C.L., Mital P., Chejara R. A study of an influenza A (H1N1)pdm09 outbreak in pregnant women in Rajasthan, India. Int J Gynaecol Obstet. 2016;132:146–150. doi: 10.1016/j.ijgo.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J.J.Y., Ong J.A., Syn N.L., et al. Extracorporeal membrane oxygenation in pregnant and postpartum women: a systematic review and meta-regression analysis. J Intensive Care Med. 2019 doi: 10.1177/0885066619892826. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Muthu V., Agarwal R., Dhooria S., et al. Epidemiology, lung mechanics and outcomes of ARDS: a comparison between pregnant and non-pregnant subjects. J Crit Care. 2019;50:207–212. doi: 10.1016/j.jcrc.2018.12.006. [DOI] [PubMed] [Google Scholar]


