Abstract
Oral candidiasis is a very common oral condition among susceptible individuals, with the main causative organism being the fungus Candida albicans. Current drug delivery systems to the oral mucosa are often ineffective because of short drug/tissue contact times as well as increased prevalence of drug-resistant Candida strains. We evaluated the potency of saturated fatty acids as antifungal agents and investigated their delivery by novel electrospun mucoadhesive oral patches using agar disk diffusion and biofilm assays. Octanoic (C8) and nonanoic (C9) acids were the most effective at inhibiting C. albicans growth on disk diffusion assays, both in solution or when released from polycaprolactone (PCL) or polyvinylpyrrolidone/RS100 (PVP/RS100) electrospun patches. In contrast, dodecanoic acid (C12) displayed the most potent antifungal activity against pre-existing C. albicans biofilms in solution or when released by PCL or PVP/RS100 patches. Both free and patch-released saturated fatty acids displayed a significant toxicity to wild-type and azole-resistant strains of C. albicans. These data not only provide evidence that certain saturated fatty acids have the potential to be used as antifungal agents but also demonstrate that this therapy could be delivered directly to Candida-infected sites using electrospun mucoadhesive patches, demonstrating a potential new therapeutic approach to treat oral thrush.
Keywords: oral medicine, candidiasis, antifungal, drug resistance, fatty acids, electrospinning
Introduction
Forming part of the normal oral microbial flora, Candida albicans is a human commensal fungal organism that can be detected in the oral cavity of approximately half of all healthy individuals.1 A balance exists between the oral microbial community and host immunity that maintains many organisms in their commensal state. This balance can be disrupted by several factors such as the prolonged use of antibiotics reducing the number of oral bacteria, leading to microbial dysbiosis and the unchecked growth of C. albicans on the surface of the oral mucosa that manifests clinically as pseudomembranous or erythematous candidiasis where Candida penetrate the oral epithelium, leading to tissue damage.2 Individuals at risk of transient or chronic immunosuppression such as those receiving corticosteroids, immunotherapy, or chemotherapy, or those with HIV infection or salivary gland dysfunction (Sjögren’s syndrome) are also highly susceptible to opportunistic Candida mucosal infection where the incidence of oral candidiasis for these patients can be as high as 95%.3−5 Indeed, C. albicans infections among the immunocompromised are increasing and are now a leading cause of nosocomial infections in the United States.6 Environmental factors also play a role in oral Candida infection where smoking is heavily linked with chronic hyperplastic candidiasis, a condition that is associated with premalignant lesions that have a high risk of transformation to oral cancer.7 Excessive C. albicans growth is also a common problem for denture wearers where the organism is able to form a biofilm between the denture and the hard palate, clinically known as chronic atrophic candidiasis or denture stomatitis, which causes significant Candida-mediated mucosal tissue damage.8 At present, candidiasis is treated with a number of antifungal agents such as nystatin or with azoles such as miconazole and fluconazole. Worryingly, research shows that C. albicans has become increasingly resistant to these common antifungal drugs,9,10 so finding alternative treatments to overcome antifungal resistance is of paramount importance.
Some alternative antifungal therapies that have been explored include the use of surfactants,11,12 synthetic peptides,13−15 and fatty acids.16 The size, hydrophilicity, and charge of these molecules determine how they interact with the C. albicans cell wall, inhibiting the cell growth and reducing the viability or the biofilm formation.12 An issue with surfactants and some peptides is their relatively high toxicity to mammalian cells, whereas fatty acids have been used as antimicrobial agents in food and dermatological products for many years and their toxicity to human cells is reported to be relatively low.17 Indeed, many fatty acids, including those naturally secreted by microbes, have been shown to display antifungal properties.18,19 While a number of fungal species appear to have evolved detoxification mechanisms for fatty acids, these do not appear to be present in Candida.20 However, the potential for the treatment of oral candidiasis using short-to-medium-chain fatty acids remains relatively unexplored.
Another key consideration is the delivery method of antifungal compounds. The majority of antifungal drugs are delivered orally and are therefore delivered to lesion sites via the circulation, even for the treatment of localized mucosal Candida infection. Such a therapy often causes undesirable side effects such as headaches, nausea, abdominal pain, vomiting, and diarrhea.21 For topical treatment, current antifungal delivery options include creams, gels, mouthwash solutions, or oral lozenges.22 These preparations provide good initial coverage but are severely limited by their quick removal from the mucosal surface to the alimentary canal by saliva flow, restricting prolonged drug availability. Therefore, a delivery system that is able to retain the antifungal compound at the disease site and release its therapeutic load over a protracted time period would be of great benefit.
We have recently developed mucoadhesive electrospun patches for therapeutic use in oral medicine.23−25 These patches are able to release drugs in vivo, are well tolerated by humans, and are currently in phase two clinical trials for the treatment of oral lichen planus. Electrospinning is a versatile fiber-manufacturing technique that combines polymers, solvents, and other molecules into microscale fibers that can be collected as mesh-like patches with a high surface area for drug availability and adhesion to the oral mucosa.26 Common polymers used in the manufacture of electrospun patches include polycaprolactone (PCL) and polyvinylpyrrolidone (PVP) as these are known to be highly compatible with this spinning technology, are nontoxic, and have been approved by the Food and Drug Administration (FDA) as ingredients in multiple pharmaceutical products for human use, allowing a quick route to clinical use. For the different clinical forms of mucocutaneous oral candidiasis, a mucoadhesive electrospun patch could not only deliver the antifungal over a prolonged period of time at the disease site but also act as a protective barrier for painful lesions. Consequently, combining the electrospun delivery system with an antifungal compound would provide many benefits for patients with oral candidiasis. Here, we identify short-to-medium-chain fatty acids with potent antifungal properties and report their successful incorporation into and release from electrospun patches with effective antifungal activity.
Materials and Methods
Electrospinning Conditions and Electrospun Patch Production
Electrospinning methods including solution preparation and patch production for both PCL and PVP/RS100 patches were performed as previously described by Santocildes et al.(23) Briefly, PCL (Mw 80 kDa; Sigma-Aldrich, Poole, UK) was added at 10 wt % to a solution of dichloromethane/N,N-dimethylformamide (93:7 w/w %; Thermo Fisher Scientific, Altrincham, UK) and mixed at room temperature until dissolved. PVP (Kollidon 90 F, Mw 1000–1500 kDa; BASF, Cheadle, UK) at 10 wt % and Eudragit RS100 (Mw 32 kDa; Evonik Industries AG, Essen, Germany) at 12.5 wt % were dissolved in 97% w/w ethanol prepared in deionized water at room temperature. In this study, short-to-medium-chain fatty acids including butanoic (C5), hexanoic (C6), heptanoic (C7), octanoic (C8), nonanoic (C9), decanoic (C10), undecanoic (C11), or dodecanoic (C12) (all from Sigma-Aldrich, Poole, UK) were added to the polymer dope solutions at a concentration of 0.2 M prior to spinning. Spinning conditions were 17 kV, 3 mL/h with a 15 cm tip-to-collector distance. 1H NMR spectroscopy was used to show that solvents were absent in the final composition of patches (Figure S1).
Scanning Electron Microscopy
Samples were analyzed by scanning electron microscopy (SEM) as previously described.23 Electrospun patches were sputter-coated with gold (5 nm thickness) and imaged using a Philips XL20 scanning electron microscope using an emission current of 15 kV.
Nuclear Magnetic Resonance To Measure Fatty Acid Content of PCL Electrospun Patches
Proton (1H) nuclear magnetic resonance (NMR) spectra of fatty acids and PCL electrospun patches dissolved in 1 mL of deuterated chloroform (CDCl3) and sealed in a 5 mm tube were recorded using an AVANCE III or AVANCE III HD spectrometer (Bruker, Coventry, UK) at 298 K and 400.2 or 500.13 MHz. The quantitative spectra were recorded with eight transients using a 90° pulse and a relaxation delay of 40 s (having previously determined the longest T1 within the mixtures to be ∼6.5 s), over an acquisition window of 20 ppm and 64k acquisition points. The spectra were analyzed using TopSpin version 3.2 software (Bruker, Coventry, UK). The 1H NMR peaks are representative of the number of hydrogens present at a particular resonance, where the normalized integrated signals give the relative number of hydrogens at a particular resonance. By comparing the normalized integrals of distinguishable PCL signals versus fatty acid signals, the mole ratio of the two components was calculated, and thereby, the mass concentration of fatty acid present in the PCL electrospun fibers was established.
Gas Chromatography To Measure Fatty Acid Content in PVP/RS100 Electrospun Patches
Electrospun PVP/RS100 patches were weighed and dissolved in 1 mL of 97% (v/v) ethanol. Standards of the fatty acids were also prepared in 97% (v/v) ethanol, ranging from 0.01 to 1 M. An Agilent DB-WAX-UI (30 m × 0.25 mm × 0.25 μm) column was used with helium flowing through the column at 1.2 mL min–1 with a pressure of 9.15 psi. The injection volume of the sample was 1 μL, and the temperature of the column was increased from 50 to 250 °C at 10 °C min–1 intervals. The mass spectrometer scan range was between 25 and 500 m/z.
Strains and Growth Conditions
The C. albicans wild-type strains used were SC5314 (provided by Dr Stephen Saville, University of Texas at San Antonio, USA) and BWP17 (provided by Prof. Julian Naglik, Kings College London, UK). The azole-resistant clinical strain CAR17 was provided by Prof. David Williams (Cardiff University, UK). Candida auris strain 8971, Candida tropicalis strain 3111 (both National Collection of Pathogenic Fungi, Public Health England, UK), and Candida glabrata strain OM146/89 (Sheffield Teaching Hospitals NHS Foundation Trust, UK) were also used. All strains were cultured on 1% w/v yeast extract, 2% w/v peptone, and 2% w/v dextrose (YPD; Oxoid, Basingstoke, UK) agar at 37 °C and stored at 4 °C. Two to three colonies of each strain were inoculated in 15 mL of YPD broth and incubated at 30 °C overnight. The colonies were then counted and resuspended in phosphate-buffered saline (PBS) or RPMI-1640 (Sigma-Aldrich, Poole, UK) for further experimentation.
Fatty Acid Susceptibility Using Agar Disk Diffusion
Agar disk diffusion tests on YPD agar plates were performed using strains SC5314, BWP17, and CAR17. Fatty acids with chain lengths of C5 to C12 were prepared at 0.2 M in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Poole, UK); 200 μL of 2 × 105 colony-forming units (CFU) per milliliter of C. albicans was diluted in PBS and spread across the agar plate; 20 μL of each fatty acid solution was pipetted onto filter paper susceptibility disks that were then placed on the C. albicans-streaked agar plate in triplicate. Fluconazole (5 μg/disk; purchased in powder form and prepared in DMSO, Sigma-Aldrich, Poole, UK) and miconazole (10 μg/disk; used as purchased from Thermo Fisher Scientific, Altrincham, UK) were used as positive controls and DMSO as the negative control. Agar plates were incubated overnight at 37 °C, imaged, and the zone of inhibition was measured using Fiji imaging software (National Institute of Health, Bethesda, USA27). Agar diffusion was also used to determine the antifungal effects of fatty acid-containing electrospun patches; here, 12.7 mm diameter disks were punched from fatty acid-containing patches, placed onto C. albicans-streaked plates, incubated overnight at 37 °C, and analyzed as previously described; patches containing no fatty acid were used as controls.
C. albicans Biofilm XTT Assay
An XTT assay was used to determine the levels of C. albicans metabolism as a measure of the biofilm viability.28C. albicans biofilms were prepared by adding 100 μL of a 1 × 106 CFU/mL suspension of SC5314 in RPMI-1640 into each well of a 96-well plate and incubating overnight at 37 °C. Wells were then washed with PBS and incubated for 18 h at 37 °C with a concentration series of each fatty acid (C7 to C12, prepared in 0.8 M DMSO as the carrier and further diluted in RPMI-1640) ranging from 0.0015 to 0.4 M. Biofilms treated with equivalent concentrations of DMSO were used as controls. Biofilms were washed with PBS, and then 50 μL of sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis-(4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT; Sigma-Aldrich, Poole, UK) was added to each well and the plate was incubated for 1 h, after which the XTT solutions were transferred to a new plate and OD was measured at 450 nm and 690 nm for background correction (Infinite 200 Pro, Tecan, Switzerland). The data were plotted after transformation by nonlinear regression, and IC50 values were calculated using GraphPad Prism v8 (GraphPad Software). A modified version of the XTT assay was also used to measure the effect of fatty acid-containing patches on the C. albicans biofilm viability. Here, C. albicans biofilms were prepared in poly-l-lysine-coated 24-well plates using strain SC5314 or CAR17. C. albicans (1 × 106 CFU in RPMI-1640) were added to each well, and the plates were incubated overnight at 37 °C. Wells were washed with PBS, and then fatty acid-containing electrospun disks (12.7 mm ø) were placed in each well, followed by 200 μL of RPMI-1640; disks containing no fatty acid were used as controls. Following incubation at 37 °C for 18 h, the liquid in each well was removed and 200 μL of XTT reagent was added to each well and incubated for 1 h, after which the XTT reagent was removed to a new plate, OD measured, and data analyzed as previously described.
Fluorescence Microscopy of the Biofilm Viability
Biofilms were prepared and treated with fatty acid preparations as previously described. Biofilms were then washed and incubated with the Live/Dead BacLight viability kit (Thermo Fisher Scientific, Altrincham, UK) according to the manufacturer’s instructions. Microscopy images were acquired using an Axiovert 200 M inverted fluorescence microscope supported with AxioVision software (Zeiss, Oberkochen, Germany).
Statistics
Statistical analysis was undertaken using GraphPad Prism v8.0 (GraphPad Software). All data are expressed as mean ± standard deviation (SD) of at least three independent experiments, each performed in triplicate unless otherwise stated. Group-wise comparisons were carried out using ANOVA Kruskal–Wallis test with Dunn’s post hoc multiple comparison analysis, and differences were considered significant when p < 0.05.
Results
Application of Medium-Chain-Saturated Fatty Acid Solutions Inhibit C. albicans Yeast Growth
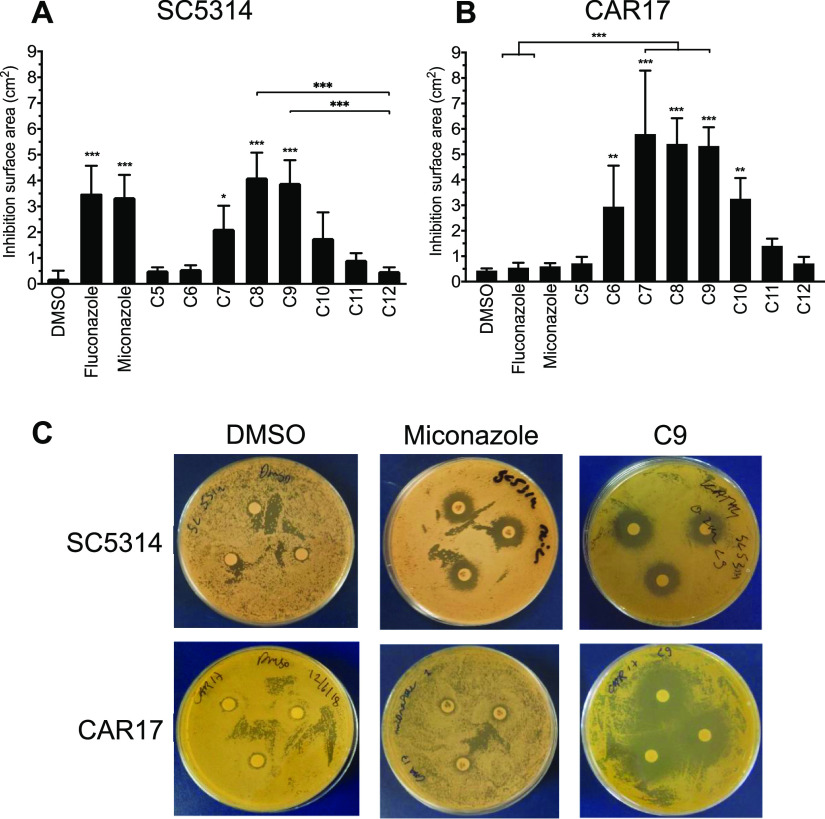
The growth inhibition properties of a range of fatty acid solutions (used at 0.2 M) were tested on both wild-type (SC5314, BWP17) strains and an azole-resistant clinical strain (CAR17) of C. albicans using an agar disk diffusion assay. For SC5314, zones of growth inhibition that were significantly different from those of DMSO controls were observed for heptanoic (C7), octanoic (C8), and nonanoic (C9) acid (p ≤ 0.05), with octanoic and nonanoic acid showing similar levels of inhibition to that of the commonly used clinical antifungal drugs, fluconazole and miconazole (Figure 1A,C). Similar data were observed for strain BWP17 that appeared to be even more susceptible to treatment with octanoic and nonanoic acids than the SC5314 strain (Figure S2). The CAR17 strain was confirmed as being azole-resistant as treatment with either fluconazole or miconazole failed to inhibit C. albicans growth (Figure 1B,C).29,30 In contrast, significant zones of inhibition were observed upon treatment with fatty acids ranging from hexanoic (C6) to decanoic (C10) acid (p ≤ 0.01; Figure 1B,C). We next extended these studies to test the activity of some of these fatty acids in other Candida species. Similar to its effect on C. albicans, nonanoic acid (C9) caused significant growth inhibition of C. auris (p < 0.01), C. tropicalis (p < 0.01), and C. glabrata (p < 0.001) in particular when compared to dodecanoic acid (C12) or DMSO controls (Figure S3). These data indicate that several short–medium-chain fatty acids have the ability to markedly inhibit the growth of both susceptible and azole-resistant forms of C. albicans as well as other disease-causing Candida species.
Figure 1.
Saturated fatty acids in DMSO solution ranging from butanoic (C5) to dodecanoic (C12) at 0.2 M loaded onto filter disks inhibit C. albicans strains streaked on agar plates. Fluconazole (0.8 mM) and miconazole disks (10 μg or 2.4 nmol) were used as positive controls and DMSO as the negative control. On the wild-type strain (A) SC5314, heptanoic (C7), octanoic (C8), and nonanoic acid (C9) have similar surface area inhibition zones as fluconazole and miconazole, whereas on the patient-specific strain (B) CAR17, C7, C8, and C9 have even greater inhibition areas, whereas the commonly used antifungals fluconazole and miconazole do not have significant inhibition zones. Results are mean ± SD (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001 vs DMSO. (C) Visual representative images of treated agar plates related to data in (A,B).
Fatty Acid Solutions Reduce the C. albicans Biofilm Viability in a Chain Length-Dependent Manner
The agar disk diffusion assay tests the ability of a compound to inhibit the growth of C. albicans from single-cell yeast forms, whereas C. albicansin vivo mainly exist as biofilm structures that are much less susceptible to antifungal treatment. Therefore, we tested whether fatty acids were able to affect the viability of preformed in vitro cultured biofilms using an XTT assay where changes in metabolism are used as a surrogate measure of the C. albicans viability. The viability of the SC5314 biofilm was dose-dependent and also decreased in a fatty acid carbon chain length-dependent manner (Figure 2A). Calculation of the 50% inhibitory concentration (IC50) showed that undecanoic (C11) and dodecanoic (C12) acids had approximately 10-fold lower IC50 values than those for heptanoic (C7) and octanoic (C8) acids (4.7 ± 1.1 and 5.4 ± 1.3 for C11 and C12 compared to 47.3 ± 1.9 and 47.5 ± 2.7 for C7 and C8, respectively) (Figure 2B). To confirm that the fatty acids were directly killing C. albicans and not just altering their cell metabolism or fungistatic, a live/dead fluorescent stain was performed on 50 mM fatty acid-treated biofilms. Image analysis showed that decanoic (C10), undecanoic (C11), and dodecanoic (C12) acids caused significantly (p < 0.001) more cell death as determined by propidium iodide-positive staining than heptanoic (C7), octanoic (C8), and nonanoic (C9), as well as medium-only controls (Figure 2C,D). These results are in agreement with those obtained for the XTT biofilm assay, confirming the chain length association with Candida killing efficiency. These data also show that nonanoic (C9) acid, which was the most efficient fatty acid at killing C. albicans in the agar diffusion assay, was much less potent at killing a C. albicans biofilm, whereas the opposite was observed for dodecanoic (C12) acid, which was the most proficient of all fatty acids tested at reducing the biofilm viability. Interestingly, fluorescence microscopy revealed that nonanoic acid mainly killed yeast forms of C. albicans, whereas dodecanoic acid killed both yeast and hyphal forms within the biofilm (Figure 2D).
Figure 2.
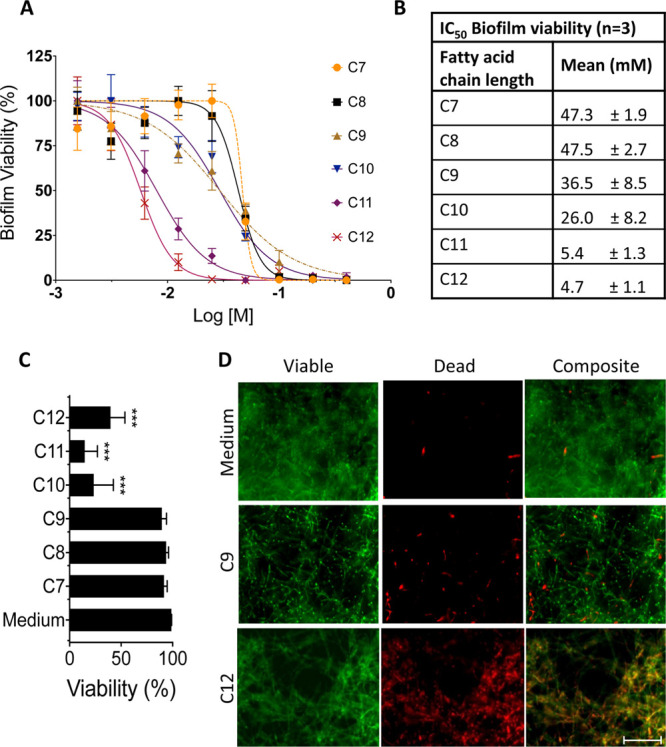
C. albicans strain SC5314 biofilm viability when subjected to fatty acids’ heptanoic acid (C7) to dodecanoic acid (C12) ranging in concentration in graph (A) from 400 to 1.56 mM using a metabolic XTT assay. The data are normalized to untreated controls (100% viability) and mean ± SD (n = 3). (B) Table showing the IC50 of fatty acids on the C. albicans SC5314 biofilm. (C) Live/dead image analysis data of the C. albicans strain SC5314 biofilms treated with 50 mM fatty acids ranging from C7 to C12 (p < 0.001 for C10–C12 compared to all other conditions, n = 5). (D) Representative fluorescence live (green)/dead (red) and composite images for conditions media-only, C9, and C12 applied to biofilms. Scale bar = 50 μm.
Incorporation of Fatty Acids into Electrospun Patches
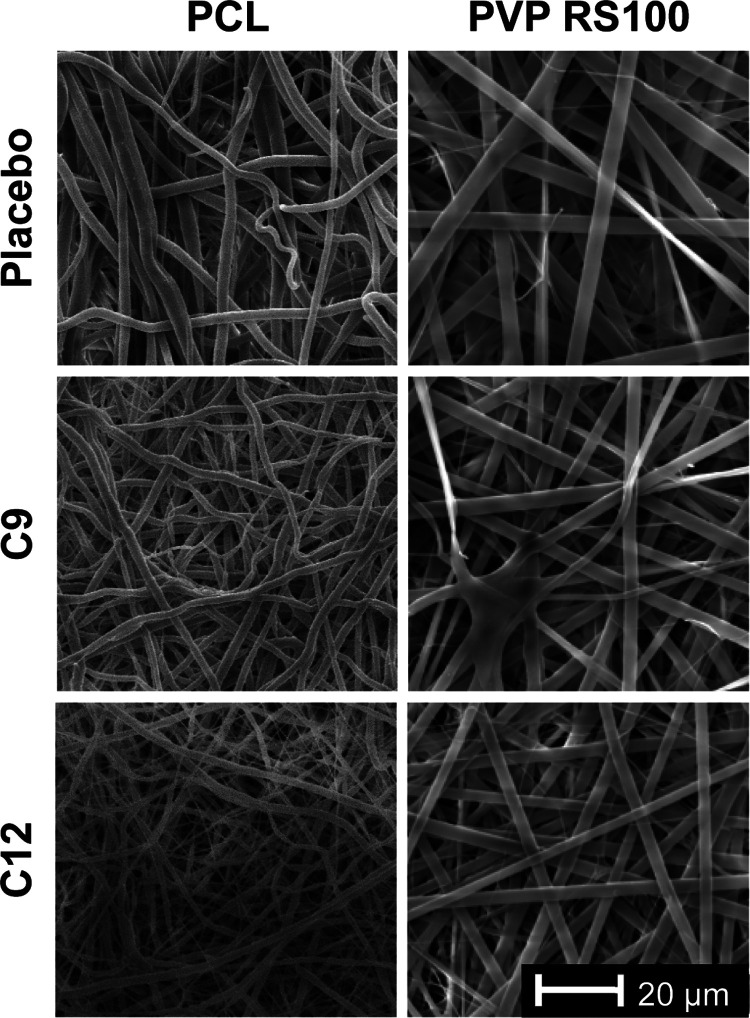
As nonanoic (C9) and dodecanoic (C12) acids were shown to have the greatest effect on the C. albicans viability in the agar and biofilm assays, respectively, these fatty acids were chosen to incorporate into PCL and PVP/RS100 electrospun patches. SEM images show that PCL and PVP/RS100 patches are composed of a mesh-like network of microfibers. The placebo patches that contained no fatty acid displayed fibers of similar width for both PCL and PVP/RS100 patches, although PVP RS100 fibers appeared straighter, flatter, and more uniform than the PCL fibers (Figure 3). Addition of nonanoic or dodecanoic acid to the PCL dope produced electrospun fibers with much smaller diameters, resulting in a more entangled mesh compared to the placebo PCL patch, whereas this was not evident when these fatty acids were incorporated into PVP RS100 fibers (Figure 3).
Figure 3.
Representative SEM images of PCL and PVP RS100 electrospun patches containing no fatty acid (placebo) or nonanoic (C9) or dodecanoic (C12) acid. The scale bar in the bottom right applies to all images.
In the process of electrospinning, the solvent system evaporates as the fibers are produced. Consequently, it is feasible that some of the volatile short-chain fatty acids may also evaporate in the procedure, leading to discrepancies between the fatty acid concentration in the pre-electrospinning polymer dope and that in the final electrospun patch. Therefore, it is important to evaluate the final fatty acid content in the electrospun patch. NMR spectroscopy was used to analyze PCL patches as the 1H NMR peaks for PCL and the fatty acid component did not overlap (Figure S4A,B). However, the PVP/RS100 patches displayed overlapping 1H NMR peaks and therefore GC–MS was used as an alternative quantification method. The mean w/w % concentration of the fatty acid compared to the total patch weight was 2.2% for nonanoic (C9) acid and 22% for dodecanoic (C12) acid for the PCL patches (Figure S4C), compared to 8.1% for nonanoic (C9) acid and 12% for dodecanoic (C12) acid in the PVP RS100 patches (Figure S4D). As the fatty acids had been added as a molar concentration in the solvent system of the electrospinning solution, the actual percentage mass of fatty acid to the polymer mass in the polymer dope solution prior to electrospinning was 28% and 18% for dodecanoic (C12) acid and 22% and 14% for nonanoic (C9) acid in PCL and PVP/RS100 solutions, respectively.
Electrospun Patches Containing Fatty Acids Inhibit C. albicans Growth
An agar disk diffusion assay was used to test if fatty acids could be released from electrospun patches and remain inhibitory to C. albicans growth. PCL placebo patches did not inhibit C. albicans growth, showing that polymers alone do not affect the C. albicans viability, whereas a small zone of inhibition was observed for PVP/RS100 patches (Figure 4A,B). It was noted that the PVP/RS100 patches are much reduced in diameter at the end of the experiment compared to their PCL counterparts, even though they were both the same size (12.7 mm diameter) at the start of the experiment. This is likely related to the hydrophilicity of the PVP/RS100 patches, which swell almost instantly when placed on the agar plate as a consequence of moisture uptake from the C. albicans lawn, resulting in an increase in volume but decrease in area, which may give rise to the observed false-positive effect (Figure 4B). For the PCL patches, both nonanoic (C9) and dodecanoic (C12) acids displayed greater zones of inhibition compared to the placebo PCL patches (p < 0.001 and p < 0.01 respectively; Figure 4A). Moreover, the disk diffusion data clearly show that the released nonanoic acid is the most potent fatty acid causing a significantly greater zone of inhibition than dodecanoic acid (C9: 7.2 ± 1.3 mm compared to C12: 2.3 ± 1.7 mm, p < 0.001; Figure 4A,B). Similarly, nonanoic acid- and dodecanoic acid-containing PVP/RS100 patches displayed greater zones of inhibition compared to placebo patches (p < 0.001 and p < 0.01 respectively; Figure 4A). In contrast to PCL patches, dodecanoic acid-containing PVP/RS100 patches were equally as potent at inhibiting C. albicans growth as nonanoic acid-containing patches. Meanwhile, nonanoic acid-containing PCL patches were significantly better at inhibiting growth than both nonanoic and dodecanoic acid-containing PVP/RS100 patches (p < 0.01; Figure 4A).
Figure 4.
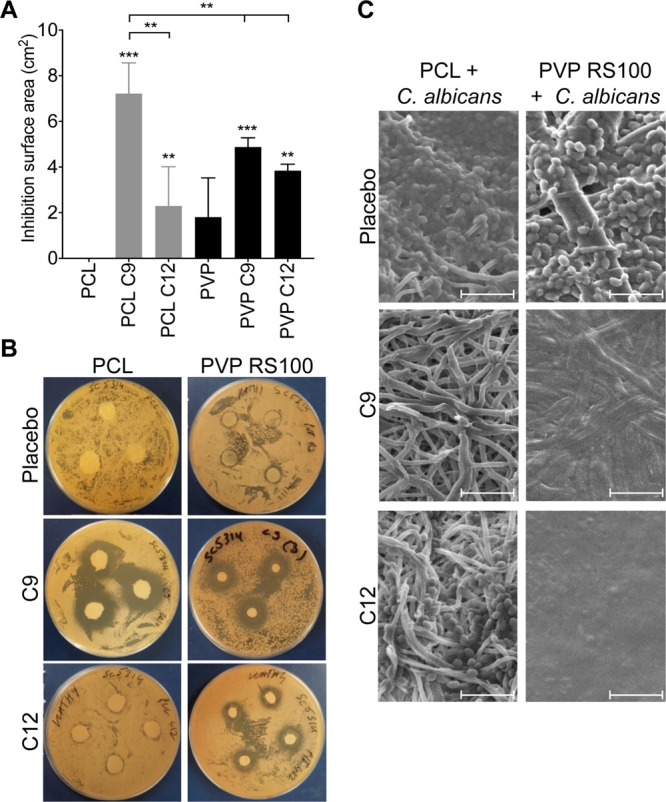
(A) Inhibition surface areas of PCL and PVP R100 fibers containing nonanoic (C9) and dodecanoic (C12) acids on C. albicans (strain SC5314) on streaked agar plates are significantly different to the respective placebo controls. Results are mean ± SD (n = 3). p-Values above SD bars are compared to the PCL or the PVP RS100 placebo patch and comparison between PCL and the PVP RS100 patch with the same fatty acid is given. **p < 0.01; ***p < 0.001. (B) Representative images of agar disk diffusion plates. (C) Representative SEM images of the electrospun patches once removed from a C. albicans-streaked agar plate are shown; the round shapes along the fibers are the yeast Candida cells, which are present in all PCL patch conditions, even with C9 and C12 content, however, are only present in the PVP/RS100 placebo patch. Scale bar = 20 μm.
SEM images of the fatty acid-containing electrospun patches that had been used to inhibit C. albicans growth gave a comparative view of whether C. albicans yeast cells are still present among the fibers of the electrospun patches (Figure 4C). In both the PCL and PVP/RS100 placebo patches, yeast forms of C. albicans can be seen adhering to the polymer fibers (Figure 4C). In contrast, no C. albicans was observed in either PCL or PVP/RS100 patches loaded with nonanoic acid, in line with its antifungal activity. In dodecanoic acid-containing PCL patches, yeast forms of C. albicans were detected but were markedly less abundant than in placebo patches, whereas no Candida were seen in SEM images of dodecanoic acid-containing PVP/RS100 patches (Figure 4C). The contrast between the PCL and PVP RS100 SEM images clearly shows the difference in hydrophilicity between the two polymer systems; the hydrophilicity of the PVP RS100 patch is greater with the inclusion of dodecanoic acid than nonanoic acid, producing a collapsed fibrous structure upon contact with the moist agar plate surface (Figure 4C).
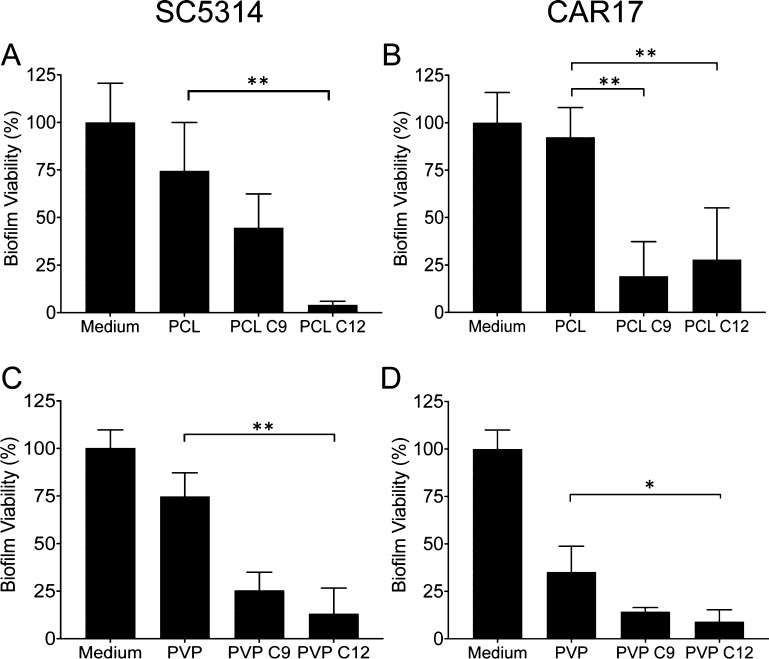
The effect of the electrospun patches containing nonanoic or dodecanoic acid on the established C. albicans biofilm viability (SC5314 and CAR17) was determined using an XTT assay. The placebo patches caused a slight reduction in the biofilm viability for both strains tested, especially PVP/RS100 on the CAR17 strain in which the viability was reduced by 60%, although this was not statistically different to C. albicans treated with a medium alone (Figure 5A–D). This reduction may be due to the presence of the quaternary amine-containing polymer RS100 in the patch as polycations are known to have an antifungal effect against Candida.31 Dodecanoic acid (C12)-containing PCL patches caused a 17-fold reduction in the SC5314 biofilm viability compared to treatment with PCL placebo patches (p < 0.001), whereas treatment with nonanoic acid-containing PCL patches showed no effect (Figure 5A). In contrast, treatment with nonanoic or dodecanoic acid-containing PCL patches significantly reduced the biofilm viability of the azole-resistant CAR17 strain by 80% and 70%, respectively (p < 0.01; Figure 5B). PVP/RS100 patches loaded with dodecanoic acid reduced both the SC5314 and the CAR17 biofilm viability by 82% (p < 0.01, Figure 5C) and by 74% (p < 0.05, Figure 5D) compared to placebo controls, whereas nonanoic acid-containing PVP/RS100 patches, although reduced the viability, showed no statistically significant effect (Figure 5C,D).
Figure 5.
Nonanoic (C9) and dodecanoic (C12) acid-loaded PCL and PVP/RS100 electrospun patches reduce the SC5314 and the CAR17 C. albicans biofilm viability. The following conditions are shown: PCL patches on the (A) SC5314 biofilm and (B) on the CAR17 biofilm, PVP/RS100 patches on the (C) SC5314 biofilm and (D) on the CAR17 biofilm. Results are mean ± SD (n = 3). *p < 0.05; **p < 0.01 compared to the placebo electrospun patch.
Discussion
As described above, oral candidiasis is relatively common amongst immunocompromised individuals, those on long-term antibiotics, and denture wearers and can cause substantial tissue damage.32 Topical, local delivery at the site of infection is preferred to directly target C. albicans growth; however, current delivery methods are transient with the drug being quickly removed from the mucosal surface via mastication and salivary flow. We have recently developed a highly mucoadhesive oral patch that can attach to mucosal surfaces for up to 2 h and have used these to deliver the anesthetic lidocaine25 or the steroid clobetasol-17-proprionate as a means to treat the autoimmune condition, oral lichen planus.23,24 Here, we examined the effectiveness of delivering antifungal therapy using similar electrospun patches.
Studies have shown that various fatty acids display antifungal properties against an array of fungal species, although data regarding their effects on C. albicans are limited.16 Nearly all of the previous studies have been performed using C. albicans strains grown in their yeast form in broth culture. However, it is widely accepted that C. albicans are present in the oral cavity as a biofilm, which is a mixture of yeast and hyphal forms within a dense extracellular matrix. Therefore, in this study we used two in vitro methods to study C. albicans growth inhibition; an agar disk diffusion assay and a biofilm assay determine if fatty acids could prevent C. albicans growth from a yeast form or affect the viability of a pre-existing biofilm.
Using the agar disk diffusion test, it was found that octanoic (C8) and nonanoic (C9) acids showed the greatest C. albicans growth inhibition on both wild-type (SC5314 and BWP17) and the azole-resistant CAR17 strain. For the wild-type strains, inhibition levels were similar to the commonly used antifungal agents fluconazole and miconazole, albeit at higher concentrations of fatty acids compared with azoles. Importantly, these data show, for the first time, that certain fatty acids can inhibit the growth of azole-resistant strains, which is of great importance given the rapid rise of antifungal resistance in many clinical isolates of C. albicans.30 Previous studies showed that only even-numbered carbon-chain-length fatty acids were effective against C. albicans.(33−36) Therefore, to our knowledge, this is the first study to show that nonanoic (C9) acid inhibits C. albicans growth as well as the growth of C. glabrata, C. auris, and C. tropicalis. Additionally, there are no previously published agar disk diffusion data for fatty acids on C. albicans. Other studies using a fatty acid as an anti-C. albicans agent measured minimum inhibitory concentration (MIC) by broth turbidity or by colony counting.33−36 Huang et al. showed that hexanoic, octanoic, and dodecanoic acid all inhibited C. albicans with a similar potency,33 whiereas both Kabara et al.(34) and Bergsson et al.(35) reported that decanoic and dodecanoic acids provided the greatest inhibition, with octanoic acid producing low inhibitory responses. Hayama et al.(36) reported the MIC of octanoic acid, decanoic acid, and dodecanoic acid as 34.7, 29.0, and 49.9 mM, respectively on a C. albicans clinical isolate. Overall, these data suggest that for yeast forms, the predominant morphological form in these assays, nonanoic and decanoic acids have the most potent antifungal activity. The reason for the large zone of inhibition for octanoic compared to decanoic, undecanoic, and dodecanoic acids in this study is likely due to its water solubility that is 10-fold higher than for decanoic acid and as the carbon chain length of the fatty acid increases the water solubility decreases further. This increased solubility allows octanoic acid to diffuse further within the agar, reaching a larger surface area to inhibit C. albicans growth.
Experiments conducted on preformed biofilms provided a different perspective. Here, undecanoic and dodecanoic acid were the most potent C. albicans inhibitors (IC50 5.4 and 4.7 mM, respectively). Only one previous study has conducted inhibition tests on biofilms, where far lower fatty acid concentrations were required for a reduction in the biofilm viability.36 The reason for this may be the use of different C. albicans strains, the number of CFU used (5 × 103 compared to 1 × 105 cells/mL used in this study), and the use of crystal violet staining compared to XTT. Crystal violet stains the entire biomass (including viable, nonviable cells and extracellular matrix), whereas XTT measures the cell metabolism as a surrogate for the viability and has been shown to have greater experimental reproducibility compared to crystal violet staining.37,38 Live/dead fluorescence staining confirmed that the fatty acids directly affected the C. albicans viability rather than the metabolism. Here, nonanoic acid showed low potency on the biofilm and appeared to selectively kill yeast forms, whereas dodecanoic acid caused significant cell death of both yeast and hyphal forms.
The main molecular mechanism by which fatty acids are thought to act is through their direct insertion into the fungal plasma membrane, resulting in increased fluidity, dysregulation of membrane proteins, and altered hydrostatic turgor pressure within the cell leading to cytoplasmic disorder and ultimately cell death.18,35,39 There is no evidence to corroborate our finding that dodecanoic acid has selectivity for hyphal forms of C. albicans, although there are reports that certain fatty acids impact hyphal formation, where McClain et al.(40) found that undecanoic acid prevented hyphal formation by inhibiting enzymes involved in lipid synthesis. Moreover, dodecanoic acid was found to affect cAMP-mediated pathways,41 whereas decanoic acid reduced the expression of the hyphal wall protein HWP-1,19 both of which lead to reduced hyphal formation in C. albicans.
There are no previous reports on incorporating fatty acids into electrospun patches for antimicrobial purposes, although dodecanoic acid has been incorporated into electrospun polymer fibers to phase-change the polymer material for thermal energy storing uses.42,43 Tonglairoum et al.(44−46) incorporated the antifungal drug clotrimazole into electrospun patches that successfully eradicated yeast forms of C. albicans in broth dilution assays. Therefore, given the potency of saturated fatty acids as antifungal agents, even for azole-resistant C. albicans strains, and the applicability of electrospun patches as mucosal drug delivery vehicles, we reasoned that these two entities be combined for antifungal therapy.
We selected nonanoic and dodecanoic acid fatty acids because of their contrasting activities in disk diffusion and biofilm experiments. Both fatty acids were successfully electrospun in PCL and PVP/RS100 fibers. There was a slight morphological change when the fatty acids were incorporated into the PCL patches compared to PVP/RS100, which is explained by the different degrees of plasticization of the polymer fibers. 1H NMR spectroscopy and GC–MS analysis confirmed fatty acid loading. The relatively volatile nonanoic acid was found at low levels in the PCL fibers, whereas the less volatile dodecanoic acid was present at a similar wt % as in the original polymer dope, which was 22 wt % in the fibers compared to the 28 wt % in the polymer dope. In contrast, in the PVP/RS100 fibers, nonanoic acid and dodecanoic acid were both incorporated at 6 wt % less in the fibers compared to what had been present in the polymer dope. Reasons for this were not explored, but the fatty acids will interact differently with the polymers; for example, hydrogen bonding between the carboxyl group in dodecanoic acid and esters in poly(d,l-lactide-co-glycolide) has previously been shown using Fourier-transform infrared spectroscopy.47 The amide groups in PVP should hydrogen-bond more strongly to the carboxyl groups of the fatty acids, which may explain why more nonanoic acid is incorporated in the PVP/RS100 fibers compared to the PCL fibers.
Agar disk diffusion tests using nonanoic and dodecanoic acid-containing electrospun patches produced comparable inhibition data to that observed with soluble fatty acids released from filter paper disks. Nonanoic acid release was found to produce the largest inhibition zones and kill C. albicans bound to the polymer fibers, particularly for PCL patches, even though the 1H NMR data showed a lower fatty acid content, once again indicating that nonanoic acid is the most potent anti-C. albicans fatty acid for multiplying yeast forms. SEM images showed that acid-containing PVP/RS100 patches did not retain their fiber structure compared to fatty acid-containing PCL patches. This is due to the difference in hydrophilicity between the two patches where the PVP/RS100 patches rapidly absorb water, resulting in swelling and loss of the fiber structure, whereas the PCL fibers remain intact.
More informative data, from a clinical perspective, were obtained in the biofilm experiments using both wild-type and azole-resistant C. albicans strains. Both PCL and PVP/RS100 patches containing dodecanoic acid displayed a remarkable reduction in the biofilm viability, suggesting that this fatty acid is the most attractive for the treatment of oral candidiasis using mucoadhesive electrospun patches.
Conclusions
The data presented in this study clearly demonstrate that medium-chain saturated fatty acids are an attractive alternative antifungal therapy to treat C. albicans compared to current drugs, for which this organism is becoming increasingly resistant. Moreover, we show here that dodecanoic acid can be successfully incorporated and released from mucoadhesive electrospun patches while still retaining potent antifungal activity in that it is able to penetrate and kill C. albicans even within a biofilm. An electrospun patch would therefore be an ideal vehicle to deliver dodecanoic acid directly to the locally infected sites for the treatment of many forms of oral candidiasis, and this might be an applicable technology for other anatomical sites at the risk of fungal infection including the vaginal epithelium.
Acknowledgments
The authors would like to thank Jason Heath for providing technical help with microbiology, Simon Thorpe for his technical help running samples using GC–MS, and Dr Sandra van Meurs for her technical help running samples using 1H NMR spectroscopy. The UK EPSRC CDT in Polymers, Soft Matter and Colloids (EP/L016281/1) and Afyx Therapeutics are acknowledged for funding this PhD research.
Glossary
Abbreviations
- C9
nonanoic acid
- C12
dodecanoic acid
- PCL
polycaprolactone
- PEO
polyethylene oxide
- PVP
polyvinylpyrollidone
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsbiomaterials.0c00614.
Representative NMR spectrum of a nonanoic acid-loaded PCL patch; agar disk diffusion assay of fatty acids on the C. albicans BWP17 strain and other Candida species; and determination of fatty acid content in electrospun patches (PDF)
Author Contributions
C.M., S.G.S., P.V.H., and J.H. conceived and designed research. K.H.C. and T.M.B. performed the research. K.H.C., C.M., and S.G.S. analyzed the data. K.H.C., C.M., S.G.S., and P.V.H. wrote and edited the paper.
The authors declare the following competing financial interest(s): JH is a former employee of Afyx Therapeutics. PVH is a member of the Research Advisory Board for Afyx Therapeutics. KHC, SGS, PVH and CM have all received funding from Ayfx Therapeutics.
Supplementary Material
References
- Odds F. C. Candida Infections: An Overview. CRC Crit. Rev. Microbiol. 1987, 15, 1–5. 10.3109/10408418709104444. [DOI] [PubMed] [Google Scholar]
- Lewis M. A. O.; Williams D. W. Diagnosis and Management of Oral Candidosis. Br. Dent. J. 2017, 223, 675–681. 10.1038/sj.bdj.2017.886. [DOI] [PubMed] [Google Scholar]
- Rodu B.; Carpenter J. T.; Jones M. R. The Pathogenesis and Clinical Significance of Cytologically Detectable Oral Candida in Acute Leukemia. Cancer 1988, 62, 2042–2046. . [DOI] [PubMed] [Google Scholar]
- Dupont B.; Graybill J. R.; Armstrong D.; Laroche R.; Touzé J. E.; Wheat L. J. Fungal Infections in AIDS Patients. J. Med. Vet. Mycol. 1992, 30, 19–28. 10.1080/02681219280000731. [DOI] [PubMed] [Google Scholar]
- Deslauriers N.; Coulombe C.; Carré B.; Goulet J.-P. Topical Application of a Corticosteroid Destabilizes the Host-Parasite Relationship in an Experimental Model of the Oral Carrier State of Candida Albicans. FEMS Immunol. Med. Microbiol. 1995, 11, 45–55. 10.1111/j.1574-695X.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H.; Bischoff T.; Tallent S. M.; Seifert H.; Wenzel R. P.; Edmond M. B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Sitheeque M. A. M.; Samaranayake L. P. Chronic Hyperplastic Candidosis/Candidiasis (Candidal Leukoplakia). Crit. Rev. Oral Biol. Med. 2003, 14, 253–267. 10.1177/154411130301400403. [DOI] [PubMed] [Google Scholar]
- Gendreau L.; Loewy Z. G. Epidemiology and Etiology of Denture Stomatitis. J. Prosthodontics 2011, 20, 251–260. 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- Sanglard D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, e11 10.3389/fmed.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J.; Mukherjee P. K.; Leidich S. D.; Faddoul F. F.; Hoyer L. L.; Douglas L. J.; Ghannoum M. A. Antifungal Resistance of Candidal Biofilms Formed on Denture Acrylic in Vitro. J. Dent. Res. 2001, 80, 903–908. 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- Haque F.; Alfatah M.; Ganesan K.; Bhattacharyya M. S. Inhibitory Effect of Sophorolipid on Candida Albicans Biofilm Formation and Hyphal Growth. Sci. Rep. 2016, 6, 23575–23585. 10.1038/srep23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q.; Zhang B.; Ma F.; Jia C.; Xiao C.; Zhang B.; Xing L.; Li M. Novel Mechanisms of Surfactants against Candida Albicans Growth and Morphogenesis. Chem.-Biol. Interact. 2015, 227, 1–6. 10.1016/J.CBI.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Lum K. Y.; Tay S. T.; Le C. F.; Lee V. S.; Sabri N. H.; Velayuthan R. D.; Hassan H.; Sekaran S. D. Activity of Novel Synthetic Peptides against Candida Albicans. Sci. Rep. 2015, 5, 9657–9668. 10.1038/srep09657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulone S.; Ardizzoni A.; Tavanti A.; Piccinelli S.; Rizzato C.; Lupetti A.; Colombari B.; Pericolini E.; Polonelli L.; Magliani W.; et al. The Synthetic Killer Peptide KP Impairs Candida Albicans Biofilm in Vitro. PLoS One 2017, 12, e0181278 10.1371/journal.pone.0181278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morici P.; Fais R.; Rizzato C.; Tavanti A.; Lupetti A. Inhibition of Candida Albicans Biofilm Formation by the Synthetic Lactoferricin Derived Peptide HLF1-11. PLoS One 2016, 11, e0167470 10.1371/journal.pone.0167470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C. H.; Kock J. L. F.; Thibane V. S.. Antifungal Free Fatty Acids: A Review. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center, 2011; Vol. 3, pp 61–71. [Google Scholar]
- Kitahara T.; Koyama N.; Matsuda J.; Aoyama Y.; Hirakata Y.; Kamihira S.; Kohno S.; Nakashima M.; Sasaki H. Antimicrobial Activity of Saturated Fatty Acids and Fatty Amines against Methicillin-Resistant Staphylococcus Aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. 10.1248/bpb.27.1321. [DOI] [PubMed] [Google Scholar]
- Avis T. J.; Bélanger R. R. Specificity and Mode of Action of the Antifungal Fatty Acid Cis-9-Heptadecenoic Acid Produced by Pseudozyma Flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. 10.1128/AEM.67.2.956-960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzyn A.; Krasowska A.; Stefanowicz P.; Dziadkowiec D.; Łukaszewicz M. Capric Acid Secreted by S. Boulardii Inhibits C. Albicans Filamentous Growth, Adhesion and Biofilm Formation. PLoS One 2010, 5, e12050 10.1371/journal.pone.0012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton P. V.; Kinderlerer J. L. Toxicity of Medium Chain Fatty Acids to Penicillium Crustosum Thom and Their Detoxification to Methyl Ketones. J. Appl. Bacteriol. 1991, 70, 401–407. 10.1111/j.1365-2672.1991.tb02956.x. [DOI] [Google Scholar]
- Garcia-Cuesta C.; Sarrion-Perez M.; Bagan J. Current Treatment of Oral Candidiasis: A Literature Review. J. Clin. Exp. Dent. 2014, 6, e576–e582. 10.4317/jced.51798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millsop J. W.; Fazel N. Oral Candidiasis. Clin. Dermatol. 2016, 34, 487–494. 10.1016/j.clindermatol.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Santocildes-Romero M. E.; Hadley L.; Clitherow K. H.; Hansen J.; Murdoch C.; Colley H. E.; Thornhill M. H.; Hatton P. V. Fabrication of Electrospun Mucoadhesive Membranes for Therapeutic Applications in Oral Medicine. ACS Appl. Mater. Interfaces 2017, 9, 11557–11567. 10.1021/acsami.7b02337. [DOI] [PubMed] [Google Scholar]
- Colley H. E.; Said Z.; Santocildes-Romero M. E.; Baker S. R.; D’Apice K.; Hansen J.; Madsen L. S.; Thornhill M. H.; Hatton P. V.; Murdoch C. Pre-Clinical Evaluation of Novel Mucoadhesive Bilayer Patches for Local Delivery of Clobetasol-17-Propionate to the Oral Mucosa. Biomaterials 2018, 178, 134–146. 10.1016/j.biomaterials.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Clitherow K. H.; Murdoch C.; Spain S. G.; Handler A. M.; Colley H. E.; Stie M. B.; Mørck Nielsen H.; Janfelt C.; Hatton P. V.; Jacobsen J. Mucoadhesive Electrospun Patch Delivery of Lidocaine to the Oral Mucosa and Investigation of Spatial Distribution in a Tissue Using MALDI-Mass Spectrometry Imaging. Mol. Pharm. 2019, 16, 3948–3956. 10.1021/acs.molpharmaceut.9b00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sill T. J.; von Recum H. a. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Schneider C. A.; Rasband W. S.; Eliceiri K. W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T.; Levitz S. M.; Christin L.; Diamond R. D. A Simplified New Assay for Assessment of Fungal Cell Damage with the Tetrazolium Dye, (2,3)-Bis-(2-Methoxy-4-Nitro-5-Sulphenyl)-(2H)-Tetrazolium-5-Carboxanilide (XTT). J. Infect. Dis. 1995, 172, 1153–1156. 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- Barry A. L.; Brown S. D. Fluconazole Disk Diffusion Procedure for Determining Susceptibility of Candida Species. J. Clin. Microbiol. 1996, 34, 2154–2157. 10.1128/jcm.34.9.2154-2157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandven P. Detection of Fluconazole-ResistantCandida Strains by a Disc Diffusion Screening Test. J. Clin. Microbiol. 1999, 37, 3856–3859. 10.1128/jcm.37.12.3856-3859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth F.; Schliemann S.; Elsner P.; Hipler U. Antifungal Effect of High- and Low-Molecular-Weight Chitosan Hydrochloride, Carboxymethyl Chitosan, Chitosan Oligosaccharide and N-Acetyl-d-Glucosamine against Candida Albicans, Candida Krusei and Candida Glabrata. Int. J. Pharm. 2007, 353, 139–148. 10.1016/j.ijpharm.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Richardson J. P.; Moyes D. L.; Ho J.; Naglik J. R. Candida Innate Immunity at the Mucosa. Semin. Cell Dev. Biol. 2019, 89, 58–70. 10.1016/j.semcdb.2018.02.026. [DOI] [PubMed] [Google Scholar]
- Huang C. B.; Alimova Y.; Myers T. M.; Ebersole J. L. Short- and Medium-Chain Fatty Acids Exhibit Antimicrobial Activity for Oral Microorganisms. Arch. Oral Biol. 2011, 56, 650–654. 10.1016/j.archoralbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabara J. J.; Swieczkowski D. M.; Conley a. J.; Truant J. P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. 10.1128/AAC.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsson G.; Arnfinnsson J.; Steingrímsson O.; Thormar H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. 10.1128/aac.45.11.3209-3212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama K.; Takahashi M.; Yui S.; Abe S. Inhibitory Effects of Several Saturated Fatty Acids and Their Related Fatty Alcohols on the Growth of Candida Albicans. Drug Discov. Ther. 2015, 9, 386–390. 10.5582/ddt.2015.01062. [DOI] [PubMed] [Google Scholar]
- Jin Y.; Yip H. K.; Samaranayake Y. H.; Yau J. Y.; Samaranayake L. P. Biofilm-Forming Ability of Candida Albicans Is Unlikely to Contribute to High Levels of Oral Yeast Carriage in Cases of Human Immunodeficiency Virus Infection. J. Clin. Microbiol. 2003, 41, 2961–2967. 10.1128/JCM.41.7.2961-2967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G.; Walle K. V.; Wickes B. L.; López-Ribot J. Standardized Method for In Vitro Antifungal Susceptibility Testing of Candida Albicans Biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyagoub M.; Willemot C.; Bélanger R. R. Influence of a Subinhibitory Dose of Antifungal Atty Acids from Sporothrix Flocculosa on Cellular Lipid Composition in Fungi. Lipids 1996, 31, 1077–1082. 10.1007/BF02522465. [DOI] [PubMed] [Google Scholar]
- Mclain N.; Ascanio R.; Baker C.; Strohaver R. A.; Dolan J. W. Undecylenic Acid Inhibits Morphogenesis of Candida Albicans. Antimicrob. Agents Chemother. 2000, 44, 2873–2875. 10.1128/aac.44.10.2873-2875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Hanna A.; Piispanen A. E.; Stateva L. I.; Hogan D. A. Farnesol and Dodecanol Effects on the Candida Albicans Ras1-CAMP Signalling Pathway and the Regulation of Morphogenesis. Mol. Microbiol. 2008, 67, 47–62. 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Wang L.; Huang Y. Morphology and Thermal Properties of Electrospun Fatty Acids/Polyethylene Terephthalate Composite Fibers as Novel Form-Stable Phase Change Materials. Sol. Energy Mater. Sol. Cells 2008, 92, 1382–1387. 10.1016/j.solmat.2008.05.013. [DOI] [Google Scholar]
- Ismar E.; Sarac A. S. Electrospun Polyacrylonitrile–Lauric Acid Composite Nanofiber Webs as a Thermal Energy Storage Material. J. Eng. Fibers Fabr. 2019, 14, 1–6. 10.1177/1558925018824890. [DOI] [Google Scholar]
- Tonglairoum P.; Ngawhirunpat T.; Rojanarata T.; Panomsuk S.; Kaomongkolgit R.; Opanasopit P. Fabrication of Mucoadhesive Chitosan Coated Polyvinylpyrrolidone/Cyclodextrin/Clotrimazole Sandwich Patches for Oral Candidiasis. Carbohydr. Polym. 2015, 132, 173–179. 10.1016/j.carbpol.2015.06.032. [DOI] [PubMed] [Google Scholar]
- Tonglairoum P.; Ngawhirunpat T.; Rojanarata T.; Kaomongkolgit R.; Opanasopit P. Fabrication of a Novel Scaffold of Clotrimazole-Microemulsion-Containing Nanofibers Using an Electrospinning Process for Oral Candidiasis Applications. Colloids Surf., B 2015, 126, 18–25. 10.1016/j.colsurfb.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Tonglairoum P.; Ngawhirunpat T.; Rojanarata T.; Panomsuk S.; Kaomongkolgit R.; Opanasopit P. Fast-Acting Clotrimazole Composited PVP/HPβCD Nanofibers for Oral Candidiasis Application. Pharm. Res. 2014, 31, 1893–1906. 10.1007/s11095-013-1291-1. [DOI] [PubMed] [Google Scholar]
- Jamuna-Thevi K.; Saarani N. N.; Abdul Kadir M. R.; Hermawan H. Triple-Layered PLGA/Nanoapatite/Lauric Acid Graded Composite Membrane for Periodontal Guided Bone Regeneration. Mater. Sci. Eng. C 2014, 43, 253–263. 10.1016/j.msec.2014.07.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.