Abstract
Background and Objectives
Informal caregivers are rarely as involved as they want to be in the housing decisions of cognitively impaired older adults. Lack of awareness of available options and their benefits and risks may lead to decisions that do not reflect older adults’ preferences, and to guilt and regret. We assessed the effect of training home care teams in interprofessional shared decision-making (SDM) on the proportion of caregivers who report being active in this decision.
Research Design and Methods
In a two-arm pragmatic cluster randomized trial with home care teams working in health centers in the Province of Quebec, we randomized health centers to receive training in interprofessional SDM (intervention) or not (control). Eligible caregivers had made a housing decision for a cognitively impaired adult aged 65 years or older who was receiving services from a home care team. The primary outcome was the proportion of caregivers reporting an active role in decision making. We performed intention-to-treat multilevel analysis.
Results
We consecutively enrolled a random group of 16 health centers and recruited 309 caregivers, among whom 296 were included in the analysis. In the intervention arm, the proportion of caregivers reporting an active role in decision making increased by 12% (95% CI −2% to 27%; p = .10). After removal of an influential cluster outlier, the proportion increased to 18% (95% CI: 7%–29%; p < .01).
Discussion and Implications
Training home care teams in interprofessional SDM increased caregiver involvement in health-related housing decisions for cognitively impaired older adults.
Keywords: Shared decision making, Interprofessional, Home care, Nursing homes, Patient engagement
When informal caregivers are not involved in health-related housing decisions about cognitively impaired older adults (e.g., should he/she move into a nursing home or stay at home with home care services?), or the caregivers have insufficient decision-making support, the decision can be poorly informed and result in decisional conflict, guilt, and regret.
In 2012 in Canada, older adults with loss of autonomy represented 40% of those receiving home care services, with the most hours of care provided to those with cognitive impairment (Sinha & Bleakney, 2014). Cognitive impairment is one of the strongest predictors of nursing home placement (Wattmo, Wallin, Londos, & Minthon, 2011). When cognitively impaired older adults face a housing decision, their informal caregivers are often asked to make the decision instead (Garvelink, Ngangue, et al., 2016). Although older adults with cognitive impairment have been shown to benefit greatly from being involved in decision-making processes about their care (Miller, Whitlatch, & Lyons, 2016), ill-health or cognitive loss may limit their ability to make the final choice. Making these decisions alone on behalf of a loved one is painful for caregivers. They report stress, doubt, interpersonal and intrapersonal conflict, uncertainty beforehand, and guilt and regret afterwards. They also report inadequate support from health care professionals, lack of involvement in the decision, not knowing what the options are, and uncertainty about the benefits and risks of the options (Adekpedjou et al., 2018; Garvelink, Ngangue, et al., 2016). Other studies show that caregivers need more support in decision making (Zarit, Reever, & Bach-Peterson, 1980) and better opportunities to participate in health-related housing decisions made for their cognitively impaired loved ones. Involving caregivers in decisions about the care of their loved ones, if that is their wish, is considered an ethical imperative (Hamann & Heres, 2019; Risco & Kelly, 2019). Moreover, caregivers are often the experts on the older adult’s condition, history, and care experiences (Buckwalter & Hall, 1987; Williams et al., 2018).
Shared decision making (SDM) is the process by which health care professionals collaborate with patients to help them identify the best options, clarify their values and preferences, and take more control over their care plan (Charles, Gafni, & Whelan, 1999). SDM has been shown to have a favorable impact on decision-making processes, patients’ experience of care, and patient outcomes (Légaré et al., 2018; Shay & Lafata, 2015). Interprofessional SDM, designed for multiprofessional team-based practices, is more likely to improve the quality of decision support provided by team-based health care practices and bridge gaps that occur between health care professionals as well as between them and their patients and families (Légaré et al., 2013).
In the United States, only one in three older adults is living in housing that matches his or her preference (Kasper, Wolff, & Skehan, 2018). Unplanned, uninformed, abrupt, and forced relocations prevent or interfere with older adults aging in a place of their choice and generate anxiety, frustration, distress, and poor quality of life (Sussman & Orav-Lakaski, 2018). If caregivers were better informed about the options, their decisions would better reflect what most older adults want: to stay at home (Caron, Ducharme, & Griffith, 2006). Inviting and supporting a caregiver to participate in decisions with home care team members about a loved one’s health-related housing thus holds promise for better-informed choices that reflect the knowledge, experiences, values, and preferences of caregivers and their loved ones. This study therefore assessed the effect of training home care teams in interprofessional SDM on the proportion of caregivers who report being active in decision making regarding health-related housing for a cognitively impaired older adult. We opted for a pragmatic cluster randomized trial with randomization at the health center level (out of which home care services are operated) to reduce the risk of contamination and facilitate future implementation of the training program in other health care organizations.
Theoretical Background
For this study, we adapted an interprofessional model that we began to develop in 2007 (see Supplementary Table 1 for a detailed history) (Dogba, Menear, Stacey, Briere, & Legare, 2016). Our model has two main axes (Supplementary Figure 1). The vertical axis is the SDM process that occurs over time: identifying the decision to be made (namely the health-related housing decision), discussing evidence about the options, clarifying older adults and caregivers’ values, considering the feasibility of each option, identifying the preferred option, and finally reaching consensus on the best option. The horizontal axis represents the key actors involved, both in the older adult team (the cognitively impaired older adult, the caregiver, and significant others) and in the health care team (health care professionals and/or decision coaches), with the older adult at the center of the process. For the SDM process to be interprofessional, at least two health care professionals from different professions collaborate with the older adult either concurrently or sequentially. The initiator of the SDM process can be any health care professional who identifies the health problem and makes explicit the decision to be made. The family category (first column, Supplementary Figure 1) includes caregivers, relatives, and/or other people who are important to the older adult and may influence the decision-making process. The caregiver participates in decision making on behalf of the older adult in situations where the older adult cannot be involved (e.g., if the older adult has severe mental illness or is unconscious). Another key role is the decision coach, a health care professional trained to support older adults or caregivers in thinking about the options, preparing for discussing the decision in a consultation with the health care professional, and implementing the chosen option (O’Connor, Stacey, & Légaré, 2008). The last column of the model refers to health care professionals. The model also shows horizontal lines of common understanding and the varying influence of those involved at each step of the decision-making process (from deliberation to choice). The whole is situated within the broader environmental influences of social norms, organizational routines, and institutional structures (Légaré, Stacey, et al. 2011). This paper presents outcomes of our implementation of an interprofessional SDM intervention in seven health jurisdictions in the Province of Quebec, Canada.
Methods
More details about the methods can be found in the study protocol (Légaré et al., 2015).
Study Design and Participants
This study was a pragmatic two-arm cluster randomized trial with post-test measures only and 1:1 allocation ratio. Before the intervention, baseline data were collected on older adults and caregivers to assess the comparability between intervention and control clusters at trial entry (Adekpedjou et al., 2018). The caregivers assessed at baseline were different from those assessed post-intervention and whose data are reported here. This post-intervention data was analyzed to compare the proportion of caregivers reporting an active role in the intervention group with the proportion in the control group (not with the baseline measures) (Campbell & Stanley, 2015). We conducted the trial in seven health jurisdictions in the Province of Quebec, Canada. Clusters were the health and social services centers (henceforth referred to as “health centers”) of these jurisdictions.
Study participants were the health centers, their interprofessional home care teams, and informal caregivers of their older adult clients with loss of autonomy and cognitive impairment. To be eligible, a health center had to (a) serve a territory of more than 10,000 inhabitants and (b) be located within 500 km of Quebec City (location of our research team) (Garvelink et al., 2015). Eligible interprofessional home care teams (a) were involved in caring for older adults with loss of autonomy, and (b) practised in one of the health centers selected to participate in the trial. To qualify as interprofessional, a minimum of two health care professionals from different professions had to be involved in the older adult’s care. We invited one interprofessional home care team per health center to participate. Eligible informal caregivers (a) had made a health-related housing decision (in the months following the intervention) for a cognitively impaired older adult aged 65 years or older who was receiving services from a home care team; (b) were able to read, understand, and write French or English; and (c) consented to participate in the study. Cognitively impaired older adults were those who were no longer able to make decisions about their own life, according to the clinical evaluation of the health care professionals (as per usual care, which involves a validated autonomy coding instrument (Hébert, Guilbault, Desrosiers, & Dubuc, 2001), in which case their potentially eligible caregivers were invited to participate instead. Given the pragmatic nature of our trial, we selected caregivers this way to align with clinical practice as much as possible, that is, we wanted health care professionals to engage with older adults, or else their proxies, in much the same way as they would in usual care, and so we did not impose additional selection criteria (Ford & Norrie, 2016; Oude Rengerink et al., 2017).
In the study protocol (Légaré et al., 2015) we based our sample size estimate on a SDM trial in primary care clinics and a review of SDM interventions (Légaré et al., 2012, 2014) as there was no relevant literature on SDM in home care. Originally our target population was older adults with loss of autonomy and, if they were cognitively impaired and the team considered them inapt to make a final decision, their informal caregivers. The first sample size we calculated was 501 older adults/caregivers (32/health center). However, analysis of the baseline data revealed that about 93% of the older adults with loss of autonomy and 71% of the caregivers reported taking an active role in the decision making (Adekpedjou et al., 2018). In addition, we observed that caregivers of cognitively impaired older adults were more often confronted with this type of decision; experienced more decisional conflict during decision making; and if they were older themselves, would be having to decide whether to move themselves in the not-so-distant future (Garvelink, Emond, et al., 2016). These observations coupled with the needs expressed by home care teams led us to focus solely on the caregivers of cognitively impaired older adults with loss of autonomy.
Randomization and Masking
The unit of randomization was the health center, stratified by rural or urban/semi-urban setting. After obtaining the consent of their directors, an independent biostatistician centrally computer-generated a random sequence for allocating the intervention to health centers. The biostatistician was not involved in data analysis. Due to the nature of the trial, there was no blinding to the group to which a health center was assigned, but those recruiting caregivers and collecting data were instructed not to disclose to caregivers the group to which their home care team had been assigned.
Procedure
We implemented the intervention at the cluster level. It consisted of an online tutorial, a live interactive workshop on the interprofessional SDM approach, and the use of a decision guide.
Members of the home care teams completed the 1.5-hr online SDM tutorial (Boland et al., 2019; Ottawa Hospital Research Institute, 2015) before attending a 3.5-hr live interactive workshop which included a review of SDM concepts, a video demonstrating the interprofessional SDM approach in the context of a home care team (Stacey et al., 2014); the presentation of a decision guide (Garvelink, Emond, et al., 2016); and a role play session in which health care professionals practised the interprofessional SDM approach using the decision guide.
The interprofessional home care teams in the control group received only the standard procedure for determining eligibility for state-provided home care or institutionalization currently in use in the Province of Quebec, to which both groups were exposed (Agence de la santé et des services sociaux du Bas-Saint-Laurent, 2011).
Data Collection
After the training, home care teams made lists of potentially eligible caregivers of their cognitively impaired older adult clients, who were identified consecutively and then invited to participate. A research assistant contacted potential participants to schedule an appointment. After obtaining written informed consent, caregivers were asked to complete a questionnaire about sociodemographic characteristics, caregiver burden, housing preference and actual health-related housing decision, preferred role and actual role in the decision making, decisional conflict, and decision regret. Questions about what role caregivers preferred to take in decision making and their housing preference for the older adult were asked after the housing decision had been made (retrospectively). In a logbook, the research assistant documented length of encounter, general ambiance, location, and participants' verbal comments about making their health-related housing decision.
Outcomes
The primary outcome was the proportion of caregivers who reported they played an active role in decision making. We assessed this role using a modified version of the Control Preference Scale (CPS) (Degner & Sloan, 1992), a single question with five responses categories: (A) I made the decision, (B) I made the decision after seriously considering the health care professionals’ opinions, (C) the health care professionals and I shared the responsibility for the decision making, (D) the health care professionals made the decision after seriously considering my opinion, and (E) the health care professionals made the decision (Degner & Sloan, 1992). For sample size calculation and analysis, we dichotomized the outcome into two response categories: (1) A, B, and C: active role (i.e., decision making controlled by the caregiver or SDM and (2) D and E: passive role (i.e., health care professional-controlled decision making).
Secondary outcomes were preferred health-related housing option and actual health-related housing decision (remain at home or move to a care facility). We also used the original Decisional Conflict scale (O’Connor et al., 2003), the Decision Regret scale (Brehaut et al., 2003), and the Zarit Burden Interview (caregiver’s burden of care) (Hébert, Bravo, & Girouard, 1993; Hébert, Bravo, & Préville, 2000; Seng et al., 2010). More details about study outcomes are reported in Supplementary Table 2.
Statistical Analysis
We recalculated our sample size when we refocused our study on caregivers of cognitively impaired older adults. We assumed that in the absence of any intervention, 70% of caregivers would take an active role and there would be a 20% difference between the intervention and control groups after implementation. With a power of 80%, a significance level of 5%, 62 caregivers per group would have been required to perform an individually randomized trial. Given that we did not have intraclass correlation coefficients (ICCs) estimates from former studies, our biostatisticians provided a number of scenarios with ICCs ranging from .02 to .05. We used a conservative value of ICC of .05 as the upper limit. With k = 8 clusters (at the health center level) in each group and an ICC of ρ = .05 for such clusters, the number of caregivers per cluster needed to achieve the same power was 12 (Eldridge & Kerry, 2012), for a total of 192 caregivers. Allowing for 10% loss to follow-up, we expected a total sample size of 208 caregivers (13 caregivers per health center). However, as a reorganization of the entire health care system disrupted the study, we did not reach our target sample size in time and some health centers recruited more than others, resulting in an imbalance in cluster sizes. To address the consequent loss of power, we extended the recruitment period and recruited more caregivers than our required sample size (Guittet, Ravaud, & Giraudeau, 2006).
We performed intention-to-treat analyses. Unit of analysis was the caregiver. We used descriptive statistics to describe characteristics of health centers, health care professionals, and caregivers, and to assure the comparability of the intervention and control groups. To take non-independence of the data (clustering effect) into account, we performed multilevel modeling, specifying a random effect at the health center level. We used the SAS statistical package for analysis. We assessed the impact of the intervention on the dichotomous outcomes using generalized estimating equations (GEEs). We assessed the impact of the intervention on continuous outcomes using linear mixed models (LMMs) with the restricted maximum likelihood (REML) estimation method. For exploratory purposes, we used GEE to perform additional analyses of the effect of the intervention on the match between preferred option and decision made and the match between role preferred and role assumed in the decision making. We modeled difference of proportion for dichotomous outcomes and mean difference for continuous outcomes. We conducted model diagnostics for each outcome analyzed according to the type of variable (continuous or dichotomous). During model diagnostics, we found that in the model estimating the effect of the intervention on decision regret, the distribution of scaled residuals was not normal and was heteroskedastic. We therefore performed a transformation of the response variable (decision regret) from continuous to dichotomous (decision regret = 0 if decision regret score = 0; decision regret = 1 if decision regret score > 0). ICCs were obtained for each outcome analyzed. We used an alpha value of .05 as the level of statistical significance for all analysis.
Ethics and Study Registration
The CHU de Québec Multicentre Ethics Committee approved the study (reference: MP-CHU-QC-14-001). All participants gave written informed consent. This trial is registered at clinicaltrials.gov (reference: NCT02244359).
Results
Trial Flow
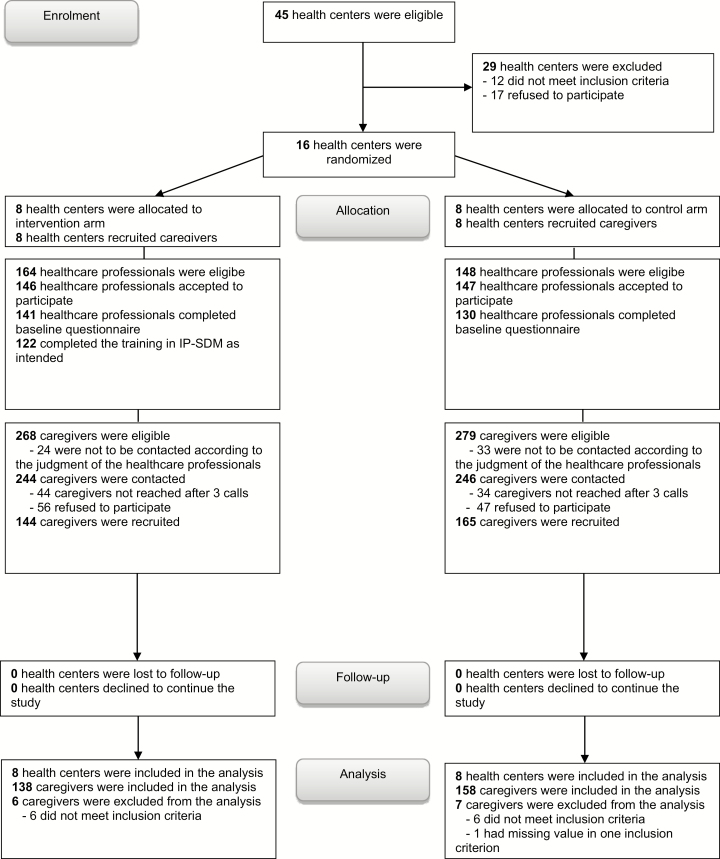
Recruitment took place between April 2014 and August 2016. Of the 45 potentially eligible health centers, 16 (35.5%) were recruited, 12 (26.7%) did not meet inclusion criteria, and 17 (37.7%) refused to participate. Eight were assigned to the intervention group and eight to the control group. One interprofessional home care team per health center was invited to participate (Figure 1).
Figure 1.
Flow chart of the study.
Characteristics of Health Centers and Health Care Professionals (Baseline Measures)
More than half of the health centers recruited were rural (68.7%). Median age of health care professionals was 36.1 years (interquartile range [IQR]: 30.1–45.9) and most were women (90.4%). Their median year of experience in home care was 6 (IQR: 2–11). Overall, health center and health care professional characteristics were well balanced between the groups except for professions, with the proportion of social workers being higher in the intervention group (46.8%) than in the control group (24.6%) (Table 1).
Table 1.
Characteristics of Health Centers and Health Care Professionals (Baseline Measures)
| Characteristics, n (%) | Health center/health care professionals | |
|---|---|---|
| Setting (health center) | Intervention group (n = 8) | Control group (n = 8) |
| Urban/semi-urban | 3 (37.5) | 2 (25) |
| Rural | 5 (62.5) | 6 (75) |
| Health care professionals | Intervention group (n = 141) | Control group (n = 130) |
| Age (years), median (IQR) | 35.1 (30.2–44.9) | 36.9 (30.0–47.3) |
| Sex | ||
| Male | 16 (11.3) | 10 (7.7) |
| Female | 125 (88.7) | 120 (92.3) |
| Profession | ||
| Social worker | 66 (46.8) | 32 (24.6) |
| Physiotherapist | 3 (2.1) | 5 (3.9) |
| Nurse | 33 (23.4) | 30 (23.1) |
| Occupational therapist | 9 (6.4) | 17 (13.1) |
| Other (e.g., nutritionists, nursing assistants, respiratory therapists) | 30 (21.3) | 46 (35.4) |
| Experience in home care (years), median (IQR) | 6.9 (2.2–11.0) | 5.5 (2.0–12.0) |
| Intention to adopt IP-SDM approacha, median score (IQR) | 6.0 (5.0–6.5) | 6.0 (5.0–6.5) |
Note: IQR = interquartile range.
aScale range: 1–7.
Characteristics of Caregivers (Post-Intervention Measures)
Median age of caregivers was 60.5 years (IQR: 54.0–69.6) (Table 2). Most caregivers were women (74.6%), were married or living with a partner (77.4%), 50.3% were retired, 20.3% had no more than a post-secondary education, and 60.5% were the adult child of the older adult. More than 80% of the caregivers in both groups reported that they preferred to play an active or collaborative role in the decision making. Overall, caregivers’ characteristics were well balanced between the groups.
Table 2.
Characteristics of Caregivers (Post-Intervention Measures)
| Characteristic, n (%) | Caregivers | |
|---|---|---|
| Intervention group (n = 138) | Control group (n = 158) | |
| Age (year), median (IQR) | 59.5 (53.9–69.3) | 62.5 (54.5–69.8) |
| Sex | ||
| Male | 38 (27.5) | 37 (23.4) |
| Female | 100 (72.5) | 121 (76.6) |
| Civil status | ||
| Single | 16 (11.6) | 14 (8.9) |
| Married/common-law partner | 105 (76.1) | 125 (79.1) |
| Separated/divorced | 9 (6.5) | 13 (8.2) |
| Widower | 8 (5.8) | 6 (3.8) |
| Employment status | ||
| Employed (full time) | 41 (29.7) | 47 (29.8) |
| Employed (part time) | 15 (10.9) | 8 (5.1) |
| At home | 10 (7.2) | 5 (3.2) |
| Unemployed | 2 (1.5) | 2 (1.3) |
| Job seeker | 0 (0) | 2 (1.3) |
| Retired | 63 (45.7) | 86 (54.4) |
| Other (e.g., business owner, on welfare, on disability pension) | 7 (5.1) | 8 (5.1) |
| Education level | ||
| Primary | 16 (11.6) | 14 (8.9) |
| Secondary | 55 (39.9) | 54 (34.2) |
| College/Cegep (grade 11–12) | 27 (19.6) | 35 (22.2) |
| Diploma, vocational studies | 8 (5.8) | 16 (10.1) |
| University undergraduate | 20 (14.5) | 29 (18.4) |
| University graduate | 4 (2.9) | 7 (4.4) |
| Other (e.g., commercial college, 1 year university) | 8 (5.8) | 3 (1.9) |
| Total family income (CAD$) | ||
| <15,000 | 6 (4.4) | 11 (7.0) |
| 15,000–29,999 | 25 (18.1) | 27 (17.1) |
| 30,000–44,999 | 22 (15.9) | 21 (13.3) |
| 45,000–59,999 | 22 (15.9) | 31 (19.6) |
| 60,000 and more | 43 (31.2) | 34 (21.5) |
| No answer | 20 (14.5) | 34 (21.5) |
| Relationship to older person | ||
| Husband/wife | 30 (21.7) | 36 (22.8) |
| Child | 88 (63.8) | 93 (58.9) |
| Other family member | 19 (13.8) | 24 (15.2) |
| Friend | 1 (0.7) | 2 (1.3) |
| Other (colleague, trustee, former common-law partner) | 0 (0.0) | 3 (1.9) |
| Decision-making preferences | ||
| Active | 118 (85.5) | 130 (82.3) |
| Passive role | 19 (13.8) | 28 (17.7) |
| No answer | 1 (0.7) | 0 (0.0) |
Note: CAD = Canadian dollars; IQR = interquartile range.
Effect of the Interprofessional SDM Program on the Primary Outcome
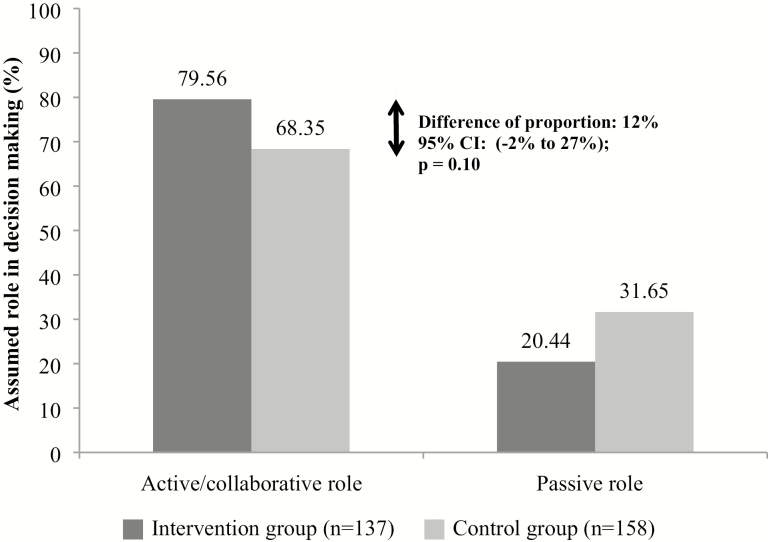
The intervention increased the proportion of caregivers who reported an active role in the decision making by 12% (95% CI: −2% to 27%; p = .10) (Figure 2). During the analysis, we noted an influential cluster outlier in the intervention group. This considerably lowered the mean proportion of active or collaborative roles in the intervention group, but also overly inflated the CI of the effect size due to an overestimation of the between-cluster variance. We performed a sensitivity analysis by removing this cluster from the analysis. The revised data suggested that the intervention increased the proportion of caregivers reporting an active or collaborative role during decision making from 12% to 18% (95% CI: 7%–29%; p < .01) (Supplementary Figure 2). In this influential cluster, few caregivers reported an active/collaborative role in the decision making (43.5%) and they had a lower preference for playing this role (52.2%) compared to caregivers in the seven other intervention clusters (85.5%); in addition, the match between the role they preferred in decision making and the role they actually played was high (90.5%). A further exploration of the characteristics of this outlier cluster revealed that they were less educated and more likely to be retired (results not shown). Removal of the cluster did not change the balance of participant characteristics between groups (results not shown).
Figure 2.
Effect of the intervention on role caregiver assumed in decision making.
Note: Generalized estimated equations (GEEs) were used and adjustment was made for clustering. Passive role was the reference category.
Effect of the Interprofessional SDM Program on Secondary Outcomes
The intervention showed no effect on the preferred health-related housing option, decision made, decisional conflict, decision regret (dichotomized), or burden of care as perceived by caregivers (Table 3 and Supplementary Table 3).
Table 3.
Effect of the Intervention on Preferred Health-Related Housing Option, Actual Health-Related Housing Decision, Decision Regret (Dichotomized), Match Between Preferred Option and Actual Decision, and Match Between Role Preferred and Role Assumed in Decision Making
| Variables | Intervention, n (%) (n = 138) | Control, n (%) (n = 158) | Absolute difference of proportion (%)a | Adjusted difference of proportion (%) (95% CI)b | p-value |
|---|---|---|---|---|---|
| Preferred option (stay home) | 70 (50.7) | 82 (51.9) | −1.2 | 0.5 (−15.2 to 16.2) | .95 |
| Actual decision (stay home) | 27 (19.6) | 26 (16.4) | 3.1 | 4.3 (−11.6 to 20.1) | .60 |
| Decision regret (dichotomized) (>0) | 73 (52.9) | 77 (48.7) | 4.2 | 4.2 (−11.4 to 19.8) | .60 |
| Match between preferred option and actual decision | 89 (64.5) | 92 (58.2) | 6.3 | 6.2 (−5.1 to 17.5) | .28 |
| Match between role preferred and assumed in decision making | 123 (90.4)c | 120 (75.9) | 14.5 | 14.4 (7.4 to 21.4) | <.0001 |
Note: Generalized estimated equations (GEE).
aProportion in intervention group – proportion in control group.
bAdjusted for clustering.
c n = 136.
Ancillary Analyses
The intervention made no difference to the match between preferred option and decision made. However, the intervention significantly increased the proportion of caregivers who reported a match between the role they preferred and the role they actually played in the decision making; this increase was 14.4% (95% CI: 7.4%–21.4%; p < .0001) (Table 3).
Intraclass Correlation Coefficient
For the primary outcome (role assumed in decision making), the ICC was .027 (Supplementary Table 4).
Discussion
We observed that training home care teams in interprofessional SDM combined with a decision guide increased the proportion of caregivers who reported being active in making health-related housing decisions for an older adult with cognitive impairment. It also increased the proportion of caregivers who reported a match between the role they preferred and the role they actually assumed in decision making. However, the data suggest that it had no effect on caregivers’ preferred health-related housing option, the decision made, the match between preferred option and decision made, decisional conflict, decision regret, or burden of care. This leads us to make the following observations.
First, as our trial is the first to involve caregivers of cognitively impaired older adults facing health-related housing decisions, and the first to assess the effect of an interprofessional SDM approach on such decisions, direct comparisons with previous research is not possible. However, trials targeting similar populations or testing similar interventions to increase patients’ or caregivers’ participation in health-related decisions have produced similar positive results (Légaré et al., 2018; Stacey et al., 2017). Interestingly, removing the influential outlier cluster significantly improved the effect of the intervention on caregivers’ role in decision making. Caregivers in that cluster had lower preference for playing an active role in decision making and were less educated. Taking an active role in decision making is particularly difficult for less literate populations (Kiesler & Auerbach, 2006). Although they report less interest in SDM (Brom et al., 2014), they are the ones who may benefit the most from it (McCaffery, Smith, & Wolf, 2010). Their reluctance to engage in the decision-making process may not reflect a lack of preference for involvement but rather a lack of self-efficacy (Légaré, St-Jacques, et al. 2011). To comply with ethical principles and avoid aggravating health inequities, developing shared decision-making tools tailored to less literate populations is essential.
Second, although the match between the role preferred and the role actually played in decision making was already high (control), this intervention improved the match and seemed to reinforce caregivers in their preferred role. Results of randomized trials assessing match between preferred and perceived participation in medical decisions have been mixed (Brom et al., 2014), which can be partly explained by differences in study design. A systematic review found mismatches less often in retrospective studies (total 37) than in prospective studies (total 12) (Brom et al., 2014). This may be due to respondents’ desire for concordance which produces a cognitive bias. Cognitive bias may also explain why we found a high level of match in our study, as preference was measured retrospectively. Still, the desire for a match did not result in a 100% match, and the fact that our intervention significantly increased the match shows that there is room for improvement.
Third, the typical caregiver was a woman, 60 years old, caring for her spouse/father/mother, who wanted to play an active or collaborative role in the decision about housing for her loved one. Our sample is representative of caregivers of people facing difficult health decisions and considered unable to make these decisions alone (Garvelink, Ngangue, et al., 2016). At the individual level, our trial results are generalizable to caregivers with similar characteristics facing a health-related housing decision for their loved one. At the cluster level, our intervention may not be applicable in every setting, since home care services are organized differently from one jurisdiction to another. However, the pragmatic nature of our trial will support flexibility in the application of the intervention.
This study has several strengths. First is our study design. Randomization allowed us to control for potential measured and non-measured confounding factors, increasing the validity of the results (Rothmans, Lash, & Greenland, 2008). Second, as this was a pragmatic trial, we tested our intervention in normal practice with all the flexibility that normal practice requires (Zwarenstein et al., 2008). This maximizes the applicability of our results. Moreover, the trial was designed to respond to the needs of home care teams, older adults, and caregivers in the actual settings in which the intervention would be implemented (interprofessional home care). Third, good collaboration among health center staff and their strong relationship with the research team mitigated obstacles encountered, such as when, in 2014–2015, the Province of Quebec entirely restructured its health care system (Government of Quebec, 2015), delaying recruitment by several months. Moreover, no clusters were lost to follow-up. This allowed us to keep a good balance of participant characteristics between groups and limit selection bias.
Our study also had some limitations. First, we assumed our sample size would give us enough power to detect a between-group difference of 20% for our primary outcome, but we detected a difference of 12% (not statistically significant). This lack of power to detect a significant difference for the primary outcome may also explain why our study failed to detect a significant difference between the study groups on the secondary outcomes given the large CIs around their point estimates. However, we suspect that the intervention reduced decision regret. Indeed, our data showed that more caregiver participation in the decision making and a better match between the role they preferred and the role they assumed in the decision making were associated with less decision regret (results not shown). Second, the restructuring of the health care system led to the merging of some of our clusters, increasing the risk of contamination. To limit that risk, we instructed the home care teams and managers of merging intervention clusters not to share any documents relating to our intervention with other clusters. Finally, recruiting caregivers after random allocation may have increased the risk of selection bias (Giraudeau & Ravaud, 2009). However, the fact that caregiver characteristics were well balanced between groups indicates that this bias was minimal.
Implications for Practice and Research
Supporting caregivers in making health-related housing decisions for older adults with loss of autonomy and cognitive impairment led to better experiences in decision making and increased quality of decision making for caregivers. In addition, our interprofessional SDM intervention standardized how home care teams will help caregivers make decisions informed by all available options and by their own and the older adults’ values and preferences. It may also reduce practice variations among health care professionals, a problem that the SDM model was conceived to address (O’Connor, Llewellyn-Thomas, & Flood, 2004).
Our study opens up several avenues for further research. First, further research could assess the effect of an intervention to support decision making in the older adult and their caregiver together as an interdependent dyad. Second, outcomes measured over a longer time would help identify long-term effects of such interventions. Third, the potential economic, logistical, organizational, and political barriers to applying this approach need further study to help policymakers create implementation strategies. Finally, further studies could identify which components are essential in all contexts and which need adapting to local situations.
Funding
The study was funded by the Canadian Frailty Network, which is supported by the Government of Canada through the Networks of Centres of Excellence program (known previously as Technology Evaluation in the Elderly Network [TVN] is the former name of Canadian Frailty Network; MFE-140842) and the Ministère de la Santé et des Services Sociaux (MSSS) du Québec (Quebec Ministry of Health and Social Services) funds research it considers essential to meeting its priorities and implementing its policies (13-SS-00431).
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
We thank Julie Emond for her contributions to designing the decision guide and giving the interactive workshop. We thank Serge Dumont, Kimberley Fraser, Henriette Bourassa, and Lise Roy for their contributions to designing the study. We thank Matthew Menear, Maria Marguerita Becerra-Perez, and Laura Boland for their contributions to designing the decision guide. We thank Louisa Blair for editing the manuscript. We thank the Canadian Frailty Network and the Ministère de la Santé et des Services Sociaux (MSSS) du Québec for their financial support. We also acknowledge the in-kind support of the following local health networks: the Consortium InterESt Santé, the CISSS de la Gaspésie (former CSSS du Rocher Percé), the CIUSSS de la Capitale Nationale (former CSSS de la Vieille Capitale), and the Agence de la Santé et des Services sociaux de la Capitale-Nationale. This study is embedded in a parent study led by Chairholder of the Tier 1 Canada Research Chair in Shared Decision Making and Knowledge Translation. F. Légaré, N. Brière, S. Desroches, L.-P. Rivest, and D. Stacey designed and wrote the parent study. R. Adekpedjou designed and wrote this study. F. Légaré, N. Brière, S. Desroches, P. J. Durand, L.-P. Rivest, and D. Stacey sought funding. F. Légaré, N. Brière, D. Stacey, and M. M. Garvelink conceived the decision guide. F. Légaré, N. Brière, and M. J. Dogba gave the interactive workshop. A. Freitas coordinated the study, including data collection. R. Adekpedjou conceived the statistical analysis plan and performed data editing. R. Adekpedjou and J. Croteau performed statistical analyses. R. Adekpedjou, J. Croteau, F. Légaré, and L.-P. Rivest interpretated the data. R. Adekpedjou drafted the manuscript. R. Adekpedjou, D. Stacey, N. Brière, A. Freitas, M. M. Garvelink, M. J. Dogba, P. J. Durand, S. Desroches, J. Croteau, L.-P. Rivest, and F. Légaré critically reviewed the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript. F. Légaré is its guarantor. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
References
- Adekpedjou R., Stacey D., Brière N., Freitas A., Garvelink M. M., Turcotte S., … Légaré F (2018). “Please listen to me”: A cross-sectional study of experiences of seniors and their caregivers making housing decisions. PLoS One, 13, e0202975. doi:10.1371/journal.pone.0202975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agence de la santé et des services sociaux du Bas-Saint-Laurent (2011). L’hébergement et ses mécanismes d’accès pour les personnes âgées en perte d’autonomie liée au vieillissement. Québec, QC: Cadre de référence régional. [Google Scholar]
- Boland L., Légaré F., Carley M., Graham I. D., O’Connor A. M., Lawson M. L., & Stacey D (2019). Evaluation of a shared decision making educational program: The Ottawa Decision Support Tutorial. Patient Education and Counseling, 102, 324–331. doi:10.1016/j.pec.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Brehaut J. C., O’Connor A. M., Wood T. J., Hack T. F., Siminoff L., Gordon E., & Feldman-Stewart D (2003). Validation of a decision regret scale. Medical Decision Making, 23, 281–292. doi:10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- Brom L., Hopmans W., Pasman H. R., Timmermans D. R., Widdershoven G. A., & Onwuteaka-Philipsen B. D (2014). Congruence between patients’ preferred and perceived participation in medical decision-making: A review of the literature. BMC Medical Informatics and Decision Making, 14, 25. doi:10.1186/1472-6947-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter K. C., & Hall G. R (1987). Families of the institutionalized older adult: a neglected resource. In Brubaker T. H. (Ed.), Aging, health, and families: Long term care (pp. 176–196). Newbury Park, CA: Sage Publications. [Google Scholar]
- Campbell D. T., & Stanley J. C (2015). Experimental and quasi-experimental designs for research. Ravenio Books, ePub [N.p.]. [Google Scholar]
- Caron C. D., Ducharme F., & Griffith J (2006). Deciding on institutionalization for a relative with dementia: The most difficult decision for caregivers. Canadian Journal on Aging, 25, 193–205. [DOI] [PubMed] [Google Scholar]
- Charles C.,, Gafni A.,, Whelan T. (1999). Decision-making in the physician–patient encounter: revisiting the shared treatment decision-making model. Social science & medicine, 49(5), 651–661. doi:10.1016/S0277-9536(99)00145-8 [DOI] [PubMed] [Google Scholar]
- Degner L. F., & Sloan J. A (1992). Decision making during serious illness: What role do patients really want to play? Journal of Clinical Epidemiology, 45, 941–950. [DOI] [PubMed] [Google Scholar]
- Dogba M. J., Menear M., Stacey D., Brière N., & Légaré F (2016). The evolution of an interprofessional shared decision-making research program: Reflective case study of an emerging paradigm. International Journal of Integrated Care, 16, 4. doi:10.5334/ijic.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge S., & Kerry S (2012). A practical guide to cluster randomised trials in health services research. Chichester, West Sussex: John Wiley & Sons. [Google Scholar]
- Ford I., & Norrie J (2016). Pragmatic trials. The New England Journal of Medicine, 375, 454–463. doi:10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- Garvelink M. M., Emond J., Menear M., Brière N., Freitas A., Boland L., … Légaré F (2016). Development of a decision guide to support the elderly in decision making about location of care: An iterative, user-centered design. Research Involvement and Engagement, 2, 26. doi:10.1186/s40900-016-0040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvelink M. M., Freitas A., Menear M., Brière N., Stacey D., & Légaré F (2015). In for a penny, in for a pound: The effect of pre-engaging healthcare organizations on their subsequent participation in trials. BMC Research Notes, 8, 751. doi:10.1186/s13104-015-1743-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvelink M. M., Ngangue P. A., Adekpedjou R., Diouf N. T., Goh L., Blair L., & Légaré F (2016). A synthesis of knowledge about caregiver decision making finds gaps in support for those who care for aging loved ones. Health Affairs (Project Hope), 35, 619–626. doi:10.1377/hlthaff.2015.1375 [DOI] [PubMed] [Google Scholar]
- Giraudeau B., & Ravaud P (2009). Preventing bias in cluster randomised trials. PLoS Medicine, 6, e1000065. doi:10.1371/journal.pmed.1000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Quebec (2015). Bill 10, Act to modify the organization and governance of the health and social services network, in particular by abolishing the regional agencies, 1st Sess, 41st Leg, Quebec, c 1. [Google Scholar]
- Guittet L., Ravaud P., & Giraudeau B (2006). Planning a cluster randomized trial with unequal cluster sizes: Practical issues involving continuous outcomes. BMC Medical Research Methodology, 6, 17. doi:10.1186/1471-2288-6-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J., & Heres S (2019). Why and how family caregivers should participate in shared decision making in mental health. Psychiatric Services, 70(5), 418–421. doi:10.1176/appi.ps.201800362 [DOI] [PubMed] [Google Scholar]
- Hébert R., Bravo G., & Girouard D (1993). Fidélité de la traduction française de trois instruments d’évaluation des aidants naturels de malades déments. Canadian Journal on Aging, 12, 324–337. doi:10.1017/S0714980800013726 [Google Scholar]
- Hébert R., Bravo G., & Préville M (2000). Reliability, validity and reference values of the Zarit Burden Interview for assessing informal caregivers of community-dwelling older persons with dementia. Canadian Journal on Aging, 19(4), 494–507. doi:10.1017/S0714980800012484 [Google Scholar]
- Hébert R., Guilbault J., Desrosiers J., & Dubuc N (2001). The functional autonomy measurement system (SMAF): A clinical-based instrument for measuring disabilities and handicaps in older people. Geriatrics Today, 4, 141–158. [Google Scholar]
- Kasper J. D., Wolff J. L., & Skehan M (2018). Care arrangements of older adults: What they prefer, what they have, and implications for quality of life. The Gerontologist. Advance online publication. doi:10.1093/geront/gny127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesler D. J., & Auerbach S. M (2006). Optimal matches of patient preferences for information, decision-making and interpersonal behavior: Evidence, models and interventions. Patient Education and Counseling, 61, 319–341. doi:10.1016/j.pec.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Légaré F., Adekpedjou R., Stacey D., Turcotte S., Kryworuchko J., Graham I. D., … Donner-Banzhoff N (2018). Interventions for increasing the use of shared decision making by healthcare professionals. The Cochrane Database of Systematic Reviews, 7, CD006732. doi:10.1002/14651858.CD006732.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré F., Brière N., Stacey D., Bourassa H., Desroches S., Dumont S., … Roy L (2015). Improving decision making on location of care with the frail elderly and their caregivers (the DOLCE study): Study protocol for a cluster randomized controlled trial. Trials, 16, 50. doi:10.1186/s13063-015-0567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré F., Labrecque M., Cauchon M., Castel J., Turcotte S., & Grimshaw J (2012). Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: A cluster randomized trial. Canadian Medical Association Journal, 184, E726–E734. doi:10.1503/cmaj.120568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré F., Stacey D., Brière N., Desroches S., Dumont S., Fraser K., … Aubé D (2011). A conceptual framework for interprofessional shared decision making in home care: Protocol for a feasibility study. BMC Health Services Research, 11, 23. doi:10.1186/1472-6963-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré F., Stacey D., Brière N., Fraser K., Desroches S., Dumont S., … Aubé D (2013). Healthcare providers’ intentions to engage in an interprofessional approach to shared decision-making in home care programs: A mixed methods study. Journal of Interprofessional Care, 27, 214–222. doi:10.3109/13561820.2013.763777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré F., Stacey D., Turcotte S., Cossi M., Kryworuchko J., & Graham I (2014). Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Systematic Reviews, 9 Art. No.: CD006732. doi:10.1002/14651858.CD006732.pub3 [DOI] [PubMed] [Google Scholar]
- Légaré F., St-Jacques S., Gagnon S., Njoya M., Brisson M., Frémont P., & Rousseau F (2011). Prenatal screening for Down syndrome: A survey of willingness in women and family physicians to engage in shared decision-making. Prenatal Diagnosis, 31, 319–326. doi:10.1002/pd.2624 [DOI] [PubMed] [Google Scholar]
- McCaffery K. J., Smith S. K., & Wolf M (2010). The challenge of shared decision making among patients with lower literacy: A framework for research and development. Medical Decision Making, 30, 35–44. doi:10.1177/0272989X09342279 [DOI] [PubMed] [Google Scholar]
- Miller L. M., Whitlatch C. J., & Lyons K. S (2016). Shared decision-making in dementia: A review of patient and family carer involvement. Dementia (London, England), 15, 1141–1157. doi:10.1177/1471301214555542 [DOI] [PubMed] [Google Scholar]
- O’Connor A. M., Drake E. R., Wells G. A., Tugwell P., Laupacis A., & Elmslie T (2003). A survey of the decision-making needs of Canadians faced with complex health decisions. Health Expectations, 6, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor A. M., Llewellyn-Thomas H. A., & Flood A. B (2004). Modifying unwarranted variations in health care: Shared decision making using patient decision aids. Health Affairs (Project Hope), Suppl Variation, VAR63–VAR72. doi:10.1377/hlthaff.var.63 [DOI] [PubMed] [Google Scholar]
- O’Connor A. M., Stacey D., & Légaré F (2008). Coaching to support patients in making decisions. British Medical Journal, 336, 228–229. doi:10.1136/bmj.39435.643275.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottawa Hospital Research Institute (2015). Ottawa decision support tutorial Retrieved from https://decisionaid.ohri.ca/odst/.
- Oude Rengerink K., Kalkman S., Collier S., Ciaglia A., Worsley S. D., Lightbourne A., … Irving E. A.; Work Package 3 of the GetReal consortium (2017). Series: Pragmatic trials and real world evidence: Paper 3. Patient selection challenges and consequences. Journal of Clinical Epidemiology, 89, 173–180. doi:10.1016/j.jclinepi.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Risco J., & Kelly A (2019). Improving medical decisions. In Creutzfeldt, CJ et al. (eds.), Neuropalliative care (pp. 171–185). [N.p.]: Springer. [Google Scholar]
- Rothmans K. J., Lash T. L., & Greenland S (2008). Modern epidemiology (3rd ed). Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Seng B. K., Luo N., Ng W. Y., Lim J., Chionh H. L., Goh J., & Yap P (2010). Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Annals of the Academy of Medicine, Singapore, 39, 758–763. [PubMed] [Google Scholar]
- Shay L. A., & Lafata J. E (2015). Where is the evidence? A systematic review of shared decision making and patient outcomes. Medical Decision Making, 35, 114–131. doi:10.1177/0272989X14551638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M., & Bleakney A (2014). Spotlight on Canadians: Results from the general social survey. In Canada S. (Ed.), Receiving care at home, 7. Ottawa, Canada: Statistics Canada. [Google Scholar]
- Stacey D., Brière N., Robitaille H., Fraser K., Desroches S., & Légaré F (2014). A systematic process for creating and appraising clinical vignettes to illustrate interprofessional shared decision making. Journal of Interprofessional Care, 28, 453–459. doi:10.3109/13561820.2014.911157 [DOI] [PubMed] [Google Scholar]
- Stacey D., Légaré F., Lewis K., Barry M. J., Bennett C. L., Eden K. B., … Trevena L (2017). Decision aids for people facing health treatment or screening decisions. The Cochrane Database of Systematic Reviews, 4, CD001431. doi:10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman T., & Orav-Lakaski B (2018). “I Didn’t Even Make My Bed”: Hospital relocations and resident adjustment in long-term care over time. The Gerontologist. Advance online publication. doi:10.1093/geront/gny141 [DOI] [PubMed] [Google Scholar]
- Wattmo C., Wallin A. K., Londos E., & Minthon L (2011). Risk factors for nursing home placement in Alzheimer’s disease: A longitudinal study of cognition, ADL, service utilization, and cholinesterase inhibitor treatment. The Gerontologist, 51, 17–27. doi:10.1093/geront/gnq050 [DOI] [PubMed] [Google Scholar]
- Williams L. A., Moeke-Maxwell T., Wiles J., Black S., Trussardi G., Kerse N., & Gott M (2018). How family caregivers help older relatives navigate statutory services at the end of life: A descriptive qualitative study. Palliative Medicine, 32, 1124–1132. doi:10.1177/0269216318765853 [DOI] [PubMed] [Google Scholar]
- Zarit S. H., Reever K. E., & Bach-Peterson J (1980). Relatives of the impaired elderly: Correlates of feelings of burden. The Gerontologist, 20, 649–655. [DOI] [PubMed] [Google Scholar]
- Zwarenstein M., Treweek S., Gagnier J. J., Altman D. G., Tunis S., Haynes B., … Moher D.; CONSORT Group; Pragmatic Trials in Healthcare (Practihc) Group (2008). Improving the reporting of pragmatic trials: An extension of the CONSORT statement. British Medical Journal, 337, a2390. doi:10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.