Abstract
Background and Objectives
There is a link between sensory and cognitive functioning across old age. However, there are no integrative measures for assessing common determinants of sensory-cognitive functioning. This study aims to develop a combined measure of sensory-cognitive functioning, and to identify heterogeneous trajectories and associated risk factors.
Research Design and Methods
Two thousand two hundred and fifty-five individuals aged 60 years and over selected from the first six waves (2002–2012) of the English Longitudinal Study of Ageing completed a set of five self-reported visual and hearing functioning items and four cognitive items. Several health-related outcomes were also collected.
Results
The common cause model presented longitudinal factorial invariance (Tucker-Lewis index [TLI] = 0.989; Comparative Fit Index [CFI] = 0.991; Root Mean Square Error of Approximation [RMSEA] = 0.026). A common factor explained 32%, 36%, and 26% of the visual, hearing, and cognitive difficulties, respectively. The developed sensory-cognitive measure predicted incident dementia over 10 years (area under the curve = .80; 95% confidence interval [CI] = .75, .86). A three-trajectory model was proved to fit better, according to growth mixture modeling. Low levels of education and household wealth, disability, diabetes, high blood pressure, depressive symptoms, and low levels of physical activity were risk factors associated with the classes showing trajectories with a steeper increase of sensory-cognitive difficulties.
Discussion and Implications
A time-invariant factor explains both sensory and cognitive functioning over 8 years. The sensory-cognitive measure derived from this factor showed a good performance for predicting dementia 10 years later. Several easily identifiable socioeconomic and health-related risk factors could be used as early markers of subsequent sensory-cognitive decline. Therefore, the proposed latent measure could be useful as a cost-effective indicator of sensory-cognitive functioning.
Keywords: Sensory functioning, Cognitive functioning, Latent classes, Structural equation modeling
Aging is a multidimensional phenomenon associated with declines in both sensory and cognitive functioning. Several cross-sectional and longitudinal studies have evidenced a relationship between sensory and cognitive functioning in the older population (Baltes & Lindenberger, 1997; Humes, Busey, Craig, & Kewley-Port, 2013; Lin et al., 2013, 2014; Lin et al., 2004; Lindenberger & Ghisletta, 2009; Maharani, Dawes, Nazroo, Tampubolon, & Pendleton, 2018; Yamada et al., 2016). Although diverse hypotheses have been proposed to address this link (Humes & Young, 2016; Roberts & Allen, 2016), the causal mechanisms underlying the pattern of relationships between perception and cognition in the older age is still debatable (Whitson et al., 2018).
According to the common cause hypothesis, cognitive and sensory functioning is closely related in older persons since they both depend on the physiological integrity of the brain, which gradually declines in functioning with aging (Roberts & Allen, 2016). Thus, a common neurodegenerative factor simultaneously affecting sensory and cognitive functioning would explain the association between age-related declines in these domains. Neurobiological age-related changes have been found to affect both sensory and cognitive functioning (Chang et al., 2015; Harris & Dubno, 2017). On the other hand, the American Geriatrics Society and the National Institute on Aging highlight that the role of cardiovascular disease and inflammation as common pathways for sensory and cognitive impairment has been overlooked (Whitson et al., 2018). In that regard, a study showed a decrease in the association between sensory impairment and risk of cognitive impairment after controlling for inflammatory and cardiovascular disease and related factors (Fischer et al., 2016). Another recent study showed that visual and olfactory impairments and cardiovascular disease were associated with cumulative incidence of cognitive impairment over 10 years (Schubert et al., 2019). Despite the available evidence, the nature of the common cause and its determinants remain unclear.
Previous research highlights the potential usefulness of developing a combined measure of sensory-cognitive difficulties to explore mechanisms of brain health (Fischer et al., 2016), allowing assessing joint trajectories of sensorineurocognitive functioning across the old age. Moreover, a single measure capturing common aging pathways of sensory and cognitive functioning could be useful for predicting important health-related outcomes, especially those that have been found to be independently associated with these domains of functioning in the older population. For instance, previous research has reported independent associations of visual, hearing, and cognitive impairment with disability (Brennan, Su, & Horowitz, 2006; Cimarolli & Jopp, 2014; Fabbri et al., 2016; Mansbach & Mace, 2018), higher mortality (Gopinath et al., 2013; Wahl et al., 2013; Wilson, Segawa, Hizel, Boyle, & Bennett, 2012), and some mental disorders, like depression (Cosh et al., 2018; Kim, Liu, Cheung, & Ahn, 2018; Lawrence et al., 2019) or dementia (Deal et al., 2017; Lin et al., 2011; Mitoku, Masaki, Ogata, & Okamoto, 2016; Panza, Solfrizzi, & Logroscino, 2015). However, none of these associations have been assessed taking into account the covariance structure underlying sensory and cognitive functioning.
Therefore, the present study had three aims. First, to assess the longitudinal invariance of a common factor accounting for the shared variance among visual, hearing, and cognitive difficulties in older population. It is important to note that although the actual common cause remains unknown, we propose a common cause model that would present factorial invariance over time. Second, we aim at developing a latent measure capturing the common variation underlying a set of individual hearing, visual, and cognitive functioning measures, assessing its ability to predict incident dementia. Third, to identify groups presenting heterogeneous trajectories of sensory-cognitive difficulties, and their associated risk factors.
Methods
Sample and Study Design
The sample comprised 2,255 participants aged 60 years and over from the first six waves (2002–2012) of the English Longitudinal Study on Ageing (ELSA) who had responded to all the self-reported items of sensory functioning and the measured test of cognition. ELSA is a biannual longitudinal study focused on nationally representative samples of people aged 50 years and over from the English population (Steptoe, Breeze, Banks, & Nazroo, 2013). All participants provided informed consent. The National Research Ethics Service granted ethical approval for all the ELSA waves (MREC/01/2/91). Further details on the specifics of ELSA can be found in the study website (https://www.elsa-project.ac.uk/).
Measures
Self-reported sensory functioning scale items and cognitive measures employed for the measurement models are given in Table 1. Visual functioning was measured by means of three self-reported items assessing eyesight in far, near, and general vision. For hearing functioning, self-reported hearing functioning and presence of difficulties following a conversation with background noise were used. These original variables were five-category questions (except self-reported difficulties following a conversation which had two categories), with the following categories: “Excellent,” “Very good,” “Good,” “Fair,” and “Poor.” For both visual and hearing functioning, participants were assessed with their visual and hearing aids if they had them. These items were highly skewed, with a great amount of responses grouped in the “best functioning” categories. In these cases, dichotomizing the values is a habitual strategy (De La Fuente et al., 2018). Thus, these variables were dichotomized, collapsing “Excellent,” “Very good,” and “Good” as “Absence of difficulties,” and “Fair” and “Poor” as indicators of “Presence of difficulties.”
Table 1.
Self-reported Sensory Functioning Scale Items and Cognitive Measures Employed for the Measurement Models
| Vision | Excellent | Very good | Good | Fair | Poor | |
|---|---|---|---|---|---|---|
| Far vision | How good is your eyesight for seeing things at a distance, like recognizing a friend across the street (using glasses or corrective lens as usual)? | 1 | 2 | 3 | 4 | 5 |
| Near vision | How good is your eyesight for seeing things up close, like reading ordinary newspaper print (using glasses or corrective lens as usual)? | 1 | 2 | 3 | 4 | 5 |
| General vision | How is your eyesight (using glasses or corrective lens as usual)? | 1 | 2 | 3 | 4 | 5 |
| Hearing | Excellent | Very good | Good | Fair | Poor | |
| General hearing | How is your hearing (using a hearing aid as usual) | 1 | 2 | 3 | 4 | 5 |
| Following conversations | Do you find it difficult to follow a conversation if there is background noise, such as TV, radio, or children playing (using a hearing aid as usual)? | No 0 |
Yes 1 |
|||
| Cognition* | ||||||
| Verbal fluency | Participants are asked to name the maximum number of animals in one minute. The total score was the number of animals named by the participant. | |||||
| Processing speed | Score obtained from a letter cancellation task where participants had to identify and mark two target letters (P and W) in a page of 65 random letters set out in rows and columns within one minute. | |||||
| Immediate recall and delayed recall | Number of words recalled by the participant from a list of 10 common words. Word recall is tested immediately and after a short delay filled with other cognitive tests |
Note: *All cognitive measures are freely available in: https://www.elsa-project.ac.uk/uploads/elsa/docs_w1/booklet.pdf.
The assessment of cognitive functioning comprised four measured tests of verbal fluency, processing speed, and immediate and delayed recall. The verbal fluency task consisted in naming the maximum number of animals in one minute. The total score was the number of animals named by the participant. The processing speed score was obtained from a letter cancellation task where participants had to identify and mark two target letters in a page of 65 random letters. Finally, the immediate and delayed recall memory scores corresponded with the number of words recalled by the participant from a list of 10 common words, immediately and after a short delay, respectively. All the scores derived from the cognitive functioning tests were dichotomized using the lower quartile of each distribution as cutoff point for indicating presence of difficulties.
Participants also provided information on sociodemographic variables, including age, sex, household wealth (net value of total wealth minus all debts), and formal qualification (having an academic certificate recognized by the English educational system). Level of physical activity was obtained by means of a self-reported item comprising four categories: “Sedentary,” “Mild,” “Moderate,” and “Vigorous.” Self-reported doctor-diagnosed diabetes and high blood pressure were also used.
Incident dementia in Wave 6 of the ELSA study was obtained following the three-way protocol described by Davies, Cadar, Herbert, Orrell, and Steptoe (2017). Participants with either: (a) a physician diagnosis of dementia, (b) a score of 3.5 or higher in the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), or (c) receiving prescriptions for N-methyl-D-aspartate receptor antagonists, anticholinesterase inhibitors, or other antidementia medications (such as galantamine, rivastigmine, memantine, donepezil, or tacrine) were categorized as presenting incident dementia if they did not present any of these characteristics in previous waves of the study.
Participants indicated the presence of difficulties to perform six activities of daily living (ADL) (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963) and six instrumental activities of daily living index (IADL) (Graf, 2008). The original variables for assessing ADL and IADL, ranged from 0 (no difficulties) to 6 (difficulties with all six activities of ADL/IADL).
The overall score of the Center for Epidemiologic Studies-Depression scale, eight-item version survey was used to assess the presence of depressive symptoms (CES-D 8) (Turvey, Wallace, & Herzog, 1999). This instrument is made up of eight items with a dichotomous (yes/no) scale of response.
Statistical Analysis
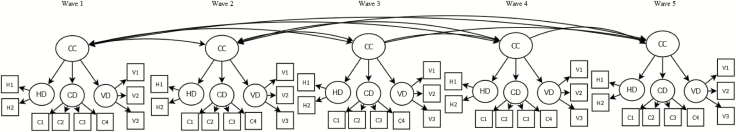
A structural equation modeling (SEM) approach was used to assess the longitudinal factorial invariance of the common cause model proposed in Figure 1 across the first five waves of ELSA. In each wave, this model comprises a second-order latent factor explaining the common variance of the visual, hearing and cognitive difficulties first-order factors. Two nested models were compared in terms of goodness-of-fit. An unconstrained model with free parameters across waves was first implemented to test configural invariance. Then, a constrained model with equal factor loadings and thresholds across waves was used to assess strong factorial invariance. Based on Widaman, Ferrer, and Conger (2010), the following constraints were imposed to identify the SEM models: (a) latent factors were standardized at baseline (M = 0, SD = 1); (b) the factor loading and threshold of the first indicator of each factor were freely estimated at baseline and constrained to be equal at subsequent waves. The residual variances of the same indicators were allowed to correlate across waves for modeling unique item effects. The goodness-of-fit of the SEM models was assessed using the cutoff points proposed by Hu and Bentler (1999); a model showed a good fit when Comparative Fit Index (CFI) and Tucker-Lewis index (TLI) values were greater than .95, and Root Mean Square Error of Approximation (RMSEA) values were lower than .05. The longitudinal factorial invariance analysis was based on a change in the CFI value lower than .01 between the nested models (Cheung & Rensvold, 2002). Means adjusted weighted least squares (WLSM) estimator for categorical data was used for the SEM models.
Figure 1.
Common cause model for explaining the relationships between hearing, visual, and cognitive difficulties. CC = Common cause; CD = Cognitive difficulties; HD = Hearing difficulties; VD = Visual difficulties.
If strong factorial invariance was achieved, latent scores on the second-order common factor in each wave were predicted using the factor score regression method. To improve interpretability, these latent scores were then transformed into a 0–100 scale, where higher values indicated higher sensory-cognitive difficulties. The ability of the metric to predict incident dementia 10 years later was assessed by means of receiver operating characteristics (ROC) curves, and the area under the ROC curve (AUC). As a sensitivity analysis, we compared the ROC curves and AUCs for predicting incident dementia of the common cause metric with latent scores of visual, hearing, and cognitive functioning estimated separately. AUC values range from .5 (representing no predictive ability) to 1 (representing perfect predictive ability).
Finally, a latent class mixed model (LCMM) (Proust-Lima, Philipps, & Liquet, 2017) was conducted to identify a finite set of groups of subjects with similar sensory-cognitive trajectories. Models with increasing number of latent classes were fitted, considering age effects up to the cubic. The model with lower sample-size-adjusted Bayesian information criterion (SABIC) was selected. As additional criteria for selecting the final model, a successful convergence, average of posterior probabilities over .70, and no less than a 5% of the overall sample in each class were considered. The highest average of the posterior probability was used for assigning class membership. A general profile of each class, comprising sociodemographic and clinical information, was obtained. One-way analysis of variances (ANOVAs) and chi-square tests were conducted to assess between-class differences in these variables. Finally, a multinomial logistic regression was conducted to identify determinants of the sensory-cognitive difficulties trajectories.
SEM analyses were conducted with the lavaan R package (Rosseel, 2012). Latent class mixed models were implemented with the lcmm R package (Proust-Lima et al., 2017). ROC curves, linear, and multinomial regressions were conducted with STATA (StataCorp, 2015).
Results
The mean age of the sample at baseline (N = 2,555) was 68.19 years (SD = 6.01), with 56.81% of them being women.
Longitudinal Factorial Invariance of the Common Cause Model
The unconstrained model for testing configural factorial invariance (χ2(838) = 2,252.55, p < .001; RMSEA = .024; TLI = .989; CFI = .991), and the constrained model assessing strong factorial invariance (χ2(883) = 2,254.90, p < .001; RMSEA = .026; TLI = .989; CFI = .991) presented an adequate fit. Longitudinal factorial invariance of the common cause model was achieved, since the difference in fit between the constrained and unconstrained models was below the cutoff point (∆CFI < .001).
All items presented significant loadings across waves (p < .001) on the hearing difficulties (ranging from .75 to .96), visual difficulties (ranging from .83 to .98), and cognitive difficulties (ranging from .50 to .82) first-order factors in the constrained model. The loadings of the visual, hearing, and cognitive difficulties first-order factors on the common cause second order factor were all statistically significant (.57, .60, and .51; p < .001). The common cause accounted for 32%, 36%, and 26% of the visual, hearing, and cognitive difficulties factors variance, respectively.
Creation of a Sensory-Cognitive Difficulties Latent Score
The strong factorial invariance common cause model was used to estimate a sensory-cognitive difficulties latent score in each wave. The following means were obtained for the sensory-cognitive difficulties score after rescaling (ranging from 0 to 100): Wave 1: M = 31.71, SD = 15.87; Wave 2: M = 32.34, SD = 16.82; Wave 3: M = 36.78, SD = 16.21; Wave 4: M = 40.14, SD = 17.16; Wave 5: M = 45.20, SD = 16.24). According to the results from the ROC curves analyses, this metric at baseline presented a good ability to predict incident dementia at Wave 6 (AUC = .80; 95% confidence interval [CI] = .75, .86). Results from the sensitivity analysis indicated that latent scores on visual and hearing difficulties factors presented a poor ability to predict dementia over 10 years (visual difficulties: AUC = .53; 95% CI = .41, .65; hearing difficulties: AUC = .50; 95% CI = .37, .63). On the other hand, latent scores on cognitive difficulties presented an appropriate ability to predict incident dementia (AUC = .70; 95% CI = .60, .80).
Trajectories of the Common Cause Metric
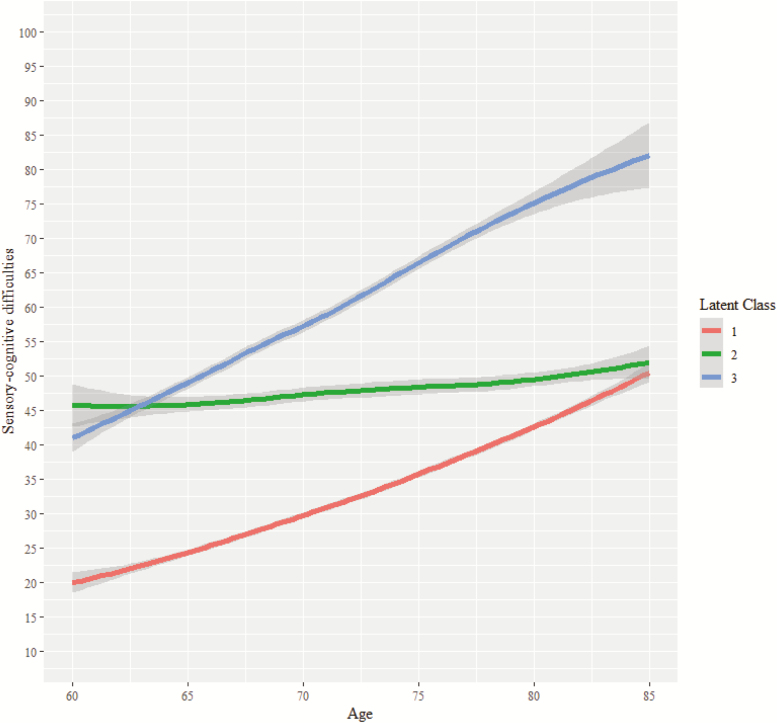
Results from the LCMM indicated that the model comprising three latent classes with quadratic fixed and random effects presented the lowest SABIC value (SABIC = 67237.59). In addition, average of posterior probabilities of class membership was over .70 in every class, with no class comprising less than a 5% of the overall sample. Supplementary Table S1 contains the sample size and growth parameters of each class. A modal class comprising a 73.44% of the sample (Class 1) was identified. This class presented the lowest sensory-cognitive difficulties at baseline (intercept = 11.88, p < .001), and a significant slope with both linear (β = 1.27, p < .001) and quadratic (β = 0.02, p < .001) shape. A class presenting a stable trajectory of high sensory-cognitive difficulties was also identified (Class 2). Although this trajectory class presented the highest levels of sensory-cognitive difficulties at baseline (intercept = 39.29, p < .001), it only showed a small but significant quadratic shape (β = 0.02, p < .001). Finally, a sensory-cognitive risk trajectory class was detected (Class 3). This class presented the highest linear slope (β = 1.55, p < .001). Figure 2 displays the observed sensory-cognitive difficulties trajectories for each class.
Figure 2.
Trajectories of the combined sensory-cognitive difficulties latent score by class.
The overall profile of the Classes identified in the LCMM is presented in Table 2. Results from multinomial logistic regressions conducted to assess baseline determinants of Class membership are presented in Table 3. Considering the modal class (Class 1) as reference, classes 2 and 3 comprised more female participants, and were associated with lower levels of education and wealth, as well as a greater presence of ADL and IADL difficulties, self-reported medical diagnoses of diabetes, lower levels of physical activity, and higher Center for Epidemiologic Studies-Depression scale (CESD) score. On the other hand, whereas Class 2 presented older participants compared with the modal class, individuals comprising Class 3 were more likely to be younger. In addition, Class 3 presented a significantly higher proportion of people with high blood pressure.
Table 2.
Baseline General Profile of the Three Classes Identified in the LCMM
| Class 1 (n = 1,656) | Class 2 (n = 332) | Class 3 (n = 267) | |
|---|---|---|---|
| Age, M (SD) | 68.36 (5.97) | 69.87 (6.48) | 65.11 (4.24) |
| Male, N (%) | 668 (40.34) | 164 (49.40) | 142 (53.18) |
| Formal qualification, N (%) | 1,075 (64.92) | 172 (51.81) | 129 (48.31) |
| Belonging to the first to second quintile of household wealth, N (%) | 428 (25.85) | 144 (43.37) | 113 (42.32) |
| Difficulties in ADLs, N (%) | 220 (13.29) | 86 (25.90) | 82 (30.71) |
| Difficulties in IADLs, N (%) | 17 (1.03) | 15 (4.52) | 13 (4.87) |
| Diabetes, N (%) | 72 (4.35) | 33 (9.94) | 28 (10.49) |
| High blood pressure, N (%) | 620 (37.44) | 128 (38.55) | 122 (45.69) |
| Physical activity, N (%) | |||
| Sedentary | 90 (5.43) | 43 (12.95) | 39 (14.61) |
| Mild | 406 (24.52) | 99 (29.82) | 74 (27.72) |
| Moderate | 817 (49.34) | 142 (42.77) | 113 (42.32) |
| Vigorous | 343 (20.71) | 48 (14.46) | 41 (15.36) |
| CESD score, M (SD) | 1.11 (1.60) | 1.59 (1.91) | 1.83 (2.02) |
Note: ADLs = Activities of daily living; CESD = Center for Epidemiologic Studies-Depression scale; IADLs = Instrumental activities of daily living; LCMM = Latent class mixed model.
Table 3.
Multinomial Logistic Regression Model for Predicting Sensory-Cognitive Classes Identified in the LCMM
| Multinomial logistic regression (reference category = Class 1) | ||
|---|---|---|
| Class 2 (n = 332) | Class 3 (n = 267) | |
| Relative risk ratio (95% CI) | Relative risk ratio (95% CI) | |
| Age | 1.03** (1.01, 1.05) | 0.86*** (0.84,0.89) |
| Sex (ref. male) | 0.58*** (0.45, 0.74) | 0.46*** (0.35, 0.62) |
| Formal qualification (ref. no) | 0.72* (0.56, 0.94) | 0.51*** (0.38, 0.68) |
| Belonging to the first to second quintile of household wealth (ref. no) | 1.77*** (1.36, 2.31) | 1.50* (1.10, 2.05) |
| ADLs difficulties (ref. no) | 1.46* (1.06, 2.02) | 2.30*** (1.61, 3.29) |
| IADLs difficulties (ref. no) | 2.80** (1.33, 5.89) | 2.77* (1.19, 6.44) |
| Diabetes (ref. no) | 2.03** (1.29, 3.20) | 1.86* (1.11, 3.09) |
| High blood pressure (ref. no) | 0.84 (0.65, 1.09) | 1.37* (1.03, 1.83) |
| Physical activity (ref. sedentary) | ||
| Mild | 0.60* (0.38, 0.93) | 0.59* (0.36, 0.96) |
| Moderate | 0.53** (0.34, 0.82) | 0.59* (0.36, 0.94) |
| Vigorous | 0.46** (0.28, 0.76) | 0.49* (0.28, 0.84) |
| CES-D 8 score | 1.08* (1.01, 1.17) | 1.15*** (1.06, 1.24) |
Note: ADLs = Activities of daily living; CESD = Center for Epidemiologic Studies-Depression scale; CI = Confidence interval; IADLs = Instrumental activities of daily living; LCMM = Latent class mixed model.
*p < .05; **p < .01; ***p < .001.
Discussion
This study presents a methodological approach for developing a combined measure of sensory-cognitive difficulties based on self-reported items of visual and hearing functioning, and a set of cognitive tests. To develop this measure, we proposed a common cause model, testing the temporal stability of a common factor accounting for the observed associations between sensory and cognitive functioning, using a sophisticated SEM approach. This measure presented a good ability to predict incident dementia 10 years later. Moreover, we identified three population groups with heterogeneous trajectories of sensory-cognitive difficulties, as well as risk factors associated with groups presenting high or increasing levels of sensory-cognitive difficulties over time.
We identified a latent factor accounting for the common variance between visual, hearing, and cognitive difficulties over 8 years. Moreover, the explanatory power of this factor as a predictor of sensory and cognitive functioning remains stable over time, accounting for 32%, 36%, and 26% of the visual, hearing, and cognitive difficulties. These results are consistent with previous research evidencing a common etiology underlying both sensory and cognitive age-related decline (Anstey, Luszcz, & Sanchez, 2001; Baltes & Lindenberger, 1997; Lindenberger & Baltes, 1994; Lindenberger & Ghisletta, 2009).
We presented a method for developing a latent measure of sensory-cognitive difficulties based on self-reported items of visual and hearing functioning, as well as a set of cognitive measured tests. Based on the proposed common cause model, this metric allows capturing individual variations in a common factor predicting both sensory and cognitive declines. As suggested previously, this factor might be reflecting senescent neurodegenerative processes affecting perceptive and cognitive functioning, in which people vary in terms of level and rate of decline (Lindenberger & Baltes, 1994; Lindenberger & Ghisletta, 2009). The estimated measure of sensory-cognitive difficulties presented an appropriate ability to predict incident dementia over 10 years. It is important to highlight that the common cause explained sensory and cognitive difficulties to a similar extent, and thus, was not overlooking any domain. The above-mentioned evidences of criterion validity regarding the metric are in line with the previous literature showing isolated associations of visual, hearing, and cognitive impairment with risk of dementia (Deal et al., 2017; Lin et al., 2011; Luo et al., 2018; Mitoku et al., 2016; Panza et al., 2015).
The LCMM-based methodology implemented in this study allowed assessing heterogeneous trajectories of sensory-cognitive difficulties. In that regard, we identified three populations groups with varying trajectories. The modal class comprising the largest proportion of the sample presented low levels of sensory-cognitive difficulties at baseline and a moderate increase over time. This class was associated with higher levels of education, household wealth, and physical activity. These results are consistent with the previous literature evidencing positive links between health status and education, income, and physical activity (De La Fuente et al., 2018). In that regard, higher education could enable people to access more qualified occupations which take place in healthier environments, thus reducing exposure to sensory-related risk factors. Similarly, a higher income might facilitate access to better healthcare services and healthy habits.
Two risk groups presenting trajectories with high levels or increases of sensory-cognitive difficulties were identified. A set of common risk factors were associated with these groups: a worse functional ability, medical-diagnose of diabetes, and depressive symptomatology. It is important to highlight that disability and depression have been previously associated with both sensory (Brennan et al., 2006; Cimarolli & Jopp, 2014; Fabbri et al., 2016; Nikolova, Demers, & Béland, 2009) and cognitive functioning (Cosh et al., 2018; Kim et al., 2018). Specific risk factors associated with the group presenting the most accelerated rates of sensory-cognitive difficulties should be noted. This group comprised younger participants, and it was associated with high blood pressure, as well as higher levels of depressive symptoms. These results are in line with previous literature suggesting that the common cause might reflect cardiovascular-related factors affecting both sensory and cognitive functioning (Fischer et al., 2016). Similarly, depression has been associated with both sensory (Cosh et al., 2018) and cognitive functioning (Kim et al., 2018).
Two major limitations of the study should be considered. First, our sample is focused on participants from the U.K. population aged 60 years and over that had responded to all the sensory and cognitive items. Thus, participants who did not survive the time frame considered were excluded from the analyses, constraining the sample and limiting the generalizability of the results. Second, the visual and hearing domains were assessed by means of self-reported items, which can be affected by response biases, and may underestimate sensory impairment in older population (Kamil, Genther, & Lin, 2015). The underestimation of sensory impairment might reduce variability in the responses to self-reports, attenuating the relationships between the sensory and cognitive domains. Subsequently, this attenuation might have a negative impact on the reliability and strength of the common cause factor. Therefore, the predictive ability of the common cause latent measure on incident dementia could be increased in case objective measures of sensory functioning are included in the structural model. Nonetheless, both self-reported measures of visual and hearing impairments present a high correspondence with objective measures of visual (Whillans & Nazroo, 2014) and hearing (Sindhusake et al., 2001) functioning. That aside, the hearing domain only comprised two indicators. Further research should be conducted to assess the temporal stability of the common cause in other populations (e.g., younger cohorts, or populations exposed to sensory or cognitive environmental risk factors), as well as the psychometric properties of the sensory-cognitive measure proposed in this study. Additionally, interdisciplinary research could be useful for identifying other potential neurobiological and genetic markers of the common cause. Neuroimaging studies could help localizing structural and functional regions of interest in the brain associated with both sensory and cognitive functioning. Such findings would be potentially valuable for identifying common neurodegenerative factors associated with declines in both sensory and cognitive functioning. Genome-wide association studies could also help identifying single-nucleotide polymorphisms and genetic correlations across these domains of functioning.
In conclusion, the longitudinal factorial invariance of a common factor accounting for the observed associations between sensory and cognitive functioning is assessed to derive a sensory-cognitive measure from the model. Our results identify a latent factor accounting for the communalities among visual, hearing, and cognitive functioning. Moreover, here we show that the predictive ability of this common factor in relation to sensory and cognitive functioning remains stable over 8 years. The sensory-cognitive measure derived from this factor outperformed both sensory and cognitive functioning isolated measures at predicting dementia over 10 years. Therefore, complementing cognitive measures with a few self-reported indicators of sensory functioning proved to be useful for enhancing the assessment of risk of dementia. Three population-based groups with different trajectories of sensory-cognitive difficulties were identified. This study suggests that older people with lower education and household wealth, more disability, higher presence of diabetes, high blood pressure, and depressive symptoms, as well as lower levels of physical activity, may present a steeper decline in sensory-cognitive functioning over time, as well as a higher risk of dementia. Considering our results, the proposed measure could be useful as a cost-effective indicator of sensory-cognitive functioning among older population.
Funding
This work was supported by the Ageing Trajectories of Health: Longitudinal Opportunities and Synergies (ATHLOS) project. The ATHLOS project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 635316. The first and fifth ELSA waves have been funded jointly by U.K. government departments and the National Institute on Aging, in the United States. Javier de la Fuente work is supported by the FPU predoctoral grant (FPU16/03276) from the Spanish Ministry of Education, Culture, and Sport.
Supplementary Material
Acknowledgment
The authors thank the ATHLOS Consortium for useful discussions.
Conflict of Interest
None reported.
References
- Anstey K. J., Luszcz M. A., & Sanchez L (2001). A reevaluation of the common factor theory of shared variance among age, sensory function, and cognitive function in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 56, P3–11. doi:10.1093/geronb/56.1.P3 [DOI] [PubMed] [Google Scholar]
- Baltes P. B., & Lindenberger U (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging, 12, 12–21. doi:10.1037/0882-7974.12.1.12 [DOI] [PubMed] [Google Scholar]
- Brennan M., Su Y. P., & Horowitz A (2006). Longitudinal associations between dual sensory impairment and everyday competence among older adults. Journal of Rehabilitation Research and Development, 43, 777–792. doi:10.1682/JRRD.2005.06.0109 [DOI] [PubMed] [Google Scholar]
- Chang L. H., Yotsumoto Y., Salat D. H., Andersen G. J., Watanabe T., & Sasaki Y (2015). Reduction in the retinotopic early visual cortex with normal aging and magnitude of perceptual learning. Neurobiology of Aging, 36, 315–322. doi:10.1016/j.neurobiolaging.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G. W., & Rensvold R. B (2002). Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling, 9, 233–255. doi:10.1207/S15328007SEM0902 [Google Scholar]
- Cimarolli V. R., & Jopp D. S (2014). Sensory impairments and their associations with functional disability in a sample of the oldest-old. Quality of Life Research, 23, 1977–1984. doi:10.1007/s11136-014-0657-0 [DOI] [PubMed] [Google Scholar]
- Cosh S., von Hanno T., Helmer C., Bertelsen G., Delcourt C., & Schirmer H (2018). The association amongst visual, hearing, and dual sensory loss with depression and anxiety over 6 years: The Tromsø Study. International Journal of Geriatric Psychiatry, 33, 598–605. doi:10.1002/gps.4827 [DOI] [PubMed] [Google Scholar]
- Davies H. R., Cadar D., Herbert A., Orrell M., & Steptoe A (2017). Hearing impairment and incident dementia: Findings from the English longitudinal study of ageing. Journal of the American Geriatrics Society, 65, 2074–2081. doi:10.1111/jgs.14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente J., Félix Caballero F., Sánchez-Niubó A., Panagiotakos D. B., Prina M. A., Arndt H., … Utrecht U (2018). Determinants of health trajectories in England and the US: An approach to identify different patterns of healthy aging. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 73, 1512–1518. doi:10.1093/gerona/gly006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal J. A.,, Betz J.,, Yaffe K.,, Harris T.,, Purchase-Helzner E.,, Satterfield S.,…, Lin F. R.; Health ABC Study Group (2017). Hearing impairment and incident dementia and cognitive decline in older adults: The health ABC study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 72, 703–709. doi:10.1093/gerona/glw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri E.,, An Y.,, Zoli M.,, Tanaka T.,, Simonsick E. M.,, Kitner-Triolo M. H.,…, Ferrucci L. (2016). Association between accelerated multimorbidity and age-related cognitive decline in older Baltimore longitudinal study of aging participants without dementia. Journal of the American Geriatrics Society, 64, 965–972. doi:10.1111/jgs.14092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M. E.,, Cruickshanks K. J.,, Schubert C. R.,, Pinto A. A.,, Carlsson C. M.,, Klein B. E.,…, Tweed T. S. (2016). Age-related sensory impairments and risk of cognitive impairment. Journal of the American Geriatrics Society, 64, 1981–1987. doi:10.1111/jgs.14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B., Schneider J., McMahon C. M., Burlutsky G., Leeder S. R., & Mitchell P (2013). Dual sensory impairment in older adults increases the risk of mortality: A population-based study. PLoS ONE, 8, e55054. doi:10.1371/journal.pone.0055054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf C. (2008). The Lawton instrumental activities of daily living (IADL) scale. American Journal of Nursing. 108, 52–62. doi:10.1017/S1041610205001547 [PubMed] [Google Scholar]
- Harris K. C., & Dubno J. R (2017). Age-related deficits in auditory temporal processing: Unique contributions of neural dyssynchrony and slowed neuronal processing. Neurobiology of Aging, 53, 150–158. doi:10.1016/j.neurobiolaging.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., & Bentler P. M (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. doi:10.1080/10705519909540118 [Google Scholar]
- Humes L. E., Busey T. A., Craig J., & Kewley-Port D (2013). Are age-related changes in cognitive function driven by age-related changes in sensory processing? Attention, Perception & Psychophysics, 75, 508–524. doi:10.3758/s13414-012-0406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes L. E., & Young L. A (2016). Sensory-cognitive interactions in older adults. Ear and Hearing, 37 (Suppl 1), 52S–61S. doi:10.1097/AUD.0000000000000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil R. J., Genther D. J., & Lin F. R (2015). Factors associated with the accuracy of subjective assessments of hearing impairment. Ear and Hearing, 36, 164–167. doi:10.1097/AUD.0000000000000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S., Ford A. B., Moskowitz R. W., Jackson B. A., & Jaffe M. W (1963). Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA, 185, 914–919. doi:10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- Kim B. J., Liu L., Cheung C., & Ahn J (2018). Effects of cognitive impairment and functional limitation on depressive symptoms among community-dwelling older Korean immigrants in the U.S. PLoS ONE. 13, e0193092. doi:10.1371/journal.pone.0193092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence B. J., Jayakody D. M. P., Bennett R. J., Eikelboom R. H., Gasson N., & Friedland P. L (2019). Hearing loss and depression in older adults: A systematic review and meta-analysis. The Gerontologist. Advance online publication. doi:10.1093/geront/gnz009 [DOI] [PubMed] [Google Scholar]
- Lin F. R., Ferrucci L., An Y., Goh J. O., Doshi J., Metter E. J., … Resnick S. M (2014). Association of hearing impairment with brain volume changes in older adults. NeuroImage, 90, 84–92. doi:10.1016/j.neuroimage.2013.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. Y.,, Gutierrez P. R.,, Stone K. L.,, Yaffe K.,, Ensrud K. E.,, Fink H. A.,…, Mangione C. M.; Study of Osteoporotic Fractures Research Group (2004). Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. Journal of the American Geriatrics Society, 52, 1996–2002. doi:10.1111/j.1532-5415.2004.52554.x [DOI] [PubMed] [Google Scholar]
- Lin F. R., Metter E. J., O’Brien R. J., Resnick S. M., Zonderman A. B., & Ferrucci L (2011). Hearing loss and incident dementia. Archives of Neurology, 68, 214–220. doi:10.1001/archneurol.2010.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Yaffe K., Xia J., Xue Q., Harris T. B., Purchase-Helzner E. … Simonsick E. M (2013). Hearing loss and cognitive decline in older adults. JAMA Internal Medicine, 173, 293–299. doi:10.1001/jamainternmed.2013.1868.Hearing [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U., & Baltes P. B (1994). Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging, 9, 339–355. doi:10.1037/0882-7974.9.3.339 [DOI] [PubMed] [Google Scholar]
- Lindenberger U., & Ghisletta P (2009). Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychology and Aging, 24, 1–16. doi:10.1037/a0014986 [DOI] [PubMed] [Google Scholar]
- Luo Y., He P., Guo C., Chen G., Li N., & Zheng X (2018). Association between sensory impairment and dementia in older adults: Evidence from China. Journal of the American Geriatrics Society. 66, 480–486. doi:10.1111/jgs.15202 [DOI] [PubMed] [Google Scholar]
- Maharani A., Dawes P., Nazroo J., Tampubolon G., & Pendleton N (2018). Visual and hearing impairments are associated with cognitive decline in older people. Age and Ageing, (April), 47, 575–581. doi:10.1093/ageing/afy061 [DOI] [PubMed] [Google Scholar]
- Mansbach W. E., & Mace R. A (2018). Predicting functional dependence in mild cognitive impairment: Differential contributions of memory and executive functions. The Gerontologist. Advance online publication. doi:10.1093/geront/gny097 [DOI] [PubMed] [Google Scholar]
- Mitoku K., Masaki N., Ogata Y., & Okamoto K (2016). Vision and hearing impairments, cognitive impairment and mortality among long-term care recipients: A population-based cohort study. BMC Geriatrics, 16, 112. doi:10.1186/s12877-016-0286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova R., Demers L., & Béland F (2009). Trajectories of cognitive decline and functional status in the frail older adults. Archives of Gerontology and Geriatrics, 48, 28–34. doi:10.1016/j.archger.2007.09.007 [DOI] [PubMed] [Google Scholar]
- Panza F., Solfrizzi V., & Logroscino G (2015). Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nature Reviews. Neurology, 11, 166–175. doi:10.1038/nrneurol.2015.12 [DOI] [PubMed] [Google Scholar]
- Proust-Lima C., Philipps V., & Liquet B (2017). Estimation of extended mixed models using latent classes and latent processes: The R Package lcmm. Journal of Statistical Software, 78, 1–56. doi:10.18637/jss.v078.i02 [Google Scholar]
- Roberts K. L., & Allen H. A (2016). Perception and cognition in the ageing brain: A brief review of the short- and long-term links between perceptual and cognitive decline. Frontiers in Aging Neuroscience, 8, 39. doi:10.3389/fnagi.2016.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. doi:10.18637/jss.v048.i02 [Google Scholar]
- Schubert, C. R., Cruickshanks, K. J., Fischer, M. E., Pinto, A. A., Chen, Y., Huang, G. H., ... Dalton, D. S. (2019). Sensorineural Impairments, Cardiovascular Risk Factors, and 10-Year Incidence of Cognitive Impairment and Decline in Midlife: The Beaver Dam Offspring Study. The Journals of Gerontology: Series A. doi: 10.1093/gerona/glz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhusake D., Mitchell P., Smith W., Golding M., Newall P., Hartley D., & Rubin G (2001). Validation of self-reported hearing loss. The Blue Mountains Hearing Study. International Journal of Epidemiology, 30, 1371–1378. doi:10.1093/ije/30.6.1371 [DOI] [PubMed] [Google Scholar]
- StataCorp (2015). Stata Statistical Software: Release 14. [computer program]. College Station, TX: StataCorp LP. doi:10.2307/2234838 [Google Scholar]
- Steptoe A., Breeze E., Banks J., & Nazroo J (2013). Cohort profile: The English longitudinal study of ageing. International Journal of Epidemiology, 42, 1640–1648. doi:10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey C. L., Wallace R. B., & Herzog R (1999). A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics, 11, 139–148. doi:10.1017/S1041610299005694 [DOI] [PubMed] [Google Scholar]
- Wahl H. W., Heyl V., Drapaniotis P. M., Hörmann K., Jonas J. B., Plinkert P. K., & Rohrschneider K (2013). Severe vision and hearing impairment and successful aging: A multidimensional view. The Gerontologist, 53, 950–962. doi:10.1093/geront/gnt013 [DOI] [PubMed] [Google Scholar]
- Whillans J., & Nazroo J (2014). Assessment of visual impairment: The relationship between self-reported vision and ‘gold-standard’ measured visual acuity. British Journal of Visual Impairment, 32, 236–248. doi:10.1177/0264619614543532 [Google Scholar]
- Whitson H. E., Cronin-Golomb A., Cruickshanks K. J., Gilmore G. C., Owsley C., Peelle J. E., … Lin F. R (2018). American geriatrics society and national institute on aging bench-to-bedside conference: Sensory impairment and cognitive decline in older adults. The Journal of the American Geriatrics Society, 66, 2052–2058. doi:10.1111/jgs.15506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widaman K. F., Ferrer E., & Conger R. D (2010). Factorial invariance within longitudinal structural equation models: Measuring the same construct across time. Child Development Perspectives, 4, 10–18. doi:10.1111/j.1750-8606.2009.00110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. S., Segawa E., Hizel L. P., Boyle P. A., & Bennett D. A (2012). Terminal dedifferentiation of cognitive abilities. Neurology, 78, 1116–1122. doi:10.1212/WNL.0b013e31824f7ff2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y.,, Denkinger M. D.,, Onder G.,, Henrard J. C.,, van der Roest H. G.,, Finne-Soveri H.,…, Topinkova E. (2016). Dual sensory impairment and cognitive decline: The results from the shelter study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 71, 117–123. doi:10.1093/gerona/glv036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.