Abstract
A significant number of severely obese adolescents undergoing bariatric surgery have evidence of early kidney damage. To determine if kidney injury is reversible following bariatric surgery, we investigated renal outcomes in the Teen-Longitudinal Assessment of Bariatric Surgery cohort, a prospective multicenter study of 242 severely obese adolescents undergoing bariatric surgery. Primary outcomes of urine albumin-to-creatinine (ACR) ratio and cystatin C-based estimated glomerular filtration rate (eGFR) were evaluated pre-operatively and up to 3 years following bariatric surgery. At surgery, mean age of participants was 17 years and median BMI was 51 kg/m2. In those with decreased kidney function at baseline (eGFR<90 mL/min/1.73m2), mean (±SD) eGFR improved from 76 ± 12 mL/min/1.73m2 to 102 ± 28 mL/min/1.73m2 at 3-year follow-up (p<0.0001). Similarly, participants with albuminuria (ACR≥30 mg/g) at baseline demonstrated significant improvement following surgery: geometric mean (95% CI) of ACR was 74 mg/g (45-121) at baseline and decreased to 17 mg/g (10-28) at 3 years (p<0.0001). Those with normal renal function and no albuminuria at baseline remained stable throughout the study period. Among subjects with a BMI ≥40 kg/m2 at follow-up, increased BMI was associated with lower eGFR (p<0.001), while no association was observed in those with a BMI <40 kg/m2. In adjusted analysis, eGFR increased by 3.9 mL/min/1.73m2 for each 10 unit loss of BMI. Early kidney abnormalities improved following bariatric surgery in adolescents with evidence of pre-operative kidney disease. Kidney disease should be considered as a selection criteria for bariatric surgery in severely obese adolescents who fail conventional weight management.
Keywords: severe obesity, bariatric surgery, kidney disease
Introduction
Severe obesity, defined as a body mass index (BMI) >120% of the 95th percentile or an absolute BMI ≥35 kg/m2, is increasing and now affects 4-6% of U.S. children and adolescents.1-3 The American Heart Association (AHA) recently issued a consensus statement to raise awareness of this growing problem and summarize available treatment options. Considering the limited effectiveness of lifestyle and pharmacologic interventions, use of bariatric surgery has been advocated for appropriately selected adolescents with severe obesity and obesity-related comorbidities.4
The obesity epidemic has been paralleled by a proportionate rise in chronic kidney disease (CKD).5 Obesity is now recognized as an independent risk factor for CKD, with recent estimates indicating that 24-33% of all kidney disease could be related to obesity.6 Obese children, moreover, have a significantly higher risk of developing CKD and end stage renal disease (ESRD) in adulthood.7, 8 To estimate the prevalence of early kidney disease in severely obese adolescents, we recently conducted a baseline analysis of the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) cohort. Teen-LABS is a prospective observational study of adolescent bariatric surgical patients aimed to understand the broad ranging outcomes following bariatric surgery. We reported that a concerning number of severely obese adolescents undergoing bariatric surgery had evidence of early kidney disease at baseline prior to surgery: 17% had albuminuria and 3% had significantly decreased estimated glomerular filtration rate (eGFR).9
Despite promising results of bariatric surgery to improve obesity and obesity-related comorbidities,10 little is known about the potential salutary effect of bariatric surgery on obesity-related kidney disease in adolescents. To determine if early kidney injury that is associated with severe obesity reverses following weight loss surgery, we analyzed kidney outcomes in the Teen-LABS cohort up to 3 years following bariatric surgery. We hypothesized that albuminuria and eGFR would improve post-operatively, with the greatest improvement occurring in those with the most severe kidney abnormalities prior to undergoing bariatric surgery.
Results
Demographic and clinical characteristics
Mean age at surgery was 17.1 years. Of the 242 subjects, 75.6% were female and 64.9% were non-Hispanic white. Procedures included Roux-en-Y gastric bypass (gastric bypass) in 66.5%, sleeve gastrectomy in 27.7%, and adjustable gastric band in 5.8%. Clinical characteristics of the cohort at baseline and at 3 years follow-up are presented in Table 1. Significant improvements occurred in BMI and other cardiovascular risk factors following bariatric surgery. Median BMI decreased from 50.5 kg/m2 at baseline to 36.2 kg/m2 at 3 years follow-up. Most of this change occurred during the first 6 months post-operatively, after which the median BMI was 38.0 kg/m2. Significantly reduced ferritin and elevation in transferrin were evident in the cohort. However, significant improvements were seen in the homeostasis model assessment of insulin resistance (HOMA-IR), C-reactive protein, and the percentage of participants with dyslipidemia, hypertension, and diabetes. The percentage of participants taking anti-hypertensive medications also decreased: at baseline, 22% compared to 7% at 3 years follow-up. Of those taking antihypertensive medications, 59% were taking ACE inhibitors at baseline and 23% at 3 years follow-up.
Table 1:
Clinical characteristics at baseline and 3 years follow-up in the Teen-LABS cohort
| Variable | Baseline (N=242) |
3 years follow-up (N=206) |
P value |
|---|---|---|---|
| BMI, kg/m2 | 50.5 (45.2, 58.3) | 36.2 (30.2,44.9) | <0.0001 |
| Hypertension (%) | 104 (43.7%) | 29 (15.3%) | <0.0001 |
| Type 2 Diabetes (%) | 30 (12.6%) | 1 (0.6%) | <0.0001 |
| Dyslipidemia (%) | 179 (75.2%) | 54 (30.0%) | <0.0001 |
| Transferrin, mg/dL | 268 (244, 292) | 313 (274, 352) | <0.0001 |
| Ferritin (μg/L) | 37.0 (23.0, 66.0) | 9.0 (5.0,24.0) | <0.0001 |
| Serum albumin (g/dL) | 4.1 (3.9, 4.4) | 4.3 (4.1, 4.5) | <0.0001 |
| HOMA-IR | 5.91 (3.60, 9.98) | 1.97 (1.20, 3.32) | <0.0001 |
| hsCRP, (mg/L) | 0.63 (0.30, 1.17) | 0.09 (0.03, 0.32) | <0.0001 |
| HbA1c (%) | 5.20 (5.00, 5.50) | 5.1 (4.9, 5.3) | <0.0001 |
Data presented as median (25th, 75th percentile) or n (%). P-values are generated from GLMM analysis.
Estimated glomerular filtration rate
The mean (±standard deviation [SD]) of cystatin C-based eGFR at baseline was 108 ± 27 mL/min/1.73m2, and this increased by 6% to 115 ± 29 mL/min/1.73m2 at 3 years post-operatively (p=0.02). In this severely obese cohort, a low eGFR (<90 mL/min/1.73m2) was observed in 24.8% (59/238) at baseline and improved to 15.5% (29/187) at 3 years follow-up (p=0.004). An abnormally high eGFR (≥150 mL/min/1.73m2) was present in 7.1% (17/238) at baseline and 7.5% (14/187) at 3 years (p = 0.83).
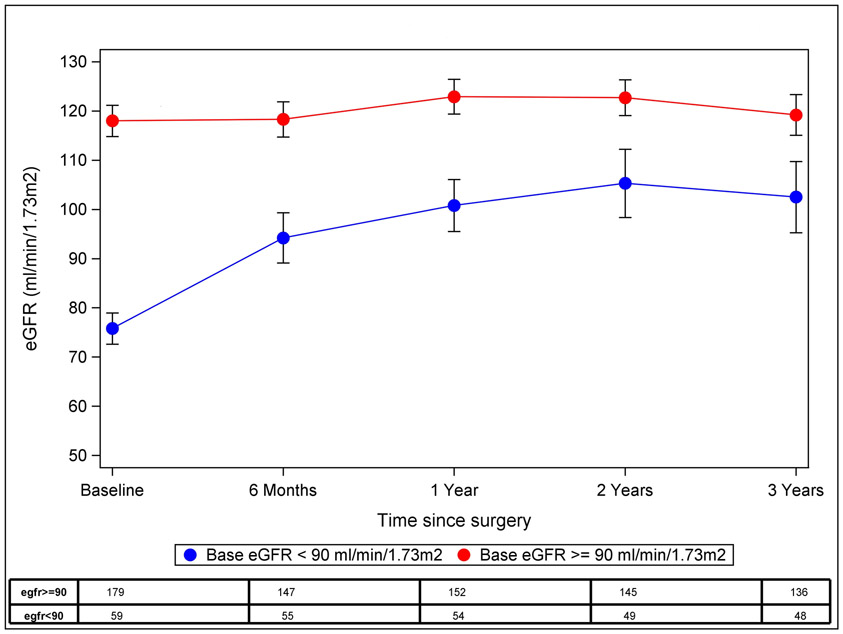
Three-year follow-up of eGFR stratified by baseline level of renal function (<90 mL/min/1.73m2 versus ≥90 mL/min/1.73m2) is presented in Figure 1. The majority of improvement in renal function occurred in those with a baseline eGFR of <90 mL/min/1.73m2, whose mean (±SD) eGFR was 76 ± 12 mL/min1.73m2. In this group, eGFR improved by 34% to 102 ± 28 mL/min/1.73m2 at 3 years follow-up (p <0.0001). Improvement in renal function was first detected at the earliest postoperative visits (6-12 months post-operatively) and then remained stable throughout the remainder of the study period. Among the seven subjects with a baseline eGFR of <60 mL/min/1.73m2, eGFR improved from 52 ± 7 mL/min/1.73m2 at baseline to 98 ± 32 mL/min/1.73m2 at 1 year (n=6, p=0.03), 103 ± 38 at 2 years (n=6, p=0.01) and 94 ± 48 at 3 years (n=5, p=0.08) follow-up. However, those with an eGFR ≥90 mL/min/1.73m2 at baseline did not change significantly over time (118 ± 21 mL/min/1.73m2 at baseline and 119 ± 28 mL/min/1.73m2 at 3 years follow-up, p=0.98). A non-statistically significant decline in eGFR was also detected in the small subgroup (n=17) with eGFR ≥150 mL/min/1.73m2 at baseline, for whom the mean eGFR of 162 ± 16 mL/min/1.73m2 decreased to an eGFR of 138 ± 32 mL/min/1.73m2 at 3 years (n=15, p=0.07).
Figure 1: Three-year follow-up of eGFR in the Teen-LABS cohort.
Least-squares means and 95% CI are presented at each time point using GLMM analysis. Participants were stratified according to baseline kidney function.
Albuminuria
Albuminuria was observed in 17.0% (39/230) of the cohort at baseline and decreased to 11.0% (19/173) at 3 years follow-up (p=0.06). Participants with albuminuria at baseline demonstrated a significant improvement of ACR: Geometric mean (95% CI) of ACR was 74 mg/g (45-121mg/g) at baseline and decreased to 17 mg/g (10-28 mg/g) at 3 years (p<0.0001). Of these subjects, 69% experienced normalization of albuminuria at 2 years, and 75% experienced resolution of albuminuria at 3 years follow-up.
Three-year follow-up of ACR ratios stratified by baseline level of albuminuria is presented in Table 2. Pre-operatively, 13.9% (32 subjects) had microalbuminuria and 3.0% (7 subjects) had macroalbuminuria. Among those with microalbuminuria, an 83% reduction in ACR was observed 1 year postoperatively (p<0.0001). The seven participants with macroalbuminuria at baseline also demonstrated a remarkable improvement during the study period, 3 of whom had complete normalization of urinary protein at 1 year follow-up (p<0.0001). Among those with normal levels of urinary albumin at baseline, there was no significant change in ACR at 3 years follow-up, although 11 of these subjects (7.6%) developed incident microalbuminuria at 3 years.
Table 2:
Three-year follow-up of proteinuria in the Teen-LABS cohort
| Baseline status | Baseline (N=230) |
6 months (N=180) |
1 year (N=189) |
2 years (n=182) |
3 years (N=173) |
|---|---|---|---|---|---|
| Normala | 5.7 (3.7, 9.5) [n=191] |
6.0 (3.8, 13.0) [n=145] |
6.3 (4.1, 10.7) [n=156] |
5.7 (4.1, 10.7) [n=149] |
5.9 (4.1, 12.2) [n=145] |
| Microalbuminuriab | 58.9 (45.2, 176.9) [n=32] |
10.5 (4.7, 19.2) [n=28] |
11.2 (6.0, 19.1) [n=26] |
8.0 (6.4, 16.6) [n=27] |
10.2 (5.5, 14.0) [n=23] |
| Macroalbuminuriab | 729 (533, 1677) [n=7] |
38.7 (4.8, 611.0) [n=7] |
80.7 (7.6, 245.1) [n=7] |
106.1 (30.4,121.4) [n=6] |
67.5 (11.2,173.8) [n=5] |
Data presented as geometric mean (95% confidence interval) of urine albumin (in mg) per gram of urine creatinine (ACR). Participants were stratified according to baseline level of albuminuria.
Baseline different from 6 months and 12 months p<0.03 (using a Tukey-Kramer adjustment to account for multiple comparisons)
Baseline different from 6-months, 1-year, 2-years and 3-years, all p<0.00001 (using a Tukey-Kramer adjustment to account for multiple comparisons)
Association of BMI with GFR during the study period
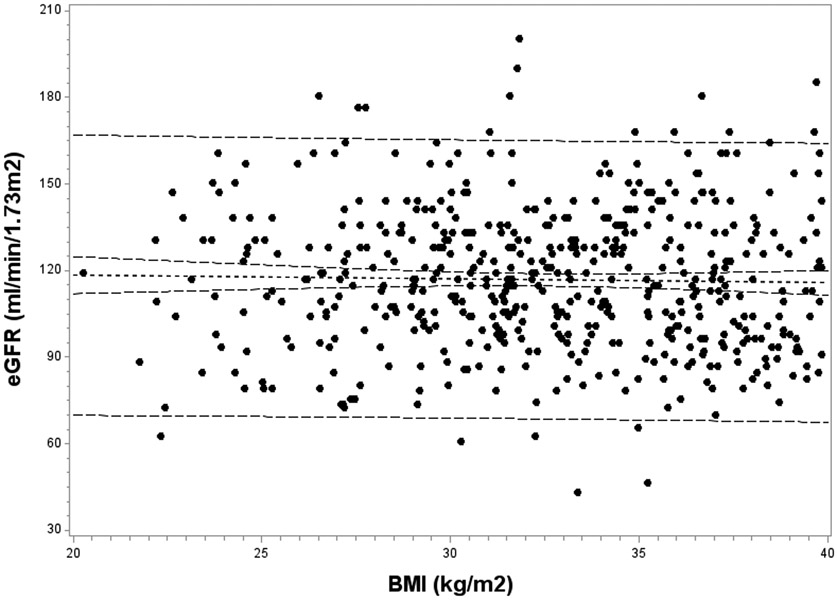
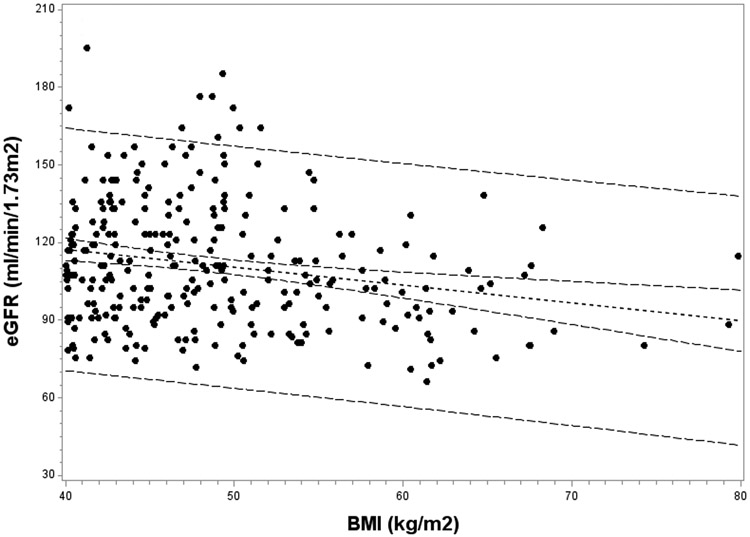
The relationship between BMI and eGFR was investigated at each study time point. At baseline, those with higher BMI values demonstrated lower eGFR; a strongly negative correlation was observed (r = −0.34; p<0.0001). However, this inverse association between BMI and eGFR dissipated postoperatively, and was no longer significant by 2 years (r = −0.11, p=0.13). To further investigate this finding, the association of BMI with eGFR was examined separately among those with BMI values of <40 kg/m2 and ≥40 kg/m2 at follow-up visits (Figure 2). Among subjects with BMI <40 kg/m2 at follow-up, no association of BMI with eGFR was observed. However, the negative association of BMI with eGFR persisted in those with a BMI ≥40 kg/m2 during the follow-up period. Among these subjects with residual severe obesity postoperatively, a mean decrease of 7.0 mL/min/1.73m2 in eGFR occurred for each 10 kg/m2 increase above 40 kg/m2 in BMI (p=0.0001).
Figure 2: Association of BMI with eGFR at follow-up in the Teen-LABS cohort.
The association of BMI with eGFR was investigated separately according to BMI at follow-up: Figure 2a, BMI <40 kg/m2 (p=0.41); and Figure 2b, BMI ≥40 kg/m2 (p =0.0001).
Multivariate analysis: Predictors of eGFR and albuminuria at follow-up
Mixed-effects linear regression modeling was performed to investigate demographic and clinical variables associated with eGFR and ACR following weight loss surgery (Table 3). Regression models were adjusted for baseline eGFR and baseline ACR, as appropriate. After adjusting for significant covariates, eGFR increased by 3.9 mL/min/1.73m2 for each 10 unit loss of BMI at follow-up (p<.0001). Higher serum albumin and transferrin levels at follow-up were associated with an increase in eGFR, while white race and male sex were associated with decreased eGFR. Increased eGFR at follow-up was also observed following gastric bypass compared to sleeve gastrectomy. Although post-operative improvement in albuminuria was observed in participants with an elevated ACR, weight loss was not linearly associated with ACR at follow-up (p=0.61). Multivariate analysis indicated that female sex, hypertension at follow-up, and increasing ferritin at follow-up were associated with a worsening (increased) ACR.
Table 3:
Multivariate analysis of 3-year outcomes
| Predictors of eGFR | Predictors of ACR | ||||
|---|---|---|---|---|---|
| Variable | Increase in eGFR* (95% CI) |
p-value | Variable | Percent Increase in ACR (95% CI) |
p-value |
| BMI loss (per 10 kg/m2 decrease) | 3.9 (2.4, 5.4) | <.0001 | BMI loss (per 10 kg/m2 decrease) | 2% (−6%, 10%) | .61 |
| Female sex (vs. males) | 4.5 (1.5, 7.5) | .0003 | Female sex (vs. male) | 57% (41%, 74%) | <.0001 |
| Non-White (vs. White race) | 10.2 (7.5, 12.9) | <.0001 | Hypertension (vs. normotensive) | 38% (21%, 56%) | <.0001 |
| Serum albumin (per 1 g/dL increase) | 6.2 (2.1, 10.4) | .003 | Ferritin (per 10 μg/L increase) | 5% (3%, 6%) | <.0001 |
| Transferrin (per 10 mg/dL increase) | 0.4 (0.1, 0.6) | .006 | |||
| Gastric bypass (vs sleeve gastrectomy)** | 7.0 (4.1, 9.8) | <0.001 | . | ||
eGFR reported in ml/min/1.73m2;
Laparoscopic adjustable band included only14 subjects and was not significantly different from sleeve gastrectomy (increase in eGFR of −0.2, p=0.96)
Models adjusted for baseline eGFR or ACR ratio, as appropriate, and adjusted for study site (included as a random variable). All other variables included in the model are reported.
Discussion
This report represents the first study to investigate kidney outcomes in severely obese adolescents undergoing bariatric surgery. Among participants with baseline kidney abnormalities, kidney function and albuminuria improved 6-12 months following bariatric surgery and remained stable for the duration of the study period. Equally important, in participants without evidence of pre-operative kidney disease, kidney function and albuminuria remained stable throughout the follow-up period.
Proteinuria and decreased kidney function in obese subjects often indicates the presence of obesity-related glomerulopathy, which is characterized by increased glomerular size, increased mesangial matrix, and glomerular sclerosis.11, 12 This condition can portend a grave prognosis, with up to 50% progressing to ESRD within 10 years of diagnosis.13 Therefore, vigilant screening of severely obese adolescents is needed to identify those with evidence of early kidney injury. In this cohort, 13 participants (5%) had evidence of significant kidney disease, defined by an ACR ≥300 mg/g (macroalbuminuria) or an eGFR <60 mL/min/1.73m2 (CKD stage 3). These participants demonstrated a marked reduction of albuminuria and an increase in eGFR following bariatric surgery. Similarly, those 63 (23%) participants with microalbuminuria or mildly reduced eGFR (<90 mL/min/1.73m2) at baseline also improved following bariatric surgery. These findings suggest that surgical intervention may be an effective treatment to improve early kidney injury and prevent progression of obesity-related kidney disease.
Our results are consistent with previous adult studies that have demonstrated improvement in albuminuria following bariatric surgery.14-19 Improvements in serum creatinine and eGFR after bariatric surgery have also been observed in adults with decreased kidney function, while function has remained stable in those without baseline kidney disease.20-22 Similar to adult studies, improvements in this adolescent cohort occurred mainly within a year of bariatric surgery.18, 22
Hyperfiltration is a proposed mechanism of early kidney injury in obesity-related glomerulopathy that precedes the development of proteinuria and decreased kidney function.23 We previously reported an increased prevalence of elevated eGFR at baseline in the Teen-LABS cohort.9 Interestingly, among the 17 participants with an elevated eGFR at baseline (>150 mL/min/1.73m2), a decreasing trend in eGFR was observed at 3 years follow-up, although this should be interpreted with caution considering the small sample size. Previous studies in adults have reported similar changes in GFR following bariatric surgery,16, 18 suggesting that weight loss may improve obesity-induced hyperfiltration.
We previously reported a strong association of increased BMI with lower eGFR at the baseline visit of Teen-LABS participants, with an adjusted 8 mL/min/1.73m2 lower eGFR for every 10 kg/m2 elevation in baseline BMI. This finding prompted the important question of whether an intervention to reduce BMI would improve kidney function and/or dissociate the link between BMI and kidney function.9 Indeed, these data optimistically suggest that bariatric surgery may prevent a progressive decline in kidney function in severely obese adolescents with evidence of obesity-related kidney disease. Renal function improved significantly in participants with a low eGFR at baseline, and this improvement in eGFR at follow-up was directly associated with a reduction in BMI. However, a concerning facet of our study findings was that those with residual severe obesity likely still face residual risks. When the kidney outcome was examined separately among patients with persistent severe obesity at follow-up, evidence of a strong residual association between BMI and eGFR was present. Conversely, no relationship between BMI and eGFR was apparent in those who were no longer severely obese. It is not clear from these preliminary analyses what factors might mediate a continued association between residual severe obesity and renal dysfunction. However, a reasonable conclusion from the data is that there is plasticity in renal function that relates resolution of severe obesity with optimal kidney outcomes. Conversely these data also prompt the physiologic hypothesis that factors which underlie obesity-related nephron damage, such as hyperfiltration and inflammation, may be insufficiently reversed in those who remain severely obese after bariatric surgery.
Our results indicate that demographic and clinical parameters other than BMI are related to albuminuria and kidney function following weight loss surgery. High serum transferrin and albumin were associated with increased eGFR at follow-up, associations which similarly have been observed in adults with moderate to advanced CKD.24, 25 It is speculated that higher albumin and transferrin may reflect improved nutritional status and increased protein intake, which are known to increase GFR.26 Increased eGFR at follow-up was also observed following gastric bypass compared to sleeve gastrectomy, which may be related to recently described metabolic differences between these two procedures, with gastric bypass having more pronounced effects than the sleeve procedure.27, 28 Interestingly, non-Caucasian participants experienced a significantly greater improvement in eGFR over the study period compared to Caucasian participants. Racial variability exists in common causes of kidney disease, including diabetes and hypertension, and remains unexplained by modifiable risk factors.29-32 It is possible that racial variability can similarly influence improvement in kidney function following bariatric surgery, and the relevance of this finding in our cohort warrants further evaluation. Increased albuminuria during the follow-up period was associated with female gender and increased ferritin. We previously reported similar associations at baseline in the Teen-LABS cohort.9 Hypertensive participants were also more likely to have worsening albuminuria, which is of particular interest given the high prevalence of hypertension pre-operatively. Taken together, these associations suggest that factors other than excess adiposity per se may be involved in the pathogenesis of obesity-related kidney disease. For example, metabolic risk factors and hypertension have been shown to modify the association of BMI with incident ESRD.33 Future studies, however, are needed to establish a cause-and-effect relationship of potential mechanisms of obesity-related kidney disease.
This study has several strengths, including the relatively large sample size, standardized methods of data collection, and centralized and uniform laboratory analysis. The study protocol was designed to collect comprehensive data on long-term outcomes of bariatric surgery, including cardiovascular risk factors and kidney disease. Specifically, cystatin C was collected as part of the Teen-LABS protocol to optimize estimation of GFR in this unique, severely obese cohort. Cystatin C has demonstrated superior performance in estimating GFR when compared with serum creatinine,34 and creatinine-based estimations of GFR have performed inconsistently in obese patients.35 Cystatin C is also less affected by demographic variables including age, sex, and race compared to serum creatinine.36
Nevertheless, several limitations of our study deserve mention. First, although cystatin C has been validated to provide an accurate estimation of kidney function,37 we did not have a direct measurement of GFR. Some have suggested cystatin C might slightly underestimate GFR in severely obese patients due to an association with adiposity.38 We cannot exclude that the performance of cystatin C-based eGFR was affected by body composition. However, eGFR remained unchanged in participants with normal renal function at baseline despite marked weight loss, suggesting that changes in body composition did not have a significant effect on GFR estimation. Serum cystatin C can also be mildly affected by factors other than renal function, which may affect GFR estimation. Gender variations in cystatin C have been reported, which may have accounted for increased eGFR at follow-up among females, who composed 76% of our study cohort.36 Also, although we report outcomes for those who presented with eGFR values ≥150 mL/min/1.73m2, there are limitations to using cystatin C-based estimates for assessment of hyperfiltration. Formal assessment of hyperfiltration requires direct GFR measurement with methods such as inulin clearance or a 24 hour urine collection, as has been performed by others who demonstrated improvement in hyperfiltration following bariatric surgery.16, 18 Second, the use of BMI as a marker of adipose tissue burden does not differentiate between fat and lean mass, or visceral and subcutaneous adiposity. Visceral adiposity in particular is a source of inflammatory cytokine secretion that has been implicated in the pathogenesis of obesity-related kidney disease.5 Third, although the Teen-LABS study had relatively high retention rates, laboratory data were missing in 24-29% of the subjects at 3 years follow-up. However, sensitivity analyses that imputed missing values were performed and supported our primary results. Finally, Teen-LABS was a prospective observational study and did not have a control arm, and our results should be interpreted in this context. Other treatment options, including caloric restriction and captopril, have been shown to decrease proteinuria in obese patients with kidney disease.39, 40 Future randomized controlled trials in patients with obesity-related kidney disease are needed compare bariatric surgery to more conventional management strategies. However, non-surgical treatment options in severely obese adolescents have demonstrated only modest improvement in weight and cardiovascular risk factors, with poor long-term sustainability.4
In conclusion, we report 3-year kidney outcomes of the largest study to date of adolescents undergoing bariatric surgery. Kidney function and albuminuria improved following weight loss surgery in those participants with evidence of pre-operative kidney disease. Furthermore, BMI levels of greater than 40 kg/m2 at follow-up were associated with a progressive decline in kidney function. These data support the addition of kidney dysfunction as a selection criterion for bariatric surgery in adolescents who reach a BMI of 40kg/m2 to optimize chances for reversal of severe obesity and kidney risks.
Methods
Study population and design
The Teen-LABS study was developed as an ancillary study to the Longitudinal Assessment of Bariatric Surgery (LABS), a prospective observational study of adults undergoing bariatric surgery. The standardized methodology of the Teen-LABS study was patterned from the second phase of LABS, the goal of which is to evaluate the longer term safety and efficacy of bariatric surgery as it relates to specific outcome domains, including kidney disease.41 Briefly, 277 consecutive adolescents undergoing bariatric surgery at each of the five Teen-LABS participating centers were offered enrollment between March 2007 and December of 2011. Clinical decision making was specified by center-specific patient care pathways. No attempt was made to standardize selection criteria for bariatric surgery, although all centers followed accepted guidelines for surgical treatment of obesity.42 Baseline characteristics and obesity-related comorbidities of the Teen-LABS cohort have been previously described.43 Thirty-five individuals either declined participation or did not undergo surgery by the end of the enrollment period, leaving a final cohort of 242 subjects. During the 3-year follow-up period, 89.2% completed postoperative visits at 6 months, 90.1% at year 1, 89.3% at year 2 and 85.1% at year 3. Written informed consent was obtained from participants over 18 years of age. In those younger than 18, written permission was obtained from caregivers and assent from the adolescent. The study protocol, assent/consent forms, and data and safety monitoring plans were approved by the institutional review board of each center.
Data collection and laboratory analysis
Data collection included demographic, anthropometric, and clinical variables to evaluate weight loss, cardiovascular, metabolic, and renal outcomes. Preoperative data were collected within 30 days of surgery, and follow-up data were obtained at 6 months and then annually following surgery. A Teen-LABS-certified clinical coordinator or surgical investigator followed standard definitions to determine the presence or absence of comorbid conditions using medical records, physical examination, interview, and laboratory values. Detailed descriptions of study definitions, including hypertension, diabetes, and dyslipidemia, have been previously published.41 All laboratory assays were performed centrally at the Northwest Lipid Research Laboratories at the University of Washington, Seattle, WA. HOMA-IR was calculated by dividing the product of serum insulin (mU/mL) and glucose (mg/dl) by a factor of 405. Estimated glomerular filtration rate (eGFR) was calculated using the cystatin C-based Larsson formula (GFR = 77.24 x [Cystatin C]−1.2623) as recommended by the assay manufacturer (Dade Behring, Deerfield, IL) and as previously described.44 Proteinuria was assessed by the urine albumin-to-creatinine ratio (ACR). Microalbuminuria was defined as an ACR ≥30 mg/g and <300 mg/g; macroalbuminuria was defined as an ACR ≥300 mg/g.
Statistical Analysis
Analysis was conducted using SAS®, version 9.3 (SAS Institute, Cary NC). Summary descriptive statistics were reported as frequency (%) for the categorical variables and median (25th percentile, 75th percentile) for continuous variables. McNemar’s test was used to examine the change from baseline in the number of subjects with an elevated ACR ratio and those with reduced or abnormally high eGFR. Generalized linear mixed models (GLMM) were used to analyze eGFR and ACR following bariatric surgery to account for the repeated nature of the data and the within subject correlation; both intercept and study center were specified as random effects and an autoregressive covariance structure specified. Study subject was treated as a repeated measure and time (visit) was treated as categorical when specified in the model. A normal distribution and identity link function was used for continuous dependent variables and binomial distribution with logit link for binary dependent variables. Log transformation of ACR was performed to fulfill the assumptions of linear regression modeling. Multivariable analyses were conducted to identify predictors of eGFR and ACR at follow-up. The independent variable of interest in all of these models was BMI decrease; site identification was retained in all models as a random effect. The initial models included the baseline variables of age, sex, race (non-Hispanic white vs all others), diagnosis of diabetes, surgery type, and the baseline value of the dependent variable (ACR or eGFR). Also included were the time-dependent variables of hypertension, dyslipidemia, transferrin, ferritin, serum albumin, HOMA-IR, hsCRP, and HbA1c. These variables were selected a priori. A backwards elimination procedure was used to find the most parsimonious model with examination of the beta coefficient of BMI decrease as variables were removed from the model. If the removal of a variable from the model changed the beta coefficient for BMI decrease by more than 10%, the variable was retained in the model. Regression diagnostics were examined when the final model was determined. Least-square means and associated 95% confidence intervals (95% CI) were reported from the models. A Tukey-Kramer adjustment was used to adjust for multiple comparisons. Sensitivity analyses were performed using multiple imputation for missing covariates using SAS PROC MI and PROC MIANALYZE; the results did not alter any conclusions drawn from the results of the analyses. The critical value for statistical significance was set a priori at p<0.05.
Exploratory analysis of the association between BMI and eGFR was pursued, as in the baseline analysis of the Teen-LABS cohort in which we reported a significant association between BMI and eGFR.9 In contrast to pre-operative data, a similar linear association was not observed after surgery. To further explore the association of BMI with eGFR during follow-up visits, generalized additive models (PROC GAM) and smoothing functions (PROC TRANSREG) were used to examine spline functions and potential influential knots. Inflection points were observed at a BMI of 40 and 50 kg/m2. The BMI of 40 kg/m2 was further examined for a relationship with eGFR as this value is clinically meaningful and used in defining eligibility for bariatric surgery.42
Acknowledgements
Funding/Support: The Teen-LABS consortium was funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases through grants U01DK072493 (Cincinnati Children’s Hospital Medical Center), UM1DK072493 (Cincinnati Children’s Hospital Medical Center), and UM1DK095710 (University of Cincinnati). The study was also supported by National Institutes of Health grants UL1TR000077-04 (Cincinnati Children’s Hospital Medical Center), UL1RR025755 (Nationwide Children’s Hospital), M01-RR00188 (Texan Children’s Hospital/Baylor College of Medicine), UL1RR024153 and UL1TR000005 (University of Pittsburgh), and UL1TR000165 (University of Alabama, Birmingham). The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Additional Contributions: Teen-LABS is an ancillary study to the Longitudinal Assessment of Bariatric Surgery (LABS) study (U01 DK066557). Data collection methodology was patterned after LABS-1 and LABS-2, with modification as needed for the adolescent population. The Teen-LABS consortium gratefully acknowledges the expertise and guidance provided by the LABS consortium and Data Coordinating Center.
Footnotes
Conflict of Interest Disclosures: Dr. Inge has received bariatric research grant funding from Ethicon Endosurgery, and has served as a consultant for Sanofi Corporation, NPS Pharma, Up To Date, and Independent Medical Expert Consulting Services, all unrelated to this project.
Previous presentation of information: Results contained in this manuscript were presented as an abstract in poster format at the American Society of Nephrology, San Diego, CA, 2015.
References
- 1.Claire Wang Y, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976-2006. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity 2011; 6: 12–20. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Wei R, Ogden CL, et al. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. The American journal of clinical nutrition 2009; 90: 1314–1320. [DOI] [PubMed] [Google Scholar]
- 3.Koebnick C, Smith N, Coleman KJ, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. The Journal of pediatrics 2010; 157: 26–31 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013; 128: 1689–1712. [DOI] [PubMed] [Google Scholar]
- 5.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN 2007; 2: 550–562. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney international 2008; 73: 19–33. [DOI] [PubMed] [Google Scholar]
- 7.Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Archives of internal medicine 2012; 172: 1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics 2013; 132: 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao N, Jenkins TM, Nehus E, et al. Kidney function in severely obese adolescents undergoing bariatric surgery. Obesity 2014; 22: 2319–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. The New England journal of medicine 2016; 374: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HM, Li SJ, Chen HP, et al. Obesity-related glomerulopathy in China: a case series of 90 patients. American journal of kidney diseases : the official journal of the National Kidney Foundation 2008; 52: 58–65. [DOI] [PubMed] [Google Scholar]
- 12.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney international 2001; 59: 1498–1509. [DOI] [PubMed] [Google Scholar]
- 13.Praga M, Hernandez E, Morales E, et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2001; 16: 1790–1798. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal V, Khan I, Rai B, et al. The effect of weight loss after bariatric surgery on albuminuria. Clin Nephrol 2008; 70: 194–202. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal V, Krause KR, Chengelis DL, et al. Relation between degree of weight loss after bariatric surgery and reduction in albuminuria and C-reactive protein. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2009; 5: 20–26. [DOI] [PubMed] [Google Scholar]
- 16.Chagnac A, Weinstein T, Herman M, et al. The effects of weight loss on renal function in patients with severe obesity. Journal of the American Society of Nephrology : JASN 2003; 14: 1480–1486. [DOI] [PubMed] [Google Scholar]
- 17.Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clinical journal of the American Society of Nephrology : CJASN 2009; 4: 1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. Journal of the American Society of Nephrology : JASN 2006; 17: S213–217. [DOI] [PubMed] [Google Scholar]
- 19.Serra A, Granada ML, Romero R, et al. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin Nutr 2006; 25: 400–408. [DOI] [PubMed] [Google Scholar]
- 20.Jose B, Ford S, Super P, et al. The effect of biliopancreatic diversion surgery on renal function--a retrospective study. Obes Surg 2013; 23: 634–637. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan SD, Yehnert H. Bariatric surgery and progression of chronic kidney disease. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2009; 5: 662–665. [DOI] [PubMed] [Google Scholar]
- 22.Schuster DP, Teodorescu M, Mikami D, et al. Effect of bariatric surgery on normal and abnormal renal function. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2011; 7: 459–464. [DOI] [PubMed] [Google Scholar]
- 23.Palatini P Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2012; 27: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 24.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney international 1997; 51: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 26.King AJ, Levey AS. Dietary protein and renal function. Journal of the American Society of Nephrology : JASN 1993; 3: 1723–1737. [DOI] [PubMed] [Google Scholar]
- 27.Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg 2014; 24: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes care 2013; 36: 2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brancati FL, Whittle JC, Whelton PK, et al. The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. Jama 1992; 268: 3079–3084. [PubMed] [Google Scholar]
- 30.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. Journal of the American Society of Nephrology : JASN 2006; 17: 2892–2899. [DOI] [PubMed] [Google Scholar]
- 31.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. Journal of the American Society of Nephrology : JASN 2002; 13: 2363–2370. [DOI] [PubMed] [Google Scholar]
- 32.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes care 2003; 26: 2392–2399. [DOI] [PubMed] [Google Scholar]
- 33.Panwar B, Hanks LJ, Tanner RM, et al. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney international 2015; 87: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation 2002; 40: 221–226. [DOI] [PubMed] [Google Scholar]
- 35.Verhave JC, Gansevoort RT, Hillege HL, et al. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. Journal of the American Society of Nephrology : JASN 2004; 15: 1316–1322. [PubMed] [Google Scholar]
- 36.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney international 2009; 75: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacchetta J, Cochat P, Rognant N, et al. Which creatinine and cystatin C equations can be reliably used in children? Clinical journal of the American Society of Nephrology : CJASN 2011; 6: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vupputuri S, Fox CS, Coresh J, et al. Differential estimation of CKD using creatinine-versus cystatin C-based estimating equations by category of body mass index. American journal of kidney diseases : the official journal of the National Kidney Foundation 2009; 53: 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales E, Valero MA, Leon M, et al. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. American journal of kidney diseases : the official journal of the National Kidney Foundation 2003; 41: 319–327. [DOI] [PubMed] [Google Scholar]
- 40.Praga M, Hernandez E, Andres A, et al. Effects of body-weight loss and captopril treatment on proteinuria associated with obesity. Nephron 1995; 70: 35–41. [DOI] [PubMed] [Google Scholar]
- 41.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. Journal of pediatric surgery 2007; 42: 1969–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity 2009; 17: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inge TH, Zeller MH, Jenkins TM, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA pediatrics 2014; 168: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsson A, Malm J, Grubb A, et al. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scandinavian journal of clinical and laboratory investigation 2004; 64: 25–30. [DOI] [PubMed] [Google Scholar]