Abstract
We investigated the distribution of antibodies neutralizing SARS-CoV-2 according to age, sex or blood group in French blood donors. In 464 samples collected before the emergence of SARS-CoV-2 (2017 and 2018), our virus neutralization assay had a 100% specificity. It was used to test 998 samples collected from blood donors during the last week of March or the first week of April 2020. As expected at this stage of the outbreak, the prevalence was low (2.7%) and, importantly, criteria for blood donation imply that the vast majority of seropositives had asymptomatic or pauci-symptomatic SARS-CoV-2 infections. Seroprevalence values did not differ significantly among age groups (but were slightly higher in donors <30yo and ≥60yo), and between males and females (2.82% vs 2.69%), unlike what has been observed regarding hospitalizations admission to ICU and death rates in France. By contrast, we observed that the proportion of seropositives was significantly lower in group O donors (1.32% vs 3.86% in other donors, p = 0.014). We conclude that virus infection seems to occur with a similar incidence in men and women among French blood donors, but that blood group O persons are less at risk of being infected and not only of suffering from severe clinical presentations, as previously suggested.
Keywords: SARS-CoV-2, COVID-19, Seroneutralisation, Blood donors, Blood groups
There has been many investigations of risk factors associated with COVID-19 clinical severity and outcome. Age, sex and various comorbidities have been identified as factors worsening the prognosis of the disease. However, little is known about possible determinants of susceptibility to infection. We wondered whether the distribution of antibodies neutralizing SARS-CoV-2 in the population may be influenced by demographic or biological parameters such as age, sex or blood group, and whether this would reflect a role on infection rather than on the clinical expression of infection. To test this hypothesis, we studied SARS-CoV-2 neutralizing antibodies in asymptomatic volunteer blood donors from different French regions in the early phase of the outbreak. The COVID-19 outbreak has spread through Metropolitan France from late February 2020, hitting in particular the Paris region and the Northeastern part of the country. As this article is being written, French authorities estimated that 140,000 people have been infected and 27,000 died. All donors tested in the current study provided informed consent for a non-therapeutic use of their blood samples. Data were analyzed according to sex, age and blood types.
Neutralizing antibodies were detected using a virus neutralization test (VNT) as previously described for Zika virus (Nurtop et al., 2018) and adapted to SARS-CoV-2. We used VeroE6 cells cultured in 96-well microplates, 100 TCID50 of the SARS-CoV-2 strain BavPat1 (courtesy of Pr. Drosten, Berlin) and serial dilutions of serum (1/20–1/160). Dilutions associated with CPE (cytopathic effect) were considered as negative (no neutralization) and those with no CPE at day 4 post-infection were considered positive (complete neutralization), respectively. The neutralization titer referred to the highest dilution of serum with a positive result. Specimens with a VNT titer ≥40 were considered positive.
We first tested 464 samples from French blood donors (M/F = 1.45; median age = 42.0) collected before the emergence of SARS-CoV-2, in 2017 (East of France, n = 287) and 2018 (South East and South West of France, n = 177), to establish the specificity of the method. No sample tested positive with a titer ≥40 (specificity = 100%), indicating that a noteworthy specificity can be obtained without using additional VNTs for other respiratory coronaviruses.
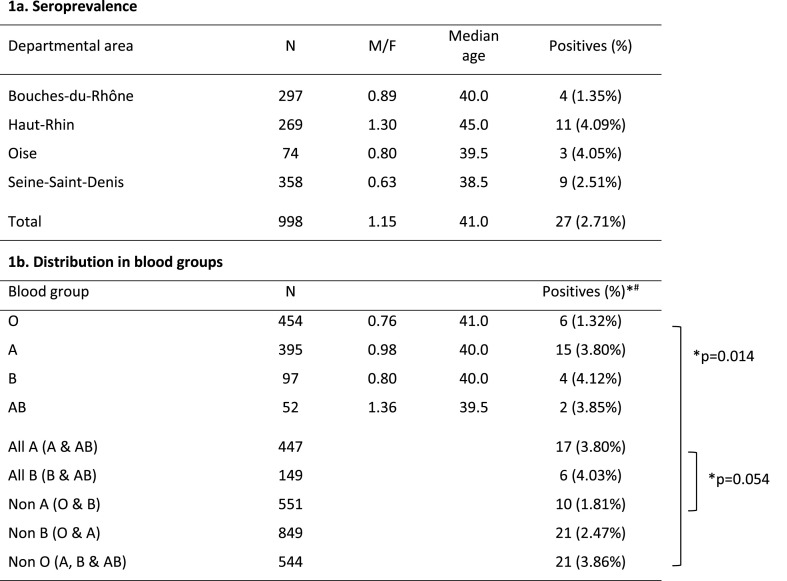
We then tested 998 samples collected from blood donors during the last week of March or the first week of April 2020 (Haut-Rhin departmental area [DA]; Seine-Saint-Denis DA; Bouches-du-Rhône DA; Oise DA; see Table 1 ). As a rule that preexisted the COVID-19 outbreak, French volunteers can give blood only if they have no history of fever or symptom of respiratory infection in the previous 2 weeks. In addition, those with confirmed or suspected COVID-19 were deferred 28 days after the end of symptoms. Accordingly, if it remains possible that some of the seropositive donors identified had been infected at the very beginning of virus spread in France, it is likely that a majority had undergone asymptomatic or pauci-symptomatic infection in March 2020. As expected at this stage of the outbreak, the prevalence values were low (Table 1) but the Haut-Rhin, Oise and Seine-Saint-Denis DAs had higher prevalence than the Bouches-du-Rhône DA, which reflects the actual epidemiological situation based on hospitalization numbers (hospitalizations per 1000 inhabitants at the end of March 2020: 5.4 in the Bouches-du-Rho^ne DA; 27.1 in the Haut-Rhin DA; 6.6 in the Oise DA; 7.9 in the Seine-Saint-Denis DA; of note, the Oise DA was the first epidemiological foci in metropolitan France and case counting was probably underestimated).
Table 1.
Prevalence of neutralizing antibodies in French blood donors (weeks 14–15 2020) according to blood types.
Statistical differences were estimated using the Chi-square tests.
#GMT (Geometric Mean Titer) in seropositives: group O: 113; group A: 80; group B: 113; group AB: 80.
Importantly, while it has been abundantly documented that severe forms of COVID and fatal outcome were more frequent in men than in women (Cai H. 2020; Guan et al., 2020; Li et al., 2020; Zhang et al., 2020), independently of age and susceptibility (Jin et al., 2020), we observed in the complete population tested similar seroprevalence values in males and females (2.82% vs 2.69%, no significant difference, M/F = 1.04). This is in agreement with the findings of Slot et al. (2020) in Dutch blood donors (non-peer-reviewed report). Accordingly, our results suggest that SARS-CoV-2 infection occurs equally among men and women, but has a different phenotypic expression according to sex, with hospital data from the French national institute of statistics (INSEE, https://www.data.gouv.fr/fr/datasets/donnees-hospitalieres-relatives-a-lepidemie-de-covid-19/, downloaded on May 8th, 2020) indicating that COVID-related deaths (M/F = 1.50), admissions to intensive care unit (ICU, M/F = 2.82), and to a lesser extent hospitalizations (M/F = 1.22) are significantly more frequent in men than in women (p < 0.001).
Seroprevalence values did not differ significantly among age groups, but it was noted that they were slightly higher (over 3%) in donors <30yo and ≥60yo, which deserves attention for future studies with larger numbers. The median age of seropositives was 36yo (range 19–64) and not different between men and women.
Finally, we analyzed the relation between sero-status and blood type. In a recent non-peer-reviewed report, Zhao et al. (2020) observed that among Chinese in-patients there was an over- and under-representation of groups A and O, respectively. Since only hospitalized patients were studied, this does not formally establish whether infection or the phenotype of infection was associated with blood type. However, it has been argued in another recent article (Dai, 2020) that ABO blood group does not represent a risk factor predisposing to the risk of getting SARS-CoV-2 infection, but rather predisposes to COVID-19 severity. Here, we observed that the proportion of seropositives was significantly lower in group O donors (1.32% vs 3.86% in other donors, p = 0.014). The M/F sex ratio was slightly lower in O group individuals, but the low seroprevalence was not driven by the higher proportion of women since seropositivity in women was higher than in men in this group (1.6% vs 1.03%). There was a trend associating group A with a higher seroprevalence (3.80% in A and AB groups vs 1.81% in other donors, p = 0.054), and a higher prevalence in group B donors (p > 0,05, no significant difference) but studying larger numbers may be required to obtain the adequate statistical power. Since it is unlikely that seropositivity in the current study is associated with clinical disease drawing medical attention (see above), our results suggest a link between ABO type and susceptibility to infection, against Dai (2020). A similar observation was previously made for the SARS virus by using an immune-fluorescence assay, with low odds of infection in O blood group exposed health personal compared to non-O individuals in a hospital outbreak that occurred in March 2003 in Hong Kong (Cheng et al., 2005). SARS coronavirus and SARS-CoV-2, share the same obligate cellular receptor (ACE2) although other receptors may participate in the infection process. It has been suggested that the ABO polymorphism could contribute to substantially reduce the virus transmission, possibly due to natural anti-A antibodies that may block the interaction between the virus and its receptor (Guillon P. et al., 2008). An association between ABO type and the infection phenotype may also exist as suggested by Dai (2020), but could not be studied here. Rhesus and Kell blood groups were not associated with seropositivity. In a paper that appeared following the submission of our manuscript, Ellinghaus D. et al. (2020) conducted a genomewide association study involving 1980 patients with Covid-19 and identified a 3p21.31 gene cluster as a genetic susceptibility locus in patients with severe clinical presentations. The association signal suggests a higher risk in blood group A and a protective effect in blood group O as compared with other blood groups.
In conclusion, our study of SARS-CoV-2 neutralizing antibodies in French blood donors suggests that virus infection occurs with a similar incidence in men and women within the French population, but with a higher frequency of hospitalizations, admissions to ICU and deaths in men. Blood group O persons are less at risk of being infected. An increased risk of infections associated with blood group A is likely but remains to be formally established in non-hospitalized persons.
Funding
This study was performed with the financial support of the Agence Nationale de la Recherche (ANR-20-COVI-0073-01, France) and of REACTing, a French multi-disciplinary collaborative network working on emerging infectious diseases.
Acknowledgments
We are indebted to blood donors who participated in the study; to directors and medical staff of each regional blood services for their implication and support (EFS Grand Est: C. Gachet, C. Claudel, C. Forny; EFS Ile de France: S. Noel and E. Jacquot; EFS Nord de France: R. Courbil and N. Delemer; EFS Provence, Cote d’Azur et Corse: C. Lazaygues); to C. Isnard for invaluable technical contribution.
References
- Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet. 2020;4:E20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Cheng G., Chui C.H., Lau F.Y. ABO blood group and susceptibility to severe acute respiratory syndrome. J. Am. Med. Assoc. 2005;293:1450–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur. J. Prev Cardiol. 2020 doi: 10.1177/2047487320922370. 2020 Apr 28:2047487320922370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., Fernández J., Prati D., Baselli G., Asselta R., Grimsrud M.M., Milani C., Aziz F., Kässens J., May S., Wendorff M., Wienbrandt L., Uellendahl-Werth F., Zheng T., Yi X., de Pablo R., Chercoles A.G., Palom A., Garcia-Fernandez A.E., Rodriguez-Frias F., Zanella A., Bandera A., Protti A., Aghemo A., Lleo A., Biondi A., Caballero-Garralda A., Gori A., Tanck A., Carreras Nolla A., Latiano A., Fracanzani A.L., Peschuck A., Julià A., Pesenti A., Voza A., Jiménez D., Mateos B., Nafria Jimenez B., Quereda C., Paccapelo C., Gassner C., Angelini C., Cea C., Solier A., Pestaña D., Muñiz-Diaz E., Sandoval E., Paraboschi E.M., Navas E., García Sánchez F., Ceriotti F., Martinelli-Boneschi F., Peyvandi F., Blasi F., Téllez L., Blanco-Grau A., Hemmrich-Stanisak G., Grasselli G., Costantino G., Cardamone G., Foti G., Aneli S., Kurihara H., ElAbd H., My I., Galván-Femenia I., Martín J., Erdmann J., Ferrusquía-Acosta J., Garcia-Etxebarria K., Izquierdo-Sanchez L., Bettini L.R., Sumoy L., Terranova L., Moreira L., Santoro L., Scudeller L., Mesonero F., Roade L., Rühlemann M.C., Schaefer M., Carrabba M., Riveiro-Barciela M., Figuera Basso M.E., Valsecchi M.G., Hernandez-Tejero M., Acosta-Herrera M., D'Angiò M., Baldini M., Cazzaniga M., Schulzky M., Cecconi M., Wittig M., Ciccarelli M., Rodríguez-Gandía M., Bocciolone M., Miozzo M., Montano N., Braun N., Sacchi N., Martínez N., Özer O., Palmieri O., Faverio P., Preatoni P., Bonfanti P., Omodei P., Tentorio P., Castro P., Rodrigues P.M., Blandino Ortiz A., de Cid R., Ferrer R., Gualtierotti R., Nieto R., Goerg S., Badalamenti S., Marsal S., Matullo G., Pelusi S., Juzenas S., Aliberti S., Monzani V., Moreno V., Wesse T., Lenz T.L., Pumarola T., Rimoldi V., Bosari S., Albrecht W., Peter W., Romero-Gómez M., D'Amato M., Duga S., Banales J.M., Hov J.R., Folseraas T., Valenti L., Franke A., Karlsen T.H. Severe covid-19 GWAS group. Genomewide association study of severe covid-19 with respiratory failure. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon P., Clément M., Sébille V., Rivain J.-G., Chou C.-F., Ruvoën-Clouet N., Le Pendu J. Inhibition of the interaction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18(12):1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.M., Bai P., He W., Wu Fei, Liu X.F., Han D.-M., Liu S., Yang J.-K. Gender differences in patients with COVID-19: focus on severity and mortality. Frontiers in Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early Transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. https://doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurtop E., Villarroel P.M.S., Pastorino B., Ninove L., Drexler J.F., Roca Y., Gake B., Dubot-Peres A., Grard G., Peyrefitte C., Priet S., de Lamballerie X., Gallian P. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol. J. 2018;15:192. doi: 10.1186/s12985-018-1105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot E., Hogema B.M., Reusken C.B.E.M., Reimerink J.H., Molier M., Karregat J.H.M., IJlst J., Novotný V.M.J., van Lier R.A.V., Zaaijer H.L. 2020. Herd Immunity Is Not a Realistic Exit Strategy during a COVID- 19 Outbreak. Research Square Preprint. [DOI] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.-D. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;2020 doi: 10.1111/all.14238. https://doi: 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- Zhao J., Yang Y., Huang H., Li D., Gu D., Lu X., Zhang Z., Liu L., Liu T., Liu Y., He Y., Sun B., Wei M., Guangyu G., Wang X., Zhang L., Zhou X., Xing M., Wang P.G. 2020. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. MedRxiv preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]