Abstract
The integration of urban green spaces into modern city planning is seen as a promising tool to offset the drawbacks of ever-expanding cities. Urban agriculture is a common method to implement such strategies and to increase urban sustainability with a special focus on food security. Due to their location, urban farms are highly influenced by past and present anthropogenic activities which can threaten both soil health and food safety. This study includes 12 urban agriculture sites in the metropolitan area of Adelaide, Australia. It is the first of its kind to focus on soil health in urban agriculture systems with a further emphasis on mycorrhizal fungi. Descriptive information about each site, the biodiversity of the selected plots and soil samples from different depths and locations were collected and analysed for chemical and biological parameters. Seven metals, total and plant-available (Colwell) phosphorus and available nitrogen were measured in soils. A glasshouse bioassay was also conducted to determine the abundance of beneficial arbuscular mycorrhizal fungi in the soils and the change of root colonization after inoculation with the mycorrhizal fungus Rhizophagus irregularis. Results showed a generally high biodiversity of plants that correlated with site activity (commercial or community garden) and which could potentially be used for urban biodiversity conservation. Metal concentrations in soils were below national guidelines levels for all samples, although sites with previous industrial history showed elevated levels when compared to sites without industrial history. The use of raised beds with introduced soils eliminated differences in previous land-use history, thereby providing a good option to support cleaner production. Gardening soils were considered highly fertile, with plant-available (Colwell) P concentrations exceeding recommended levels for most horticultural crops, while soils were adequately supplied with nitrogen. Most plant nutrients were derived from freely available urban waste streams and integrated via composting. Various urban waste streams could be used to counter-act imbalanced soil nutrients. Arbuscular mycorrhizal fungi were present in all sites, indicating that the practiced soil management is sustainable from a microbial perspective. Given their important role in supporting plant nutrition, and potential to reduce the need for external nutrient inputs, they provide an important focal point for achieving clean and sustainable urban food production. The results were incorporated into a framework for the management of urban soil health.
Keywords: Arbuscular mycorrhizal fungi, Biological, Chemical, South Australia, Soil, Urban agriculture
Graphical abstract
Highlights
-
•
Urban agriculture is a promising solution for food supply but not well researched.
-
•
We investigated 12 urban sites regarding soil contamination and fertility.
-
•
The formation of mycorrhizas in soils was quantified in a greenhouse bioassay.
-
•
Metal contamination was identified, but not to levels posing concerns.
-
•
Nutrient imbalances were identified, in particular overfertilization with P.
1. Introduction
The global population is expected to reach more than 9 billion by 2050, with most of this increase to occur within urban areas (United Nations, 2019). In terms of land use, urban areas are projected to grow up to 80% by the year 2030, with most of this increase happening in developing countries (Mahendra and Seto, 2019). As a consequence, around 2% of the world’s current arable land will be lost due to urbanisation (Bren d’Amour et al., 2017). These developments lead to various social, economic and environmental challenges that need to be addressed accordingly in the context of urban planning. The integration of urban green spaces is seen as a promising strategy to offset many drawbacks of ever-expanding cities and to increase urban sustainability. Urban green spaces can also contribute to food security, which is of special importance for developing countries. This implementation is called urban agriculture (Skar et al., 2019).
Urban agriculture refers to food production systems inside city boundaries or densely populated areas. As such, it makes significant contribution to social, economic and ecological quality (Miccoli et al., 2016). It is a global phenomenon which is of special importance for food security in developing countries. Estimates suggest that the scale of urban agriculture grows linearly with the urban growth of countries in equatorial Africa (Lee-Smith, 2010). Developing countries in Asia show a similarly high participation of urban dwellers in agriculture, which is considered an important source of livelihood (Zezza and Tasciotti, 2010). Urban agriculture in more developed countries has a stronger focus on social components rather than food production and is often associated as a leisure activity or as a form of ecological activism. To date, the driving forces behind urban agriculture appear to be less concerned with food security, and more so with social, cultural and ecological factors (Mok et al., 2014). However, in the context of climate change, and other shocks to the food system (e.g. the recent Covid-19 pandemic), there is renewed interest in urban agriculture as a means to secure a supply of clean food in all regions of the world. Especially when regional transport of foods may be affected by pandemic-induced controls on movement. In the pursuit of urban sustainability and sustainable food production, there is further need to re-evaluate urban agriculture on a global scale (Skar et al., 2019).
There are many mechanisms involved through which urban agriculture is able to contribute to sustainable food production and urban sustainability. One of the biggest advantages is its ability to produce food locally and to reduce transportation routes (Lee et al., 2015). The greatest reductions in greenhouse gas emissions can be achieved by growing high-yielding seasonal food that would otherwise be imported (Kulak et al., 2013). The integration of urban waste streams for nutrients and organic matter enables urban agriculture systems to reach a high self-sufficiency for most plant nutrients and even up to 100% for phosphorus (Wielemaker et al., 2018). Many case studies have shown that urban agriculture is highly adaptable and often tailor-made for the specific needs of the local residents and their surrounding environment. This multifunctionality allows for efficient land-use in densely populated areas (Lovell, 2010) and even in areas with poor or unknown soil conditions (Armar-Klemesu, 2000). It can also be shaped to specifically provide urban ecosystem services such as pollination, pest control or climate resilience (Lin et al., 2015).
Urban agriculture comes in various forms and shapes, and especially developed countries see an increase in more advanced systems such as hydroponics in combination with vertical gardening and LED light systems. However, the most common form of urban agriculture is using either the natural soil or a soil-based medium in raised beds or containers (Mok et al., 2014). The importance of urban agriculture for sustainable urban planning and food security warrants the need for detailed investigations of urban soil health in the context of food production. To this day, most of this research focused on soil contamination due to anthropogenic activities. Such results are often individual to each sampling location, and show high variability according to their particular surroundings (Säumel et al., 2012). Information on soil fertility in terms of available plant-nutrients and soil microbial activity is scarce. The available studies agree that urban agriculture sites have an ample supply of plant nutrients which are derived from various forms of urban waste streams (Wielemaker et al., 2019). Research also indicates that using organic fertilizers rather than inorganic forms is associated with higher microbial activity due to carbon inputs to soil (Igalavithana et al., 2017). In terms of yield-efficiency, high outputs have been reported, however, often at benefits-to-cost ratios similar to conventional farms. In a hypothetical scenario, most inputs could have been substituted with local renewables, thereby increasing the sustainability of those systems (McDougall et al., 2019).
Maintaining and enhancing soil health is commonly cited as a high priority in urban farming communities. While measuring soil health is difficult, one approach that can be used is to assess impacts on key soil biota. To this end, arbuscular mycorrhizal fungi (AMF) are a near-ubiquitous group of soil fungi that colonise the roots of the majority of terrestrial plant species (Siddiqui and Pichtel, 2008). These resulting associations, arbuscular mycorrhizas (AM), can provide many important ecosystem services, including improved nutrient uptake and decreased nutrient losses caused by leaching or soil erosion (Rillig et al., 2019) and are oftien cited as an important indicator of good ‘soil health’. Colonization of roots by AMF can also alleviate effects of metal toxicity in plants (Watts-Williams and Cavagnaro, 2012), increase plant pathogen resistance and improve the soil structure. All of the aforementioned benefits of AMF are relevant in the contexts of both urban and conventional agriculture practices (Siddiqui and Pichtel, 2008). Although AM have an important role to play in sustainable production systems, the status of AM in urban agricultural systems has not, to our knowledge, been studied previously.
This study includes the results of a survey of physiochemical and biological properties of soil from urban agricultural sites across a major metropolitan city (see Supp. Fig. 1). The research involved 12 urban sites, which were described according to their design and plant biodiversity. Soil samples were collected and tested for a number of different soil parameters. This analysis answers questions regarding soil nutrient potential and contamination with potentially toxic metals. Soil collected for the sites was also used in a greenhouse bioassay to gain information on the soil’s biological properties, namely its mycorrhizal potential. Following, the term “mycorrhizal potential” is used to describe the soil’s potential to promote colonization of roots by AMF.
2. Material and methods
The selected sites were dominated by community gardens (n = 10), but also included two commercial production sites in an urban setting. The sites were surveyed in September–October (Austral Spring), 2017 and soil physicochemical properties were measured. The same soils were used in a glasshouse bioassay experiment with the aim to assess their mycorrhizal potential.
2.1. Site selection
All sites were within a 15 km radius of the City of Adelaide (see Supp. Fig. 2A). The City of Adelaide (Longitude S-34.93°, Latitude E138.60°) has a population of approximately 1.3 million people with a varied history (post-European settlement in 1836) of urban, agricultural, and industrial land use (see below). Using publicly available data, a total of 17 urban agriculture sites were identified as potential survey sites. Selection criteria were a minimum size of 200 m2 and evidence of active food production. Of the 17 sites identified, representatives of 12 sites agreed to being included in this study. For confidentially, the precise locations and names of some sites are not identified here.
2.2. Survey: site characterization and sampling
Prior to visiting sites, further information was gathered using publicly available web sites as well as current and historical satellite imagery. This contextual information includes local land use context, garden size and number of garden beds. Information on historical land use was supplemented and/or confirmed during site visits. Upon arrival at each site, the number of beds was recorded and if production took place in raised beds or not. At each site, gardening beds with evidence of active farming were identified and four or five representative beds randomly selected for more detailed investigation and sampling (see Supp. Fig. 2B).
The dimensions of the beds sampled at each site were measured, and the identity and abundance of plants species being grown at the time was recorded. The source of the soil (i.e., indigenous or imported potting soil) in the production areas was recorded, and where possible, information on the nature of amendments (e.g., manure, compost, etc.) was recorded. Although no sites were formally certified as organic, all sites followed basic principles and ethos of organic farming. These principles mainly included the use of organic pesticides over synthetic ones and abstinence of any mineral fertilizers.
Soil was collected from each bed by taking five soil cores from the 0–10 cm soil layer using a 10 cm diameter auger. Those five cores were then combined at the bed level to produce one composite sample per bed. At two of the sites, cropping was in rows rather than beds, thus soil samples were taken from an area of 1.5 × 2.5 m, which was equivalent to the typical bed size at the other sites.
In an effort to characterise underlying soil conditions at each site, soil samples were also taken from across the site in the non-cultivated area (e.g. in the space between the beds), later referred to as the ‘underlying soil’. These samples were taken from four separate locations randomly distributed across the site (i.e. n = 4). Samples were taken from the underlying soil for the 0–10 cm and 10–30 cm soil layers using a 5 cm diameter auger; at some sites it was not possible to sample to a depth of 30 cm due to high soil strength. All soils collected were stored in air-tight plastic bags and placed in a travel refrigerator at 4 °C until their return to the laboratory, where they were processed immediately.
2.3. Survey: soil physicochemical analysis
Upon return to the laboratory, soil samples were carefully mixed and any coarse woody (or other) debris removed using a 2 cm sieve. The sieved soil was then divided into subsamples for analysis as follows. The first sub-sample was used for determination of soil gravimetric moisture content after drying at 105 °C for 48 h. The second sub-sample was used for colorimetric determination of mineral N (ammonium and nitrate) on 2 M KCl soil extracts as described in Cavagnaro et al. (2006). The third sub-sample was air-dried at 40 °C for at least 48 h and used for further physicochemical analysis: soil pH and EC (1:5 water extract) was measured using a TPS WP-81 pH, TDS, Temperature & Conductivity Meter (EnviroEquip Biolab, Australia). Plant-available (Colwell) P was determined colorimetrically in soil samples collected from the garden beds, using Murphey & Riley colour reagent after extraction in 0.5 M sodium bicarbonate solution for 16 h (Cavagnaro et al., 2006). Total Dumas carbon (C) and N analysis was performed by Australian Precision Ag Laboratory (see http://www.apal.com.au/, last accessed May 2019). The concentration of metals in the soil was determined on soil digests in aqua regia and perchloric acid, followed by analysis for the individual elements: Arsenic (As), cadmium (Cd), copper (Cu), manganese (Mn), nickel (Ni), phosphor (P), lead (Pb) and zinc (Zn), by inductively coupled plasma optical emission spectroscopy (ICP-OES, PerkinElmer Avio 200). The reference soil ACU-4 was used as certified reference material with recovery rates between 89% and 106%. The instrument detection limits (on a soil basis) were 0.028 mg kg−1 for As, 0.012 mg kg−1 for Cd, 0.1 mg kg−1 for Cu and Mn, 0.028 mg kg−1 for Ni and 0.1 mg kg−1 for Pb and Zn.
2.4. Bioassay
Mycorrhizal fungi are often cited as a key indicator of soil health and as having a role to play in clean and sustainable production systems. In order to investigate the potential for indigenous and introduced (Rhizophagus irregularis, see below) AMF to colonise the roots of plant grown in the soils collected from the sites, a glasshouse bioassay experiment was undertaken. Due to the limited amount of soil from some sites following physicochemical analysis, it was not possible to conduct the supplemented inoculation (i.e. R. irregularis) treatment on every collected sample; however, 80% of the soils could be inoculated, with n = 50 in the test of indigenous AMF inoculum potential, and n = 40 in the test of impacts on soil after supplemental inoculation with R. irregularis.
The culture of R. irregularis (WFVAM10) has been used in previous studies and was found to result in good mycorrhizal root colonization (Watts-Williams and Cavagnaro, 2012). The culture is regularly propagated in a closed pot culture system with Tagetes patula nana as a host plant. On average, 7 spores g−1 inoculum were present, as well as a variable number of infected root pieces. This source of mycorrhiza inoculum has previously been found to provide high levels of AM colonization under a range of conditions.
The glasshouse bioassay was performed as follows: tomato (Lycopersicon esculentum cv. 76R) seeds were surface-sterilized and pre-germinated on double autoclaved sand mixture, before being transplanted into the final substrate after the development of the first true leaf. The final substrate consisted of 150 g of the collected garden bed soils mixed with 150 g of double autoclaved fine sand. R. irregularis inoculum was added (10% w/w) for the supplemented treatment while keeping the same final weight. Plants were grown in an environmentally controlled greenhouse from November to December 2017 (Austral Spring-Summer) and randomized weekly. Plants were watered daily using reverse osmosis (RO) water, and no other nutrients were added.
Plants were destructively harvested 36 days after transplanting, and roots and shoots were separated before being dried at 65 °C. At harvest, a subsample of the fresh roots was taken and stored in 50% ethanol for 24 h. Mycorrhizal colonization was quantified using the gridline intersect method after staining with ink and vinegar (Vierheilig et al., 1998). Shoots were ground to a fine powder before being analysed for the elements calcium (Ca), copper (Cu), iron (Fe), potassium (K), sulphur (S), magnesium (Mg), manganese (Mn), phosphor (P) and zinc (Zn) by ICP-AES (as described above). To obtain information about the presence of indigenous mycorrhizal spores in the collected soil samples, a subsample of the collected soils (n = 27) was processed according to (Merryweather and Moyersoen, 1997) as follows: depending on the available soil, between 10 and 30 g dry soil was weighed as biological triplicates and wet-sieved on 27 μm and 450 μm sieves for spore extraction. The extract was then centrifuged in a 50% sugar solution for further cleaning. The supernatant was separated and washed three times with RO water. Spores were then placed onto a 45 mm glass dish with four circular walls in between (nematode counting dish) and counted using a dissecting microscope (Olympus SZ-PT) between 80–100× magnification.
2.5. Statistical analysis
Survey: the data was not normally distributed and was therefore analysed using the non-parametric Kruskal–Wallis one-way analysis of variance with Bonferroni correction. In order to identify differences between the variables ‘location’ (garden beds, underlying soil 0–10 and 10–30 cm) or ‘previous industrial history’ (yes/no) (see below), site means (e.g. averaged across beds) were used as replicates. However, when comparing sites, individual samples were used as replicates. Where significant differences were identified, post hoc tests were performed using Fisher’s Least Significant Difference. In order to explore the relationship between different variables (e.g. total P and plant-available (Colwell) P), simple linear regression modelling was undertaken.
Bioassay: data was not normally distributed and Kruskal–Wallis one-way analysis of variance with Bonferroni correction was used in order to reveal differences between groups. Where significant differences were identified, post hoc tests were performed using Fisher’s Least Significant Difference. Individual samples were used as replicates and analysed with the grouping factor Inoculation (none/R. irregularis). Again, simple linear regression modelling was used to explore relationship between different variables (e.g., shoot P concentration and soil P concentration).
All data was analysed with the software R in the version 3.5.0, using the package ‘agricolae’ 1.2 (CRAN, 2018) for non-parametric Kruskal-Wallis analysis with Fisher’s Least Significant Difference as post hoc test. Principal component analysis was performed using the function ‘prcomp’ and ‘lm’ was used for the coefficient of determination R2.
3. Results
3.1. Site characterization
The sites included in this study (Table 1 ), were on average approximately 0.1 ha in size, but ranged from 210 to 15,000 m2. Whereas at nine of the sites production was predominantly conducted in raised beds using introduced soil or potting mix, at two of the sites it was in beds formed from the natural soil and supplemented with self-made or externally sourced compost. The remaining site grew crops in the natural soil without any organic amendments. Across all sites, an average of 35% of the available area was dedicated to production (as garden beds, chickens, beekeeping and fruit trees), and the remainder was used for pathways, storage facilities (e.g. sheds), and other non-production oriented activities. There was an average of 29 beds at each of the 10 community garden sites, which was similar to the average number of gardeners (23) at each of these sites. At the two commercial sites, production was set up in rows rather than beds. While the community gardens provided a mix of activities ranging from food production to social inclusion and educational activities, the two commercial enterprises focused solely on food production. Compost was produced and used at all but two of the sites (one commercial and one community garden). Further nutrients were imported, typically in the form of commercially available municipal green-waste compost and/or animal (predominantly horse) manure. Most sites were located between residential allotments and often in close proximity to park lands or other nature reserves (see Table 1).
Table 1.
Descriptive information about sites included in this study and their correlating plant biodiversity.
| Site | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (m2) | 680 | 210 | 880 | 15,000 | 2100 | 710 | 700 | 1000 | 2400 | 600 | 300 | 1100 |
| Year established | 2011 | 2010 | 2005 | 1907 | 1992 | 2003 | 2014 | 2010 | 1994 | 2016 | 2012 | 2011 |
| Previous land use | Plant nursery | Parkland | School yard | Paddock | Factory | Tennis court | Car park | Parkland | Paddock | Junkyard | Vacant lot (former blacksmith) | Bowling area |
| Surrounding land use | Residential allotment and park | Park | Residential allotment | Residential allotment and park | Residential allotment | Residential allotment and community center | CBD | Residential allotment and park | Residential allotment and park | Park | Residential allotment | Residential allotment |
| Number of gardeners | 30 | 20 | 35 | 3 | 25 | 20 | 10 | 35 | 30 | 6 | 2 | 45 |
| Use of raised beds | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| On-Site composting | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Livestock | No | No | No | No | Bees and Chickens | No | Chickens | No | No | No | No | No |
| Plant richness per site | 14 | 16 | 13 | 1 | 12 | 19 | 13 | 18 | 21 | 4 | 7 | 11 |
| Plant richness per bed mean (median) | 6.75 (6.5) | 6.25 (6.5) | 4 (3.5) | 1 (1) | 5 (5) | 5.75 (7) | 4 (4) | 6.75 (7) | 6.25 (6.5) | 2 (2) | 2.25 (2.5) | 3.25 (2.5) |
| Dominant species | Cabbage | Beans | Onions | Vine | Beans | Garlic | Parsley | Onions | Onions | Onions | Onions | Onions |
| Shannon-Index H | 9.1 | 11.1 | 9.7 | 2.0 | 8.4 | 14.9 | 10.6 | 13.7 | 15.0 | 2.0 | 6.2 | 4.3 |
All sites together had a total plant species richness of 73 species in the production areas surveyed, and at the individual site level, ranging from one to 21 species (Table 1). On the bed level, species richness ranged from one to twelve species. The most abundant crops were varieties of onions, lettuce, cabbage, broad beans and carrots, all of which are typical winter crops grown in South Australia. Plant richness and biodiversity (Shannon-Index) varied greatly between the sites and in some cases beds only contained one plant species. The Shannon-Index was used as a biodiversity index which accounts for both species abundance and evenness (Tuomisto, 2010). However, most garden beds showed a high plant species richness with different crops grown in close proximity. This likely reflects the fact that beds typically service the needs of an individual grower.
3.2. Potentially toxic metals
In an attempt to identify potential contamination of these urban soils (referred to as garden beds) and in the underlying soil (sampled from between the beds, using soil layers 0–10 and 10–30 cm, referred to as ‘underlying soil 10’ and ‘underlying soil 30’), soil elemental concentrations were compared to National Environmental Protection Measure Health Investigation “A” Guideline Levels (NEPM-HIL) as stated by NEPM (1999) (Table 2 ). Across all sites, the concentrations of As, Cu, Cd, Mn, Ni, Pb and Zn were well below the NEPM-HIL A guideline levels, indicating that minimal risks to human health are posed by the soil either in the beds or the underlying soils (see Table 2). Importantly, for As and Cd, concentrations were below detection limits (0.028 mg kg−1) in the majority of samples and were therefore omitted from statistical analysis.
Table 2.
Summary description of soil metal concentrations of all collected samples (beds and natural soil).
| Summary statistic | As [mg kg−1] | Cd [mg kg−1] | Cu [mg kg−1] | Mn [mg kg−1] | Ni [mg kg−1] | Pb [mg kg−1] | Zn [mg kg−1] |
|---|---|---|---|---|---|---|---|
| Sample size (n = ) | 1 | 9 | 133 | 133 | 123 | 133 | 133 |
| Below detection limit/NAs | 132 | 124 | – | – | 10 | – | – |
| Detection limit | 0.028 | 0.012 | 0.1 | 0.1 | 0.028 | 0.1 | 0.1 |
| Minimum | 0.6 | 0.01 | 0.3 | 0.1 | 0.5 | 0.1 | 0.6 |
| Median | 0.6 | 0.08 | 25.2 | 168.7 | 7.8 | 30 | 103.1 |
| Mean | 0.6 | 0.13 | 32.6 | 213.1 | 8.6 | 45.8 | 114.9 |
| Max | 0.6 | 0.38 | 183.4 | 750.1 | 32.6 | 267.7 | 661.7 |
| SD | – | 0.1 | 29.3 | 134.3 | 4.6 | 46.9 | 91.5 |
| HIL-A Guidelines (NEPM) | 100 | 20 | 6000 | 3800 | 400 | 300 | 7400 |
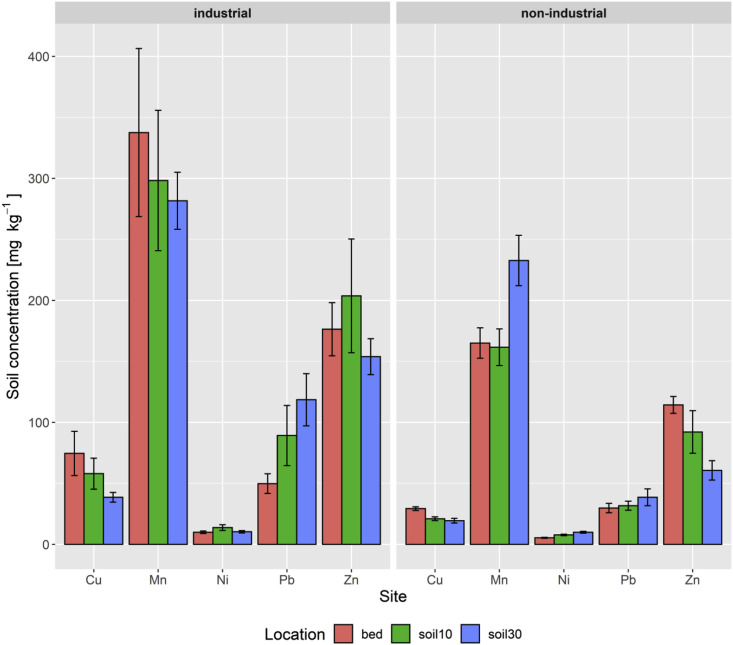
One of the motivations for undertaking production in raised beds was a perceived risk that there may be contamination in soil at the site(s), as a legacy of previous land use (e.g. industrial or unknown) at the site. To explore this concern, the results were compared for concentrations of Cu, Mn, Ni, Pb and Zn between sample locations (garden beds, underlying soil 0–10 and 10–30 cm) using the sites as replicates (Fig. 1 ). Whereas this analysis revealed significantly higher concentrations of Ni in the underlying 10–30 cm soil layer than in the garden beds (p = 0.04), there were no significant differences between the sampling locations for Cu, Mn, Pb and Zn. Variability within sites was high with a number of outliers identified (see Supp. Fig. 3).
Fig. 1.
Soil concentrations of tested heavy metals between sites with industrial and non-industrial history and the different sampling locations ‘garden bed’ (red bar), ‘underlying soil 0–10 cm’ (green bar) and ‘underlying soil 10–30 cm’ (blue bar). Values are mean ± SE, N = 133. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Sites were further classified on the basis of their prior land use; industrial (n = 3) or non-industrial (n = 9) (Fig. 1). When comparing metals in the garden beds at sites with industrial versus non-industrial land use histories, there were no significant differences detected. However, for the underlying soil layers (0–10 and 10–30 cm, respectively), there were significant differences for Cu, Ni, Pb and Zn, with the industrial sites having higher concentrations than the non-industrial ones (see Fig. 1 and Supp. Fig. 3).
3.3. Phosphorus and nitrogen
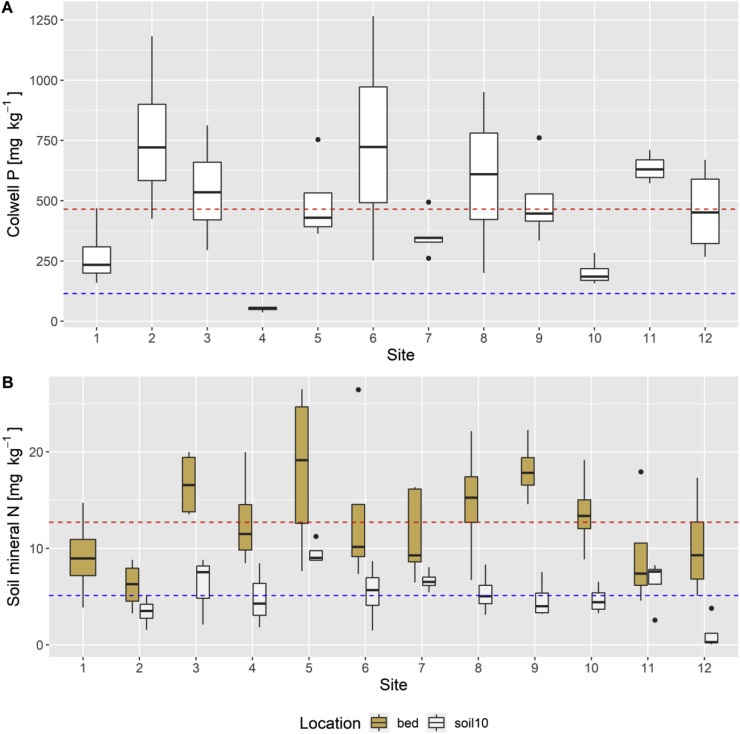
Concentrations of plant-available (Colwell) P in the garden beds ranged from 36 to 1265 mg kg−1 soil and showed high variability within and between sites. Most of the garden beds contained relatively high concentrations of plant-available (Colwell) P (median = 442.5 mg kg−1 soil), exceeding the critical concentration of plant-available (Colwell) P for most horticultural crops (e.g. lettuce = 115 mg kg−1 soil, Hartemink (2000)) (see Fig. 2 A). Only sites 4 and 10 differed significantly from all other sites, with these beds having significantly lower concentrations of plant-available (Colwell) P than at all other sites. Concentrations of total P in the soil were also measured and were significantly higher in the garden beds than in the underlying soil layers (0–10 and 10–30 cm) (Supp. Fig. 4). A regression analysis between concentrations of total P and plant-available (Colwell) P in the garden beds resulted in a positive, albeit moderate, correlation (R2 = 0.43).
Fig. 2.
2A: Plant-available (Colwell) P concentration of garden beds over all tested sites. Dashed lines indicating critical Colwell P of 115 mg kg−1 for lettuce (blue) as a reference and the median of all samples (red). 2B: Mineral-N concentrations of garden beds (brown) and underlying soil (white) over the tested sites. Dashed lines indicating median for location ‘garden bed’ (bed) (red) ‘underlying soil 0–10 cm’ (soil10) (blue). N = 80. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Total nitrogen (N) in the soil collected from the garden beds was generally high (median = 0.7%). Mineral N in the garden beds was comprised from an approximate equimolar ratio of ammonium and nitrate (median = 6.2 mg kg−1 and 6.1 mg kg−1 soil, respectively), and did not differ significantly between sites (Table 3 and Fig. 2B). However, variability within sites was high; for example, at site 5 mineral N ranged from 7.6 to 26.5 mg kg−1 soil. Total N in the underlying soil (0–10 cm) was lower than in the beds (median = 0.4%). Mineral N in the underlying soil was dominated by ammonium rather than nitrate (median = 4.3 mg NH4-N kg−1 soil and 0.6 mg NO3-N kg−1 soil).
Table 3.
Summary description of Colwell P, total P, Ammonium, Nitrate, total C, total N, C/N, pH and EC of all collected samples (beds and underlying soil).
| Location | Sample size | Min | Median [mg kg−1] | Mean [mg kg−1] | Max [mg kg−1] | |
|---|---|---|---|---|---|---|
| Colwell P [mg kg−1] | Garden bed | 49 | 36.3 | 442.6 | 465.3 | 1265.7 |
| Total P [mg kg−1] | Garden bed | 50 | 338.8 | 1866.0 | 2296.0 | 6490.0 |
| Natural soil 0-10 | 48 | 0.6 | 665.3 | 852.5 | 4859.0 | |
| Natural soil 10-30 | 35 | 191.1 | 372.8 | 561.9 | 1440.0 | |
| Ammonium [mg kg−1] | Garden beds | 49 | 1.2 | 6.2 | 6.2 | 15.8 |
| Natural soil 0-10 | 41 | 0.1 | 4.3 | 4.4 | 9.6 | |
| Nitrate [mg kg−1] | Garden beds | 49 | 0.2 | 6.1 | 9.1 | 16.2 |
| Natural soil 0-10 | 42 | 0.1 | 0.6 | 1.0 | 4.6 | |
| Total C [%] | Garden beds | 49 | 1.5 | 7.3 | 7.6 | 16 |
| Natural soil 0-10 | 49 | 0.2 | 5.5 | 7.5 | 29 | |
| Total N [%] | Garden beds | 49 | 0.03 | 0.7 | 0.7 | 1.3 |
| Natural soil 0-10 | 49 | 0.03 | 0.4 | 0.5 | 1.8 | |
| C/N | Garden beds | 49 | 8.3 | 10.9 | 12.8 | 50 |
| Natural soil 0-10 | 49 | 1.5 | 14.5 | 16.5 | 130 | |
| pH | Garden bed | 50 | 6.4 | 7.1 | 7.1 | 7.9 |
| Natural soil 0-10 | 42 | 6.5 | 6.9 | 7.0 | 7.8 | |
| Natural soil 10-30 | 35 | 6.3 | 7.1 | 7.1 | 7.9 | |
| EC [mS] | Garden bed | 50 | 75 | 341 | 402 | 2057 |
| Natural soil 0-10 | 43 | 29 | 261 | 288 | 1234 | |
| Natural soil 10-30 | 35 | 31 | 154 | 156 | 528 |
Principal component analysis (PCA) revealed that sites 4, 5 and 11 had distinct physico-chemical soil characteristics (see Supp. Fig. 5A), while the 95% confidence limits of the remaining sites overlapped and were thus more closely related to each other. The variation within sites was often small, such as for sites 7, 10 and 12. The first two principal components explain about 56% of the variance of the data set.
3.4. Bioassay
Of the 90 plants included in the bioassay, 13 died within the first 14 days after transplantation, with symptoms of tomato stem rot evident on those seedlings. One seedling was omitted from further analysis due to a mutated growth phenotype. Of the 76 remaining plants, 27 were inoculated with the AMF R. irregularis.
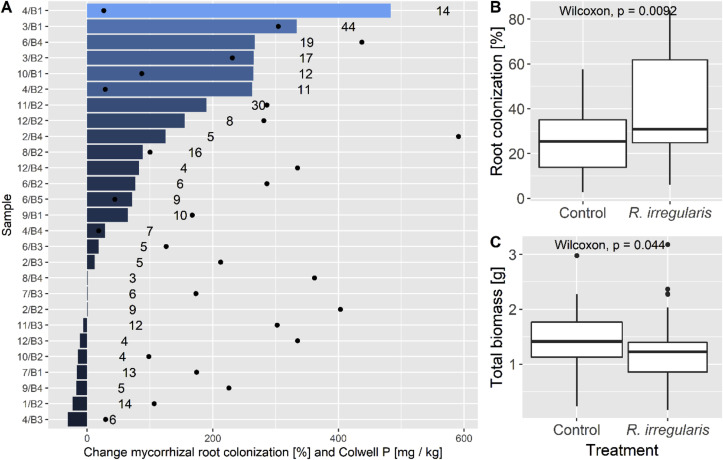
Plants growing in the indigenous soil without the R. irregularis treatment showed a mycorrhizal root colonization between 3 and 56%. Inoculation with R. irregularis increased average colonization significantly from 26 to 31% (Fig. 3 B). Altogether, 17 samples had increased colonization, three samples had a neutral response and seven were negatively affected. This change in root colonization was highly variable between samples collected from beds within a given site. For example, two separate beds within site 4 showed the greatest increase (4/B1) and decrease (4/B3) in mycorrhizal colonization with inoculation with R. irregularis (Fig. 3A).
Fig. 3.
3A: Change of mycorrhizal root colonization after addition of R. irregularis in percentage, number of spores present per gram of dried indigenous soil (numbers) and corresponding plant-available Colwell P (dots). 3B: Mycorrhizal root colonization between indigenous soil (Control) and treatment with R. irregularis. 3C: Shoot biomass between non-inoculated soil (Control) and R. irregularis treatment.
Correlation between plant-available (Colwell) P and mycorrhizal root colonization was low (R2 = 0.08), and some samples with high concentrations of plant-available (Colwell) P showed a strong increase in mycorrhizal root colonization with inoculation (e.g., samples 2/B4 or 6/B4). The abundance of AMF spores in the tested subsample ranges from 3 to 44 spores g−1 dry soil with a mean of 11 spores (Fig. 3A).
Shoot biomass varied greatly between samples, similar to the measured variability of soil mineral N and plant-available (Colwell) P. However, shoot biomass was significantly lower in the R. irregularis inoculated plants (mean = 0.8 mg kg−1), than in the non-inoculated control (mean = 1.0 mg kg−1) (Fig. 3C).
Shoot P concentrations were significantly higher in the R. irregularis treatment (mean = 4.0 mg kg−1) than in the non-inoculated treatment (mean = 3.3 mg kg−1). Conversely, concentrations of Fe were lower in the R. irregularis treatment (mean = 0.05 mg kg−1) than in the non-inoculated control treatment (mean = 0.07 mg kg−1). There were no significant differences for Zn (Supp. Fig. 6). Regression analysis between concentrations of P, Mn and Zn in the plant tissue and soil resulted in R2 < 0.01 for P and Zn and R2 = 0.55 for Mn.
The PCA showed that shoot biomass was most closely correlated to soil total N, total P, Colwell P, and total C (see Supp. Fig. 5B). Strong negative correlations were found between shoot biomass and mycorrhizal root colonization and, to a lesser degree, soil nitrate.
4. Discussion
The sites included in this urban agriculture study ranged in size, number of participants, and their focus (commercial and community gardens). The nature of most sites was relatively uniform with plants being grown in raised beds with relatively high plant biodiversity compared to conventional agriculture systems. While concentrations of potentially toxic metals in soils were well below guideline levels, they were higher on sites with a history of industrial land use. Whereas systems had relative low levels of mineral N and adequate levels of total N, plant-available (Colwell) P was very high. Collected soils were abundant in AMF spores and a greenhouse bioassay showed high mycorrhizal root colonization, even in soils with high P concentration. Following, these results are discussed in the context of soil health and safety as well as their significance towards sustainable and clean food production.
4.1. Site characterization
There were two broad types of sites identified in this study: community gardens and commercial sites. Both types differed in their configuration, farming methods and plant biodiversity. All community gardens showed a strong multifunctional character by combining mainly social and ecological functions. As such, they allocated more space to non-production areas and wheelchair accessible pathways to allow social gatherings for the community. Food production in most community gardens took place in raised beds, while both commercial sites were growing plants in the natural soil. The decision to use raised beds and imported soil was in many cases due to perceived concerns around potential soil contamination and was in some cases mandated by local government. In general, plant biodiversity in the community garden was higher than in the commercial sites and included many ornamental plants and perennials such as Rosmarinus officinalis or Physalis peruviana. The higher diversity of crops grown in the community gardens is likely due to using the garden as a kitchen garden, whereas the commercial sites put an emphasis on producing saleable amounts of product. Those results suggest that especially the community gardens present a big potential for urban biodiversity conversation and provide important ecosystem functions (Goddard et al., 2010). The sustainable character of the commercial sites lies mainly within their focus on food production, combined with their proximity to the consumers and short transportation routes. Although not part of this study, it is likely that food produce of both commercial sites is associated with less greenhouse gas emissions than conventionally produced food (Lee et al., 2015). Both the community gardens and the commercial sites made efficient use of valuable urban space in a densely populated area. Their actual configuration is a reflection of their surroundings and the needs of the local residents and they all followed a strong multifunctional character (Lovell, 2010). This multifunctionality allows all sites to mitigate various challenges that arise from expanding cities (Mahendra and Seto, 2019).
4.2. Potentially toxic metals
One of the main reasons for the use of raised beds in urban environments are concerns over possible soil contamination. Previous studies showed that those concerns are justified and concentrations of metals in urban agriculture soils (Mitchell et al., 2014) and products (Sung and Park, 2018) can exceed regulated guideline concentrations. Anthropogenic input of metals into the soil occurs through various mechanisms such as atmospheric deposition, runoff from metal surfaces, bonfires, burial of metal-containing waste, pesticides, or fertilizers (Alloway, 2004). All samples in this study were below the NEPM HIL-A guidelines for the tested metals, however, sites with industrial historical land use had significant higher concentrations of Cu, Ni, Pb and Zn in the underlying soil layer than sites with non-industrial history. In contrast, there was no significant difference in concentrations of metals in soils from gardening beds when sites with and without industrial land use histories were compared. The use of raised beds with introduced soils appears to have been an effective way to safely (from a metal perspective) undertake food production in sites with industrial histories. Although it is unlikely for developed countries to undertake any form of food production in areas with known soil contamination, raised beds represent one option to help ensure a safe and secure food supply system, in countries facing food shortages (Kessler, 2013).
Concentrations of Zn in site 11 (mean = 258 mg kg−1) were well above the typical levels of about 57–100 mg kg−1 in organically managed soils (Noulas et al., 2018). This finding might not only be caused by its industrial history, but also the use of Zn-based pesticides or the application of municipal composts (Heiger-Bernays et al., 2009). While speculative, this highlights the need to consider potential introduction of heavy metals, and indeed other contaminants, with external inputs. These levels of Zn are of interest from an agricultural perspective but are still within the critical guideline levels by a factor of 28. Although the re-use of urban waste products comes with certain reservations, it did not negatively affect the sites included in this study (from a metal perspective). On the contrary, it is likely that the use of organic amendments from urban waste streams saved a substantial amount of energy due to the omission of mineral fertilizer (Favoino and Hogg, 2008), however, that was not a focus in this study.
4.3. Phosphorus and nitrogen
With the exception of two sites, plant-available (Colwell) P in the soil collected from the garden beds was very high, and well in excess of required levels for horticultural production (Hartemink, 2000). High levels of plant available P in these soils is likely a reflection of easily accessible nutrient sources that are high in P, such as horse manure (Airaksinen et al., 2001), coupled with the highly immobile nature of P in the soil (Hartemink, 2000). Similar results were found in various urban agriculture projects in Portugal (Arrobas et al., 2017) and the Netherlands (Wielemaker et al., 2019), where the nutrient inputs would even exceed the fertilizer application limits of conventional farming. Nitrogen analysis of the collected garden beds revealed similar and low concentrations of ammonium and nitrate. However, most plant N is derived (following mineralization) from organic forms in the soil which is also represented in the total N analysis. Concentrations of total N in the soil from the garden beds ranged from 0.03% to 1.3% with a median of 0.7%. When comparing those values against the critical concentrations for wheat (0.1%) (Hartemink, 2000), most garden bed soils can be considered adequately supplied with N. This divergence between high amounts of total N and low amount of mineral N might be caused by the highly dynamic cycling of N in soils which is affected by many environmental factors (Hartemink, 2000). All things considered, nutrient management in urban agriculture systems is characterised by an over-supply of urban waste products which leads to excess or imbalanced soil nutrient concentrations. Such imbalances between nutrient inputs and outputs should be closely monitored to avoid build-up in the soil. Excess nutrients may pose a risk due to run-off or can interfere with the uptake of other plant nutrients (Fageria, 2001). However, the use of mainly organic urban waste products also resulted in high total N concentrations which is a significant parameter for good soil health (Hartemink, 2000). One solution to counteract excess or imbalanced nutrients in the context of urban agriculture is to either reduce nutrient inputs or to use a blend of different organic materials with different nutrient profiles. For example, after communicating the issue of high P concentrations to participants of the study, one community garden incorporated spent coffee ground as nutrient source which has a broad N:P ratio of about 30:1 (Liu and Price, 2011). Other common composting materials with high N:P ratios are straw (N:P = 8:1) or wood chips (N:P = 7:1) (Wurff et al., 2016). The results of the PCA revealed that all sites which used raised beds with introduced soils shared a close relationship. This indicates that most soils and composts originate from a similar source, probably due to its easy accessibility. However, most developed cities provide a variety of freely available organic materials with different nutrient profiles. In order to use this resource in a sustainable way, it is necessary for gardeners to familiarize themselves with the principles of balanced nutrient management.
4.4. Bioassay
Mycorrhizal fungi were present in all soils collected in this survey. On average, 11 AMF spores g−1 dry soil were present in the samples that were used in the bioassay. Such spore abundance is similar to organic agriculture soils where up to 14 AMF spores g−1 soil were found (Oehl et al., 2004). The true mycorrhiza potential of the soil samples is probably still higher, as root pieces or extraradical hyphae in the soils act as another inoculum source but were not measured in this study. The mycorrhizal potential is also reflected by the high percentage root colonization of plants without R. irregularis inoculation. The inoculation with R. irregularis suggested that most soils have higher mycorrhizal potential and can support higher root colonization. In that way, the addition of R. irregularis further bolstered the mycorrhizal root colonization for most samples which might be explained by the fast-growing nature of this AMF species (Malbreil et al., 2014). Interestingly, site 4 showed a high variability in its response to inoculation with R. irregularis as those samples showed either a positive, neutral or negative response. This response to inoculation cannot be explained within the methodology of this study and might be linked to other microbial processes that impact mycorrhizal growth (Miransari, 2011). Such a spatial variability of soil microorganisms has been reported previously by Štursová et al. (2016). To this date it is not possible to compare the AMF spore numbers of this study with other urban agriculture sites, as no such data are available.
Given the ample supply of plant nutrients at most sites, it is surprising to find such an abundance of AMF in the soil. Most scientific literature even described an inhibition of mycorrhizal development at high levels of soil P. The results of this study might suggest that nutrient uptake is not the major driver behind mycorrhizal symbiosis in urban agriculture soils, or, is at least redundant from a nutrient perspective. Still, shoot P concentrations in the bioassay were higher in the R. irregularis treatment than non-inoculated and significantly exceeded values reported by Watts-Williams and Cavagnaro (2012). This discrepancy between the studies is likely due to the far higher soil P concentration in the urban agriculture soils. Plant shoot weight was decreased in the R. irregularis treatment which is commonly found in plants with high mycorrhizal root colonization (Johnson et al., 1997) when compared to non-mycorrhizal control plants. However, it is important to consider that the importance of AMF in ecosystems should not be questioned over the decrease of shoot and root dry weights with higher root colonization. As mentioned by Rillig et al. (2019), AMF provide a broad range of services that positively affect sustainability in food production.
Similarly, the natural establishment of AMF in urban agriculture soils is likely because they provide important ecosystem functions besides nutrient uptake, such as disease resistance or improved soil structure. Direct inoculation of AMF was not practised at any of the sites, suggesting that common management practices of urban agriculture, such as high plant biodiversity and principles of organic farming, lead to high levels of AMF propagules. If needed, most soils could sustain even higher levels of mycorrhizal root colonization after inoculation with a fast-growing mycorrhizal species such as R. irregularis. Those result suggest that, although urban agriculture soils are prone to excess soil nutrient concentrations, they are managed sustainably from a microbial perspective. Direct inoculation of urban agriculture soils with mycorrhizal inoculum is not necessary and common urban agriculture practices are naturally selecting for an abundant mycorrhizal assemblages (Verbruggen and Toby Kiers, 2010). Although not addressed in this study, it is likely that this process provides a variety of functions to the host plants and soils that goes beyond the uptake of plant nutrients. If gardeners seek to accelerate the establishment of mycorrhizal communities, it is possible to use small amounts of soil from an established garden bed as inoculum source for new garden plots. If need be, inoculum could even be produced on-site using a variety of organic substrates (Douds et al., 2010). Those options provide low-cost and sustainable alternatives to commercial mycorrhizal inoculants that have been shown to be of variable quality (Salomon et al., unpublished).
4.5. Urban soil health framework
Healthy soils are the foundation of urban green spaces, regardless of whether those spaces are intended for leisure or food production. As such, protecting urban soils from anthropogenic influences and improving soils wherever possible should be a priority in every urban planning framework. The following preliminary framework outlines the main steps involved in managing urban soil health based on the results of this study and with an emphasis on urban agriculture and urban green spaces (see Supp. Fig. 7).
The basis of this framework is to minimize the impacts of anthropogenic activities on urban soils, for example through environmental policies (De Kimpe and Morel, 2000). Future urban development is then classified as “hazardous” or “safe” depending on the expected effect on the surrounding soil. Hazardous activities are such that are likely to result in adverse soil properties that can only be fixed at high cost (e.g. organic soil pollutants or potentially toxic metals). Activities that only have limited effects on soil health or effects that can be overcome in the context of urban green spaces are considered “safe”. Most community gardens in this study were operating on “safe” zones, where soil compaction due to previous urban development was an issue that could be overcome by using raised beds.
The soil health of the urban surrounding is mapped according to the two categories (De Kimpe and Morel, 2000). Where soil contamination is of no concern, urban green spaces are encouraged, for example, through the use of government initiatives such as increased funding (van den Nouwelant et al., 2015), and environmental policies which support community gardens and other environmentally focused communities (Middle et al., 2014). Hazardous areas that are conveniently located for green space development are prioritized for remediation efforts (Yao et al., 2012). Hazardous areas that are unattractive for green spaces are used for clustering activities that are hazardous to soil health. Where urban soils need to be improved, for example in the context of urban agriculture, municipal compost is made available. Such compost blends should be nutrient-balanced and free of contaminants. The composts would ideally be based on a variety of high-quality urban waste streams which are collected city-wide to close the nutrient-loop and increase the city’s self-sustainability (Farrell and Jones, 2009).
“The 30-Year Plan for Greater Adelaide” is a critical component of the planning strategy for South Australia, established by the Development Act 1993 (Government of South Australia, 2010). The 30-Year Plan identifies specific goals which are consistent with the goals of this proposed framework, such as increasing the liveability of Adelaide by planting 20 million trees by 2020 and transforming Adelaide into a “green liveable city”. However, the importance of healthy urban soil is only briefly mentioned in the Plan and is not bolstered by any specific strategies to achieve this goal. This framework could be implemented as an addition to the Plan to solve this deficit and ultimately improve the overall soil quality of Adelaide for future generations.
5. Recommendations and conclusions
The urban agriculture sites in this study provided multiple benefits towards the local community which included social services, eco-biodiversity, food production and recycling of urban waste streams. All sites showed strong multi-functional characteristics that allowed for efficient space use in a densely populated area. All soil samples were within the national guidelines for concentrations of potentially toxic metals, although higher concentrations were observed in industrially affected soils than in non-industrial soil. The use of raised beds and introduced soil was a successful method to offset those differences caused by the previous industrial legacy. Soils that were used for plant production had an adequate supply of N and very high levels of plant-available P, which mostly stemmed from freely accessible urban waste streams rather than mineral fertilization. One case example showed that organic amendments can be sourced from different urban waste streams with different nutritional values to avoid such imbalanced nutrient concentrations. Although most soils had imbalanced concentrations of plant nutrients, they were managed sustainably from a microbial perspective and contained a high abundance of mycorrhizal propagules. This naturally developed mycorrhizal assemblage is likely to provide important ecosystem functions in the context of urban agriculture. The findings of this study were incorporated into a preliminary framework for the management of urban soil health. This framework aims to facilitate the planning and implementation of urban green spaces by mapping the soil health of urban areas.
CRediT authorship contribution statement
M.J. Salomon: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Visualization. S.J. Watts-Williams: Conceptualization, Validation, Writing - review & editing, Supervision. M.J. McLaughlin: Conceptualization, Validation, Resources, Writing - review & editing, Supervision. T.R. Cavagnaro: Conceptualization, Validation, Resources, Writing - review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
MJS acknowledges support from the University of Adelaide and the provided Adelaide Scholarship International. SJWW acknowledges support from the University of Adelaide Ramsay Fellowship. We thank Ms. Bogumila Tomczak and Mr. Colin Rivers for technical assistance. Further, we would like to express our gratitude towards the gardeners and representatives of all sites involved in this study. The following sites agreed to being acknowledged by name:Common Grounds Community Garden. Fern Ave Community Garden. Glandore Community Garden. Glenelg North Community Garden. Linde Community Garden. Lochiel Park Community Garden. Marion Vineyard (managed by Patritti) Prospect Community Garden. Walyu Yarta Community Garden. Wynn Vale Community Garden.
Handling editor: Yutao Wang
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclepro.2020.122900.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Airaksinen S., Heinonen-Tanski H., Heiskanen M.L. Quality of different bedding materials and their influence on the compostability of horse manure. J. Equine Vet. Sci. 2001;21(3):125–130. doi: 10.1016/S0737-0806(01)70108-6. [DOI] [Google Scholar]
- Alloway B.J. Vol. 12. 2004. (Contamination of soils in domestic gardens and allotments: a brief overview). 3. [DOI] [Google Scholar]
- Armar-Klemesu M. 2000. Urban Agriculture and Food Security, Nutrition and Health. [Google Scholar]
- Arrobas M., Lopes H., Rodrigues M.Â. Urban agriculture in Bragança, Northeast Portugal: assessing the nutrient dynamic in the soil and plants, and their contamination with trace metals. Biol. Agric. Hortic. 2017;33(1):1–13. doi: 10.1080/01448765.2016.1172345. [DOI] [Google Scholar]
- Bren d’Amour C., Reitsma F., Baiocchi G., Barthel S., Güneralp B., Erb K.-H., Haberl H., Creutzig F., Seto K.C. Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114(34):8939–8944. doi: 10.1073/pnas.1606036114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagnaro T.R., Jackson L.E., Six J., Ferris H., Goyal S., Asami D., Scow K.M. Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil. 2006;282(1):209–225. doi: 10.1007/s11104-005-5847-7. [DOI] [Google Scholar]
- CRAN . 2018. Agricolae: Statistical Procedures for Agricultural Research.https://cran.r-project.org/web/packages/agricolae/index.html Accessed March 2020. [Google Scholar]
- De Kimpe C.R., Morel J.-L. Urban soil management: a growing concern. Soil Sci. 2000;165(1) [Google Scholar]
- Douds D.D., Nagahashi G., Hepperly P.R. On-farm production of inoculum of indigenous arbuscular mycorrhizal fungi and assessment of diluents of compost for inoculum production. Bioresour. Technol. 2010;101(7):2326–2330. doi: 10.1016/j.biortech.2009.11.071. [DOI] [PubMed] [Google Scholar]
- Fageria V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001;24(8):1269–1290. doi: 10.1081/PLN-100106981. [DOI] [Google Scholar]
- Farrell M., Jones D.L. Critical evaluation of municipal solid waste composting and potential compost markets. Bioresour. Technol. 2009;100(19):4301–4310. doi: 10.1016/j.biortech.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Favoino E., Hogg D. The potential role of compost in reducing greenhouse gases. Waste Manag. Res. 2008;26(1):61–69. doi: 10.1177/0734242X08088584. [DOI] [PubMed] [Google Scholar]
- Goddard M.A., Dougill A.J., Benton T.G. Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol. Evol. 2010;25(2):90–98. doi: 10.1016/j.tree.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Government of South Australia . Department of Planning and Local Government Adelaide; 2010. The 30-Year Plan for Greater Adelaide. [Google Scholar]
- Hartemink A.E. CSIRO Publishing; Collingwood, Victoria: 2000. Soil Analysis - an Interpretation Manual. [DOI] [Google Scholar]
- Heiger-Bernays W., Fraser A., Burns V., Diskin K., Pierotti D., Merchant-Borna K., McClean M., Brabander D., Hynes H.P. Characterization and low-cost remediation of soils contaminated by timbers in community gardens. Int J Soil Sediment Water. 2009;2(3) [PMC free article] [PubMed] [Google Scholar]
- Igalavithana A.D., Lee S.S., Niazi N.K., Lee Y.-H., Kim K.H., Park J.-H., Moon D.H., Ok Y.S. Assessment of soil health in urban agriculture: soil enzymes and microbial properties. Sustainability. 2017;9(2):310. doi: 10.3390/su9020310. [DOI] [Google Scholar]
- Johnson N.C., Graham J.H., Smith F.A. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 1997;135(4):575–585. doi: 10.1046/j.1469-8137.1997.00729.x. [DOI] [Google Scholar]
- Kessler R. Urban gardening: managing the risks of contaminated soil. Environ. Health Perspect. 2013;121(11-12):A326–A333. doi: 10.1289/ehp.121-A326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak M., Graves A., Chatterton J. Reducing greenhouse gas emissions with urban agriculture: a Life Cycle Assessment perspective. Landsc. Urban Plann. 2013;111:68–78. doi: 10.1016/j.landurbplan.2012.11.007. [DOI] [Google Scholar]
- Lee-Smith D. Cities feeding people: an update on urban agriculture in equatorial Africa. Environ. Urbanization. 2010;22(2):483–499. doi: 10.1177/0956247810377383. [DOI] [Google Scholar]
- Lee G.-G., Lee H.-W., Lee J.-H. Greenhouse gas emission reduction effect in the transportation sector by urban agriculture in Seoul, Korea. Landsc. Urban Plann. 2015;140:1–7. doi: 10.1016/j.landurbplan.2015.03.012. [DOI] [Google Scholar]
- Lin B.B., Philpott S.M., Jha S. The future of urban agriculture and biodiversity-ecosystem services: challenges and next steps. Basic Appl. Ecol. 2015;16(3):189–201. doi: 10.1016/j.baae.2015.01.005. [DOI] [Google Scholar]
- Liu K., Price G.W. Evaluation of three composting systems for the management of spent coffee grounds. Bioresour. Technol. 2011;102(17):7966–7974. doi: 10.1016/j.biortech.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Lovell S.T. Multifunctional urban agriculture for sustainable land use planning in the United States. Sustainability. 2010;2(8):2499. doi: 10.3390/su2082499. [DOI] [Google Scholar]
- Mahendra A., Seto K.C. World Resources Institute; 2019. Upward and Outward Growth: Managing Urban Expansion for More Equitable Cities in the Global South, World Resources Report. [Google Scholar]
- Malbreil M., Tisserant E., Martin F., Roux C. Genomics of arbuscular mycorrhizal fungi: out of the shadows. In: Martin F.M., editor. Advances in Botanical Research. Academic Press; 2014. pp. 259–290. [DOI] [Google Scholar]
- McDougall R., Kristiansen P., Rader R. Small-scale urban agriculture results in high yields but requires judicious management of inputs to achieve sustainability. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116(1):129–134. doi: 10.1073/pnas.1809707115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merryweather J., Moyersoen B. 1997. Working with Mycorrhizas in Forestry and Agriculture. [DOI] [Google Scholar]
- Miccoli S., Finucci F., Murro R. Feeding the cities through urban agriculture the community esteem value. Agricult and Agricult Sci Procedia. 2016;8:128–134. doi: 10.1016/j.aaspro.2016.02.017. [DOI] [Google Scholar]
- Middle I., Dzidic P., Buckley A., Bennett D., Tye M., Jones R. Integrating community gardens into public parks: an innovative approach for providing ecosystem services in urban areas. Urban For. Urban Green. 2014;13(4):638–645. doi: 10.1016/j.ufug.2014.09.001. [DOI] [Google Scholar]
- Miransari M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biotechnol. 2011;89(4):917–930. doi: 10.1007/s00253-010-3004-6. [DOI] [PubMed] [Google Scholar]
- Mitchell R.G., Spliethoff H.M., Ribaudo L.N., Lopp D.M., Shayler H.A., Marquez-Bravo L.G., Lambert V.T., Ferenz G.S., Russell-Anelli J.M., Stone E.B., McBride M.B. Lead (Pb) and other metals in New York City community garden soils: factors influencing contaminant distributions. Environ. Pollut. 2014;187:162–169. doi: 10.1016/j.envpol.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H.-F., Williamson V.G., Grove J.R., Burry K., Barker S.F., Hamilton A.J. Strawberry fields forever? Urban agriculture in developed countries: a review. Agron. Sustain. Dev. 2014;34(1):21–43. doi: 10.1007/s13593-013-0156-7. [DOI] [Google Scholar]
- NEPM . 1999. National environment protection (assessment of site contamination) measure. [Google Scholar]
- Noulas C., Tziouvalekas M., Karyotis T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018;49:252–260. doi: 10.1016/j.jtemb.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Oehl F., Sieverding E., Mäder P., Dubois D., Ineichen K., Boller T., Wiemken A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia. 2004;138(4):574–583. doi: 10.1007/s00442-003-1458-2. [DOI] [PubMed] [Google Scholar]
- Rillig M.C., Aguilar-Trigueros C.A., Camenzind T., Cavagnaro T.R., Degrune F., Hohmann P., Lammel D.R., Mansour I., Roy J., van der Heijden M.G.A., Yang G. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019;222(3) doi: 10.1111/nph.15602. [DOI] [PubMed] [Google Scholar]
- Säumel I., Kotsyuk I., Hölscher M., Lenkereit C., Weber F., Kowarik I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012;165:124–132. doi: 10.1016/j.envpol.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Siddiqui Z.A., Pichtel J. Mycorrhizae: an overview. In: Siddiqui Z.A., Akhtar M.S., Futai K., editors. Mycorrhizae: Sustainable Agriculture and Forestry. Springer Netherlands; Dordrecht: 2008. pp. 1–35. [DOI] [Google Scholar]
- Skar S.L.G., Pineda-Martos R., Timpe A., Pölling B., Bohn K., Külvik M., Delgado C., Pedras C.M.G., Paço T.A., Ćujić M., Tzortzakis N., Chrysargyris A., Peticila A., Alencikiene G., Monsees H., Junge R. Urban agriculture as a keystone contribution towards securing sustainable and healthy development for cities in the future. Blue-Green Systems. 2019;2(1):1–27. doi: 10.2166/bgs.2019.931. [DOI] [Google Scholar]
- Štursová M., Bárta J., Šantrůčková H., Baldrian P. Small-scale spatial heterogeneity of ecosystem properties, microbial community composition and microbial activities in a temperate mountain forest soil. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol. 2016;92(12) doi: 10.1093/femsec/fiw185. [DOI] [PubMed] [Google Scholar]
- Sung C.Y., Park C.B. The effect of site- and landscape-scale factors on lead contamination of leafy vegetables grown in urban gardens. Landsc. Urban Plann. 2018;177:38–46. doi: 10.1016/j.landurbplan.2018.04.013. [DOI] [Google Scholar]
- Tuomisto H. A consistent terminology for quantifying species diversity? Yes, it does exist. Oecologia. 2010;164(4):853–860. doi: 10.1007/s00442-010-1812-0. [DOI] [PubMed] [Google Scholar]
- United Nations . 2019. World Population Prospects: the 2019 Revision, Key Findings and Advance Tables. [Google Scholar]
- van den Nouwelant R., Davison G., Gurran N., Pinnegar S., Randolph B. Delivering affordable housing through the planning system in urban renewal contexts: converging government roles in Queensland, South Australia and New South Wales. Aust. Plan. 2015;52(2):77–89. doi: 10.1080/07293682.2014.914044. [DOI] [Google Scholar]
- Verbruggen E., Toby Kiers E. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evolutionary Applicat. 2010;3(5-6):547–560. doi: 10.1111/j.1752-4571.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierheilig H., Coughlan A.P., Wyss U., Piché Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998;64(12):5004–5007. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts-Williams S.J., Cavagnaro T.R. Arbuscular mycorrhizas modify tomato responses to soil zinc and phosphorus addition. Biol. Fertil. Soils. 2012;48(3):285–294. doi: 10.1007/s00374-011-0621-x. [DOI] [Google Scholar]
- Wielemaker R., Oenema O., Zeeman G., Weijma J. Fertile cities: nutrient management practices in urban agriculture. Sci. Total Environ. 2019;668:1277–1288. doi: 10.1016/j.scitotenv.2019.02.424. [DOI] [PubMed] [Google Scholar]
- Wielemaker R.C., Weijma J., Zeeman G. Harvest to harvest: recovering nutrients with new sanitation systems for reuse in urban agriculture. Resour. Conserv. Recycl. 2018;128:426–437. doi: 10.1016/j.resconrec.2016.09.015. [DOI] [Google Scholar]
- Wurff A.W.G.v.d., Fuchs J.G., Raviv M., Termorshuizen A. BioGreenhouse; Netherlands: 2016. Handbook for Composting and Compost Use in Organic Horticulture. [Google Scholar]
- Yao Z., Li J., Xie H., Yu C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ Sci. 2012;16:722–729. doi: 10.1016/j.proenv.2012.10.099. [DOI] [Google Scholar]
- Zezza A., Tasciotti L. Urban agriculture, poverty, and food security: empirical evidence from a sample of developing countries. Food Pol. 2010;35(4):265–273. doi: 10.1016/j.foodpol.2010.04.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.