Abstract
Many parallel studies of convalescent plasma with modest enrolment projections have been launched for the treatment of COVID-19. By pooling data from multiple parallel studies that are similar, we can increase the effective sample size and achieve enough statistical power to determine effectiveness more quickly through meta-analysis. A scoping review of registered clinical trials of convalescent plasma for COVID-19 was conducted to assess the feasibility of performing a rapid and timely meta-analysis that will support accelerated review for approval and implementation. ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform were searched April 23, 2020. Trials were included if they utilized convalescent plasma to treat or prevent COVID-19. Forty-eight registered trials (projected to enroll more than 5000 subjects) of convalescent plasma were identified and included for analysis. The majority of studies (33 studies with 4440 projected enrolment) will address the treatment of severe and/or critical cases of COVID-19. Twenty-nine studies are controlled and 17 of these are reported as actively recruiting. The combined enrolment of patients from similar studies should be sufficient to determine meaningful improvements in mortality, rates of admission to intensive care and need for mechanical ventilation by the end of 2020—sooner than any individual study could determine effectiveness. Accessing supplemental outcome data from investigators may be needed; however, to align reporting of some outcomes from these studies. Heterogeneity in product potency due to different antibody titers is anticipated and studies using conventional treatment as controls instead of placebo may complicate our understanding of efficacy. Convalescent plasma is being tested in ongoing controlled studies, largely to treat severe and/or critical cases of COVID-19. Sufficient combined power to detect clinically important reductions in multiple outcomes, including mortality, is expected by September 2020. Regulatory approval, funding and implementation by blood operators could be accelerated by planned meta-analysis as study results become available.
Keywords: Convalescent plasma, COVID-19, Acute respiratory distress syndrome, Clinical trials, Systematic review
COVID-19 represents one of the most significant global health crises in recent times [1]. Effective therapies are lacking, and a significant proportion of patients experience progressive respiratory failure and death. Treatment with convalescent plasma from donors who have recovered from SARS-CoV-2 infection could provide passive immunity to treat patients with COVID-19 to prevent further progression and promote recovery. Convalescent plasma has been used in previous viral outbreaks including SARS and the influenza pandemic of 1918, although no randomized trials were conducted. While randomized controlled trials have been conducted more recently for influenza, the evidence is inconclusive, and no clear benefit has been determined [[6], [7], [8], [9]]. Initial published studies in COVID-19 are small and lack appropriate control groups but report reduced levels of virus, radiographic improvement, and encouraging clinical responses [[2], [3], [4], [5]]. Standardized plasma collection and manufacturing methods for preparing convalescent plasma have been advanced by the Working Party on Global Blood Safety of the International Society of Blood Transfusion [10]. Consistent implementation of these methods around the world should provide confidence regarding transferability of trial results, although characterizing biological parameters such as specific titers of anti-SARS-CoV-2 antibodies may be variable. Given the relative safety of plasma therapy, it may be difficult to enroll patients in placebo-controlled trials and the use of conventional therapy arms as controls may complicate determination of efficacy. Randomization of subjects will provide the best approach to minimize the impact of concomitant therapies and allow an interpretation of results [11]. Moreover, the dosage and frequency of administration may vary between studies and will need to be considered when pooling data from studies for meta-analysis.
Given the urgency and magnitude of the health challenge posed by COVID-19, health research funding agencies around the world and many blood operators are dedicating significant efforts and funds towards clinical trials of convalescent plasma for the treatment of COVID-19. Identifying registered studies that are actively recruiting allows us to assess the feasibility and timing of performing a rapid meta-analysis to accelerate the assessment of efficacy and safety of this therapy. A Framework for Accelerated Synthesis of Trial evidence, or FAST Evidence, will identify studies that share sufficient homogeneity for inclusion in a planned meta-analysis that can be continuously updated to provide the required knowledge synthesis for timely approval and delivery to patients if convalescent plasma is effective in the treatment of COVID-19.
Methods
Data Sources and Searches
The registry of clinical trials at clinicaltrials.gov and the WHO International Clinical Trials Registry Platform using the COVID-19 registry of trials were searched on April 23, 2020. The registers were searched for trials of convalescent plasma of COVID-19 using the following search strategy (for www.clinicaltrials.gov): (COVID OR “COVID-19” OR “2019-nCoV” OR “novel coronavirus” OR Coronavirus OR “SARS-CoV-2” OR SARS) AND (“convalescent plasma” OR plasma OR globulin OR “hyperimmune serum”); and for the WHO registry of COVID-19 studies (www.who.int/ictrp/en/): Convalescent OR plasma OR hyperimmune.
Study Selection
Registered clinical studies were included for analysis if they utilized plasma collected from patients with prior documented infection with SARS-CoV-2 to treat or prevent COVID-19. Registered studies were not included if they did not address the role of convalescent plasma to treat or prevent COVID-19, and if the study was listed as withdrawn or canceled. Registered studies were reviewed in duplicate for assessment of inclusion and exclusion criteria.
Data Extraction and Quality Assessment
For each included record, the following data were extracted in duplicate (if available): Trial ID, registry and date of registration, recruiting status, phase, title, planned start date, anticipated primary completion (data collection complete), anticipated study completion, inclusion and exclusion criteria, disease severity, age range of eligible study participants, anticipated number for patient enrolment for both the intervention and controls (if any), whether randomization is planned, antibody titer information, dosage, manufacturing method, route of administration, primary and secondary outcomes, country of origin of primary or lead investigator.
Data Synthesis and Analysis
We described the characteristics of all included trials. In order to determine the required sample size to determine efficacy for different levels of reduction in mortality, we assumed an alpha error of 5% and used a power calculation of 80% and assumed enrolment of subjects in the intervention group was 1:1 compared to control groups. Statistical modeling was done by comparing proportions of two independent groups using an on-line a calculator from (https://www.stat.ubc.ca/~rollin/stats/ssize/b2.html, Department of Statistics, University of British Columbia, Vancouver, Canada) [12]. Graphs were produced using Microsoft Excel (Microsoft Corporation, Redmond, Washington).
Results
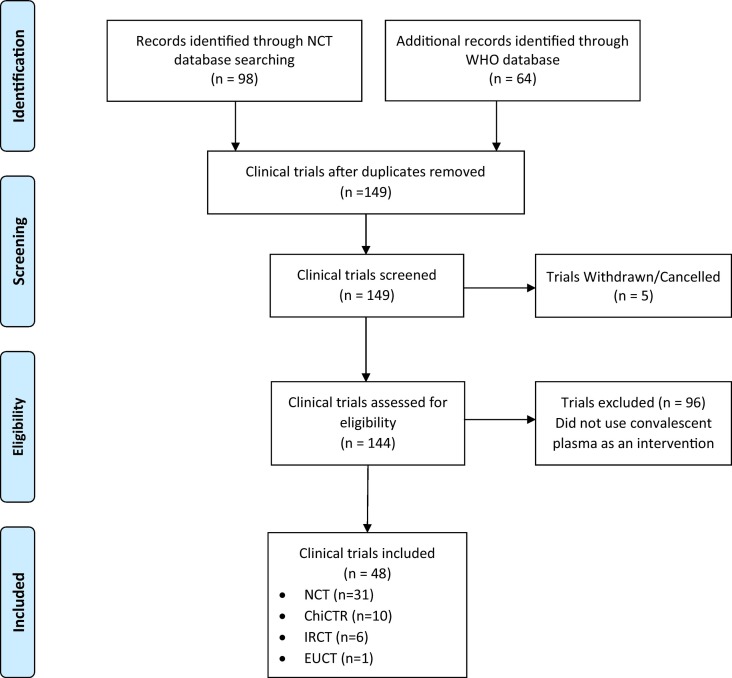
A total of 98 records were identified from Clinicaltrials.gov and 64 records from the WHO International Clinical Trials Registry Platform. After removing duplicates, 149 records were reviewed. There were 5 studies that were withdrawn or canceled, leaving 144 for determination of eligibility. After reviewing the records in detail, 69 were excluded (did not treat patients with convalescent plasma). A total of 48 studies were included in our analysis (see Fig. 1 , and Table A.1 for a list of all trials). Study characteristics are summarized in Table 1 .
Fig. 1.
PRISMA search diagram. NCT, clinicaltrials.gov; WHO, World Health Organization; ChiCTR, Chinese Clinical Trial Register; IRCT, Iranian Registry of Clinical Trials; EUCTR, European Union Clinical Trials Register.
Table 1.
Characteristics of clinical trials of COVID-19 convalescent plasma
| Total trials (n = 48) |
Controlled studies (n = 29) |
|||||
|---|---|---|---|---|---|---|
| Trials | n, treatment armsb | Trials | n, treatment armsb | Planned completion by Dec 31, 2020 (trials) | n, completed trials by Dec 31, 2020b | |
| Country | ||||||
| China | 11 | 345 | 9 | 320 | 7 | 210 |
| USA | 11 | 906 | 4 | 971 | 0 | 0 |
| Iran | 7 | 302 | 6 | 272 | 5 | 257 |
| Othera | 19 | 3861 | 10 | 1632 | 8 | 1322 |
| Critical/severe cases | 33 | 4440 | 21 | 1965 | 16 | 1530 |
| Actively recruiting as of April 23, 2020 | 23 | 1364 | 17 | 1199 | 13 | 779 |
Other: Mexico, Ireland, Mexico, Pakistan, Egypt, Canada, Saudi Arabia, Italy, India, France, Hungary, Spain, Denmark, Netherlands, Columbia, Germany.
If the study did not specify the sampling ratio, a 1:1 ratio was assumed per arm.
Studies will be conducted in multiple countries, including China (11 trials), the USA (11 studies) Iran (7 studies), and 14 other countries (19 studies combined) with the largest planned studies located in Pakistan (NCT04352751; 2000 projected subjects, no control group) and Canada (NCT043486636; 1200 projected subjects, with a control group of 400). A total of 29 trials describe a planned control group, with 6 of these trials describing normal plasma as the control (total of 518 patients to be enrolled in the intervention groups of these studies) and almost all of the remaining studies describing conventional therapy as the control group which will likely represent a range of evolving therapies (See Table 2 ). Details of additional trials are provided in Table A.2. With regards to potential risk of bias, we noted that while randomization was described in 50% of studies, information regarding allocation concealment and blinding of assessors was provided in 48% and 15% of the registered protocols, respectively (see Table A.3).
Table 2.
Controlled trials of convalescent plasma for treatment of COVID-19. Controlled trials included both randomized and non-randomized trials which had at least one intervention arm and one control arm that used either placebo (normal plasma) or conventional treatment
| Trial ID | Country | Date of Registration | Phase | Severity | Comparison | Enrolment (n) |
Intervention (n) |
Randomized (Y/N) | Antibody titer | Dose or volume | Treatment schedule |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT04356534 | Ireland | 2020-04-22 | N/A | Not mild | Conventional Treatment | 40 | 20 | Y | Unspecified | 400 mL | 200 ml over 2 hours in 2 consecutive days |
| NCT04355767 | USA | 2020-04-21 | 2 | Symptomatic | Ordinary plasma | 206 | 103 | Y | >1:80 | 1–2 U; ~200-600 mL | Unspecified |
| NCT04348656 | Canada | 2020-04-16 | 3 | Severe | Conventional treatment | 1200 | 800 | Y | Unspecified | 500 mL | Single infusion over 4 h |
| NCT04347681 | Saudi Arabia | 2020-04-15 | 2 | Critical, Severe | Conventional treatment | 40 | 40 | Y | Unspecified | 10-15 mL/kg | At least once, daily up to five sessions |
| NCT04346446 | India | 2020-04-15 | 2 | Severe | Conventional treatment, Ordinary plasma | 20 | 10 | Y | To be experimentally determined | 200-600 mL | Once |
| NCT04345991 | France | 2020-04-15 | 2 | Mild | Conventional treatment | 120 | 60 | Y | Unspecified | 200-220 mL | 2 U 24 h after first 2 U, <10d from clinical symptom onset |
| NCT04345523 | Spain | 2020-04-14 | 2 | Moderate | Conventional treatment | 278 | 139 | Y | Unspecified | Unspecified | Unspecified |
| NCT04345289 | Denmark | 2020-04-14 | 3 | Severe, Moderate | Sarilumab, Normal saline, hydroxychloroquine, oral placebo, baricitinib | 1500 | 250 | Y | Unspecified | 2 x 300 mL | Single infusion |
| NCT04344535 | USA | 2020-04-14 | 1,2 | Unspecified | Ordinary plasma | 500 | 250 | Y | >1:320 | 450-550 mL | Once |
| NCT04342182 | Netherlands | 2020-04-10 | 2,3 | Severe | Conventional treatment | 426 | 213 | Y | Unspecified | 300 mL | Unspecified |
| NCT04333251 | USA | 2020-04-03 | 1 | Symptomatic | Conventional treatment | 115 | 57 | Y | >1:64 | 1-2 U | Unspecified |
| NCT04332835 | Colombia | 2020-04-03 | 2,3 | Moderate | Hydroxychloroquine, azithromycin | 80 | 40 | Y | Unspecified | 250 mL/day | Day 1 & 2 |
| NCT04323800 | USA | 2020-03-27 | 2 | Exposed | Ordinary plasma | 150 | 75 | Y | >1:64 | ~200-250 mL | Unspecified |
| IRCT20200413047056N1 | Iran | 2020-04-17 | 3 | Severe, Critical | Conventional treatment, IVIG | 15 | 5 | Y | Unspecified | 200 mL | Twice |
| IRCT20200409047007N1 | Iran | 2020-04-12 | N/A | Severe | Conventional treatment | 32 | 16 | Y | Unspecified | 500 mL | Every other day up to 3 times |
| IRCT20200404046948N1 | Iran | 2020-04-15 | 3 | Severe, Critical | Conventional treatment | 60 | 30 | Y | Unspecified | 200-500 mL | Twice in two consecutive days |
| IRCT20200325046860N1 | Iran | 2020-03-30 | N/A | Severe | Conventional treatment | 200 | 200 | N | Unspecified | 500 mL | Single infusion over 4 h |
| IRCT20200310046736N1 | Iran | 2020-04-01 | 2,3 | Severe, Critical, Moderate, Mild | Conventional treatment, plasma-derived immunoglobulin | 45 | 15 | Y | Unspecified | 200 mL | Infusion over 1-4 h, for 1-4 days |
| IRCT20151228025732N53 | Iran | 2020-04-10 | 3 | Critical | Conventional treatment | 12 | 6 | N | >1:320 | 2 U | Infusion 2 hours with 1 hour between the 2 U given |
| EUCTR2020-001310-38-DE | Germany | 2020-03-31 | 2 | Severe | Conventional treatment | 120 | 60 | Y | Unspecified | Unspecified | Unspecified |
| ChiCTR2000031501 | China | 2020-04-02 | 0 | Critical, Severe | Conventional treatment | 20 | 10 | N | Not specified | Unspecified | Unspecified |
| ChiCTR2000030929 | China | 2020-03-17 | N/A | Severe | Ordinary plasma | 60 | 30 | Y | Unspecified | Unspecified | Unspecified |
| ChiCTR2000030702 | China | 2020-03-10 | 0 | Severe, Critical | Conventional treatment | 50 | 25 | Y | Unspecified | Unspecified | Day 1 |
| ChiCTR2000030627 | China | 2020-03-08 | 0 | Critical, Severe | Conventional treatment | 30 | 15 | Y | Unspecified | Unspecified | Unspecified |
| ChiCTR2000030179 | China | 2020-02-24 | ? | Critical, Severe | Conventional treatment | 100 | 50 | Y | Unspecified | Unspecified | Unspecified |
| ChiCTR2000030039 | China | 2020-02-21 | N/A | Severe, Critical, Normal | Conventional treatment | 90 | 30 | N | Unspecified | 200-500 mL | Two infusions |
| ChiCTR2000030010 | China | 2020-02-19 | N/A | Severe | Ordinary plasma | 100 | 50 | Y | Unspecified | Unspecified | Unspecified |
| ChiCTR2000029850 | China | 2020-02-15 | 0 | Critical | Conventional treatment | 20 | 10 | N | Unspecified | Unspecified | Unspecified |
| ChiCTR2000029757 | China | 2020-02-12 | 0 | Critical, Severe | Conventional treatment | 200 | 100 | Y | Unspecified | Unspecified | Day 1 |
With regards to plasma manufacturing and product characterization, 7 studies (15%) reported specific targets for antibody titers in the product, although the testing details and specifics of the antibodies were not provided in all cases. Other plasma manufacturing details were generally not available from the information provided at registration. In terms of the dose and schedule of administration, the controlled studies describe a dose ranging from 100 – 600 mL given either once or up to 5 infusions (daily or every other day) and in some cases, this information was not specified.
Amongst the controlled trials of convalescent plasma, the severity of disease of patients to be enrolled was severe or critical [11,12] in 21 studies (72%) and the remaining studies described the target populations as patients with mild, symptomatic, or unspecified severity of illness. The most commonly assessed outcomes amongst these trials are clinical improvement (primary outcome in 10 [34%] trials and secondary outcome in 11 [38%] trials), viral load or reverse transcriptase polymerase chain reaction results (primary in 5 [17%] trials and secondary in 11 [38%] trials), duration of hospital stay (primary in 2 [7%] trials and secondary in 13 [45%] trials), need for mechanical ventilation (primary in 7 [24%] trials and secondary in 7 [24%] trials), duration of intensive care unit stay (primary in 1 [3%] trial and secondary in 10 [34%] trials), and 28-day mortality (primary in 1 [3%] trial and secondary in 9 [31%] trials). Taken together, these outcomes represent the most promising candidates for pooling in future meta-analysis, although additional information from investigators should be requested to augment the alignment of outcome reporting for meta-analysis, which can be particularly relevant for dichotomous and objective outcomes such as mortality, need for admission to intensive care, and need for mechanical ventilation.
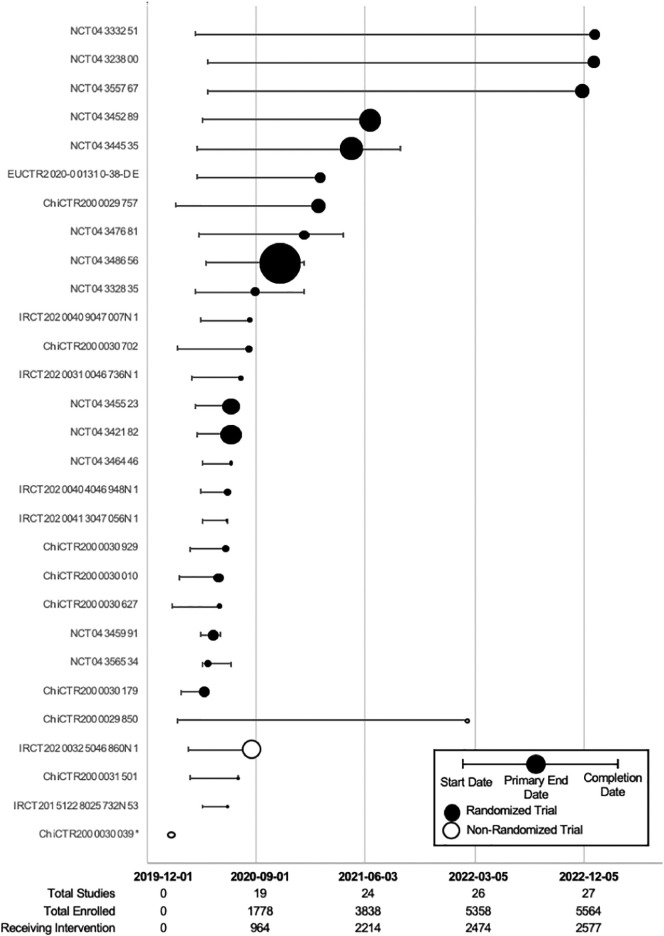
We then determined the required sample size required for different levels of significant reduction in key outcomes that could be determined through pooling of data from similar studies (See Table 3 ). Reported mortality rates for patients with severe COVID-19 are approximately 10% to 20% [[13], [14], [15]] and for critical disease, the reported mortality rates are 40% to 60% [13,[15], [16], [17], [18], [19]]. For clinically meaningful absolute reductions of 2% to 10% in mortality and other dichotomous outcomes, such as rates of intensive care unit admission (10% to 20% for hospitalized patients) and need for mechanical ventilation (20% to 80% of patients admitted to intensive care), a cumulative sample size of 74 to 9493 subjects would be required for the intervention and control arms, depending on the outcome rates in the control arms (see Table 3). Based on the projected date of completion of registered controlled trials (see Fig. 2 ), we can expect sufficient numbers of patients to be enrolled by as early as September 2020 for an assessment of efficacy, depending on the mix of patients with severe and critical COVID-19. A timeline of all registered convalescent plasma trials can be found in Fig. A.1.
Table 3.
Required sample size needed to determine a significant absolute reduction in the proportion of study subjects experiencing a dichotomous outcome in the intervention group compared with a control group. Outcomes are aligned with observed mortality rates for severe COVID-19 (10%-20%), critical COVID-19 (20%-60%) [[13], [14], [15], [16], [17], [18], [19]], need for ICU admission (10%-20% of hospitalized patients), or for the need for mechanical ventilation amongst patients admitted to the ICU (20%-80%). Type I error = 0.05, Type II error = 0.2; two-tailed comparison of proportions in independent groups (https://www.stat.ubc.ca/~rollin/stats/ssize/b2.html)
| Proportion in Control Outcome | Absolute % outcome Reduction in intervention | n (intervention group) to detect delta, 1:1 enrolment |
|---|---|---|
| 10% | 2 | 4724 |
| 5 | 686 | |
| 7.5 | 278 | |
| 10 | 74 | |
| 20% | 2 | 6039 |
| 5 | 906 | |
| 7.5 | 379 | |
| 10 | 199 | |
| 40% | 2 | 9336 |
| 5 | 1471 | |
| 7.5 | 644 | |
| 10 | 356 | |
| 60% | 2 | 9493 |
| 5 | 1534 | |
| 7.5 | 686 | |
| 10 | 388 | |
| 80% | 2 | 6510 |
| 5 | 1094 | |
| 7.5 | 505 | |
| 10 | 294 |
Fig. 2.
Timeline of all included controlled COVID-19 convalescent plasma trials (n = 29) divided into randomized and non-randomized study designs. Y-axis lists the trial identification number; X-axis represents the date. The date of study completion may be same as primary end date if this was not detailed in the protocol. The size of the date of primary trial completion icon is proportional to the anticipated total enrolment. MSC, mesenchymal stem cell. *Only a start date was provided.
Discussion
The number of newly registered studies addressing the role of convalescent plasma to treat COVID-19 is significant with many launching in early 2020 or soon afterwards and many expected to complete data collection before or soon after the end of 2020. Many of the actively recruiting trials describe randomization of subjects to treatment or control groups, and many control groups appear similar such that meta-analysis will be feasible and informative. The characterization of plasma product will be important in terms of specific antibody titers and other testing related to potency. Heterogeneity will need to be accounted for in future analysis, potentially by adopting a binary low vs. high antibody titer approach using the Food and Drug Administration 2020 guidelines for convalescent plasma in COVID-19, which recommends titers of >1:160 [24]. Manufacturing details are lacking from the limited information available for most trials, but it is likely that standard methods will be observed across the studies given the International Society of Blood Transfusion's ISBT128 standards used by most blood operators that will prepare the product, allowing for sufficient homogeneity to pool data from enough studies [20]. Other aspects related to treatment include the dose and timing of treatment that varies across studies and will need to be controlled for in any meta-analysis.
While meta-analyses and summaries of early studies have been published already [5,21], the collective power of published case series is insufficient to assess effectiveness, control groups are not appropriate for meta-analysis and the lack of randomization of study subjects introduces significant potential bias. Meta-analyses will not be insightful until there is sufficient power and unless the aspects of study design appear largely similar, including the definition of disease severity, patient age and sex, and consistent reporting of clinical outcomes such rates of admission to ICU, proportion of patients requiring mechanical ventilation and mortality rates. Other outcomes, including radiographic responses, reverse transcription polymerase chain reaction results, and measurement of inflammatory markers may be harder to combine depending on the timeframe for measuring these outcomes and specifics of the methods that will be used. While some of these outcomes may not be reported, we suggest that contacting investigators for additional data to align with the reported outcomes of other studies could mitigate against this issue and allow insightful meta-analysis. Our approach, termed FAST Evidence, can provide answers as quickly as possible regarding efficacy and builds upon previous notions of continuously updating systematic reviews and combines pre-emptive identification of active studies that allows for alignment of outcome measures to enhance the utility of meta-analyses.
While blinding is not described for all studies, the use of objective outcomes should help to limit assessor bias. Moreover, randomization of subjects is described for many studies with a planned control group which limits allocation bias. Use of placebo instead of conventional therapy as a control group will be an important consideration for assessing potential bias. Concomitant administration of other experimental or other therapies in the control groups may confound analysis in some studies. Pooling data will increase power of these similar studies and should allow for earlier understanding of efficacy compared to individual studies. If efficacious, regulatory bodies in affected countries could move more quickly to approve convalescent plasma therapy.
It is possible that studies will not accrue patients at the rate anticipated. The incidence of severe COVID-19 disease will be difficult to predict in the months ahead, especially since many studies are centered in China where the disease has slowed significantly in recent weeks [22,23]. The approach outlined in this proposed framework reduces bias as it would include all published data as soon as available, from studies that share sufficient similarity to be pooled. As more study results become available, the meta-analysis would be updated to refine initial estimates regarding efficacy and to confirm rates of adverse events. Additional new trials are anticipated from new regions of the global research community which will expand the international reach of this proposed framework. The predominance of trials that lack a control arm such as normal plasma, saline, or a placebo will be a challenge for interpretation. Conventional therapy arms commonly allow patients to enroll in other therapeutic trials which will confound the interpretation of results.
In conclusion, our scoping review of registered clinical trials of convalescent plasma for COVID-19 highlights an opportunity to perform rapid meta-analysis from placebo-controlled RCTs in particular, and from additional studies that will enroll patients receiving standard or conventional therapy in a control arm. Sufficient power to detect important improvements in outcomes is anticipated by performing meta-analysis before the end of 2020. Regulatory bodies should prepare for assessing pooled data using this framework to evaluate applications for approval.
Disclosures
No conflicts of interest to disclose.
Acknowledgements
We acknowledge the important contribution from the many patients, families and donors that will participate in these clinical trials. We also acknowledge the climate of global cooperation in this challenging time that will be required to propel research efforts towards clinical treatments for more patients. GL, KZ, and DSA designed the project, contributed to data acquisition and analysis and contributed to writing of the manuscript. ML, AT, and DF contributed to data interpretation and analysis. All authors approved the final version of the manuscript and all persons who made substantial contributions to the manuscript are listed as authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmrv.2020.06.005.
Appendix A. Supplementary data
Supplementary material
References
- 1.Walker PGT, Whittaker C, Watson O, Baguelin M, Ainslie KEC, Bhatia S, et al. The Global Impact of COVID-19 and Strategies for Mitigation and Suppression. Imperial College COVID-19 Response Team, 26 March 2020:1–19. https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-Global-Impact-26-03-2020v2.pdf [accessed 22 June 2020]
- 2.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol 2020: epub ahead of print on 15 April 2020. [DOI] [PMC free article] [PubMed]
- 4.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajendran K., Dm N.K., Rangarajan J., Glasgow F., Medicine P. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 2020:1–9. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel J.H., Aga E., Elie-Turenne M.C., Cho J., Tebas P., Clark C.L., et al. Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2019;7:941–950. doi: 10.1016/S2213-2600(19)30199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel J.H., Tebas P., Elie-Turenne M.-C., Bajwa E., Bell T.E., Cairns C.B., et al. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med. 2017;5:500–511. doi: 10.1016/S2213-2600(17)30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung I.F.N., To KKW, Lee C.-K., Lee K.-L., Yan W.-W., Chan K., et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144:464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 9.Davey R.T., Fernández-Cruz E., Markowitz N., Pett S., Babiker A.G., Wentworth D., et al. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial. Lancet Respir Med. 2019;7:951–963. doi: 10.1016/S2213-2600(19)30253-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein J., Burnouf T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Intern J Transfus Med. 2020:1–3. doi: 10.1111/vox.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzik S. COVID-19 convalescent plasma: now is the time for better science. Transfus Med Rev. 2020:2–5. doi: 10.1016/j.tmrv.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brant R. Inference for Proportions: Comparing Two Independent Samples. https://www.stat.ubc.ca/~rollin/stats/ssize/b2.html (accessed May 12, 2020).
- 13.Aylward (WHO) B, Liang (PRC) W. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 2020;2019:16–24. https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) [accessed 22 June 2020]
- 14.Cheng Z.J., Shan J. 2019 novel coronavirus: where we are and what we know. Infection. 2020;48:155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Distler P. ISBT 128 : a global information standard. Cell Tissue Bank 2010:365–373. [DOI] [PMC free article] [PubMed]
- 21.Sullivan H.C., Roback J.D. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transfus Med Rev. 2020:6–11. doi: 10.1016/j.tmrv.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:P475–P481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Drug Administration. Investigational COVID-19 convalescent plasma guidance for industry. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigational-covid-19-convalescent-plasma [accessed 22 June 2020]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material