Abstract
In December 2019, a novel SARS-CoV-2 coronavirus emerged, causing an outbreak of life-threatening pneumonia in the Hubei province, China, and has now spread worldwide, causing a pandemic. The urgent need to control the disease, combined with the lack of specific and effective treatment modalities, call for the use of FDA-approved agents that have shown efficacy against similar pathogens. Chloroquine, remdesivir, lopinavir/ritonavir or ribavirin have all been successful in inhibiting SARS-CoV-2 in vitro. The initial results of a number of clinical trials involving various protocols of administration of chloroquine or hydroxychloroquine mostly point towards their beneficial effect. However, they may not be effective in cases with persistently high viremia, while results on ivermectin (another antiparasitic agent) are not yet available. Interestingly, azithromycin, a macrolide antibiotic in combination with hydroxychloroquine, might yield clinical benefit as an adjunctive. The results of clinical trials point to the potential clinical efficacy of antivirals, especially remdesivir (GS-5734), lopinavir/ritonavir, and favipiravir. Other therapeutic options that are being explored involve meplazumab, tocilizumab, and interferon type 1. We discuss a number of other drugs that are currently in clinical trials, whose results are not yet available, and in various instances we enrich such efficacy analysis by invoking historic data on the treatment of SARS, MERS, influenza, or in vitro studies. Meanwhile, scientists worldwide are seeking to discover novel drugs that take advantage of the molecular structure of the virus, its intracellular life cycle that probably elucidates unfolded-protein response, as well as its mechanism of surface binding and cell invasion, like angiotensin converting enzymes-, HR1, and metalloproteinase inhibitors.
Keywords: Coronavirus, SARS-CoV-2, Chloroquine, Lopinavir, Remdesivir, Ribavirin, Ritonavir
Introduction
Coronaviruses (CoVs) are single-stranded RNA viruses that belong to the Coronaviridae family. They spread among a wide range of hosts, presenting clinically with an array of symptoms, ranging from common cold-like to severe, sometimes lethal, respiratory infection. The new virus, responsible for the pandemic, was initially termed as “2019-nCoV”, but it has since been renamed “SARS-CoV-2” by the Coronavirus Study Group (CSG), a body that belongs to the International Committee on Taxonomy of Viruses (ICTV), as it is believed to be familiar with the SARS-CoV, a pathogen that causes severe acute respiratory syndrome (SARS). The recent SARS-CoV-2 is closely associated with SARS-CoV, sharing 80 % identity in RNA sequence (Gorbalenya et al., 2020; Chan et al., 2020). With first cases in humans being recorded in December 2019, SARS-CoV-2 is responsible for an outbreak of respiratory disease called COVID-19 (Coronavirus Disease 2019). The full spectrum of COVID-19 ranges from benign, self-resolving respiratory distress to severe progressive pneumonia, multiple organ failure, and death (Huang et al., 2020a). The city of Wuhan, in the province of Hubei in central China has been declared as the epicenter of the pandemic, with Huanan seafood market being one of the first locations where SARS-CoV-2 potentially crossed the species barrier at the animal-human interface. Pioneering research undertaken in Shenzhen, near Hong Kong, by a group of clinicians and scientists from the University of Hong Kong, provided the first piece of evidence, that SARS-CoV-2 can been transmitted from human-to-human (Chan et al., 2020). The new threat quickly spread from China and is currently classified as a pandemic by the World Health Organization (WHO). Many countries are implementing extraordinary measures in order to provide their societies with adequate strategies of disease prevention and monitoring (Chan et al., 2020; Zhou et al., 2020).
For the time being, there is neither a vaccination or a specific SARS-CoV-2 targeted antiviral treatment available. Multiple countries have attempted varying pharmacologic strategies to combat the disease, involving currently established antivirals, different modes of oxygen therapy or mechanical ventilation. COVID-19 pandemic requires rapid development of efficacious therapeutic strategies, in the pursuit of which three concepts are being applied: (i) The first approach relies on testing currently known antiviral agents and verifying their clinical usefulness (Kim et al., 2016; Lu, 2020). (ii) Another modality is based on molecular libraries and databases, allowing for high computing power and simultaneous verification of millions of potential agents (Lu, 2020; Channappanavar et al., 2017). (iii) Lastly, the third strategy involves targeted therapy, intended to disrupt the genome and functioning of the virus. Precisely designed particles would disrupt the crucial steps of viral infection, such as cell surface binding and internalization. Unfortunately, in vitro activity does not necessarily translate into efficacy in the in vivo setting, due to differing pharmacodynamic and pharmacokinetic properties (Lu, 2020; Zumla et al., 2016). The main groups of therapeutic agents that can be useful in COVID-19 treatment involve antiviral drugs, selected antibiotics, antimalarials, and immunotherapeutic drugs. In the present paper, we aim to summarize current progress and insights that have emerged from the use of pharmaceuticals in COVID-19.
Hydroxychloroquine and other antimalarials
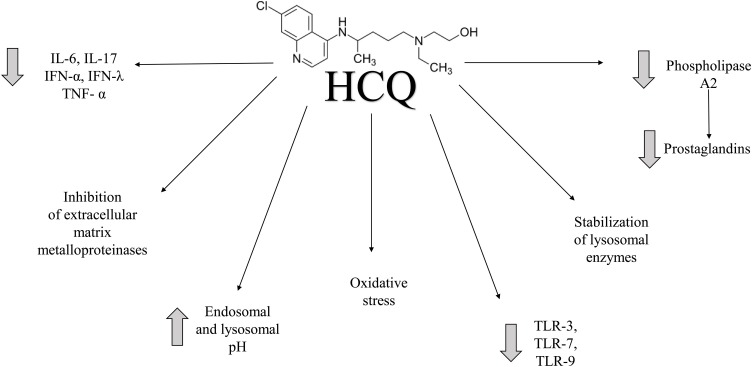
In one of the newest dissertations published by a French team of doctors, a positive influence of hydroxychloroquine (HCQ) in patients infected by SARS-CoV-2 was observed (Gautret et al., 2020). Furthermore, another in vitro trial showed that both chloroquine (CQ) and its hydroxylated derivative, HCQ, possess beneficial properties. HCQ, an agent with universally established antimalarial, anti-inflammatory, and analgesic properties, is widely used in the treatment of malaria. The US Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) are currently working on establishing randomized clinical trials that aim to confirm the usefulness of CQ and its derivatives in combating CoV-2 virus infection (Anon, 2020a, b). In the beginning of February 2020, China included CQ with its derivatives as one of the therapeutic options in SARS-CoV-2 treatment, with South Korea soon following this path (Gao et al., 2020; Sung-sun, 2020). The mechanism of action of antimalarial agents has not been well elucidated – it is believed to be pleiotropic, affecting T-cells, cytokine production, and others. Graphical representation of HCQ action can be seen in Fig. 1 . Additional anti-inflammatory effect can be attributed to the inhibition of extracellular matrix metalloproteinases (Nowell and Quaranta, 1985; Lafyatis et al., 2006; Wozniacka et al., 2006). In this case, the potential mechanism of action of CQ and its hydroxylated derivative is attributed to the blockade of viral infection via an alkalization of endosomal (and lysosomal) pH; it should be emphasized that the above acidic pH is required for virus-host cell fusion (Adar et al., 2012; Zhitomirsky and Assaraf, 2016, 2015). Furthermore, the agents are believed to disrupt SARS-CoV cell receptor glycosylation (Wang et al., 2020a).
Fig. 1.
Graphical representation of HCQ action.
It is believed that most important pathways involve lysosomal enzyme stabilization, antigen presentation suppression, T-cell stimulation inhibition, or cytokine cascade blockade. HCQ inhibits the proliferation of T-cells and monocytes, and decreases the production of pro-inflammatory cytokines (Il-6, Il-17, IFN-α, IFN-λ, TNF-α). Additionally, it inhibits antibody and prostaglandin (PG) production. It decreases thrombocyte aggregation, lipid levels, insulin secretion, as well as oxidative stress (Nowell and Quaranta, 1985). Another mechanism that contributes to its antimalarial properties involves the inhibition of toll-like-receptors, namely TLR-3, TLR-7, and TLR-9, in response to microbial antigens that under normal conditions induce inflammatory response. Furthermore, antimalarial drugs inhibit PG production and lipid peroxidation. Decreasing PG production involves the inhibition of phospholipase A2 activity.
It has been shown that HCQ presents in vitro antiviral properties against SARS-CoV (Biot et al., 2006). Its clinical safety profile is superior to that of CQ (in a long-term setting), which allows for higher daily dose, and results in fewer drug-drug interactions (Yao et al., 2020; Marmor et al., 2016). A clinical trial aiming to assess the influence of HCQ on the outcome of patients infected with SARS-CoV-2 by Gautret et al., compared patients receiving HCQ and controls, concentrating on viral load reduction (Gautret et al., 2020) (all clinical trials are summarized in Table 1 and Fig. 2 ). The study enrolled hospitalized patients with confirmed COVID-19. Patients were stratified into three categories: asymptomatic (16.7 %); upper respiratory tract infection (URTI; 61.1 %), presenting as rhinitis, pharyngitis, or isolated fever and muscle pain; lower respiratory tract infections (LRTI; 22.2 %), who suffered from symptoms of pneumonia or bronchitis. Twenty patients were administered HCQ sulfate orally, and 16 served as the control group. Among patients treated with HCQ, 6 were also treated with azithromycin, in order to prevent superimposed bacterial infection. The percentage of patients with absence of viral loads on nasopharyngeal swab sample RT-PCR was significantly higher in the treatment group than in controls, on days 3, 4, 5 and 6 of follow-up. On day 6, which was considered the endpoint, in 70 % of patients treated with HCQ viral load disappearance was observed, in comparison with 12.5 % in the control group (p = 0.001) (Gautret et al., 2020).
Table 1.
Summary of the clinical trials on COVID-19 treatment to date (16th of April 2020).
| Therapeutic agents | Clinical Trial ID | Number of participants | Comments |
|---|---|---|---|
| Adalimumab | ChiCTR20000 30089 | 60 | compared to standard treatment |
| Adamumab + Tozumab | ChiCTR20000 30580 | 60 | compared to standard treatment |
| Anakinra | NCT04341584 | 240 | - |
| Anakinra | NCT04339712 | 20 | compared to tocilizumab |
| Anakinra | NCT04324021 | 54 | compared to emapalumab and standard treatment |
| Angiotensin 1-7 | NCT04332666 | 60 | - |
| ASC09 | NCT04261270 | 60 | compared to ritonavir; combined with oseltamivir |
| ASC09 | NCT04261907 | 160 | compared to lopinavir/ritonavir; combined with ritonavir |
| Atovaquone | NCT04339426 | 25 | combined with azithromycin |
| Azithromycin | NCT04341727 | 500 | compared to chloroquine and hydroxychloroquine |
| Azithromycin | NCT04324463 | 1500 | compared to chloroquine |
| Azithromycin | NCT04339816 | 240 | combined with hydroxychloroquine |
| Azithromycin | NCT04336332 | 160 | compared to hydroxychloroquine; combined with hydroxychloroquine |
| Azithromycin | NCT04332107 | 2271 | - |
| Azithromycin + Hydroxychloroquine | NCT04322123 | 630 | compared to hydroxychloroquine |
| Azithromycin + Hydroxychloroquine | NCT04321278 | 440 | compared to hydroxychloroquine |
| Azvudine | ChiCTR20000 29853 | 20 | compared to standard treatment |
| Azvudine | ChiCTR20000 30041 | 40 | - |
| Azvudine | ChiCTR20000 30424 | 30 | - |
| Azvudine | ChiCTR20000 30487 | 10 | - |
| Baloxavir marboxil | ChiCTR20000 29544 | 30 | compared to favipiravir and standard treatment |
| Baloxavir marboxil | ChiCTR20000 29548 | 30 | compared to favipiravir and lopinavir/ritonavir |
| Baricitinib | NCT04320277 | 60 | - |
| Baricitinib | NCT04340232 | 80 | - |
| Baricitinib | NCT04321993 | 1000 | compared to hydroxychloroquine, lopinavir/ritonavir and sarilumab |
| BLD-2660 | NCT04334460 | 120 | - |
| Camostat Mesylate | NCT04321096 | 180 | - |
| CD24Fc | NCT04317040 | 230 | - |
| CD24Fc | NCT04317040 | 230 | - |
| Chloroquine | ChiCTR20000 29542 | 20 | compared to standard treatment |
| Chloroquine | ChiCTR20000 29609 | 200 | compared to lopinavir/ritonavir |
| Chloroquine | ChiCTR20000 29741 | 112 | compared to lopinavir/ritonavir |
| Chloroquine | ChiCTR20000 29826 | 45 | - |
| Chloroquine | ChiCTR20000 29837 | 120 | - |
| Chloroquine | ChiCTR20000 29935 | 100 | - |
| Chloroquine | ChiCTR20000 29939 | 100 | compared to standard treatment |
| Chloroquine | ChiCTR20000 29975 | 10 | - |
| Chloroquine | ChiCTR20000 29988 | 80 | compared to standard treatment |
| Chloroquine | ChiCTR20000 29992 | 100 | compared to standard treatment; combined with hydroxychloroquine |
| Chloroquine | ChiCTR20000 30031 | 120 | - |
| Chloroquine | ChiCTR20000 30417 | 30 | - |
| Chloroquine | ChiCTR20000 30718 | 80 | compared to standard treatment |
| Chloroquine | ChiCTR20000 29898 | 100 | compared to hydroxychloroquine |
| Chloroquine | ChiCTR20000 29899 | 100 | compared to hydroxychloroquine |
| Chloroquine | NCT04341727 | 500 | compared to azithromycin and hydroxychlorquine |
| Chloroquine | NCT04324463 | 1500 | compared to azithromycin |
| Chloroquine | NCT04323527 | 440 | - |
| Chloroquine | NCT04333628 | 210 | compared to standard treatment |
| Chloroquine | NCT04331600 | 400 | - |
| Chloroquine | NCT04328493 | 250 | compared to standard treatment |
| Ciclesonide | NCT04330586 | 141 | compared to standard treatment; combined with hydroxychloroquine |
| Colchicine | NCT04328480 | 2500 | - |
| Colchicine | NCT04322682 | 6000 | - |
| Colchicine | NCT04322565 | 100 | - |
| CSA0001 | ChiCTR20000 30939 | 10 | - |
| Danoprevir/Ritonavir | ChiCTR20000 30000 | 50 | compared to IFN-α, peginterferon α-2a and standard treatment |
| Danoprevir/Ritonavir | ChiCTR20000 30259 | 60 | compared to standard treatment |
| Danoprevir/Ritonavir | ChiCTR20000 30472 | 20 | compared to standard treatment |
| Darunavir/Cobicistat | NCT04252274 | 30 | compared to standard treatment |
| Darunavir/Cobicistat | NCT04304053 | 3040 | - |
| Darunavir/Ritonavir | NCT04291729 | 50 | compared to IFN-α, lopinavir/ritonavir and peginterferon α-2a; combined with IFN-α |
| DAS181 | NCT04324489 | 4 | - |
| Deferoxamine | NCT04333550 | 50 | compared to standard treatment |
| Defibrotide | NCT04335201 | 50 | - |
| Dexamethasone | 2020-001113-21 (EU-CTR) | 2000 | compared to IFN β-1a and lopinavir/ritonavir |
| Dexamethasone | NCT04327401 | 290 | - |
| Dihydroartemisinin/Piperaquine | ChiCTR20000 30082 | 40 | compared to IFN-α + umifenovir; combined with antiviral treatment |
| Ebastine | ChiCTR20000 30535 | 100 | combined with IFN-α and lopinavir |
| Emapalumab | NCT04324021 | 54 | compared to anakinra and standard treatment |
| Emtricitabine/Tenofovir + Lopinavir/Ritonavir | ChiCTR20000 29468 | 120 | - |
| Favipiravir | ChiCTR20000 29544 | 30 | compared to baloxavir marboxil and standard treatment |
| Favipiravir | ChiCTR20000 29548 | 30 | compared to baloxavir marboxil and lopinavir/ritonavir |
| Favipiravir | ChiCTR20000 29600 | 90 | compared to lopinavir/ritonavir; combined with IFN-α |
| Favipiravir | ChiCTR20000 29996 | 60 | - |
| Favipiravir | ChiCTR20000 30113 | 20 | compared to ritonavir |
| Favipiravir | ChiCTR20000 30254 | 240 | compared to umifenovir |
| Favipiravir | ChiCTR20000 30987 | 150 | combined with chloroquine |
| Favipiravir | JPRN jRCTs041190120 | 86 | - |
| Favipiravir | NCT04273763 | 60 | combined with bromohexine, IFN α-2b and umifenovir |
| Favipiravir | NCT04310228 | 150 | compared to tocilizumab; combined with tocilizumab |
| Favipiravir | NCT04336904 | 100 | - |
| Fingolimod | NCT04280588 | 30 | compared to standard treatment |
| Fluvoxamine | NCT04342663 | 152 | - |
| GD31 | ChiCTR20000 29895 | 160 | - |
| Hydroxychloroquine | 2020-000890-25 (EU-CTR) | 25 | - |
| Hydroxychloroquine | ChiCTR20000 29559 | 300 | - |
| Hydroxychloroquine | ChiCTR20000 29740 | 78 | compared to standard treatment |
| Hydroxychloroquine | ChiCTR20000 29868 | 200 | compared to standard treatment |
| Hydroxychloroquine | ChiCTR20000 29898 | 100 | compared to chloroquine |
| Hydroxychloroquine | ChiCTR20000 29899 | 100 | compared to chloroquine |
| Hydroxychloroquine | ChiCTR20000 30054 | 100 | compared to standard treatment |
| Hydroxychloroquine | NCT04261517 | 30 | compared to standard treatment |
| Hydroxychloroquine | NCT04315896 | 500 | - |
| Hydroxychloroquine | NCT04315948 | 3100 | compared to IFNβ-1a, lopinavir/ritonavir and remdesivir |
| Hydroxychloroquine | NCT04316377 | 202 | compared to standard treatment |
| Hydroxychloroquine | NCT04342221 | 220 | - |
| Hydroxychloroquine | NCT04340544 | 2700 | - |
| Hydroxychloroquine | NCT04338698 | 500 | compared to azithromycin and oseltamivir |
| Hydroxychloroquine | NCT04335552 | 500 | compared with azithromycin, hydroxychloroquine and standard treatment; combined with azithromycin |
| Hydroxychloroquine | NCT04334512 | 600 | combined with azithromycin |
| Hydroxychloroquine | NCT04334382 | 1550 | combined with azithromycin |
| Hydroxychloroquine | NCT04329832 | 300 | combined with azithromycin |
| Hydroxychloroquine | NCT04329572 | 400 | combined with azithromycin |
| Hydroxychloroquine | NCT04328272 | 75 | combined with azithromycin |
| Hydroxychloroquine | NCT04323631 | 1116 | compared to standard treatment |
| Hydroxychloroquine | NCT04321993 | 1000 | compared to baricitinib, lopinavir/ritonavir and sarilumab |
| Hydroxychloroquine | NCT04342169 | 400 | - |
| Hydroxychloroquine | NCT04341727 | 500 | compared to azithromycin and chloroquine |
| Hydroxychloroquine | NCT04341493 | 86 | compared to nitazoxanide |
| Hydroxychloroquine | NCT04334967 | 1250 | compared to standard treatment |
| Hydroxychloroquine | NCT04333654 | 210 | compared to standard treatment |
| Hydroxychloroquine | NCT04332991 | 510 | - |
| Hydroxychloroquine | NCT04321616 | 700 | compared to remdesivir and standard treatment |
| Hydroxychloroquine + IFN β-1b + Lopinavir/Ritonavir | IRCT20100228 003449N27 | 30 | - |
| Hydroxychloroquine + IFN β-1b + Lopinavir/Ritonavir | IRCT20100228 003449N28 | 30 | - |
| Hydroxychloroquine + Lopinavir/Ritonavir | JPRN jRCTs031190227 | 50 | - |
| Hydroxychloroquine + Lopinavir/Ritonavir + Sofosbuvir/Ledipasvir | IRCT20100228 003449N29 | 50 | - |
| Hydroxychlorquine + Camostat Mesylate | NCT04338906 | 334 | - |
| IFN α-1b | ChiCTR20000 29989 | 300 | - |
| IFN α-1b | NCT04293887 | 328 | compared to standard treatment |
| IFN α-1b + Lopinavir/Ritonavir + Ribavirin | ChiCTR20000 29387 | 108 | - |
| IFN α-2b | NCT04273763 | 60 | combined with bromohexine, favipiravir and umifenovir |
| IFN α-2b + Lopinavir/Ritonavir | ChiCTR20000 30166 | 20 | - |
| IFN β-1a | 2020-001023-14 (EU-CTR) | 400 | - |
| IFN β-1a | 2020-000936-23 (EU-CTR) | 3000 | compared to lopinavir/ritonavir and remdesivir |
| IFN β-1a | 2020-001113-21 (EU-CTR) | 2000 | compared to dexamethasone and lopinavir/ritonavir |
| IFN β-1a | NCT04343768 | 60 | compared to hydroxychloroquine + lopinavir / ritonavir and IFN β-1b; combined with hydroxychloroquine + lopinavir / ritonavir |
| IFN β-1b | NCT04343768 | 60 | compared to hydroxychloroquine + lopinavir / ritonavir and IFN β-1a; combined with hydroxychloroquine + lopinavir / ritonavir |
| IFN β-1b + Ribavirin | NCT04276688 | 70 | combined with lopinavir/ritonavir |
| IFN-α | ChiCTR20000 29496 | 90 | compared to lopinavir/ritonavir; combined with lopinavir/ritonavir |
| IFN-α | ChiCTR20000 29600 | 90 | compared to lopinavir/ritonavir and favipiravir |
| IFN-α | ChiCTR20000 29638 | 100 | compared to rSIFN-co |
| IFN-α | ChiCTR20000 30000 | 50 | compared to danoprevir/ritonavir, peginterferon α-2a and standard treatment |
| IFN-α | NCT04291729 | 11 | compared to darunavir/ritonavir, lopinavir/ritonavir and peginterferon α-2a |
| IFN-α and Lopinavir/Ritonavir | NCT04251871 | 150 | - |
| IFN-α and Lopinavir/Ritonavir | NCT04275388 | 348 | - |
| IFX-1 | NCT04333420 | 130 | compared to standard treatment |
| Interleukin-2 | ChiCTR20000 30167 | 80 | compared to standard treatment |
| Ivermectine | NCT04343092 | 50 | combined with Hydroxychloroquine; compared to placebo |
| Ixekizumab | ChiCTR20000 30703 | 40 | compared to antiviral therapy; combined with antiviral therapy |
| Leflunomide | ChiCTR20000 30058 | 200 | compared to standard treatment |
| Leronlimab | NCT04343651 | 70 | - |
| Levamisole | NCT04331470 | 30 | compared to standard treatment; combined with budesonide, formeterol and hydoxychloroquine + lopinavir/ritonavir |
| Lopinavir/Ritonavir | 2020-000936-23 (EU-CTR) | 3000 | compared to IFN β-1a and remdesivir |
| Lopinavir/Ritonavir | 2020-001113-21 (EU-CTR) | 2000 | compared to dexamethasone and IFN β-1a |
| Lopinavir/Ritonavir | ChiCTR20000 29308 | 160 | compared to standard treatment |
| Lopinavir/Ritonavir | ChiCTR20000 29400 | 60 | - |
| Lopinavir/Ritonavir | ChiCTR20000 29496 | 90 | compared to IFN-α; combined with IFN-α |
| Lopinavir/Ritonavir | ChiCTR20000 29539 | 328 | compared to standard treatment |
| Lopinavir/Ritonavir | ChiCTR20000 29548 | 30 | compared to baloxavir marboxil and favipiravir |
| Lopinavir/Ritonavir | ChiCTR20000 29573 | 480 | combined with IFN-α and umifenovir |
| Lopinavir/Ritonavir | ChiCTR20000 29600 | 90 | compared to favipiravir; combined with IFN-α |
| Lopinavir/Ritonavir | ChiCTR20000 29609 | 200 | compared to chloroquine |
| Lopinavir/Ritonavir | ChiCTR20000 30187 | 60 | compared to standard treatment |
| Lopinavir/Ritonavir | ChiCTR20000 30218 | 80 | - |
| Lopinavir/Ritonavir | NCT04252885 | 125 | compared to standard treatment and umifenovir |
| Lopinavir/Ritonavir | NCT04255017 | 400 | compared to oseltamivir and umifenovir |
| Lopinavir/Ritonavir | NCT04261907 | 160 | compared to ASC09 |
| Lopinavir/Ritonavir | NCT04291729 | 11 | compared to darunavir/ritonavir, IFN-α and peginterferon α-2a |
| Lopinavir/Ritonavir | NCT04315948 | 3100 | compared to hydroxychloroquine and remdesivir; combined with IFN β-1a |
| Lopinavir/Ritonavir | NCT04330690 | 440 | compared to standard care |
| Lopinavir/Ritonavir | NCT04321993 | 1000 | compared to baricitinib, hydroxychloroquine and sarilumab |
| Losartan | NCT04340557 | 200 | - |
| LY3127804 | NCT04342897 | 200 | - |
| Meplazumab | NCT04275245 | 28 | - |
| Methylprednisolone | NCT04263402 | 100 | - |
| Methylprednisolone | ChiCTR20000 29386 | 48 | compared to standard treatment |
| Methylprednisolone | ChiCTR20000 29656 | 100 | compared to standard treatment |
| Methylprednisolone | NCT04244591 | 80 | compared to standard treatment |
| Methylprednisolone | NCT04273321 | 400 | compared to standard treatment |
| Methylprednisolone | NCT04323592 | 104 | compared to standard treatment |
| Naproxen | NCT04325633 | 584 | compared to standard treatment |
| Nitazoxanide | NCT04341493 | 86 | compared to hydroxychloroquine |
| Nivolumab | NCT04343144 | 92 | compared to standard treatment |
| Oseltamivir | NCT04255017 | 400 | compared to lopinavir/ritonavir and umifenovir |
| Oseltamivir | NCT04261270 | 60 | compared to ASC09 and ritonavir |
| Oseltamivir | NCT04303299 | 80 | compared to favipiravir, lopinavir/ritonavir and standard treatment; combined with chloroquine, darunavir/ritonavir and lopinavir/ritonavir |
| PD-1 monoclonal antibody | ChiCTR20000 30028 | 40 | compared to standard treatment |
| PD-1 monoclonal antibody | NCT04268537 | 120 | compared to standard treatment and thymosin |
| Peginterferon Lambda-1a | NCT04331899 | 120 | - |
| Peginterferon α-2a | ChiCTR20000 30000 | 50 | compared to danoprevir/ritonavir, IFN-α and standard treatment |
| Peginterferon α-2a | NCT04291729 | 11 | compared to darunavir/ritonavir, IFN-α and lopinavir/ritonavir |
| Piclidenoson | NCT04333472 | 40 | compared to standard treatment |
| Polyinosinic polycytidylic acid | ChiCTR20000 29776 | 40 | compared to standard treatment |
| PUL-042 | NCT04312997 | 100 | - |
| Remdesivir | 2020-000841-15 (EU-CTR) | 400 | compared to standard treatment |
| Remdesivir | 2020-000842-32 (EU-CTR) | 600 | compared to standard treatment |
| Remdesivir | 2020-000936-23 (EU-CTR) | 3000 | compared to IFN β-1a and lopinavir/ritonavir |
| Remdesivir | NCT04252664 | 308 | - |
| Remdesivir | NCT04257656 | 453 | - |
| Remdesivir | NCT04280705 | 394 | - |
| Remdesivir | NCT04292730 | 600 | compared to standard treatment |
| Remdesivir | NCT04292899 | 400 | compared to standard treatment |
| Remdesivir | NCT04315948 | 3100 | compared to hydroxychloroquine, IFN β-1a and lopinavir/ritonavir |
| Remdesivir | NCT04321616 | 700 | compared to hydroxychloroquine and standard treatment |
| RhACE2 APN01 | NCT04335136 | 200 | - |
| rhG-CSF | ChiCTR20000 30007 | 200 | compared to standard treatment |
| Ribavirin | ChiCTR20000 30922 | 30 | combined with IFN α-2a and umifenovir |
| Ritonavir | ChiCTR20000 30113 | 20 | compared to favipiravir |
| rSIFN-co | ChiCTR20000 29638 | 100 | compared to IFN-α |
| Ruxolitinib | NCT04338958 | 200 | - |
| Ruxolitinib | NCT04331665 | 64 | - |
| Sarilumab | NCT04327388 | 300 | - |
| Sarilumab | NCT04322773 | 200 | compared to standard treatment and tacilizumab |
| Sarilumab | NCT04341870 | 60 | combined with azithromycin and hydroxychloroquine; compared with sarilumab |
| Sarilumab | NCT04315298 | 400 | - |
| Sarilumab | NCT04321993 | 1000 | compared to baricitinib, hydroxychloroquine and lopinavir/ritonavir |
| Sildenafil | NCT04304313 | 10 | - |
| Siltuximab | NCT04329650 | 100 | compared to methylprednisolone |
| Sirolimus | NCT04341675 | 30 | - |
| Sofosbuvir/Daclatasvir | IRCT20200128 046294N2 | 70 | compared to standard treatment |
| Tacrolimus | NCT04341038 | 84 | compared to standard treatment; combined with methylprednisolone |
| Thymosin | ChiCTR20000 29541 | 100 | combined with darunavir/cobicistat or lopinavir/ritonavir |
| Thymosin | ChiCTR20000 29806 | 120 | compared to Camrelizumab and conventional treatment |
| TJ003234 | NCT04341116 | 144 | - |
| Tocilizumab | ChiCTR20000 29765 | 188 | compared to standard treatment |
| Tocilizumab | ChiCTR20000 30196 | 60 | - |
| Tocilizumab | ChiCTR20000 30442 | 100 | - |
| Tocilizumab | NCT04310228 | 150 | compared to favipiravir; combined with favipiravir |
| Tocilizumab | NCT04315480 | 30 | - |
| Tocilizumab | NCT04317092 | 400 | - |
| Tocilizumab | NCT04339712 | 20 | compared to anakinra |
| Tocilizumab | NCT04331808 | 240 | - |
| Tocilizumab | NCT04322773 | 200 | compared to sarilumab and standard treatment |
| Tocilizumab | NCT04335305 | 24 | compared to standard treatment; combined with pembrolizumab |
| Tocilizumab | NCT04335071 | 100 | - |
| Tocilizumab | NCT04332913 | 30 | - |
| Tocilizumab | NCT04332094 | 276 | compared with azithromycin + hydroxychloroquine; combined with azithromycin + hydroxychloroquine |
| Tocilizumab | NCT04331795 | 50 | - |
| Tocilizumab | NCT04330638 | 342 | compared with anakinra and siltuximab; combined with anakinra and siltuximab |
| Tocilizumab | NCT04320615 | 330 | - |
| Tofacitinib | NCT04332042 | 50 | - |
| Tradipitant | NCT04326426 | 300 | - |
| Tranexamic acid | NCT04338126 | 60 | - |
| Tranexamic acid | NCT04338074 | 100 | - |
| Tranilast | ChiCTR20000 30002 | 60 | compared to standard treatment |
| Triazavirin | ChiCTR20000 30001 | 240 | compared to standard treatment |
| Ulinastatin | ChiCTR20000 30779 | 100 | compared to standard treatment |
| Umifenovir | ChiCTR20000 29573 | 480 | combined with IFN-α and lopinavir/ritonavir |
| Umifenovir | ChiCTR20000 29621 | 380 | compared to standard treatment |
| Umifenovir | ChiCTR20000 29993 | 40 | - |
| Umifenovir | ChiCTR20000 30254 | 240 | compared to favipiravir |
| Umifenovir | NCT04252885 | 125 | compared standard treatment and tolopinavir/ritonavir |
| Umifenovir | NCT04254874 | 100 | combined with peginterferon α-2a |
| Umifenovir | NCT04255017 | 400 | compared to lopinavir/ritonavir and oseltamivir |
| Umifenovir | NCT04273763 | 60 | combined with bromohexine, favipiravir and IFN α-2b |
| Valsartan | NCT04335786 | 651 | - |
IFN - interferon
rSIFN-co - Recombinant Super-Compound IFN
RhACE2 - Recombinant Human Angiotensin-converting Enzyme 2
rhG-CSF - Recombinant human granulocyte colony-stimulating factor
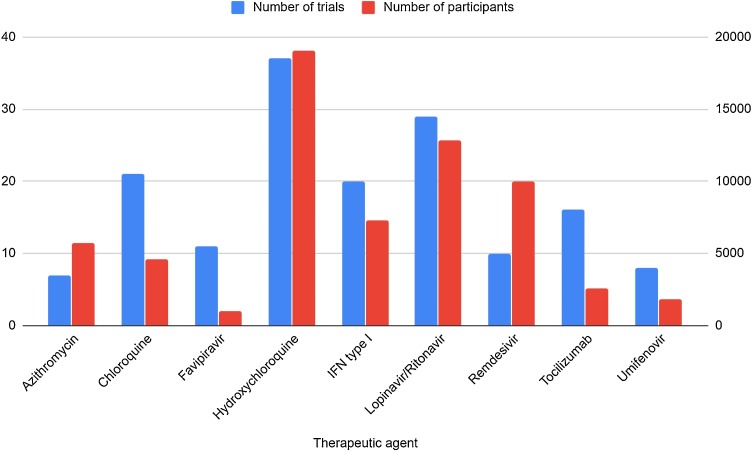
Fig. 2.
Summary of clinical trials on COVID-19 treatment.
The chart summarizes the clinical trials to date (16th of April 2020), which verify the effectiveness of different potential anti−COVID therapeutic agents, with regard to the therapeutic agent and the number of participating patients.
Another study compiling the results of over 100 patients showed that the addition of CQ phosphate is superior to standard supportive care and hence contributing to prevention of the deterioration of pneumonia. Investigators observed improved lung imaging findings, improved negative conversion, and shortening of the disease course. No severe adverse events were noted in the study. CQ phosphate was recommended to be introduced into the next edition of National Health Commission of the People’s Republic of China guidelines on prevention, diagnosis, and treatment of pneumonia caused by COVID-19 (Gao et al., 2020).
In February 2020, a randomized clinical trial on 62 patients was established in Renmin Hospital of Wuhan University to determine the efficacy of HCQ in patients with COVID-19. The trial involved 5-day HCQ treatment (400 mg/day), during which patients were examined 3 times a day, including temperature measurement and assessment of cough. CT was performed at baseline and once again after 5 days. In the HCQ arm, significantly shorter body temperature normalization and cough remission times were noted. In addition, radiological improvement in pneumonia was observed more frequently in patients from the HCQ group (80.6 % vs 54.8 %). Despite the rather limited sample size, the trial demonstrated that the use of HCQ can improve patient prognosis, accelerate remission, and improve clinical status (Chen et al., 2020a).
Teng et al., showed that administration of HCQ in patients with persistent mild to moderate COVID-19 did not improve the probability of negative conversion, in comparison with standard of care alone. One hundred and fifty patients were included in this study, with 75 assigned to HCQ plus standard of care, whereas the remaining 75 patients were treated with standard of care only. Results of HCQ group did not differ significantly from the results of the standard of care group (Tang et al., 2020).
In a recent study, HCQ administration resulted in earlier recovery, without affecting overall mortality. The study was conducted on a group of 522 patients, 127 of which were symptomatic, while the remaining 395 patients had no clinical manifestations at baseline. Their COVID-19 status was confirmed by RT-PCR. Asymptomatic patients treated with HCQ recovered earlier (average recovery time = 5.4 days) compared to asymptomatic patients who did not receive any treatment (average recovery time = 7.6 days) (Bhandari et al., 2020).
In conclusion: CQ is a cheap and relatively safe drug that has been in clinical use for over 70 years (Ciak and Hahn, 1966; Chu et al., 2018), therefore can be a potential candidate for SARS-CoV-2 treatment (Cortegiani et al., 2020). Despite promising results, it is essential to consider all safety measures and treat with this medication only as a supplementary form of treatment. Moreover, the initial enthusiasm surrounding HCQ and CQ was curbed after both were discontinued from SOLIDARITY trial due to the lack of benefit (WHO, 2020). This, along with other promising treatment schemes that have emerged in the recent months, are summarized in Table 2 .
Table 2.
10-day treatment algorithms of COVID-19, according to 6th edition of Guidelines for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced Pneumonia (Dong et al., 2020; China, 2020).
| Drug | Dose |
|---|---|
| Chloroquine phosphate | 500 mg every 12 hours, orally |
| IFN-α | 5 million units every 12 hours, nebulized solution |
| Ribavirin | 500 mg every 8 - 12 hours, iv - in combination with IFN-α (5 million units every 12 hours, nebulized solution) or lopinavir/ritonavir (200 mg/50 mg every 12 hours, orally) |
| Umifenovir | 200 mg every 8 hours, orally |
IFN-α - interferon-α.
The antiparasitic agent ivermectin is another drug worth exploring further. In an in vitro study, it showed a 99.98 % reduction in viral load after 48 h of treatment (Caly et al., 2020). The drug is not toxic at a standard dose, and is safe for pregnant women, which makes it a strong candidate for evaluation in clinical trials (Caly et al., 2020). So far, one study has been established to verify its clinical efficacy, in combination with HCQ (NCT04343092) (US National Library of Medicine, 2020).
Corticosteroids
The WHO states in his recommendations that systemic steroids should not be routinely administered in treatment of viral pneumonia or acute respiratory distress syndrome (ARDS), unless recommended for other medical reasons, or as part of a clinical trial (World Health, 2020). In a systemic review of observational studies that focused on the effects of corticoid administration to patients with SARS, no clinical benefit was noted in terms of overall survival. In the case of influenza, steroid administration was associated with higher mortality rate and superimposed infections (Hui et al., 2018). General quality of evidence advocating for the use of steroids is considered weak. Another study, adjusted for confounding factors, did not present any association of steroid therapy with lower mortality rates. Finally, the latest study on steroids administration in patients with MERS, no effect on survival was disclosed, but steroids may have been responsible for halting the disease progression in severe forms of LRTI. The use of steroids was associated with delayed clearance of viral RNA from the respiratory tract (Arabi et al., 2018a) and blood (Lee et al., 2004). Given the evidence presently available, it is recommended to avoid the routine administration of steroids, unless recommended for the treatment of another comorbidity, e.g. shock or as continuation of treatment (Who, 2020; Russell et al., 2020).
Antibiotics
There are several studies that present potential benefits of antibiotic therapy in coronavirus infection. It is challenging to elucidate the potential underlying mechanism of action that might be of benefit in monotherapy, therefore most researchers turn their attention to combination therapy. Azithromycin, a macrolide antibiotic, in combination with HCQ, might yield clinical benefit as an adjunctive. The insights from the French study (described in the section concerning CQ) presents the thesis that azithromycin potentiates the effects of therapy (Gautret et al., 2020). Among patients treated with HCQ, 6 of them were given azithromycin (500 mg initially, then 250 mg per day for the next 4 days), in order to prevent superimposed bacterial infections. When comparing HCQ monotherapy to combination therapy with azithromycin, the percentage of patients who presented with negative PCR viral load was significantly different, at 3, 4, 5 and 6 days of follow-up, in the favor of dual therapy. On day 6, 100 % of patients were declared viral load-negative, in comparison with 57.1 % in HCQ monotherapy group and 12.5 % in control group (p < 0.001). The effect of treatment was significantly more pronounced in patients with URTI and LRTI in comparison with asymptomatic patients (p < 0.05) (Gautret et al., 2020).
Teicoplanin is a glycopeptide antibiotic routinely used in the treatment of bacterial infections. In an in vitro setting it exerts anti-SARS-CoV activity. Therefore, it might be used as one of potential therapeutic agents against COVID-19. While it is most commonly used in Gram-positive bacterial infections, especially of Staphylococcal etiology, it did present some anti-viral properties in past studies. It is effective in vitro against Ebola virus, Influenza virus, Flavivirus, Hepacivirus C (HCV), human immunodeficiency virus (HIV), and coronaviruses – MERS-CoV and SARS-CoV (Baron et al., 2020). In 2016, a patent application was submitted for the use of teicoplanin in MERS-CoV infection. According to Zhou et al., teicoplanin influences the early stages of viral replication cycle, inhibiting viral detachment, thereby preventing the release of viral RNA, halting further virus-cycle progression (Baron et al., 2020). Latest studies carried out by the same researchers, suggested that it is likewise effective against SARS-CoV-2 (as the target sequence, the molecular target for cathepsin L is identical to that of SARS-CoV). The teicoplanin concentration that is required to inhibit viral replication by 50 % (IC50; 50 % inhibitory concentration) in vitro was 1.66 μM, a value significantly lower than that reached in human blood (8.78 μM for a daily dose of 400 mg). These results require further confirmation in randomized clinical trials (Baron et al., 2020).
Viral entry inhibitors
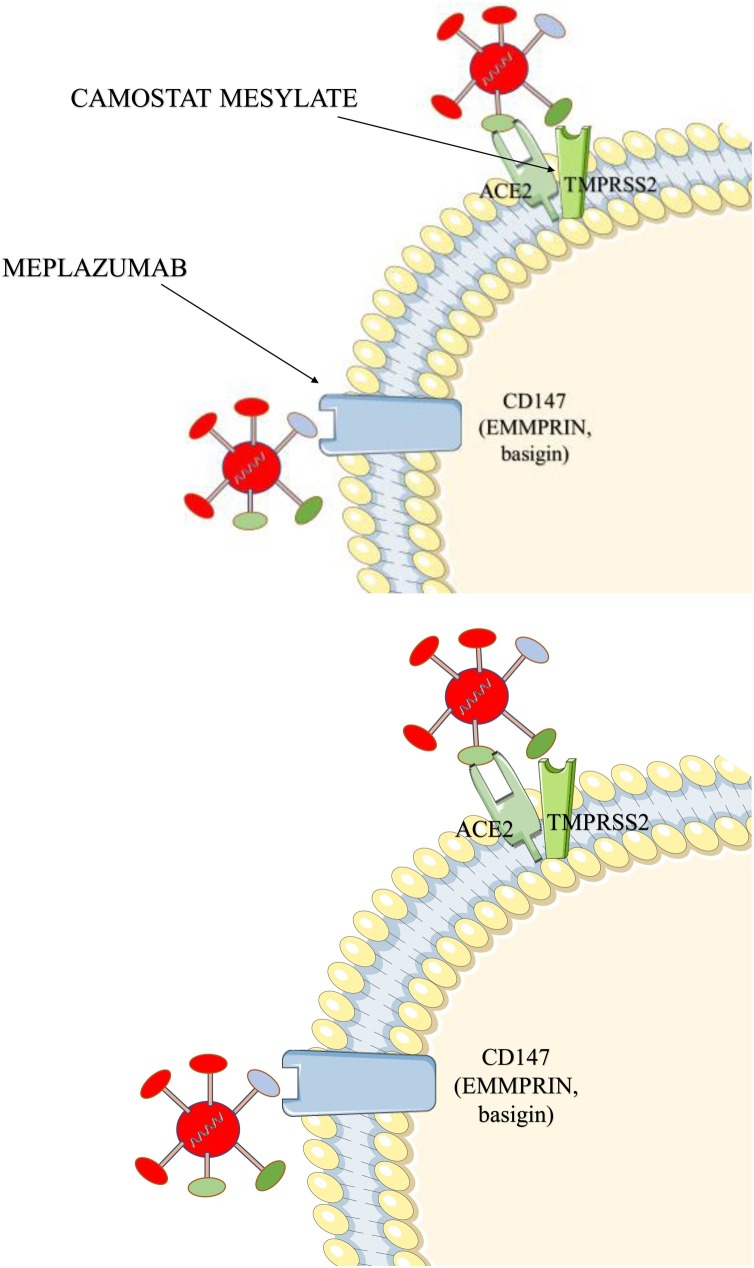
Angiotensin converting enzyme 1 (ACE1) is a monocarboxypeptidase, which is responsible primarily for the conversion of angiotensin I (ATI) into angiotensin II (ATII), while angiotensin converting enzyme 2 (ACE2) is an enzyme that catalyzes the conversion of ATII into Angiotensin 1–7 that possesses vasodilatory properties. Type 2 pneumocytes present in the alveoli belong to the group of ACE2 expressing cells (Hamming et al., 2004). Full-length ACE2 contains a structural transmembrane domain, which anchors its extracellular domain to the plasma membrane. The extracellular domain has been demonstrated as a receptor for the spike (S) protein of SARS-CoV-2 (Fig. 3 ) (Batlle et al., 2020).
Fig. 3.
Molecular targets of therapeutic agents disrupting viral invasion.
ACE2 (angiotensin converting enzyme 2) - the virus fuses with host cells and multiplies by binding to the ACE2 membrane receptor, with the aid of the receptor binding domain encoded in the SARS-fusion protein S (Spike) 2-CoV (Ou et al., 2020); TMPRSS2 – the protease activates the process of cell fusion when protein S binds to ACE2; it induces receptor-dependent syncytium formation [143]; CD147 – potential alternative mediator of invasion of host cells (its role is not well established) (Wang et al., 2020c).
ACE inhibitors (ACE-I) are the basis for the treatment of heart failure with impaired left ventricular systolic function (ejection fraction <40 %) of classes II—IV according to the New York Heart Association (Ponikowski et al., 2016). They owe their popularity in clinical practice to well-established effects on reducing all-cause mortality and heart failure hospitalization rate (Ponikowski et al., 2016; Schwartz et al., 2003; Effects of enalapril on mortality in severe congestive heart failure, 1987). An alternative to ACE-I, mainly used in the case of side effects associated with inhibition of bradykinin degradation - including persistent dry cough, are AT1 receptor antagonists (AT1-A). Both groups belong to the most basic drugs used in the treatment of hypertension, which makes them two of the most commonly used medications in the world, especially in the elderly population.
Recent analysis of SARS-CoV-2 infected populations presents a relationship between increased age of the population and more severe disease course (Guan et al., 2020). Some researchers associate this phenomenon with the universal use of drugs that affect the renin-angiotensin-aldosterone system (RAA). In the early stages of the pandemic, a hypothesis was proposed where chronic use of ACE-I and AT1-A could lead to an increase in ACE2 in the pulmonary circulation, which in turn increases the number of receptors available for the virus (Ferrario et al., 2005), thus the risk of severe COVID-19 increases (Diaz, 2020; Xu et al., 2020a). However, the results of the recent animal and human studies do not support this theory (Cappuccio and Siani, 2020; Sriram and Insel, 2020; Morales et al., 2020; Fosbøl et al., 2020; Alexandre et al., 2020).
On the other hand, a hypothesis has been proposed that the attachment of the virus to ACE2 during the development of pneumonia disrupts the homeostasis by violating the RAA system, which further aggravates the patient's condition. Thus, when used in patients developing fully-blown COVID-19, ACE-I and AT1-A can reduce symptoms and even reduce mortality (Dhama et al., 2020; Sun et al., 2020). A trial on 651 patients (NCT04335786) aiming to verify whether the antihypertensive agent valsartan influences COVID-19 treatment outcomes is currently in progress (Gommans et al., 2020). The knowledge gained thus far does not allow stating that long-term therapy with drugs inhibiting the cascade of reactions in the renin-angiotensin-aldosterone (RAA) system is associated with worse patient prognosis, while their immediate supply can save the patient. There is no reason to discontinue therapy with these groups of drugs after the infection is diagnosed (Cappuccio and Siani, 2020; Sriram and Insel, 2020; Morales et al., 2020; Fosbøl et al., 2020; Alexandre et al., 2020; American Heart, 2020).
The information obtained from mechanistic studies concerning the entry of the virus into cells, as well as the presumed association between the use of ACE-I and AT1-A with the cases, leads to the hypothesis implying potential effectiveness of controlling the virus by supplying the soluble form of ACE2. Soluble ACE2 would competitively compete for SARS-CoV attachment with receptors present on cell surfaces, preventing virion invasion of pneumocytes. In vitro studies support the above assumptions - soluble ACE2 limited the proliferation of SARS-CoV on the Vero-E6 cell line (Li et al., 2003; Ksiazek et al., 2003). In in vitro tests, ACE2 combined with the Fc fragment of the antibody neutralized the virus (Lei et al., 2020). The method described is currently not feasible, with numerous obstacles that need to be removed before this therapy can enter human testing phase. The development of bioinformatics sparks hope that the analysis of protein data banks will allow for faster discovery of a receptor for which SARS-CoV-2 proteins will be a high-affinity ligand (Morse et al., 2020). Currently, studies on an animal model are not being carried out, however, transgenic mice expressing the human form of ACE2 are achievable and it is likely a matter of time before research in this model begins (Batlle et al., 2020).
The inhibitor for transmembrane serine protease 2 (TMPRSS2) would act similarly to the described soluble ACE2. The enzyme, together with the virus receptor (ACE2) is responsible for the virion's entry into the cell (Fig. 3) (Hoffmann et al., 2020a).
Another point of focus for COVID-19 treatment associated with the mechanism of SARS-CoV-2 entry into the cell may be HR1 - a fragment of the S protein that is important for the virus in order to attach to the cell. For now, the results of in vitro and animal model tests are encouraging. OC43-HR2P peptide successfully inhibits coronavirus invasion. Its modified form - EK1 possesses even more desirable properties. Intranasal peptide administration has been shown to be effective in a murine model, while not causing any organ dysfunction (Xia et al., 2019).
Nucleotide and nucleoside analogs
This group of drugs has been routinely used in the treatment of viral infections for many years (Luo et al., 2018; Keam, 2007; Rachlis, 1990; Jordan et al., 2018; Churchill et al., 2016; Organization, 2018). It is characterized by high affinity to viral enzymes and low affinity to human enzymes. Because of that feature, nucleotide and nucleoside analogs are capable of inhibiting viral DNA replication, reverse transcription, and virion protein biosynthesis. This effect is possible due to many mechanisms, of which premature termination and inhibition of nitrogenous bases synthesis are most notable (Lu, 2020; Arabi et al., 2018b).
SARS-CoV and SARS-CoV-2 RNA-dependent RNA polymerases are structurally similar – they share 95 % identity in amino acid sequence (Morse et al., 2020). This fact accelerates research, as some substances previously tested during SARS epidemic might be found equally effective against COVID (Morse et al., 2020).
Remdesivir (GS-5734) is widely known from trials on patients infected with Ebola virus (Weston and Frieman, 2020; Sheahan et al., 2017; Brown et al., 2019). This adenosine analog binds to viral RNA, leading to premature termination (Warren et al., 2016; Ko et al., 2020). Its effectiveness has already been proven in vitro (Wang et al., 2020a). Remdesivir was used in the rhesus macaque model of MERS infection. It was effective if administered either before or after MERS-CoV infection. Remdesivir restricted lung injury, inhibited viral replication and improved medical condition (Yuen et al., 2020; de Wit et al., 2020). It was more effective than combined therapy lopinavir/ritonavir and interferon-1β in the animal model (Sheahan et al., 2017). Remdesivir was further introduced into clinical trials. Preliminary results suggest that it is safe for humans (Lu, 2020; Agostini et al., 2018). The first COVID-19 patient in the USA presented clinical improvement following remdesivir administration (Holshue et al., 2020).
Grein et al., reported on the results of a clinical trial with remdesivir, which began on January 25th, and ended on March 7th 2020. Remdesivir was given to patients with confirmed SARS-CoV-2 infection and oxygen saturation ≤94 % (either breathing atmospheric air or receiving oxygen support). Patients were treated with remdesivir intravenously for 10 days - 200 mg on the first day, and 100 mg daily over the next 9 days. Sixty-one patients from the USA, Canada, Japan, and Europe were initially included in the treatment group, 8 of which were subsequently excluded. During the median follow-up of 18 days, 36 patients (68 %) displayed an improved oxygen maintenance class. Moreover, 17 of 30 patients (57 %) assisted by mechanical ventilation were extubated. A total of 25 patients (47 %) were discharged and 7 patients (13 %) died. The mortality rate was 18 % (6 out of 34) among patients receiving invasive ventilation and 5 % (1 out of 19) among patients not receiving invasive ventilation. The risk of death was greater in patients aged 70 years or older (risk ratio compared to patients under 70 years old, 11.34; 95 % confidence interval (CI): 1.36–94.17) and among patients with higher serum creatinine at baseline (risk ratio per milligram per deciliter, 1.91; 95 % CI: 1.22–2.99). The risk ratio for patients receiving invasive ventilation compared to patients receiving non-invasive oxygen support was 2.78 (95 % CI: 0.33–23.19). Clinical improvement was seen in 36 of 53 patients (68 %) (Grein et al., 2020). However, other researchers have raised concerns regarding the methodology of this study and question its results (Compassionate Use of Remdesivir in Covid-19, 2020).
Beigel et al., verified the effectiveness of remdesivir in a randomized trial involving a group of 1063 patients (NCT04280705). Their preliminary results are promising, as patients receiving this agent recovered significantly sooner than those who received placebo (Beigel et al., 2020). Moreover, remdesivir has a positive recommendation of The European Medicines Agency in the treatment of COVID-19 (Wise, 2020). However, not all trials (NCT04257656) reported such favorable results – in 237 patients, remdesivir was not associated with any clinical benefits (Wang et al., 2020b).
Another drug - favipiravir (T-705, Avigan, Favipira) has been under investigation since mid-February 2020. Clinical Medical Research Center of the National Infectious Diseases, together with the Third People's Hospital of Shenzhen reported the first promising results. The trial conducted on 80 patients with COVID-19 indicated better results in patients treated with favipiravir than the group treated with lopinavir/ritonavir (Cai et al., 2020). Additionally, less side effects were noted in the treatment group (Cai et al., 2020; Dong et al., 2020). Pharmacokinetics of favipiravir are a cause of concern. This agent reaches significantly lower serum concentrations in critically ill patients than in healthy individuals (Irie et al., 2020). Nevertheless, favipiravir seems to be a safe therapeutic option (Pilkington et al., 2020). Other nucleotide analogs, which are under investigation for their potential effectiveness against SARS-CoV-19 include triazavirin, emtricitabine, and tenofovir (Table 1) (Lythgoe and Middleton, 2020).
Lopinavir/ritonavir
The protease inhibitor lopinavir and its booster ritonavir were verified in trial ChiCTR2000029308 on 199 patients with laboratory-confirmed COVID-19 infection. Cao et al., did not observe any benefit of lopinavir/ritonavir treatment in comparison with standard care (Cao et al., 2020). Adverse effects, such as nausea, vomiting, and hypokalemia might lead to deterioration of the clinical condition, consequently causing discontinuation of treatment (Cao et al., 2020; Liu et al., 2020; Dybul et al., 2002). Nevertheless, it is too soon to reject lopinavir/ritonavir altogether (Trial of, 2020; Osborne et al., 2020).
This drug might be by far more effective if combined with ribavirin or interferon-1β to reduce side effects and increase therapeutic potential (Xie et al., 2020). The first of aforementioned combinations has proven its effectiveness against SARS (Chu et al., 2004). The second led to better results than no antiviral treatment in an animal model (Chan et al., 2015). It is also under scrutiny in the MIRACLE trial, which seeks for an effective medication against highly fatal MERS (Dhama et al., 2020; Arabi et al., 2018b). A phase 2 trial (NCT04276688) including 127 COVID-19 patients showed superiority of triple therapy (lopinavir/ritonavir, ribavirin and interferon-β1b) over lopinavir/ritonavir. The combined therapy alleviated symptoms sooner and accelerated viral clearance (Hung et al., 2020). Most recently, the WHO has announced that it will be discontinuing its lopinavir/ritonavir arm of SOLIDARITY trial, due to no clinical benefit in terms of mortality reduction (WHO, 2020).
Since the beginning of 2020 another HIV protease inhibitor - darunavir has been in the process of verification, with early results being promising (Dong et al., 2020).
Umifenovir
Umifenovir (arbidol) has been investigated in the past as a potential drug for SARS and MERS (Lu, 2020). Its mechanism of action is similar to Imatinib, an Abelson kinase inhibitor (Abl), the anchor drug in the treatment of Chronic Myeloid Leukemia. Both of these molecules prevent virus binding to the cell membrane (Dong et al., 2020; Coleman et al., 2016).
A trial on 33 adults with laboratory proven COVID-19, who had not been invasively ventilated has reached encouraging favorable results – joint therapy of umifenovir and lopinavir/ritonavir was more efficacious than lopinavir/ritonavir only (Deng et al., 2020). Patients treated not only with protease inhibitor, but also with umifenovir became sooner SARS-CoV-19-negative (nasopharyngeal specimens) and more of them were found to improve radiologically, according to CT scans (Deng et al., 2020). As reported by Deng et al., umifenovir might decrease both the risk of SARS-CoV-19 transmission and the risk of acute respiratory distress syndrome (ARDS) (Deng et al., 2020). Other studies on the effectiveness of umifenovir showed its superiority in comparison with lopinavir/ritonavir (Zhu et al., 2020), potency to reduce COVID-19 symptoms and accelerate the recovery time (Chen et al., 2020b), but also underlined the lack of significant differences between umifenovir combined with IFN-α2b and IFN-α2b alone (Xu et al., 2020b) or lack of SARS-CoV-2 clearance acceleration (Lian et al., 2020). However, meta-analysis of 12 studies with 1052 patients reached statistical significance only in higher negative rate of PCR after 14 days of treatment (RR = 1.27, 95 % CI = 1.04–1.55). Huang et al., concluded that there is no evidence that umifenovir improves COVID-19 outcomes (Huang et al., 2020b).
TMPRSS2 inhibitor (Camostat mesylate)
SARS-CoV-2 infection depends on ACE2 and TMPRSS2 host cell factors (Fig. 3) (Zhang et al., 2020a). TMPRSS2 is believed to be involved in the process of S protein priming, a vital step in SARS-CoV-2 viral entry (Hoffmann et al., 2020a, b). This cellular protease can be blocked by the clinically proven protease inhibitor camostat mesylate. This drug is theoretically capable of preventing viral infection of the host cell. Thus, it should be considered as a potential therapeutic agent for COVID-19 infection (Lei et al., 2020). Camostat mesylate is approved in Japan for the treatment of pancreatitis. During a study on SARS-CoV-2 isolated from a patient, camostat mesylate managed to prevent the virus from entering lung cells (Hoffmann et al., 2020b). Currently, seven clinical trials (NCT4353284, NCT04455815, NCT04435015, NCT04321096, NCT04338906, NCT04374019, NCT04355052; earliest estimated completion date: December 2020) are ongoing that evaluate its clinical efficacy.
Tocilizumab
Roche Pharmaceuticals reported on a collaboration with FDA to launch a randomized, double-blind, placebo-controlled phase III clinical trial to assess the safety and efficacy of tocilizumab with standard care in hospitalized adult COVID-19 patients with severe pneumonia, compared to placebo in combination with standard care. Tocilizumab, a humanized monoclonal antibody against interleukin-6, is an immunosuppressive drug intended primarily for the treatment of rheumatoid arthritis (Cna, 2020). In China, it is expected to have a beneficial effect on coronavirus patients with severe lung damage and elevated interleukin 6 levels (Cna, 2020; Harrison, 2020).
A non-randomized, open-label clinical study involved 21 patients with severe or critical COVID-19 infection treated intravenously with tocilizumab. The clinical stage of four patients was classified as critical (19 %). All patients received standard of care, including lopinavir and methylprednisolone, as well as tocilizumab at a dose of 400 mg intravenously either in one or two doses. Eighteen patients (85.7 %) received tocilizumab once, and three patients (14.3 %) received tocilizumab twice, with the second dose being administered due to recurrent fever within 12 h of first administration. After receiving tocilizumab, all patients experienced fever resolution within 24 h, with reported improvement of clinical symptoms. In 15 out of 20 patients (75 %), there was a statistically significant decrease in oxygen demand from the fifth day after receiving tocilizumab. Additionally, in 19 patients (90.5 %), CT scan showed resolution of radiological abnormalities and mean CRP levels markedly decreased on day 5. None of the patients died during the course of the study. Nineteen patients (90.5 %) survived until discharge, and 2 remained in the hospital until the end of the follow-up period. During the clinical trial period, no significant adverse reactions related to tocilizumab treated were reported (Xu et al., 2020c).
In a retrospective cohort study on 51 COVID–19 patients, individuals with lung infiltrates and elevated inflammatory markers received a single dose of tocilizumab, if no contraindications were present. Additionally, systemic steroid, HCQ, and azithromycin were concomitantly used for the majority of patients. During the course of the study, 28 patients (55 %) received tocilizumab and 23 (45 %) did not receive. Tocilizumab cohort required more invasive ventilation (68 % vs 22 %) at baseline, as well as during the entire time of hospitalization (75 % vs 48 %). The median duration of vasopressor support and invasive mechanical ventilation in tocilizumab vs no tocilizumab cohorts was 2 days (IQR: 1.75–4.25 days) vs 5 days (IQR: 4–8 days), p = 0.039. Similar rates of hospital–acquired infections occurred in both cohorts. The authors concluded that tocilizumab administration was followed by rapid clinical improvement of COVID-19 pneumonia with ARDS (Toniati et al., 2020).
Meplazumab
H. Bian et al., have recently published the results of a clinical trial investigating the new humanized anti-CD147 monoclonal antibody - meplazumab. CD147 (extracellular matrix metalloproteinase inducer – EMMPRIN; basigin), a protein crucial for Plasmodium falciparum invasion (Crosnier et al., 2011), possibly plays a role in the interaction between spike protein of SARS-CoV-2 and lung epithelial cells (Fig. 3) (Wang et al., 2020c). In the study, 17 patients were given 10 mg meplazumab intravenously on day 1, 2 and 5, while 11 patients served as the control group. Patients treated with meplazumab, were discharged significantly faster and the severity of the disease was decreased. The time to negative viral load was also reduced. No side effects were noted during the study. Due to the small group, this drug requires further research, but the initial results are promising (Bian et al., 2020).
Other therapeutic options
The use of interferon α and β is heavily disputed (Sallard et al., 2020). Both substances are associated with serious side effects. While their administration in the early stages of the disease is associated with the expected positive effect, a delayed administration may intensify the cytokine storm, causing inflammation and consequentially worsening the patient's condition (Yuen et al., 2020).
Coronaviruses require two proteases for successful protein biosynthesis: 3CLpro and PLpro (Nascimento et al., 2020). Without them, replication and generation of virions is impossible. These proteins, like RNA polymerases, are characterized by great sequence similarity between the forms found in SARS-CoV and SARS-CoV-2 (Morse et al., 2020). The use of inhibitors to these proteases, previously tested in the context of SARS, is currently under consideration (Kumar et al., 2017; Zhou et al., 2015). Summary of research into the most important drugs is presented in Table 3 .
Table 3.
Summary of the progress in research on COVID-19 drugs. Source: drugvirus.info (Fan et al., 2020).
| Drug | Stage of research | Source |
|---|---|---|
| umifenovir | IV phase | (Deng et al., 2020) |
| lopinavir/ritonavir | III/IV phase | (Cao et al., 2020) |
| hydroxychloroquine | III phase | (Gautret et al., 2020) |
| remdesivir | III phase | (Ko et al., 2020) |
| favipiravir | II phase | (Dong et al., 2020) |
| chloroquine | research on cell lines | (Anon, 2020a; Anon, 2020b) |
| ribavirin | research on cell lines | (Dhama et al., 2020) |
| cepharanthine | research on cell lines | (Coutard et al., 2020) |
| mefloquine | research on cell lines | (Coutard et al., 2020) |
Statins are some of the most commonly prescribed drugs, especially in elderly patients. They induce ACE2 expression, which raises concerns about potentially increased risk of SARS-CoV-2 infection. Zhang et al., conducted a retrospective study in which they showed that the risk for 28-day all-cause mortality was 5.2 % and 9.4 % in the matched statin and non-statin groups of COVID-19 patients, respectively, with an adjusted hazard ratio of 0.58 (Zhang et al., 2020b).
In March 2020, the pharmaceutical company PharmaMar announced that Aplidine (Plitidepsin), a medicine commonly used to treat multiple myeloma, has antiviral activity (Pharmamar, 2020). In vitro studies showed that Plitidepsin affects EF1A (eukaryotic translation elongation factor 1 alpha 1), which is key to multiplication and spread of the virus (Pharmamar, 2020). The antiviral activity of plitidepsin was initially analyzed in a human hepatoma cell line infected with the HCoV-229E-GFP virus, which is similar to SARS-CoV-2. The preliminary results are promising, but a multicenter, randomized proof of concept (Phase 1) clinical trial is ongoing and patients are currently being recruited (NCT04382066) (PharmaMar, 2020).
Scientists are beginning to consider utilizing immunomodulatory therapies to treat COVID-19 infection (Lythgoe and Middleton, 2020). The use of drugs that increase the inflammatory response and reactivity of leukocytes can, on one hand, aid in combating the infection, but, on the other hand, could expose the body to the negative effects due to exacerbation of the inflammatory response. There are numerous clinical trials investigating drugs such as anti-PD1 antibodies, recombinant IL-2, recombinant human granulocyte colony-stimulating factor (rhG-CSF), all of which are summarized in Table 1. Previously used in cancer therapy, they can alter the inflammatory response, thereby reducing the negative effects of infection such as pulmonary fibrosis or sepsis.
Concluding remarks and future perspectives
In addition to the drugs discussed in the current review, many antiviral drugs have been explored for COVID-19 treatment for several months, without any positive effect. Neuraminidase inhibitors, known from influenza therapy: baloxavir marboxil, oseltamivir, paramivir, and zanamivir were used, especially in the first weeks of the epidemic (Lythgoe and Middleton, 2020; Li et al., 2020). Other drugs tested to date include thymidine kinase inhibitors (acyclovir and ganciclovir), translation-inhibiting mRNA encapsulation inhibitor - ribavirin, nafamostat - successful in the treatment of MERS, nitazoxanide - used to control helminthiasis and currently tested for viability in viral infections, another nucleotide analog – penciclovir, as well as drugs known from HCV therapy (azvudine, danoprevir/ritonavir, sofosbuvir/daclatasvir, and sofosbuvir/ledipasvir) (Wang et al., 2020a; Dhama et al., 2020; Lythgoe and Middleton, 2020). None of the above drugs is currently recommended for the treatment and support of treatment in SARS-CoV-2 infection (Li et al., 2020). Due to the lack of an effective COVID-19 therapeutic protocol, prevention of infection is pivotal.
In addition to isolating the sources of infection and following thorough hygienic measures, it seems essential to focus on the development of a vaccine. Effective inactivated or recombinant vaccines, possibly developed in one of currently conducted trials (NCT04283461, NCT04299724 or NCT04313127), that could also be used in immunocompromised individuals, thanks to the advancement of biotechnology, are likely to be achieved much sooner than the registration of the first SARS-CoV-2 drugs. The insights gained by researchers during the development of vaccines against MERS and SARS might prove invaluable. All of these ideas are very compelling, but more research is needed, especially on large, randomized and controlled trials to confirm the efficacy of agents in the combat against the new coronavirus.
The COVID-19 pandemic is an unprecedented health, economic and humanitarian crisis that has major and ongoing impact on people in every country around the world. It is also an example of unprecedented cooperation of scientists from every country united to find a cure and vaccine against one single pathogen, but also searching for future solutions. Apart from finding efficacious treatments against COVID-19, it is necessary to accommodate the needs of patients suffering from the complications of COVID-19, including pulmonary fibrosis (Lechowicz et al., 2020), central and peripheral neuropathies, delirium (Kotfis et al., 2020a, b), depression and many other complications. Further research towards in-depth understanding of the pathological mechanisms of SARS-CoV-2 in humans is necessary to develop novel therapeutic drugs for COVID-19.
The complex aspects of SARS-CoV-2 infection mandates collaboration of scientists from different disciplines including basic, clinical and engineering fields to enhance the probability of success against this life-threatening pandemic (Moradian et al., 2020). As viral infection hijacks fundamental mechanisms of mammalian cell physiology (Alavian et al., 2011; Yeganeh et al., 2015), besides potential vaccine development strategy, combination of antiviral treatment in the presence of targeting these pathways could have the highest rate of success on overcoming this COVID-19 pandemic. Among these mechanisms, autophagy and unfolded protein response (UPR) received the attention of many research groups and important commentary papers and suggestions have been recently published (Shojaei et al., 2020a; Bonam et al., 2020; Vallamkondu et al., 2020; Sureda et al., 2020). SARS-CoV-2 infection probably involves autophagy pathway like many other respiratory viral infections (Yeganeh et al., 2018, 2013). Some useful adjuvant therapy strategies like chloroquine or statins has common effects including regulation of cytokine storm and inflammation and inhibition of autophagy flux (Shojaei et al., 2020a; Bonam et al., 2020; Shojaei et al., 2020b). On the other hand, SARS-CoV-12 infection probably induces UPR in the infected cells as it significantly increases protein biosynthesis in the infected cells like other coronaviruses (Sureda et al., 2020). UPR is involved in regulation of autophagy and in the cellular secretome (Ghavami et al., 2014; Logue et al., 2018). Therefore, simultaneous targeting of autophagy/UPR pathway using FDA-approved compounds including chloroquine, statins, or drugs that are on their Phase-I or Phase-II clinical trials (MKC8866 to target IRE1 RNase activity) and antiviral therapy regimens could be an ideal strategy to control COVID-19 pandemic and help high risk patients to increase survival. This strategy will also potentially decrease significant amount of cost for taking care of COVID-19 patients in ICU units and decrease the demand for ventilators. The COVID-19 pandemic is likely to be controlled in the future with close collaboration of different science disciplines.
The WHO SOLIDARITY trial is ongoing and the results of this trial are anxiously expected by both researchers and clinicians. This large international multicenter study with thousands of COVID-19 patients will have the statistic power to finally prove or reject many hypotheses about COVID-19 therapy (Kupferschmidt and Cohen, 2020). This SOLIDARITY trial will test remdesivir, lopinavir/ritonavir, lopinavir/ritonavir combined with interferon-β, HCQ/CQ. As of 5th of July 2020, two of those arms have been discontinued – lopinavir/ritonavir and HCQ/CQ, due to the lack of benefit (WHO, 2020).
References
- Adar Y., et al. Imidazoacridinone-dependent lysosomal photodestruction: a pharmacological Trojan horse approach to eradicate multidrug-resistant cancers. Cell Death Dis. 2012;3:e293. doi: 10.1038/cddis.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M.L., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian S.M., et al. Virus-triggered autophagy in viral hepatitis - possible novel strategies for drug development. J. Viral Hepat. 2011;18(12):821–830. doi: 10.1111/j.1365-2893.2011.01530.x. [DOI] [PubMed] [Google Scholar]
- Alexandre J., et al. Drugs acting on renin angiotensin system and use in ill patients with COVID-19. Therapie. 2020 doi: 10.1016/j.therap.2020.05.009. p. S0040-5957(20)30099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Heart A. 2020. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon 2020 https://www.thecardiologyadvisor.com/home/topics/practice-management/fda-studies-underway-to-evaluate-chloroquine-for-covid-19/.
- Anon 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html.
- Arabi Y.M., et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Arabi Y.M., et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S.A., et al. Teicoplanin: an alternative drug for the treatment of coronavirus COVID-19? Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105944. p. 105944-105944. The antibiotic teicoplanin that displays activity against other coronaviruses retained its activity against SARS-CoV-2 in vitro. This study presents the principles, which suggest that this agent has a potential to succeed in clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. 2020:543–545. doi: 10.1042/CS20200163. (c) 2020 The Author(s). England. [DOI] [PubMed] [Google Scholar]
- Beigel J.H., et al. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2022236. p. NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- Bhandari S., et al. Characteristics, treatment outcomes and role of hydroxychloroquine among 522 COVID-19 hospitalized patients in Jaipur City: an epidemio-clinical study. J. Assoc. Physicians India. 2020;68(6):13–19. [PubMed] [Google Scholar]
- Bian H., et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. 2020 p. 2020.03.21.20040691. Novel study on the use of meplazumab in COVID in 17 Chinese patients suggests that blocking the infection of SARS-CoV-2 might be a potent therapeutic approach. It should be noted that host-cell-expressed CD147 could bind the pike protein of SARS-CoV-2 which is involved in host cell invasion. Meplazumab, a humanized an antibody against CD147, could block the infection of SARS-CoV-2. [Google Scholar]
- Biot C., et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 2006;49(9):2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- Bonam S.R., et al. Autophagy as an emerging target for COVID-19: lessons from an old friend, chloroquine. Autophagy. 2020:1–7. doi: 10.1080/15548627.2020.1779467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J., et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., et al. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio F.P., Siani A. Nutrition, metabolism, and cardiovascular diseases : NMCD; 2020. Covid-19 And Cardiovascular Risk: Susceptibility to Infection to SARS-CoV-2, Severity and Prognosis of Covid-19 and Blockade of the Renin-angiotensin-aldosterone System. An Evidence-based Viewpoint. p. S0939-4753(20)30206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., et al. Treatment with Lopinavir/Ritonavir or Interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., et al. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 p. 2020.03.22.20040758. [Google Scholar]
- Chen W., et al. A study on clinical effect of Arbidol combined with adjuvant therapy on COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T.N.H.C.O.T.P.S.R.O. China . drugvirus.info; 2020. Guidelines for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced Pneumonia (6 Th Edition) [Google Scholar]
- Chu C.M., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.S., et al. Comparison of the cumulative efficacy and safety of chloroquine, artesunate, and chloroquine-primaquine in Plasmodium vivax malaria. Clin. Infect. Dis. 2018;67(10):1543–1549. doi: 10.1093/cid/ciy319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill D., et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. HIV Med. 2016;17(S4):s2–s104. doi: 10.1111/hiv.12426. [DOI] [PubMed] [Google Scholar]
- Ciak J., Hahn F.E. Chloroquine: mode of action. Science. 1966;151(3708):347–349. doi: 10.1126/science.151.3708.347. [DOI] [PubMed] [Google Scholar]
- Cna . 2020. China Approves Use of Roche arthritis Drug for COVID-19 Patients. [Google Scholar]
- Coleman C.M., et al. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and middle east respiratory syndrome coronavirus fusion. J. Virol. 2016;90(19):8924–8933. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compassionate Use of Remdesivir in Covid-19 . 2020. New England Journal of Medicine. 382(25): p. e101. [Google Scholar]
- Cortegiani A., et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C., et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480(7378):534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019:a retrospective cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin. Immunother. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J. Travel Med. 2020 doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. Excellent review providing insight into the use of drugs, in the early stages of the pandemic, that rely on inhibition of receptors that are utilized by the virus to enter the host cell (ACE2, TMPRSS2, and CD147) [DOI] [PubMed] [Google Scholar]
- Dybul M., et al. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm. Rep. 2002;51(Rr-7):1–55. [PubMed] [Google Scholar]
- Effects of enalapril on mortality in severe congestive heart failure Results of the cooperative north scandinavian enalapril survival study (CONSENSUS) N. Engl. J. Med. 1987;316(23):1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- Fan H.H., et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus (2019-nCoV) related coronavirus model. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fosbøl E.L., et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020 doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:1–24. doi: 10.1016/j.ijantimicag.2020.105949. One of the first clinical trials in the world that evaluated HCQ in the treatment of COVID by limiting the release of proinflammatory cytokines and increasing the endosomal and lysosomal pH. Its combination with azithromycin may become one of pivotal therapeutic strategies. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ghavami S., et al. Airway mesenchymal cell death by mevalonate cascade inhibition: integration of autophagy, unfolded protein response and apoptosis focusing on Bcl2 family proteins. Biochim. Biophys. Acta. 2014;1843(7):1259–1271. doi: 10.1016/j.bbamcr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Gommans D.H.F., et al. Rationale and design of the PRAETORIAN-COVID trial: a double-blind, placebo-controlled randomized clinical trial with valsartan for PRevention of Acute rEspiraTORy dIstress syndrome in hospitAlized patieNts with SARS-COV-2 Infection Disease. Am. Heart J. 2020;226:60–68. doi: 10.1016/j.ahj.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses - a statement of the Coronavirus Study Group. bioRxiv. 2020 [Google Scholar]
- Grein J., et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. This paper reports on the results of one of the first clinical trials assessing the use of remdesivir in a clinical setting, conducted on 61 patients, with median follow up of 18 days. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. 2020. Coronavirus Puts Drug Repurposing on the Fast Track. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020 p. 2020.01.31.929042. [Google Scholar]
- Hoffmann M., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., et al. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.26256. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., et al. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. 2018;150:202–216. doi: 10.1016/j.antiviral.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.-N., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet (London, England) 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie K., et al. Pharmacokinetics of Favipiravir in critically ill patients with COVID-19. Clin. Transl. Sci. 2020 doi: 10.1111/cts.12827. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P.C., Stevens S.K., Deval J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir. Chem. Chemother. 2018;26 doi: 10.1177/2040206618764483. p. 2040206618764483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keam S.J. Telbivudine. Drugs. 2007;67(13):1917–1929. doi: 10.2165/00003495-200767130-00011. [DOI] [PubMed] [Google Scholar]
- Kim Y., et al. Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathog. 2016;12(3):e1005531. doi: 10.1371/journal.ppat.1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W.C., et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020:105933. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., et al. COVID-19: what do we need to know about ICU delirium during the SARS-CoV-2 pandemic? Anaesthesiol. Intensive Ther. 2020;52(2):132–138. doi: 10.5114/ait.2020.95164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care. 2020;24(1):176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kumar V., et al. Identification and evaluation of potent Middle East respiratory syndrome coronavirus (MERS-CoV) 3CL(Pro) inhibitors. Antiviral Res. 2017;141:101–106. doi: 10.1016/j.antiviral.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt K., Cohen J. WHO launches global megatrial of the four most promising coronavirus treatments. Science. 2020;22 [Google Scholar]
- Lafyatis R., York M., Marshak-Rothstein A. 2006. Antimalarial Agents: Closing the Gate on Toll-like Receptors? pp. 3068–3070. [DOI] [PubMed] [Google Scholar]
- Lechowicz K., et al. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J. Clin. Med. 2020;9(6):1917. doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C., et al. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig. bioRxiv. 2020 p. 2020.02.01.929976. [Google Scholar]
- Li W., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., et al. [Potential antiviral therapeutics for 2019 Novel Coronavirus] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):170–172. doi: 10.3760/cma.j.issn.1001-0939.2020.03.004. [DOI] [PubMed] [Google Scholar]
- Lian N., et al. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020;26(7):917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue S.E., et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 2018;9(1):3267. doi: 10.1038/s41467-018-05763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Luo A., Jiang X., Ren H. Lamivudine plus tenofovir combination therapy versus lamivudine monotherapy for HBV/HIV coinfection: a meta-analysis. Virol. J. 2018;15(1) doi: 10.1186/s12985-018-1050-3. p. 139-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 2020 doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M.F., et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123(6):1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- Moradian N., et al. The urgent need for integrated science to fight COVID-19 pandemic and beyond. J. Transl. Med. 2020;18(1):205. doi: 10.1186/s12967-020-02364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales D.R., et al. Renin-angiotensin system blockers and susceptibility to COVID-19: a multinational open science cohort study. medRxiv : the preprint server for health sciences. 2020 p. 2020.06.11.20125849. [Google Scholar]
- Morse J.S., et al. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento J.A.C., Junior, et al. SARS, MERS and SARS-CoV-2 (COVID-19) treatment: a patent review. Expert Opin. Ther. Pat. 2020:1–13. doi: 10.1080/13543776.2020.1772231. [DOI] [PubMed] [Google Scholar]
- Nowell J., Quaranta V. Chloroquine affects biosynthesis of Ia molecules by inhibiting dissociation of invariant (gamma) chains from alpha-beta dimers in B cells. J. Exp. Med. 1985;162(4):1371–1376. doi: 10.1084/jem.162.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. 2018. Guidelines for the Care and Treatment of Persons Diagnosed With Chronic Hepatitis C Virus Infection. [PubMed] [Google Scholar]
- Osborne V., et al. Lopinavir-ritonavir in the treatment of COVID-19: a dynamic systematic benefit-risk assessment. Drug Saf. 2020:1–13. doi: 10.1007/s40264-020-00966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmamar . Madrit; 2020. PharmaMar Reports Positive Results for Aplidin ® Against Coronavirus HCoV-229E. p. 1-1. [Google Scholar]
- PharmaMar . 2020. Proof of Concept Study to Evaluate the Safety Profile of Plitidepsin in Patients With COVID-19 (APLICOV-PC) [Google Scholar]
- Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir–a potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020;6(2):45. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski P., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- Rachlis A.R. Zidovudine (Retrovir) update. CMAJ. 1990;143(11):1177–1185. [PMC free article] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., et al. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J.S., et al. High- versus low-dose angiotensin converting enzyme inhibitor therapy in the treatment of heart failure: an economic analysis of the Assessment of Treatment with Lisinopril and Survival (ATLAS) trial. Am. J. Manag. Care. 2003;9(6):417–424. [PubMed] [Google Scholar]
- Sheahan T.P., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei S., et al. Autophagy and SARS-CoV-2 infection: apossible smart targeting of the autophagy pathway. Virulence. 2020;11(1):805–810. doi: 10.1080/21505594.2020.1780088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei S., et al. Simvastatin increases temozolomide-induced cell death by targeting the fusion of autophagosomes and lysosomes. FEBS J. 2020;287(5):1005–1034. doi: 10.1111/febs.15069. [DOI] [PubMed] [Google Scholar]
- Sriram K., Insel P.A. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.L., et al. [Inhibitors of RAS might Be a good choice for the therapy of COVID-19 pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):219–222. doi: 10.3760/cma.j.issn.1001-0939.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Kwak Sung-sun Physicians work out treatment guidelines for coronavirus. [cited 2020 27.03]; http://www.koreabiomed.com/news/articleView.html?idxno=7428.
- Sureda A., et al. Endoplasmic reticulum as a potential therapeutic target for covid-19 infection management? Eur. J. Pharmacol. 2020;882:173288. doi: 10.1016/j.ejphar.2020.173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1849. p. m1849-m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102568. p. 102568-102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trial of A. Lopinavir–Ritonavir in Covid-19. N. Engl. J. Med. 2020;382(21):e68. doi: 10.1056/NEJMc2008043. [DOI] [PubMed] [Google Scholar]
- US National Library of Medicine . 2020. ClinicalTrials.gov. [Google Scholar]
- Vallamkondu J., et al. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim Biophys Acta Mol Basis Dis. 2020:165889. doi: 10.1016/j.bbadis.2020.165889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., et al. Cell Res.; England: 2020. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-nCoV) in Vitro; pp. 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1038/s41392-020-00426-x. p. 2020.03.14.988345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.K., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston S., Frieman M.B. COVID-19: knowns, unknowns, and questions. mSphere. 2020;5(2) doi: 10.1128/mSphere.00203-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who . 2020. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected; pp. 1–21. [Google Scholar]
- WHO . 2020. WHO Discontinues Hydroxychloroquine and lopinavir/ritonavir Treatment Arms for COVID-19.https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 [cited 2020 5th of July 2020]; Available from: [Google Scholar]
- Wise J. Covid-19: remdesivir is recommended for authorisation by European Medicines Agency. BMJ. 2020;369:m2610. doi: 10.1136/bmj.m2610. [DOI] [PubMed] [Google Scholar]
- World Health O. 2020. Clinical Management of COVID-19. [Google Scholar]
- Wozniacka A., et al. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus. 2006;15(5):268–275. doi: 10.1191/0961203306lu2299oa. [DOI] [PubMed] [Google Scholar]
- Xia S., et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019;5(4) doi: 10.1126/sciadv.aav4580. p. eaav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., et al. Combination antiviral therapy with lopinavir/ritonavir. arbidol and interferon-α1b for COVID-19. 2020 doi: 10.3851/IMP3362. [DOI] [PubMed] [Google Scholar]
- Xu X., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020:457–460. doi: 10.1007/s11427-020-1637-5. China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., et al. Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microbes Infect. 2020;22(4-5):200–205. doi: 10.1016/j.micinf.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020;202003(00026):v1. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh B., et al. Asthma and influenza virus infection:focusing on cell death and stress pathways in influenza virus replication. Iran. J. Allergy Asthma Immunol. 2013;12(1):1–17. [PubMed] [Google Scholar]
- Yeganeh B., et al. Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(3):L270–86. doi: 10.1152/ajplung.00011.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh B., et al. Autophagy activation is required for influenza A virus-induced apoptosis and replication. Biochim Biophys Acta Mol Cell Res. 2018;1865(2):364–378. doi: 10.1016/j.bbamcr.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Yuen K.S., et al. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-J., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.06.015. p. S1550-4131(20)30316-30318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitomirsky B., Assaraf Y.G. Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget. 2015;6(2):1143–1156. doi: 10.18632/oncotarget.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitomirsky B., Assaraf Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist. Updat. 2016;24:23–33. doi: 10.1016/j.drup.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Zhou Y., et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., et al. 2020. Discovery of a Novel Coronavirus Associated With the Recent Pneumonia Outbreak in Humans and Its Potential Bat Origin. [Google Scholar]
- Zhu Z., et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., et al. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]