To the Editor:
Several reports have suggested ultrastructural evidence of direct infection of different types of kidney cells by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in postmortem analysis and kidney biopsy specimens in patients with proven viral reverse-transcriptase polymerase chain reaction (RT-PCR) from nasopharyngeal smears. Detection of supposed viral particles by transmission electron microscopy (TEM) was used as sufficient evidence for viral invasion of renal tissue but data regarding detection of viral RNA or other valuable methods for viral detection of kidney specimens were missing.1S1 Likewise, an additional study proposed direct SARS-CoV-2 infection of endothelial cells in multiple organs of patients with coronavirus disease 2019 (COVID-19) on the basis of TEM solely.2 The presented particles in the aforementioned studies exhibit a diameter of 50 to 150 nm and crown-like electron-dense coat, so they may appear similar to Coronavirus but also similar to ubiquitous coated vesicles, such as clathrin-coated vesicles, or COPI- or COPII-coated vesicles. Moreover, clusters of viral particles inside the vacuole might resemble multivesicular bodies (MVBs), which are regular structures of the endocytic pathway.
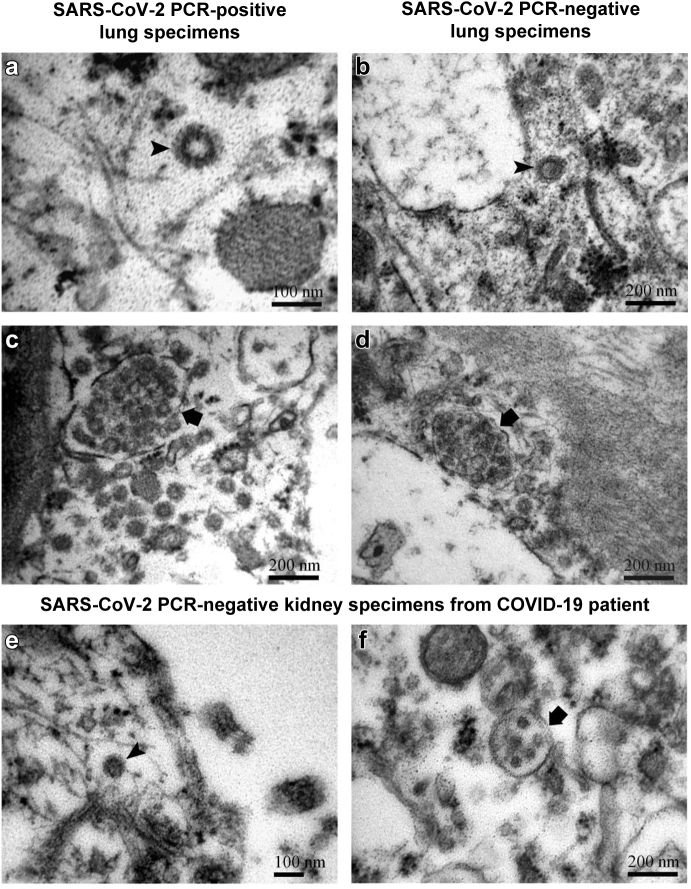
Herein, to detect direct invasion of SARS-CoV-2 in the kidney, we performed RT-PCR on fresh postmortem lung and kidney specimens of 4 patients with COVID-19. In all 4 patients, viral RNA was confirmed in all lung samples, but was negative in all kidney samples. However, ultrastructural examination revealed intracellular vesicular structures of similar size and morphology in lung with proven viral RNA and in kidney with no viral RNA. In lung specimens with proven viral RNA, we observed many structures that could be either viral particles with typical corona or coated vesicles with electron-dense protein coat (Figure 1a). In addition, in the same SARS-CoV-2–positive lung specimens, we found vacuoles that could be either membrane-bound clusters of viral particles or MVBs with intralumenal vesicles inside (Figure 1c). On the other hand, ultrastructural examination of the lung specimens of 2 patients without SARS-CoV-2 (1 autopsy specimen of lung with negative RT-PCR for viral RNA and 1 biopsy specimen of lung before COVID era) revealed the same structures, resembling viral particles, coated vesicles, or MVBs, as in a specimen with positive SARS-CoV-2 (Figure 1b and d).
Figure 1.
Individual vesicle with electron-dense coat (arrowhead) located freely in the cytosol of endothelial cell in lung with positive reverse-transcriptase polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA (a) and in lung with negative RT-PCR for SARS-CoV-2 RNA (b). Note similar morphology of the 2 structures in images (a) and (b), which could be virus or coated vesicle. In view of the RT-PCR results, the observed structures might be virus in image (a) but not in image (b). Vacuole with many small vesicles inside the limiting membrane (arrow) in the cytosol of endothelial cell in lung with positive RT-PCR for SARS-CoV-2 RNA (c) and in lung with negative RT-PCR for SARS-CoV-2 RNA (d). Note again similar morphology of the 2 structures in images (c) and (d), which could be a cluster of viral particles or multivesicular bodies (MVBs) with intralumenal vesicles inside. In view of the RT-PCR results, the observed structures might be a cluster of viral particles in (c) but not in (d). (e,f) Structures resembling virions, coated vesicles or MVBs were observed in the cytosol of kidney podocytes in a SARS-CoV-2–positive patient but with negative RT-PCR for SARS-CoV-2 RNA. In view of the RT-PCR results, the presented structures are not viruses but ubiquitous coated vesicles and MVBs.
TEM analysis of postmortem kidney specimens of patients with COVID-19 revealed numerous individual vesicles and clusters of vesicles in different types of kidney cells, despite negative RT-PCR for SARS-CoV-2 RNA (Figure 1e and f). We additionally performed semiquantitative analysis of these intracellular structures in different types of renal cells from 20 preimplantation donor kidney biopsies, before and during the outbreak of SARS-CoV-2, with negative RT-PCR for SARS-CoV-2 RNA to demonstrate that these cell structures are numerous and ubiquitous in various types of cells (Table 1).
Table 1.
Semiquantitative analysis of multivesicular bodies and clathrin-coated vesicles in 20 preimplantation donor kidney biopsies before (2018) and during COVID-19 era (2020)
| No. | Year of biopsy | Multivesicular bodies |
Clathrin-coated vesicles |
||||
|---|---|---|---|---|---|---|---|
| Podocytes | Endothelial cells | Proximal tubular cells | Podocytes | Endothelial cells | Proximal tubular cells | ||
| 1 | 2020 | + | + | + | ++ | + | ++ |
| 2 | 2020 | + | – | – | ++ | ++ | + |
| 3 | 2020 | ++ | + | + | + | + | + |
| 4 | 2020 | + | + | + | + | + | ++ |
| 5 | 2020 | + | + | – | ++ | + | ++ |
| 6 | 2020 | + | – | + | + | + | ++ |
| 7 | 2020 | + | + | + | + | + | ++ |
| 8 | 2020 | + | + | – | + | + | ++ |
| 9 | 2020 | + | + | – | + | + | ++ |
| 10 | 2020 | + | + | – | + | + | ++ |
| 11 | 2018 | + | + | – | ++ | + | ++ |
| 12 | 2018 | + | + | – | + | + | + |
| 13 | 2018 | + | + | – | + | + | ++ |
| 14 | 2018 | + | + | – | ++ | + | + |
| 15 | 2018 | + | + | – | + | + | + |
| 16 | 2018 | + | – | + | + | + | ++ |
| 17 | 2018 | + | + | – | + | + | ++ |
| 18 | 2018 | + | + | – | + | + | ++ |
| 19 | 2018 | + | – | – | + | + | + |
| 20 | 2018 | + | + | + | + | + | + |
–, no structures per cell; +, 1 to 5 structures per cell; ++, more than 5 structures per cell.
Specimens contained at least 1 glomerulus and 20 proximal tubules. Five coincidentally selected podocytes, 5 endothelial cells, and 5 proximal tubular cells were examined.
There has been increasing evidence to indicate that coated vesicles and MVBs may mimic viral particles.3S2S3 However, it is known that the budding of enveloped viruses (to which SARS-CoV-2 belongs) from the plasma membrane, or the limiting membrane of the endosome, resembles the formation of intralumenal vesicles inside MVBs. Moreover, the 2 processes share some components of the same protein machinery.4S4 Indeed, we detected intralumenal vesicles budding from discontinued limiting membrane of the vacuole in lung endothelial cells with positive SARS-CoV-2 RNA (Figure 1a). This finding strongly suggests that this structure is not MVBs but rather a cluster of viral particles with some of budding virions. Indisputable evidence of virions would thus be provided only by immunoelectron microscopy. In addition, coated vesicles might also faintly resemble viral particles, but it is necessary to be cautious about the intracellular location of coated vesicles. Specifically, coated vesicles are transient and are therefore mostly found in close vicinity to the membrane from which they bud, because they shed their coat within seconds after their formation.
To conclude, although TEM may serve as a useful diagnostic method for the detection of viral infection, caution should be exercised when confirmation of viral invasion relies only on TEM. Additional convincing methods, including immunoelectron microscopy, immunohistochemistry, and viral genetic material analysis, are needed for indisputable proof of viral invasion in organs.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller S.E., Brealey J.K. Visualization of putative coronavirus in kidney. Kidney Int. 2020;98:231–232. doi: 10.1016/j.kint.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth A.M., Fang Y., Fallon J.K. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.