Abstract
Background
Cystic fibrosis (CF) pulmonary exacerbations can be serious respiratory events and reduction in exacerbation rate or risk are important efficacy endpoints for CF therapeutic trials. Variability in exacerbation diagnoses and treatment have led drug developers to employ “objective” exacerbation definitions combining antimicrobial treatment (AT) and the presence of ≥4 of 12 respiratory criteria (first published by Fuchs et al. [NEJM 1994;331(10):637–42]). Assumptions underlying this approach have yet to be formally evaluated.
Methods
Respiratory events (RE) observed during a 48-week trial of ataluren (NCT02139306), a read-through agent for premature nonsense codons, were compared across six exacerbation definitions: any AT, intravenous AT (IVAT), ≥4 Fuchs criteria present, AT plus ≥4 Fuchs criteria, IVAT plus ≥4 Fuchs criteria, and investigator assessment. Fuchs definitions were evaluated by assessing missingness of individual criteria and associations between criteria presence and clinician exacerbation assessment.
Results
Among 751 RE, more than one third had ≥4 Fuchs criteria present but were not assessed as exacerbations by investigators. Data for ≥1 and for 4 Fuchs criteria, respectively, were missing for ~ 90% and >30% of RE. Only 6/12 Fuchs criteria were present more often when investigators assessed RE as exacerbations than when they did not.
Conclusions
“Objective” definitions have shortcomings inconsistent with their purpose of optimizing exacerbation capture in clinical trials : 1) they capture events clinicians do not consider exacerbations, 2) are prone to data missingness which can bias the likelihood of meeting the definition, and 3) employ criteria that are not associated with investigator assessment of exacerbation.
1. Background
People with cystic fibrosis (CF) commonly experience acute respiratory events (RE) characterized by worsening of signs and symptoms of airway infection [1]. When RE onset is communicated to a clinician, he or she must decide whether to intervene, at times without the benefit of physical examination or diagnostic testing. RE are frequently managed with antimicrobials, increased airway clearance, and nutritional and psychosocial support [2,3], and are commonly referred to as “pulmonary exacerbations.”[1,4] CF pulmonary exacerbations have been associated with decreased quality of life [5,6], increased resource utilization [7,8], and increased mortality risk [9,10]. For these reasons, reduction in exacerbation rate and/or risk have become important clinical efficacy endpoints for trials of chronic CF therapies [11].
Clinical trial design requires prospective agreement as to what will constitute a given clinical event, including a pulmonary exacerbation. Exacerbation definitions can rely on investigator assessment or use pre-defined criteria indicating presentation of a threshold set of clinical signs and/or symptoms. In CF, there is no consensus as to what clinical presentations define a pulmonary exacerbation [12], with clinicians differing with respect to both which RE presentations warrant intervention, as well as what those interventions might be [13]. Further, assessments of CF exacerbation have not been static, as clinical thresholds for treatment have changed over time [14]. For these reasons, clinical investigators and regulators have sought more “objective” indicators of pulmonary exacerbation.
Some sort of clinical presentation should define the start of an exacerbation, however assigning an exact date of initiation is difficult in the absence of consensus as to what clinical presentations constitute an exacerbation. Because antimicrobial treatment start- and stop-dates are more commonly captured in medical records and a clinician's decision to treat with antimicrobials suggests a change in respiratory status justifying intervention, many prospective exacerbation definitions have included a requirement for antimicrobial treatment. This approach provides a record of exacerbation start time as well as confirmation that an RE has met a minimum severity threshold warranting treatment [11]. Because the nature of RE intervention (e.g., outpatient versus inpatient, treatment with IV versus oral antimicrobials) is to some degree indicative of presentation severity, CF clinical trial exacerbation definitions have at times also stipulated intervention types, such as a requirement that IV (as opposed to any) antimicrobials be administered for an event [15,16] or that an event be treated in hospital [16].
In addition to these treatment-based criteria, drug developers and regulators have gravitated towards pre-defined objective criteria-based methods, rather than clinician assessment-based methods, for identifying pulmonary exacerbations. This approach has been driven by a desire to avoid variability in clinician assessment [13] and is intended to ensure a minimum threshold of clinical importance when a RE is counted as an exacerbation. For instance, in the dornase alfa Phase 3 study [15], a pulmonary exacerbation was defined as a RE treated with IV antimicrobials in which at least 4 of 12 specific sign and symptom criteria were present (Table 1 ). Following publication by Fuchs and colleagues [15], these have become referred to as the Fuchs criteria, and the exacerbation definition used in the study as the (original) Fuchs definition.
Table 1.
Fuchs sign and symptom criteria [15].
| • Increased cough |
| • Change in sputum |
| • New or increased hemoptysis |
| • Increased dyspnea |
| • Malaise, fatigue, or lethargy |
| • Temperature above 38 °C |
| • Anorexia or weight loss |
| • Sinus pain or tenderness |
| • Change in sinus discharge |
| • Change in physical examination of the chest |
| • Decrease in pulmonary function by 10% percent or more from a previously recorded value |
| • Radiographic changes indicative of pulmonary infection |
The original Fuchs definition provided regulators assurance that only IV antimicrobial treatments administered for RE (as opposed to those administered for bowel surgeries, etc.) were counted as pulmonary exacerbations. Because the probability of IV antimicrobial treatment for exacerbation is age- and lung function-dependent [4], while the overall rate of RE assessed by clinicians as exacerbations is not [17], exacerbation definitions have subsequently “expanded” to include any antimicrobial treatments. Meanwhile, inclusion of the Fuchs criteria in exacerbation definitions has become entrenched; an expanded Fuchs definition substituting any antimicrobial treatment for IV antimicrobial treatment was utilized to study rate/risk of exacerbation in recent cystic fibrosis transmembrane conductance regulator (CFTR) modulator clinical trials [18]. Regulators have even requested use of an exacerbation definition based solely on the presence of 4 or more Fuchs criteria irrespective of an investigator's decision to treat with antimicrobials when counting exacerbations in an inhaled antimicrobial program [19] and a CFTR modulator program [20]. Importantly, CF study protocols that have included criteria-based exacerbation definitions have not dictated that investigators managing RE complete all examinations and diagnostic tests necessary for a complete accounting of definition criteria. Rather, criteria accounting has been based on convenience: a given criterion may or may not have been met if collected and if not collected was not met.
Criteria-based CF exacerbation definitions include assumptions of standardization and clinical relevance that have never been formally tested. It has been assumed that adding respiratory sign and symptom requirements increases an exacerbation definition's stringency and promotes standardization across clinicians: in other words, RE meeting criteria-based exacerbation definitions will constitute a subset of events assessed by investigators as exacerbations, creating a more consistent and standardized set of exacerbation events. There are also assumptions that all criteria included in definitions are evaluated for a given RE and that criteria positivity is consistently indicative of clinical importance.
A 48-week Phase 3, randomized, placebo-controlled trial of the premature nonsense codon ribosomal read-through agent ataluren (NCT02139306) in 279 subjects with CF collected Fuchs criteria, antimicrobial treatments, and investigator assessments of each recorded RE [21]. The study was conducted between August 2014 and November 2016 in subjects at least 6 years old and with percent predicted forced expiratory volume in 1 s (ppFEV1) between 40 and 90 at 75 sites in 16 countries.
In this communication, we describe retrospective analyses of these ataluren trial data and evaluate the validity of assumptions underlying definition-based exacerbation endpoints in CF clinical trials. Differences in exacerbation rates defined by investigator assessments, decision to treat with antimicrobials, and previous criteria-based exacerbation definitions are described. In addition, completeness of Fuchs criteria collection is assessed, as are associations between Fuchs criteria positivity and investigator assessment of RE as a pulmonary exacerbation.
2. Methods
RE occurring among study subjects were captured on dedicated case report forms (online supplement). For each RE, investigators were asked to record event start and finish dates, symptoms, whether a physical exam was performed (including exam findings when performed), whether any diagnostic tests were conducted (with test results when conducted), whether antimicrobials were administered, whether treatment included IV antimicrobials, and presence/absence of each of the 12 Fuchs criteria (Table 1). In addition, investigators were asked to choose a diagnosis for each RE from among 13 possibilities, which in addition to “Pulmonary Exacerbation of CF,” included “No Specific Diagnosis” and “Other”, with an accompanying open text field.
In the current analysis, RE were characterized with respect to whether they met or did not meet each of 6 pulmonary exacerbation definitions: (1) Investigator Assessment (if the investigator's chosen diagnosis was that an RE was a pulmonary exacerbation, (2) Original Fuchs (if the RE presented with ≥4 Fuchs criteria and was treated with IV antimicrobials), (3) Expanded Fuchs (if the RE presented with ≥4 Fuchs criteria and was treated with any antimicrobial), (4) Modified Fuchs (if the RE presented with ≥4 Fuchs criteria), (5) IV Antimicrobial Treatment (if the RE was treated with an IV antimicrobial), and (6) Any Antimicrobial Treatment (if the RE was treated with any antimicrobial). A given RE could meet as few as zero to as many as all 6 exacerbation definitions. Elapsed times from first study visit to first exacerbation per study subject were determined separately for each exacerbation definition. Elapsed time for subjects who did not experience an exacerbation based on a given definition were censored at their last study visit. Exacerbation incidence rates derived using different definitions were compared, as were survival curves for time to first exacerbation. Area-proportional diagrams of exacerbation definition overlap were generated [22]. Missingness of individual Fuchs criteria were assessed and Fuchs criteria prevalence rates were compared across RE that had been assessed versus not assessed as pulmonary exacerbations. Univariate prevalence comparisons were made by studying juxtaposition of point estimate confidence intervals. Confidence intervals were generated using a percentile cluster bootstrap (with each individual as a cluster) to account for repeated measures in individual study subjects and multiplicity of testing (12 Fuchs criteria among two groups) was corrected for by use of 99.8% confidence intervals. Confidence intervals were calculated using the R Statistical Programming Language Version 3.6.2. Other statistical analyses were conducted with MedCalc Statistical Software version 19.2 (Ostend, Belgium). This study was reviewed by the University Hospitals Institutional Review Board (UH IRB Study 20,200,554).
3. Results
3.1. Frequencies of respiratory and pulmonary exacerbation events
In all, 751 RE were recorded among 244 of 272 study subjects (89.7%) over up to 48 weeks of observation. Baseline demographics did not differ between subjects experiencing versus not experiencing RE (Table 2 ). Exacerbation frequencies, numbers of subjects experiencing exacerbations, and times to first exacerbation varied consistently by exacerbation definition, with the Original Fuchs definition identifying the fewest exacerbations (106; 14.1% of RE) among the fewest subjects (71; 26.1% of subjects) and Any Antimicrobial Treatment identifying the most exacerbations (596; 79.4% of RE) in the greatest number of subjects (211; 77.6%) (Table 3 ). A minority of RE (126; 16.8%) did not meet any exacerbation definitions, having not been treated with antimicrobials, not been assessed as a pulmonary exacerbation by the clinician, and having not presented with at least 4 Fuchs criteria.
Table 2.
Subject baseline demographics by RE incidence.
| Subjects with No RE (N = 28) | Subjects with 1 to 2 RE (N = 112) | Subjects with ≥3 RE (N = 132) | |
|---|---|---|---|
| Mean age, years (SD) | 23.6 (13.5) | 20.9 (10.2) | 22.5 (10.5) |
| Age range, years | 6, 52 | 6, 51 | 7, 52 |
| Mean ppFEV1 (SD) | 64.1 (15) | 62.2 (14) | 62.4 (13.7) |
| ppFEV1 range | 39.5, 86 | 39.5, 91 | 38.5, 91 |
| Female N, (%) | 14 (50%) | 50 (44.6%) | 69 (52.3%) |
Table 3.
Pulmonary exacerbations observed using different definitions, ranked by incidence.
| Exacerbation definition | Description | Exacerbations, N (% of RE) | Subjects, N (%) |
|---|---|---|---|
| Any Antimicrobial Treatment (AT) | RE treatment with any antimicrobial | 596 (79.4%) | 211 (77.6%) |
| Investigator Assessment | Investigator opinion that RE was a pulmonary exacerbation | 352 (46.9%) | 172 (63.2%) |
| Modified Fuchs | RE with ≥4 Fuchs criteria recorded | 254 (33.8%) | 138 (50.7%) |
| Expanded Fuchs | Any antimicrobial treatment of RE with ≥4 Fuchs criteria recorded | 231 (30.8%) | 128 (47.1%) |
| IV Antimicrobial Treatment (IVAT) | RE treatment with IV antimicrobial | 192 (25.6%) | 106 (39.0%) |
| Original Fuchs | IV antimicrobial treatment of RE with ≥4 Fuchs criteria recorded | 106 (14.1%) | 71 (26.1%) |
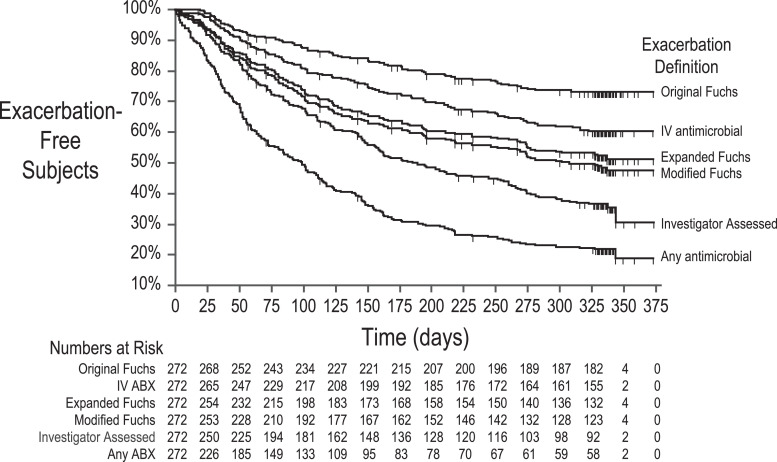
Median times to first exacerbation were 99 [95% CI 72, 115] days for Any Antimicrobial Treatment, 185 [150, 253] days for Investigator Assessment, and 307 [231, 337] days for Modified Fuchs exacerbations (Fig. 1 ). Less than 50% of subjects experienced exacerbations as defined by either Expanded Fuchs, IV Antimicrobial Treatment, or Original Fuchs.
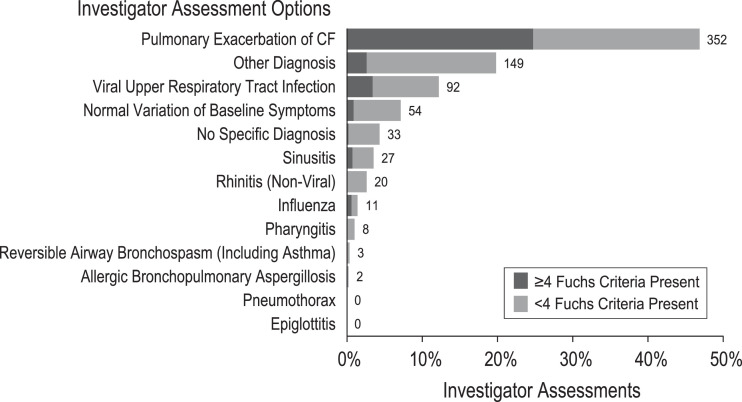
Fig. 2.
Investigator RE assessments. Assessment categories provided to study investigators on the RE case report form are shown on the ordinate and proportions of RE assigned to assessment categories are shown on the abscissa. Assessments made for RE presenting with ≥4 Fuchs criteria are shown in dark gray; those made for RE presenting with <4 criteria are shown in light gray. Numbers of total assessments for each category are provided.
Fig. 1.
Time to pulmonary exacerbation for 6 exacerbation definitions. Proportions of study subjects remaining exacerbation-free are plotted against time from study start in days for six exacerbation definitions. Subjects censored before experiencing an exacerbation are noted with vertical hashes. Numbers of subjects remaining at risk for exacerbation at given times are shown below the graph.
3.2. Consistency of criteria-based and investigator assessed pulmonary exacerbations
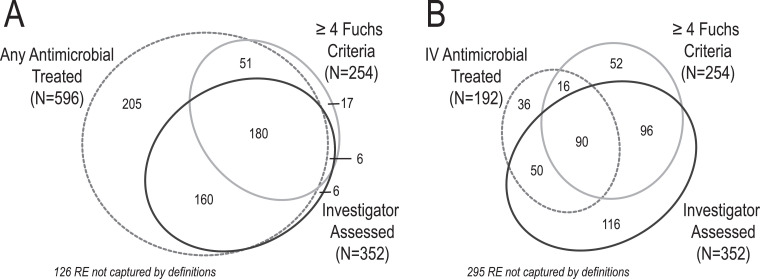
In all, about one third of 751 RE (N = 254; 33.8%) presented with ≥4 Fuchs criteria. Of these, more than a quarter (N = 68, 26.8%) were not assessed as exacerbations by investigators, despite three quarters of these events (N = 51) being treated with antimicrobials (Fig. 3 ). Twenty-six RE not assessed as exacerbations despite presenting with ≥4 Fuchs criteria were assessed as viral upper respiratory tract infections; the remainder were assessed as normal variations of baseline symptoms (N = 7), sinusitis (N = 6), influenza (N = 5), pharyngitis (N = 1), ABPA (N = 1), other diagnosis (N = 20), and no specific diagnosis (N = 2). In addition to the 186 RE presenting with ≥4 Fuchs criteria that were assessed as exacerbations by investigators, 166 additional RE that did not present with ≥4 Fuchs criteria were also assessed as exacerbations by investigators (Fig. 3).
Fig. 3.
RE meeting at least one of three exacerbation definitions. Panel A, area-proportional diagram of RE for which any antimicrobials were administered (hashed gray ellipse), for which ≥4 Fuchs criteria were recorded (gray ellipse), and which were assessed as pulmonary exacerbations by investigators (black ellipse). 126 RE did not meet any of these definitions. Panel B, RE for which IV antimicrobials were administered (hashed gray ellipse), for which ≥4 Fuchs criteria were recorded (gray ellipse), and which were assessed as pulmonary exacerbations by investigators (black ellipse). 295 RE did not meet any of these definitions.
More than three quarters of RE (N = 596; 79.4%) were treated with antimicrobials (Table 3), including almost all RE assessed as exacerbations by investigators (340, 96.6%) (Fig. 3). Addition of a requirement of the presence of ≥4 Fuchs criteria to the definition of any antimicrobial treatment of RE improved agreement between the objective exacerbation definition and investigator assessment (from 57% for any antimicrobial treatment to 78% for the Expanded Fuchs definition) but reduced the total number of exacerbations identified by 61% (from 596 to 231 exacerbations).
3.3. Completeness of individual Fuchs criteria
Greater than one third of RE (291; 38.7%) had no associated physical exam (necessary to capture the Fuchs criteria of temperature above 38 °C and change in physical examination of the chest). Almost half (375; 44.9%) had no associated diagnostic testing (necessary to capture the Fuchs criteria of >10% lung function drop and radiographic changes indicative of pulmonary infection) and most RE (668; 89.9%) lacked record of a chest x-ray (necessary to capture the Fuchs criterion of radiographic changes indicative of pulmonary infection). Similar proportions of RE presenting with ≥4 Fuchs criteria and RE assessed as exacerbations by clinicians were missing physical exams (28.3% versus 33.2%), diagnostic exams (39.8% versus 40.9%), and chest x-rays (76.8% versus 81.5%).
3.4. Assessment of clinical relevance of individual Fuchs criteria
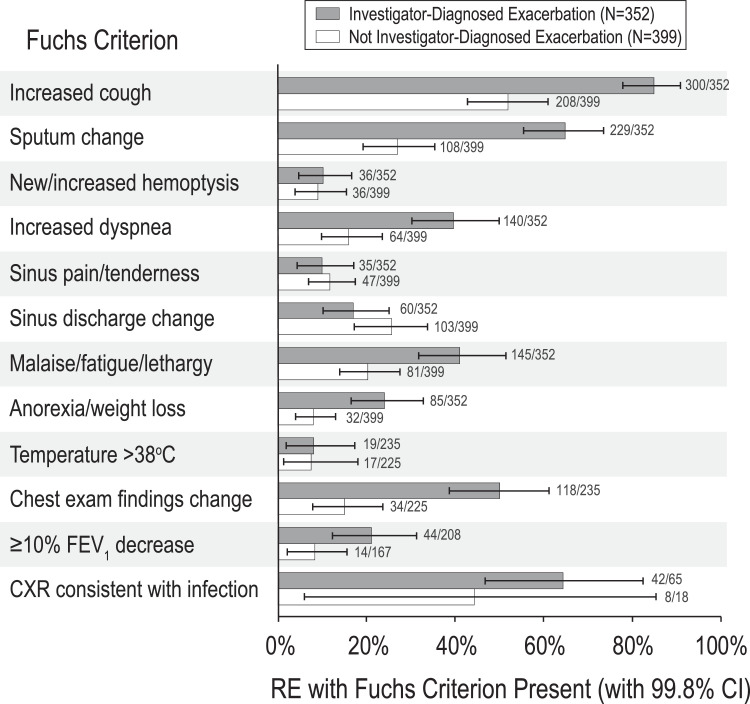
Only 6 of 12 Fuchs criteria were significantly more present (when data were available) in RE assessed by investigators as pulmonary exacerbations than in RE not assessed as exacerbations: increased cough, sputum change, increased dyspnea, malaise/fatigue/lethargy, anorexia/weight loss, and changes identified on chest examination (Fig. 4 ). Although >10% ppFEV1 drop and radiographic changes on chest x-ray were also more commonly associated with RE assessed as exacerbations by investigators, differences in proportions were not significant. In contrast, four Fuchs criteria (new/increased hemoptysis, sinus pain/tenderness, sinus discharge change, and temperature >38 °C) were not associated with investigator assessment of pulmonary exacerbation (Fig. 4).
Fig. 4.
Proportions of RE presenting with each Fuchs criterion stratified by investigator pulmonary exacerbation assessment. RE assessed by investigators as pulmonary exacerbations are dark gray and others are white. Numbers of RE presenting with each criterion divided by the total number of RE with criterion data are shown. Bars represent 99.8% confidence intervals for proportions, which account for 24 separate univariate measures.
4. Discussion
Treatment-associated pulmonary exacerbation rate and/or risk remains an important endpoint for evaluating the efficacy of chronic CF therapies, despite the lack of a consensus CF pulmonary exacerbation definition. To date, drug developers and regulators have relied on exacerbation definitions that have combined a minimum threshold of investigator-reported respiratory signs and symptoms, such as those of the Fuchs criteria, with an investigator's decision to treat RE with antimicrobials. Unfortunately, this “objective” approach to defining pulmonary exacerbations deemphasizes two important information sources: patients and their treating clinicians. Unlike health complications such as myocardial infarct or bone fracture, which people may experience only once in their lifetimes, people with CF (and their families) experience RE repeatedly and are aware of their past experiences. For the most part, they have also established a personal and longitudinal relationship with their CF clinicians, where nearly all RE are first identified by the person with CF or a family member in the home and clinician awareness follows patient/clinician communication. Further, specific RE management approaches (e.g., treatment in hospital with IV antimicrobials versus as an outpatient with oral antimicrobials) are a product of negotiation between these parties and may only partially reflect RE clinical presentation.
Although we expected criteria-based exacerbation definitions to identify only a subset of RE assessed as exacerbations by investigators, we did not expect the converse: that a substantial number of Fuchs-based exacerbations would not be assessed as exacerbations by investigators. This discrepancy appears to have at least partly resulted from an incorrect assumption that clinicians would assess all RE they treated with antimicrobials as exacerbations, which clearly was not the case. Although clinicians treated about 4 in 5 RE with antimicrobials, they assessed <60% of these RE to be exacerbations: investigators treated more than a quarter of RE they did not consider to be exacerbations with IV antimicrobials. Adding objective criteria requirements to antimicrobial treatment requirements improved agreement between objective exacerbation definitions and investigator assessments, but at a substantial cost in number of exacerbations identified.
Two fundamental problems observed with use of the Fuchs criteria in objective definitions were a) data missingness and b) apparent differences in association between presence of individual criteria and clinician assessment of exacerbation. Our results suggest that tests and procedures necessary to generate a “complete” set of Fuchs criteria exceeded those routinely employed by clinicians to diagnose and manage RE, resulting in substantial missingness of Fuchs criteria data. In our analysis, ~90% of RE were missing data for at least one Fuchs criterion and more than a third were missing data for four criteria. However, this missingness is not a reflection of poor performance by research coordinators, investigators, or the study sponsor, but results from an exacerbation definition that includes data elements unnecessary for routine RE management. These data were not missing at random (data for some criteria were nearly complete while nearly completely absent for others) and this missingness will have impacted criteria-based exacerbation definitions differently than investigator-based assessments. Since criteria-based definitions require the counting of positive criteria, data missingness with respect to criteria measure presumably systematically biases these definitions towards reduced exacerbation incidence. In contrast, an investigator may feel comfortable assessing an event without diagnostic testing, or conversely feel that diagnostic testing is necessary to rule out exacerbation. Thus, data missingness would not be expected to introduce the same degree of bias for investigator assessment of exacerbation.
Although a clinic visit is required to collect all 12 Fuchs criteria, studies of chronic CF treatments include relatively few planned study visits. The ataluren study analyzed here included only 6 planned study visits over the 48-week post-randomization treatment period. When subjects on study experienced a RE and contacted their study sight, a RE CRF was initiated remotely, but not all events resulted in a subsequent unscheduled study visit (and thus could not have had all 12 Fuchs criteria collected). Our results suggest that investigators did not require a clinic visit to assess a RE as an exacerbation, and we believe that this is generally reflective of how CF clinical care is provided today: people with CF may not need to be seen at their care center before receiving outpatient treatment for exacerbation. It is worth noting that RE presentation severity may not be the only driver of whether a given RE resulted in an unscheduled clinic visit. Anecdotally, it appears that unscheduled CF clinic visits and hospitalizations for exacerbations have substantially fallen during the global Covid-19 pandemic, but concerns of novel coronavirus infection, as opposed to reduction in the frequency and/or severity of CF exacerbation, are likely responsible for these changes.
It seems likely that past CF studies employing Fuchs-based exacerbation definitions suffered from data missingness similar to what we have reported, which in turn would have introduced bias towards lower pulmonary exacerbation incidence than would have been assessed by investigators (and likely by study subjects themselves). Although it is clear that the likelihood of meeting an objective exacerbation definition is increased with data completeness, it is not clear whether previous studies tested for systematic differences in Fuchs criteria missingness between treatment groups or conducted sensitivity analyses to address this missingness by, for instance, imputing data for missing Fuchs criteria.
Our analyses have important limitations. The NCT02139306 RE case report form asked investigators to identify an underlying RE cause from a menu of possible diagnoses (which included pulmonary exacerbation), but it is not clear that this list was optimized with respect to content, wording, or explanation. Given that about 20% of investigator assessments were listed as “other,” additional assessment categories were probably warranted. We also recognize that different investigators might disagree on individual RE assessments, a concern that has motivated movement towards criteria-based exacerbation definitions. Unfortunately, this is not a readily testable hypothesis. Moving away from criteria-based exacerbation definitions could have the effect of increasing event rates, but whether this would result in increased power to detect treatment-associated exacerbation differences is dependent on whether the true effects of treatment are identical across definitions, which has yet to be demonstrated. Moving towards investigator assessment of exacerbation as a clinical trial definition would not in and of itself lead to standardization of exacerbation diagnosis or treatment, but rather to better estimation of real-world treatment effects than are afforded by criteria-based definitions. Importantly, our results only pertain to study of a systemic treatment without direct respiratory complications. Experience suggests that exacerbation definitions that include the counting of respiratory signs and symptoms can be confounded when studying topical respiratory treatments such as inhaled antimicrobials [19], but we have no parallel experience with investigator diagnosis with which to compare outcomes. We have rationalized that investigator assessment is useful in evaluating the validity of Fuchs-based exacerbation definitions because of the clinician/patient relationship, but investigator opinion remains only a surrogate for the true information source: the person with CF. Our future goal should be to receive respiratory health information directly from those experiencing RE, rather than from their surrogates. This might take the form of simply asking study subjects for their assessment after an RE has been identified by other means or a more laborious surveillance approach of identifying changes in prospectively collected subject signs and symptoms using a validated tool such as the CF Respiratory Symptom Diary (CFRSD) [23].
In conclusion, there appear to be notable disagreements between criteria-based CF pulmonary exacerbation definitions and clinical assessments provided by study investigators and missingness of Fuchs criteria data appears common and is a likely source of bias, as examinations and diagnostic testing required to generate a complete complement of criteria are not part of routine exacerbation management. Our results suggest that a central rationale for the use of criteria-based exacerbation definitions: that they are rigorous and introduce selectivity for clinically important subsets of RE assessed by clinicians as exacerbations, must be questioned. Although adding sign and symptom criteria requirements to antimicrobial treatment definitions reduces sensitivity by excluding many RE assessed by investigators as exacerbations, it does not appear to increase selectivity, as many other events are captured by criteria-based definitions that investigators did not consider exacerbations. Fortunately, the RE signs and symptoms CRF used in this study did not generate a Fuchs score, which might have introduced bias towards assessing RE presenting with ≥4 Fuchs criteria as exacerbations, particularly since difference in criteria-based exacerbations was as an efficacy endpoint for the study.
Fuchs-based exacerbation diagnosis has been embraced by regulators but is rarely, if ever, used by CF clinicians as part of patient care, which raises questions as to the clinical relevance of treatment-associated changes in exacerbation rate or risk reported from studies employing Fuchs exacerbation definitions. Although presentations of only 6 of 12 Fuchs criteria were more common when investigators assessed RE as exacerbations than when they did not, this observation is insufficient to inform a “revised” Fuchs definition hewing closer to clinician behavior and consisting of only those 6 criteria. Questions remain as to whether other criteria (e.g., biomarkers such as C-reactive protein or calprotectin [24]) should be included in a revised criteria-based definition, how best to create a valid “score” based on criteria presentation, and how to deal with missingness, which will remain a problem. As newer chronic CF therapies are introduced into standard of care, assessments by people with CF of what does versus does not constitute healthiness is likely to evolve, as will clinician assessment of who is versus is not clinically stable. It seems unlikely that current criteria-based exacerbation definitions, which only poorly correlate with clinician assessments today, will be useful for drug developers in the future.
CRediT authorship contribution statement
Donald R. VanDevanter: Conceptualization, Methodology, Software, Formal analysis, Writing - original draft. Nicole Mayer Hamblett: Methodology, Writing - review & editing, Funding acquisition. Noah Simon: Formal analysis, Writing - review & editing. Joseph McIntosh: Data curation, Writing - review & editing. Michael W. Konstan: Resources, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
DRV reports personal fees from AbbVie, Aradigm, Arrevus, Calithera, Cystic Fibrosis Foundation, Chiesi, Eloxx, Enbiotix, Galephar, Matinas, Armata, Horizon, IBF, Ionis, Kala, Microbion, Recida, Respirion, Vast, and aMoon, outside the submitted work. NMH reports personal fees from Calithera and Kala outside the submitted work. NS has nothing to disclose. JM was an employee of PTC Therapeutics during the conduct of the study. MWK reports grants and personal fees from Anthera, AzurRx, Corbus Pharmaceuticals, Laurent Pharmaceuticals, PTC Therapeutics, Savara, and Vertex Pharmaceuticals, as well as personal fees from Albumedix, Celtaxsys, Chiesi, Genentech, Kala, Novartis, Pharmaceuticals, Merck, Paranta Biosciences, pH Pharma, Protalix Biotherapeutics, and Santhera and grants from the Cystic Fibrosis Foundation and the National Institutes of Health outside the submitted work.
Acknowledgments
The authors would like to thank PTC Therapeutics for providing access to anonymized NCT02139306 trial data. This work was supported by grants from the Cystic Fibrosis Foundation (NMH, NS, and MWK) and the National Institutes of Health: P30 DK 089507 (NMH), UL1 TR002319 (NMH), P01 HL128192 (MWK), and UL1 TR002548 (MWK).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcf.2020.07.008.
Appendix. Supplementary materials
References
- 1.Ratjen F., Döring G. Cystic fibrosis. Lancet. 2003;361(Feb 22 (9358)):681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Treatment of pulmonary exacerbation of cystic fibrosis. Clinical practice guidelines for cystic fibrosis, Chapter 2, 27-39. Cystic Fibrosis Foundation, Bethesda. MD. 1997 [Google Scholar]
- 3.Flume P.A., Mogayzel P.J., Jr, Robinson K.A., Goss C.H., Rosenblatt R.L., Kuhn R.J. Marshall BC; clinical practice guidelines for pulmonary therapies committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 4.Goss C.H., Burns J.L. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orenstein D.M., Pattishall E.N., Nixon P.A., Ross E.A., Kaplan R.M. Quality of well-being before and after antibiotic treatment of pulmonary exacerbation in patients with cystic fibrosis. Chest. 1990;98(5):1081–1084. doi: 10.1378/chest.98.5.1081. [DOI] [PubMed] [Google Scholar]
- 6.Britto M.T., Kotagal U.R., Hornung R.W., Atherton H.D., Tsevat J., Wilmott R.W. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 7.Lieu T.A., Ray G.T., Farmer G., Shay G.F. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics. 1999;103(6):e72. doi: 10.1542/peds.103.6.e72. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang L., Grosse S.D., Amendah D.D., Schechter M.S. Healthcare expenditures for privately insured people with cystic fibrosis. Pediatr Pulmonol. 2009;44(10):989–996. doi: 10.1002/ppul.21090. [DOI] [PubMed] [Google Scholar]
- 9.Liou T.G., Adler F.R., Fitzsimmons S.C., Cahill B.C., Hibbs J.R., Marshall B.C. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer-Hamblett N., Rosenfeld M., Emerson J., Goss C.H., Aitken M.L. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 11.VanDevanter D.R., Konstan M.W. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin Invest. 2012;2(2):163–175. doi: 10.4155/cli.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flume P.A., VanDevanter D.R. In: Acute exacerbations of pulmonary diseases (ERS monograph) Burgel P.R., Contoli M., López-Campos J.L., editors. European Respiratory Society; Sheffield: 2017. Cystic fibrosis: definition, severity and impact of pulmonary exacerbations; pp. 25–37. [Google Scholar]
- 13.Kraynack N.C., Gothard M.D., Falletta L.M., McBride J.T. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol. 2011;46(Sep (9):870–881. doi: 10.1002/ppul.21442. [DOI] [PubMed] [Google Scholar]
- 14.VanDevanter D.R., Elkin E.P., Pasta D.J., Morgan W.J., Konstan M.W. For the investigators and coordinators of the epidemiologic study of cystic fibrosis. Changing thresholds and incidence of antibiotic treatment of cystic fibrosis pulmonary exacerbations, 1995–2005. J Cyst Fibros. 2013;12(4):332–337. doi: 10.1016/j.jcf.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs H.J., Borowitz D.S., Christiansen D.H., Morris E.M., Nash M.L., Ramsey B.W., Rosenstein B.J., Smith A.L., Wohl M.E. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey B.W., Pepe M.S., Quan J.M., Otto K.L., Montgomery A.B., Williams-Warren J., Vasiljev-K M., Borowitz D., Bowman C.M., Marshall B.C., Marshall S., Smith A.L. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(Jan 7 (1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 17.Wagener J.S., VanDevanter D.R., Pasta D.J., Regelmann W., Morgan W.J., Konstan M.W. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48(7):666–673. doi: 10.1002/ppul.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsey B.W., Davies J., McElvaney N.G., Tullis E., Bell S.C., Dřevínek P., Griese M., McKone E.F., Wainwright C.E., Konstan M.W., Moss R., Ratjen F., Sermet-Gaudelus I., Rowe S.M., Dong Q., Rodriguez S., Yen K., Ordoñez C., Elborn J.S., VX08-770-102 Study Group A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(Nov 3 (18):1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flume P.A., VanDevanter D.R., Morgan E.E., Dudley M.N., Loutit J.S., Bell S.C., Kerem E., Fischer R., Smyth A.R., Aaron S.D., Conrad D., Geller D.E., Elborn J.S. A phase 3, multi-center, multinational, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT-1026) in stable cystic fibrosis patients. J Cyst Fibros. 2016;15(Jul (4):495–502. doi: 10.1016/j.jcf.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Kerem E., Konstan M.W., De Boeck K., Accurso F.J., Sermet-Gaudelus I., Wilschanski M., Elborn J.S., Melotti P., Bronsveld I., Fajac I., Malfroot A., Rosenbluth D.B., Walker P.A., McColley S.A., Knoop C., Quattrucci S., Rietschel E., Zeitlin P.L., Barth J., Elfring G.L., Welch E.M., Branstrom A., Spiegel R.J., Peltz S.W., Ajayi T., Rowe S.M., Cystic Fibrosis Ataluren Study Group. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2(Jul (7):539–547. doi: 10.1016/S2213-2600(14)70100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konstan M.W., VanDevanter D.R., Rowe S.M., Wilschanski M., Kerem E., Sermet-Gaudelus I., DiMango E., Melotti P., McIntosh J., De Boeck K., ACT CF Study Group Efficacy and safety of ataluren in patients with nonsense-mutation cystic fibrosis not receiving chronic inhaled aminoglycosides: the international, randomized, double-blind, placebo-controlled Ataluren Confirmatory Trial in Cystic Fibrosis (ACT CF) J Cyst Fibros. 2020;19(Jul (4):595–601. doi: 10.1016/j.jcf.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micallef L., Rodgers P. EulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS ONE. 2014;9(Jul 17 (7) doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechtzin N., Mayer-Hamblett N., West N.E., Allgood S., Wilhelm E., Khan U., Aitken M.L., Ramsey B.W., Boyle M.P., Mogayzel P.J., Jr, Gibson R.L., Orenstein D., Milla C., Clancy J.P., Antony V., Goss C.H. eICE Study Team. Home monitoring of patients with cystic fibrosis to identify and treat acute pulmonary exacerbations. eICE Study Results. Am J Respir Crit Care Med. 2017;196(Nov 1 (9):1144–1151. doi: 10.1164/rccm.201610-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung D., Dong K., Jang J., Lam G.Y., Wilcox P.G., Quon B.S. Circulating CRP and calprotectin to diagnose CF pulmonary exacerbations. J Cyst Fibros. 2020;(May 28) doi: 10.1016/j.jcf.2020.04.016. S1569-1993(20)30129-6Online ahead of print. PMID. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.