Abstract
Objective
To study the effect of COVID-19 on pregnancy and neonatal outcomes.
Study design
Prospective cohort study in a large tertiary maternity unit within a university hospital with an average annual birth of over 10,000 births. We prospectively collected and analysed data for a cohort of 23 pregnant patients including singleton and multiple pregnancies tested positive for COVID-19 between February 2020 and April 2020 inclusive to assess the effect of COVID-19 on pregnancy, and neonatal outcomes.
Results
Twenty-three pregnant patients tested positive for COVID-19, delivering 20 babies including a set of twins, with four ongoing pregnancies at the time of manuscript submission. 16/23 (70 %) whom tested positive were patients from Asian (Indian sub-continent) background. The severity of the symptoms ranged from mild in 13/23 (65.2 %) of the patients, moderate in 2/23 (8.7 %), and severe in 8/23 (34.8 %). Four out of total 23 COVID-19 pregnant patients (17.4 %) developed severe adult respiratory distress syndrome complications requiring ICU support, one of whom led to maternal death 1/23 (4.3 %). 11/23 (48 %) of the patients had pre-existing co-morbidities, with morbid obesity 5/23 (21.7 %) and diabetes 4/23 (17.4 %) being the more commonly represented. Of the 23 pregnant patients 19 were in their third trimester of pregnancy and delivered; 7/19 (36.8 %) had preterm birth, 3/19 (15.8 %) developed adult respiratory distress syndrome before delivery, and 2/19 (10.5 %) had pre-eclampsia. 16/19 (84 %) of patients delivered by C-section. Out of the 20 new-borns, 18 were singletons with a set of twin.
Conclusion
COVID-19 is associated with high prevalence of preterm birth, preeclampsia, and caesarean section compared to non−COVID pregnancies. COVID-19 infection was not found in the newborns and none developed severe neonatal complications.
Keywords: COVID-19, Coronavirus, Pregnancy, Maternal morbidity/mortality, Neonatal morbidity/mortality, Pandemic
Introduction
The World Health Organisation (WHO) was alerted on the 31 st of December 2019 by Chinese authorities of a series of pneumonia-like cases in the city of Wuhan [1]. The Chinese Centre for Disease Control and Prevention identified this infection as a novel coronavirus infection on Jan 7, 2020 and on Feb 11, 2020, the WHO announced a new name for the pandemic disease as 2019-new coronavirus disease (COVID-19). Symptoms of the infection had included fever, malaise, dry cough, shortness of breath and respiratory distress [2].
Studies from Europe, China, and USA on COVID‐19 have consistently shown that older age and comorbidity are major risk factors for adverse outcomes and mortality. Although most reported COVID-19 cases in China were mild (81 %), approximately 80 % of deaths had occurred among adults population older than 60 years of age; only one (0.1 %) death had occurred in a person under 19 years of age [[3], [4], [5]].
Data from MERS-CoV and SARS-CoV, indicate that infection in pregnancy tends to be severe and associated with adverse neonatal outcomes, including increased risk of miscarriage, fetal growth restriction, and preterm birth [[6], [7], [8], [9]]. Data from the UK [10] of more than 400 pregnant patients hospitalised with COVID-19 suggest an increased potential for adverse maternal outcomes in pregnant patients hospitalised with confirmed COVID-19 infection; while the risk of an intrauterine vertical transmission is inconclusive.
Royal college of Obstetrics and Gynaecology recommends that delivery in COVID-19 patients should be determined primarily by obstetric indication and recommends against routine separation of affected mothers and their babies [11]. Our study aims to provide additional emerging information for maternity and neonatal services planning their response to COVID-19.
Materials and methods
Prospective clinical information was collected at the time of presentation to the maternity unit from February 2020 to April 2020 inclusive. For each patient, a proforma was attached to the clinical note which was completed at each stage of the hospital stay. Telephone follow-up of maternal recovery and neonatal conditions were carried out by community midwives following hospital discharge for completion, and was recorded on electronic maternal notes [Badgernet maternity information system]. The infection was confirmed based on positive RT-PCR results supplemented by clinical symptoms, chest x ray, chest computed tomography (CT) information. RT-PCR for SARS-CoV-2 nucleic acid was used to determine COVID-19 in suspected infection from both maternal and neonatal nasopharyngeal samples. Sample collection, processing, and laboratory testing followed guidance from Public Health England [12].
Results
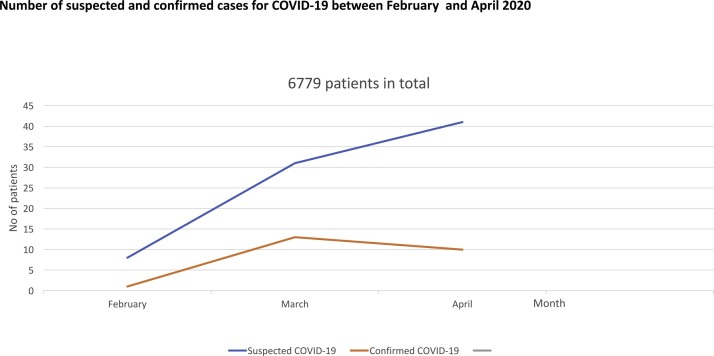
A total of 6779 pregnant patients attended our Maternity unscheduled triaging system (Fig. 1 ), of which 79 had suspected COVID-19 symptoms for which nasopharyngeal samples for RT-PCR for SARS-CoV-2 nucleic acid were taken. 23/79 (29 %) of patients had confirmed COVID-19 infection based on the RT-PCT test. For all the 23 patients, chest radiography also showed scattered multiple patchy infiltrates alongside ground glass appearances in both lungs consistent with COVID-19. The majority 13/23 (57 %) had mild symptoms (afebrile, new cough), 2/23 (8.7 %) of patients had moderate symptoms (new fever, cough), whilst 8/23 (21.7 %) were more severe (febrile, chest pain, shortness of breath). Most of the cases 14/23 (60 %) were of Asian ethnic backgrounds (i.e. Bangladeshi, Indian, or Pakistani), 2/23 (8.7 %) of Arabian Peninsula, 1/23 (4.3 %) is of black African-Caribbean ethnic background, whilst 3/23 (13 %) of UK Caucasian, and the remaining 3/23 (13 %) were Europeans (Table 1 ).
Fig. 1.
Number of suspected and confirmed cases for COVID-19 between February and April 2020.
Table 1.
Clinical data and follow-up data of 23 cases of pregnant women infected with nCoV in 2019.
| Clinical features (23 pregnancies) | |
| Maternal age (years) | Varies from 16 to 40 years with mean of 29.3 ± 2.9 |
| Ethnicity | 16 Asian, 3 Eastern Europe, 1 Black, 3 White British |
| Pregnancy comorbidities (11 pregnancies) | DM/gestational diabetes (4), acute kidney injury (3), Asthma (2), hyperthyroidism (1), pylonephritis (1) |
| Adverse pregnancy outcome (17 pregnancies) | Preterm delivery (7), RFM (6), PPROM (4), preeclampsia (2), HELLP (2), DIC (2), OC (1), fetal distress (1) |
| meconium (2), missed miscarriage (1) | |
| Signs and symptoms (23 patients) | |
| Antenatal/intrapartum Pyrexia | 14 antenatal, 2 intrapartum |
| Postpartum Pyrexia | 1 patient (day 1 post c-section) |
| Shortness of breath, chest pain | 8 patients |
| Dry cough | 12 patients |
| Diarrhea, abdominal pain | 4 patients |
| Chest x ray/ Chest CT scan evidence of 2019-nCoV pneumonia | Chest x ray positive in 20 patients, Chest CT positive in 1 patient |
| Delivery process (19 patients) | |
| Gestation at delivery | Varies from 29 weeks 3 days up to 40 weeks 2 days with mean of 38.7 ± 1.4 |
| Delivery Method | C-section in 16 patients, normal vaginal delivery in 3 patients |
| Steroids | Adminstrated in 5 patients (2 prior to ELCS < 39 weeks; 3 for PPROM) |
| Indication for c-section | 3 ELCS, 13 EMCS (2 fetal distress, 4 failure to progress, 7 maternal request/PPROM/sepsis) |
| Neonatal outcome (20 neonates [1twins]) | |
| Apgars at 1 & 5 min | (9, 10) in all of them except 1 baby (3,7) |
| Birthweight | Varies from 2240 to 4450 g with mean of 3139 g ± 437 |
| Neonatal outcome | Bacterial pneumonia (1), fetal asphyxia (Resuscitated using positive pressure/endotracheal tube) (1) |
| Neonatal rt-PCR for SARS-CoV | Negative |
| IV Antibiotics | Adminstrated in 4 neonates with potential infection |
| Maternal outcome (19 patients) | |
| Antenatal/intrapartum | Pylonephritis (1), PPH (1) |
| Postnatal | PPH (1), ECMO (1), death (1) |
Abbreviations: ECMO-Extracorporeal membrane oxygenation; OC- obstetrics Cholestasis; PPROM- Preterm premature rupture of the membranes; SGA-small for gestational age; DIC-disseminated intravascular coagulation; ELCS- elective c-section; EMCS- emergency c-section; CVA- cerebrovascular accident; HTN- hypertension.
Maternal outcomes
Mean age of patients was 29 [16;40] years, 4/23 (17.3 %) were admitted to intensive care unit (ICU), 3/23 (13 %) required mechanical ventilation, and 1/23 (4.3 %) required ECMO-Extracorporeal membrane oxygenation (ECMO). Comorbidities were diabetes mellitus 4/23 (17.3 %), Asthma 2/23 (8.7 %), pre-eclampsia 2/23 (8.7 %). One pregnant woman had hypertension, one had Anti-S antibodies, one had well-controlled hyperthyroidism, and hepatitis B. One patient died from basilar artery thrombosis, a co-existing pulmonary embolism, and complicated by diabetic ketoacidosis during ICU admission (Table 1)
In our cohort of 23 women, we had 19 pregnant women with confirmed COVID-19 in their 3rd trimester delivering 20 neonates (18 singleton, and 1 set of twins), and 4 women with confirmed COVID-19 in their second trimester. Of the later, one had missed miscarriage at 13 weeks’ gestation, while one developed acute pyelonephritis with consequent acute kidney injury. Of the 19 patients who delivered, 7/19 (36.4 %) were preterm. The gestation at delivery varied from 29 weeks to 36 weeks with mean of 33.1 weeks’ gestation. Four of these patients were preterm delivery following preterm pre-labour rupture of membrane (PPROM). Three patients required early delivery due to development of maternal severe adult respiratory distress syndrome; two were at 31 weeks’ gestation and another at 35weeks’ gestation. The majority had caesarean deliveries 16/19 (68.4 %) (Table 1). 13/16 (81 %) had an emergency C-sections while 3/16 (11.8 %) had an elective C-sections. Indications for the C-sections included pathological CTG (2), failure to progress (4), PPROM including subsequent unsuccessful induction of labour (3), maternal request (2) and severe sepsis (2) (Table 1). Two of these patients were admitted to ICU, intubated and ventilated prior to delivery. Pre-eclampsia occurred in 2/19 of patients (10.5 %), one of whom progressed to develop liver dysfunction, HELLP and DIC. For 6/19 (31.6 %) patients there were concurrent complaint of reduced fetal movement (RFM) but only one of whom had a pathological antenatal cardiotocograms (CTG) at her 40 weeks’ gestation presentation (Table 1).
In our cohort, we had one maternal death of a 29-year-old Asian patient with a history of poorly controlled type-2 diabetes. She was admitted with pyrexia and severe breathlessness requiring 100 % oxygen. Her infection was complicated by diabetic ketoacidosis. She was delivered by an emergency C-section under general anaesthetic. She was started on amoxicillin and thromboprophylactic dose of enoxaparin. Although she was extubated initially, she had to be re-intubated after 4 days due to worsening respiratory function. Her CT pulmonary angiogram confirmed pulmonary embolism, and showed bilateral solid pulmonary consolidations which was consistent with COVID-19. Her CT head showed basilar artery thrombosis. Following multidisciplinary discussion including the neurosurgical team into her care, end of life care was commenced before she passed away soon thereafter.
Neonatal outcomes
In terms of fetal and neonatal outcomes, the majority 19/20 (95 %) did not require resuscitation with 1 min Apgar scores of 8–9, and 5 min Apgar scores of 9−10. One new-born, who was delivered at 35 weeks by emergency C-section to black African patient due to severe COVID-19 respiratory symptoms requiring ventilation, had low Apgar score of 3 and 5, at 1 and 5 min respectively following delivery (Table 1). The baby was resuscitated with positive oxygen pressure following delivery, intubated and transferred to a special care baby unit (SCBU). The baby was extubated on day 3 and subsequently was discharged from hospital without further adverse, either mother or infant outcomes reported to date.
Nasal swabs were performed to screen for COVID-19 in seven infants 7/19 (37 %) who were delivered to mothers with severe symptoms (one test was not performed as guidance for not routinely performing screening test on the neonates born to COVID-19 patient was changed to routine screening soon after the data collection started). The swabs were taken on day 0, and day 3 following delivery. All 7 infants were started on antibiotics following delivery whilst awaiting swabs results. Additional neonatal pharyngeal swab testing was taken based on index of suspicion, there were four neonates where these additional swabs were taken. All neonatal swabs were, however, negative for COVID-19. None of the infants presented any respiratory symptoms as of the submission of the manuscript.
Discussion
The immune function of pregnant patients is relatively suppressed during pregnancy. At the same time, physiological changes during pregnancy will also expose pregnant patients to a higher risk, which will lead to a more adverse outcomes [13,14]. It is reported in the literature that pregnant patients infected with SARS-CoV, and MERS-CoV indeed have more adverse outcomes (spontaneous miscarriage, intrauterine growth restriction and premature delivery); the mortality rate of pregnant patients is as high as 25 % compared to 10 % in ordinary infected people [15,16]. Recently, Chen et al. [5], and Zhu et al. [17] reported that the perinatal infection COVID-19 may have adverse effects on new-borns, but compared with SARS-CoV, the adverse mother-to-child outcomes are fewer.
Analyses of our prospectively collected data of a cohort of pregnant patients infected with COVID-19 in their second and third trimester seems to bear these. Of all patients presenting in the 2nd or 3rd trimester, most cases had mild manifestation with eight severe cases. Chest imaging including CT and x ray examination showed typical patchy solid consolidation consistent with COVID-19 pneumonia in 20/23 (87 %) of the patients. Three of the severe cases progressed to requiring intubation and ventilation. All three were in the 3rd trimester.
In our cohort, there was a relatively higher rate of preterm birth, preeclampsia, and C-section. 7/19 (37 %) of the patients who acquired the infection in the 3rd trimester had preterm delivery, which remains higher compared to the national rate of preterm delivery (7.3 %) [18]. Furthermore, the rate of C-section in our cohort was 16/19 (84 %) which is significantly higher than the national C-section rate in the UK (26.2 %) [19]. Out of all patients 2/19 (10.5 %) had severe pre-eclampsia compared to (1–2 %) risk in general population [19], out of which one patient developed HELLP and DIC. One pregnancy with confirmed COVID-19 infection in the third trimester had a neonate with intrauterine growth restriction.
Four neonates with suspected COVID-19 infection had additional pharyngeal swabs taken. SARS-CoV-19 was not detected in either the nasal or throat swab by RT-PCR in any of their neonates. The negative neonatal swabs coupled with subsequent telephone follow-up enquiry, suggest that there was no direct evidence of maternal-fetal vertical transmission in COVID-19 infection in late pregnancy in our cohort.
Strengths and limitations
We are acknowledging that our study is limited by the small sample size, and incomplete information on the outcome of the infants beyond the end date of data collection, however, our findings are important for understanding the characteristics of the disease in pregnant patients, and their infants.
Conclusion
Although our cohort of 23 patients with confirmed COVID-19 was relatively small in absolute numbers, we have prospectively collected data for the three months’ period covered. The incidence of COVID-19 in our cohort mirrored the national UK trend. There is a relatively higher rate of preterm birth, preeclampsia, and C-section for patients with COVID-19 but vertical transmission including development of severe neonatal COVID-19 complications seemed reassuringly rare. Our findings can provide an additional guidance to enhance prenatal counselling of patients with COVID-19 infection during pregnancy.
Funding
The study was not funded by a grant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies
Dr Lina Antoun, is an ST6 Speciality Registrar, University Hospitals Birmingham, and clinical research fellow in the institute of genomics and cancer sciences, University of Birmingham.
Dr Nashwa El Taweel, is an Obstetrics and Gynaecology Registrar. University Hospitals of Birmingham.
Mr Irshad Ahmed, is a consultant Obstetrician, Special interest in maternal medicine. Obstetric, labour ward high dependency and elective caesarean section lead at University Hospitals of Birmingham.
Miss Shalini Patni, is a consultant Obstetrician (with special expertise in Fetomaternal medicine), University Hospitals of Birmingham. Assistant Medical Director (Division 1 –Governance). She is an Honorary Senior Lecturer at University of Birmingham, and West Midlands Ultrasound Lead.
Mr Honest Honest, is a consultant OB/GYN specialist, currently still active clinically in delivering both obstetrics and gynaecology care to his patients. His practice included in-patient care, operative lists, and out-patient consults in tertiary maternity units. In addition, he is also active in delivering education and training, including research methodologies to OB/GYN medical students and residents.
References
- 1.WHO . 2020. Pneumonia of unknown cause—China.https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ [Google Scholar]
- 2.Organization, W.H . Vol. 28. 2020. (Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance). Published January-2020. [Google Scholar]
- 3.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . Vol. 41. 2020. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China [Chinese] pp. 145–155. (Chinese Center for Disease Control and Prevention Weekly). [Google Scholar]
- 4.Karadag E. Increase in COVID-19 cases and case-fatality and case-recovery rates in Europe: a cross-temporal meta-analysis [published online ahead of print, 2020 May 21] J Med Virol. 2020 doi: 10.1002/jmv.26035. doi:10.1002/jmv.26035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. February 12-March 16, 2020, Published 2020 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2) doi: 10.3390/v12020194. pii: e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H.J., Guo J.J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 doi: 10.1016/S0140-6736(20)30360-3. [2020-02-12]. DOI: 10.1016 / s0140-6736 (20) 30360-30363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao J. What are the risks of COVID-19 infection in pregnant women. Lancet. 2020 doi: 10.1016/S0140-6736(20)30365-2. [2020-02-13]. DOI: 10.1016 / S0140-6736 (20) 30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Mascio D., Khalil A., Saccone G. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight M., Bunch K., Vousden N., et al. NPEU; 2020. Characteristics and outcomes of pregnant women hospitalised with confirmed SARS-CoV-2 infection in the UK: a national cohort study using the UK Obstetric Surveillance System (UKOSS). Oxford.https://www.npeu.ox.ac.uk/downloads/files/ukoss/annualreports/UKOSS%20COVID-19%20Paper%20pre-print%20draft%2011-05-20.pdf 11 May Available from: [Google Scholar]
- 11.Royal College of obstetricians and Gynaecologists (RCOG) 2020. COVID 19 pregnancy guidelines RCOG.https://www.rcog.org.uk/globalassets/documents/guidelines/2020-03-21-covid19-pregnancy-guidance-2118.pdf United Kingdom, Available from: [Google Scholar]
- 12.World Health Organization . 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance 2020.https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases Posted January 17, (Accessed 4 February 2020) [Google Scholar]
- 13.Silasi M., Cardenas I., Kwon J.Y., et al. Viral infection during pregnancy. Am J Reprod Immunol. 2015;73(March 3):199–213. doi: 10.1111/aji.12355. Epub 2015 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil M., Ingrid C. Immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(June 6):425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AJOG-MFM . 2020. Outcome of coronavirus Spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis.Https://Www.Ajog.Org/Coronavirus_guidance_ajog_mfm.; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2) doi: 10.3390/v12020194. pii: e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H.P., Wang L., Fang C.Z., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoVpneumonia [J / OL] Transl Pediatr. 2020 doi: 10.21037/tp.2020.02.06. (2020-02-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence [NICE], Preterm labour and birth (NG25) available on the NICE website: https://www.nice.org.uk/guidance/ng25. [PubMed]
- 19.NHS Digital Annual Maternity Statistics Publications. Summary Report 3: Percentage of deliveries by method of onset from 2008-09 to 2018-19 (HES). Publised 31st October 2019.