Highlights
-
•
Electroconvulsive therapy (ECT) is an essential psychiatric service which has an important role in treating older adult patients with severe or treatment resistant depression (TRD).
-
•
During the COVID-19 pandemic ECT services may be less accessible due to infection control measures, and alternative treatment options are needed.
-
•
Accelerated intermittent theta burst stimulation (aiTBS) treatment protocols represent an important advancement in neurostimulation and older adults should be thoughtfully included in future clinical trials.
Key Words: Accelerated intermittent theta burst stimulation, COVID-19, depression, elder
Abstract
Objective
Electroconvulsive therapy (ECT) is an essential psychiatric service with an important role in treating older adults with severe or treatment-resistant depression. During the COVID-19 pandemic, ECT services have be constrained by infection control measures. We report a case of a 66-year-old female patient with a severe major depressive episode who had previously responded to right unilateral ECT and was treated with two modified accelerated intermittent theta-burst stimulation (aiTBS) protocols.
Methods
The two aiTBS courses consisted of eight daily sessions over five consecutive days, followed by gradual tapering, using 1,800 pulses per session pre-COVID-19 (first course), and 600 pulses per session during the pandemic (second course).
Results
Moderate to severe baseline depressive symptoms reached remission levels after both courses.
Conclusion
The 600-pulses aiTBS treatment protocol reported here warrants further study and evaluation, but may be a potential option in cases where older adults with severe depressive symptoms cannot access ECT during the COVID-19 pandemic.
Electroconvulsive therapy (ECT) is an essential psychiatric service that continues to be a lifesaving treatment for severe major depressive disorder (MDD) and has an important role in treating older adult patients with treatment-resistant depression (TRD).1 The COVID-19 pandemic has caused massive shifts in healthcare delivery for patients with depression with a rapid shift to telemedicine. In-person care has been restricted to urgent cases, and though ECT is not an elective procedure in the majority of patients, many centers have had to reduce capacity due to enhanced infection control measures.2 The measures to flatten the curve of the pandemic and economic consequences will undoubtedly lead to exacerbation and onset of severe depression in many patients. Older adults may experience more stress due to isolation and loss, leading to a need for ECT. The World Health Organisation has warned that older adults and people with comorbid medical conditions are at higher risk for severe illness from COVID-19.3 Since patients receiving ECT tend to be older and have a high chance of comorbid medical conditions, there is an increased risk of mortality after COVID-19 infection.4 This risk is compounded by the need for general anesthesia and muscle paralysis resulting in close contact with patient's oral and airway secretions and bag-mask ventilation, which carries a risk of aerosolization.5 Even if this risk can be mitigated and reduced, bag-mask ventilation can potentially create an increased risk of exposure from patients who may be asymptomatic carriers, given that there is evidence supporting the transmission of COVID-19 while presymptomatic or asymptomatic.6 Importantly, ECT patients are frequently kept in close contact within small, confined rooms for several hours before and after their treatment, and their interaction with the team can last for several hours regularly over a period of weeks to months. Given these precautions, ECT practitioners have had to adapt and adhere to changing policies that reduce overall treatment capacity. In this context, ECT services are likely to be less accessible for patients, and other alternative treatment options are needed.
Intermittent theta-burst stimulation (iTBS) is a noninvasive brain stimulation treatment that has been approved by the U.S. Food and Drug Administration for treatment-resistant depression (TRD) in younger adults.7 Recent methodological advances suggest that an accelerated iTBS (aiTBS) protocol may be highly effective, rapid-acting, well-tolerated, and safe.8 The rationale for an accelerated approach is based on the idea of suboptimal dosing during once-daily treatment. The effects of treatment can be accelerated into a shorter course by repeated application of stimulation with a sufficient time interval to capitalize on neuroplastic mechanisms.9 An accelerated iTBS protocol has several advantages compared to the delivery of ECT treatment in the time of COVID-19. First and most important, aiTBS does not involve anesthesia with muscle paralysis or manual ventilation, thus removing the risk of aerosol generation. Second, the short duration of the contact (less than 10 minutes per session) between the technician and the patient and the limited number of contacts, whereas approximately four to six healthcare providers will come into close contact with the patient per ECT session. The World Health Organisation defines contact as face-to-face interaction with a probable or confirmed case of COVID-19 within 1 meter and for more than 15 minutes.3 Brief interactions are less likely to result in transmission; however, patients will still require screening for clinical symptoms and use of facemasks to mitigate the risk of viral transmission. Lastly, a large proportion of aiTBS participants met remission criteria, and no adverse cognitive side effects were reported.8 However, the previously reported protocol involves neuroimaging-guided treatment, which is impractical during the pandemic and consists of 10 hourly sessions/day, which poses clinic staffing challenges.
We report a case of a 66-year-old female patient who was treated with a modified aiTBS protocol that does not require neuroimaging. Ms. A experienced recurrent and severe MDD since early adulthood. Her medical morbidity includes a dermoid cyst and unilateral oophorectomy in the remote past. There was no history or evidence of a personality disorder. Previous episodes of depression were refractory to numerous pharmacotherapy interventions (SSRIs, SNRIs, TCAs, MAOIs, lithium, antipsychotics, stimulants, benzodiazepines, and St. John's wort), an acute course of magnetic seizure therapy (11 sessions without response), a once-daily deep TMS treatment course of 20 sessions, and once-daily bilateral TBS (once daily continuous TBS [cTBS] over the right dorsolateral prefrontal cortex [DLPFC] followed by left DLPFC iTBS) without sufficient response. A good response was achieved with an acute course of right unilateral ultra-brief pulse width ECT for 12 sessions, and 38 sessions of a maintenance course (intervals up to 3 weeks). However, the improvement was not sustained, and subjective cognitive impairment was also reported as a side effect.
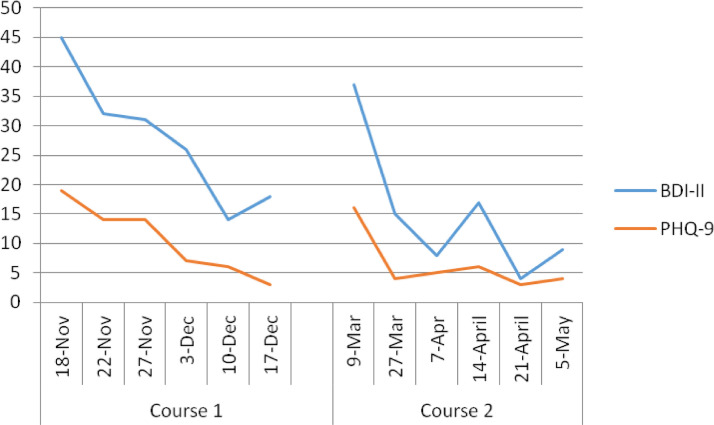
Ms. A initially presented five months before the COVID-19 pandemic, (fall of 2019), with severe depressive symptoms despite adherence to psychopharmacological treatment, which included aripiprazole 2 mg, and desipramine 100 mg. Before treatment, her Patient Health Questionnaire-9 (PHQ-9) score was 19, and the Beck Depression Inventory-II (BDI-II) score was 45. Despite nonresponse to once-daily cTBS/iTBS in the past, a modified aiTBS protocol was offered as the patient was reluctant to consider another course of ECT. The protocol consisted of eight sessions per day over five consecutive days (total of 40 iTBS sessions) during the first week, followed by a period of gradual tapering consisting of three consecutive treatment days for one week, two consecutive treatment days for one week, and two weekly treatment days, for a total of seven treatment days (intervals up to seven days), and 56 iTBS sessions. This tapering schedule was instituted due to concerns around rapid relapse after rapid-acting treatments.10 In each session, the patient received 1,800 pulses per session in triplet 50 Hz bursts, repeated at 5 Hz with train duration of 2 seconds and an intertrain interval of 8 seconds; 110% of the resting motor threshold (rMT) over the left DLPFC localized with the modified BeamF3 method.11 No adverse effects were reported, and remission was achieved during the tapering phase (PHQ-9, 3; BDI-II, 18). After two months of sustained remission, Ms. A. experienced a relapse of severe depressive symptoms (PHQ-9, 16; BDI-II, 37) and was offered another course of aiTBS. Unfortunately, this relapse coincided with the onset of the COVID-19 pandemic. In order to minimize the duration of contact between the technician and the patient, a modified aiTBS protocol of 600 pulses per session was instituted, eight sessions per day over five consecutive days. Infection control measures were taken to reduce the potential risk of viral transmission, including active screening of staff and patient for symptoms or COVID-19 contacts, physical distancing in wait areas, face shield and gloves for the technician and surgical mask for both technician and patient. The total duration of each iTBS session is approximately 5 minutes (including set-up time), a timeframe that, along with the above precautionary elements, reduces the risk of exposure. The Beam F3 method was used to localize the treatment spot once, before the initial treatment and a re-usable tape (wiped down with antiviral wipes) to minimize set-up was used at the start of each subsequent day. Ms. A ultimately responded and achieved remission in a similar and rapid way with this abbreviated aiTBS protocol (PHQ-9, 4; BDI-II, 9). This time, the tapering phase consisted of three consecutive treatment days for one week, four weekly treatment days, and one bi-weekly treatment day for a total of eight treatment days (intervals up to 14 days), and 64 iTBS sessions (Fig. 1 ).
FIGURE 1.
Beck Depression Inventory (BDI-II) and Patient Health Questionnaire-9 (PHQ-9) scores during the two courses of the accelerated intermittent Theta Burst Stimulation (aiTBS) treatment.
Sheltering at home is likely to be an essential public health approach for older adults throughout the pandemic. Furthermore, older adults and people who have severe underlying medical conditions seem to be at higher risk for developing more severe complications from COVID-19. Therefore, the lowest possible exposure, with the highest possible benefit, is required for this population. Telepsychiatry interventions may be able to successfully address symptoms in older adults with mild to moderate depression. Those with moderate to severe symptoms may require ECT, yet infection control procedures have affected ECT capacity globally.2 , 4 AiTBS represents an important advancement in neurostimulation, and older adults should be thoughtfully included in future clinical trials that examine the efficacy of this intervention. Our case not only illustrates the potential for this approach to be effective in older adults with moderate to severe depression but also highlights that a methodological modification to current aiTBS protocols that deliver 1,800 pulses per session (i.e., reducing the number of pulses to 600) may not sacrifice effectiveness.
During the COVID-19 pandemic, this modified protocol might provide relief to those patients who cannot access care and relief on ECT services that do not have the capacity. Travel to an rTMS clinic for fewer days, and brief exposure during a 600 pulses aiTBS session likely represents a lower risk of COVID-19 exposure than receiving ECT, albeit with the additional risk of being in a clinic all day. Considering the burden of providing care to as many people as possible during the pandemic, there is a potential for aiTBS to help patients in an at-risk population that may not be able to access care. Undoubtedly, aiTBS is in its infancy, and further, large-scale, controlled clinical trials are an essential next step in the development of this protocol. Nevertheless, gathering data on this modified approach, specifically in older adults with severe depression and the impact of this approach on ECT service capacity during the pandemic, may inform future randomized controlled trials (clinialtrials.gov identifier NCT04384965).
Author Contributions
GNK: Writing - Original Draft, Visualization, Data Curation, Writing - Review & Editing; JD: Writing - Review & Editing; ZJD: Writing - Review & Editing, Supervision; DMB: Conceptualization, Visualization, Methodology, Validation, Supervision, Project administration, Writing - Review & Editing, Corresponding Author.
Disclosure
GNK declares no conflict of interest.
JD has received research support from CIHR, NIMH, Brain Canada, the Canadian Biomarker Integration Network in Depression, the Ontario Brain Institute, the Klarman Family Foundation, the Arrell Family Foundation, the Edgestone Foundation, a travel stipend from Lundbeck and from ANT Neuro, an advisor to BrainCheck and in-kind equipment support for this investigator-initiated trial from MagVenture.
ZJD has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc and Magventure Inc, in the last 5 years. His work is supported by the Canadian Institutes of Health Research (CIHR), the National Institutes of Mental Health (NIMH), Brain Canada and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute.
DMB has received research support from the CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd., and he is the principal site investigator for three sponsor-initiated studies for Brainsway Ltd. He receives in-kind equipment support from Magventure for investigator-initiated research. He received medication supplies for an investigator-initiated trial from Indivior.
References
- 1.Blumberger DM, Hsu JH, Daskalakis ZJ. A review of brain stimulation treatments for late-life depression. Curr Treat Options Psychiatry. 2015;2:413‐421. doi: 10.1007/s40501-015-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sienaert P, Lambrichts S, Popleu L. Electroconvulsive therapy during COVID-19-times: our patients cannot wait. Am J Geriatr Psychiatry. 2020 doi: 10.1016/j.jagp.2020.04.013. S1064-7481(20)30297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 66. Available at https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200326-sitrep-66-covid-19.pdf?sfvrsn=9e5b8b48_2. Accessed May 10, 2020. [Google Scholar]
- 4.Tor PC, Phu AHH, Koh DSH. ECT in a time of COVID-19. J ECT. 2020 doi: 10.1097/YCT.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flexman AM, Abcejo A, Avitisian R. Neuroanesthesia practice during the COVID-19 pandemic: recommendations from Society for Neuroscience in Anesthesiology & Critical Care (SNACC) J Neurosurg Anesthesiol. 2020 doi: 10.1097/ANA.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumberger DM, Vila-Rodriguez F, Thorpe KE. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–1692. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 8.Cole EJ, Stimpson KH, Bentzley BS. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020 doi: 10.1176/appi.ajp.2019.19070720. appiajp201919070720. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Nettekoven C, Volz LJ, Kutscha M. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849‐6859. doi: 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caulfield KA. Is accelerated, high-dose theta burst stimulation a panacea for treatment-resistant depression? J Neurophysiol. 2020;123:1–3. doi: 10.1152/jn.00537.2019. [DOI] [PubMed] [Google Scholar]
- 11.Mir-Moghtadaei A, Caballero R, Fried P. Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul. 2015;8:965–973. doi: 10.1016/j.brs.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]