Abstract
The coronavirus disease 2019 pandemic will be remembered for the rapidity with which it spread, the morbidity and mortality associated with it, and the paucity of evidence-based management guidelines. One of the major concerns of hospitals was to limit spread of infection to health-care workers. Because the virus is spread mainly by respiratory droplets and aerosolized particles, procedures that may potentially disperse viral particles, the so-called “aerosol-generating procedures” were avoided whenever possible. Included in this category were noninvasive ventilation (NIV), high-flow nasal cannula (HFNC), and awake (nonintubated) proning. Accordingly, at many health-care facilities, patients who had increasing oxygen requirements were emergently intubated and mechanically ventilated to avoid exposure to aerosol-generating procedures. With experience, physicians realized that mortality of invasively ventilated patients was high and it was not easy to extubate many of these patients. This raised the concern that HFNC and NIV were being underutilized to avoid intubation and to facilitate extubation. In this article, we attempt to separate fact from fiction and perception from reality pertaining to the aerosol dispersion with NIV, HFNC, and awake proning. We describe precautions that hospitals and health-care providers must take to mitigate risks with these devices. Finally, we take a practical approach in describing how we use the three techniques, including the common indications, contraindications, and practical aspects of application.
Key Words: awake proning, coronavirus disease 2019, COVID-19, helmet mask, high-flow nasal cannula, noninvasive ventilation
Abbreviations: AGP, aerosol-generating procedure; COVID-19, coronavirus disease 2019; HEPA, high-energy particulate accumulator; HFNC, high-flow nasal cannula; NIV, noninvasive ventilation; NRB, nonrebreather; PEEP, positive end-expiratory pressure; PPE, personal protective equipment; ROX, ratio of oxygen saturation as measured by pulse oximetry/Fio2 to respiratory rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SO, standard oxygen
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which has swept through much of the world. The disease is spread through respiratory droplets and contact with fomites.1 Airborne transmission has occasionally been implicated in patients with COVID-19 during procedures that are capable of generating aerosols.2 , 3
Approximately 5% of the patients who contract COVID-19 require admission to ICUs.4 They tend to be older, generally over the age of 60 years, with comorbidities such as hypertension, diabetes, cardiac disease, and obesity.5 , 6 The rate of intubation and mechanical ventilation among patients admitted to the ICU has variously been reported as 71% to 90%.7, 8, 9 When these patients develop hypoxemic respiratory failure, they are often on a fast track to proceed from low-flow oxygen supplementation via nasal cannula to a nonrebreather (NRB) face mask, and then directly to intubation and mechanical ventilation. The reasons for rapidly resorting to invasive mechanical ventilation include concerns that a rapid decline in respiratory status may take place, that mitigation of viral spread necessitates limiting entry to an infected patient’s room, and that other respiratory modalities such as a high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), and awake (nonintubated) proning may result in dispersal of viral particles in the atmosphere.10 In the presence of bilateral lung opacities and hypoxemic respiratory failure, most of the intubated patients are placed on the low tidal volume and adequate positive end-expiratory pressure (PEEP) lung-protective strategy recommended by the ARDS Network trial.11
Among patients with COVID-19 with ARDS, Gattinoni et al12, 13, 14 have described an “L” phenotype (for low elastance) among patients who demonstrate relatively preserved respiratory system compliance of > 50 mL/cm H2O with focal areas of ground-glass opacity on CT scanning. In contrast, others manifest the low compliance (“H” phenotype—for high elastance) that is typically seen in non-COVID-19 patients with ARDS.
The concerns about aerosol dispersion have led to calls for early intubation,15 leading many hospitals to discourage use of noninvasive modalities. A significant number of patients with COVID-19-induced pneumonia and ARDS could avoid invasive mechanical ventilation and its attendant risks of ventilator-induced lung injury5 , 7 , 16 and health care-acquired pneumonia.17 In this article, we discuss the potential role of HFNC, NIV (including helmet), and awake proning in the management of COVID-19-induced acute respiratory failure. We enumerate the indications, contraindications and “How I Do It” techniques, in the context of the limited but emerging safety data available.
High-Flow Nasal Cannula
HFNC refers to high-flow oxygenated gas, heated and humidified to body conditions, that is delivered via nasal cannula at maximum flows ranging from 40 to 80 L/min depending on the manufacturer.18 It has not been around for as long as NIV, having gained traction over the past 8 years or so. The heating and humidification make it tolerable; a dry cool gas at those flow rates would rapidly desiccate the nasal mucosa, causing an uncomfortable burning sensation. Also enhancing tolerability is the soft, loosely fitting nasal interface that does not impede speech or eating during use. The heat and humidification also help to maintain hydration and mobility of secretions and to preserve mucociliary function. HFNC helps with oxygenation by flushing the nasopharynx during exhalation so that the first bolus of air during inspiration is not just expired air but is partly freshened by the oxygenated HFNC gas. Also, compared with standard oxygen (SO) techniques, the high flow rate of HFNC comes closer to the inspiratory flow rates encountered in dyspneic patients, which may exceed 60 L/min. For example, the NRB mask provides oxygen flows up to only 15 L/min, so that air entrainment and dilution of Fio 2 are greater than with HFNC. The flushing of the nasopharynx also washes out anatomic dead space, improving ventilatory efficiency. That, and the reduction in respiratory rate probably caused by the slowing of exhalation by the inflowing gas, contribute to a reduction in work of breathing per minute. The expiratory impedance also creates positive expiratory pressure that peaks early during exhalation, amounting to roughly 1 cm H2O/10 L/min high flow and has been shown to increase end-expiratory lung volume. Thus, HFNC is more than just oxygen supplementation; it is a very well-tolerated ventilatory assist device with multiple potentially advantageous physiologic attributes that is also easy and safe to apply.
Physiologic Comparison With NIV
HFNC is primarily a flow generator and it is via high flow that it achieves its main beneficial effects of more reliably delivering a targeted Fio 2 than standard oxygen supplementation (although in a clinical setting, accurate measurement of actual Fio 2 delivered to the lungs is currently not possible) and reducing dead space to improve ventilatory efficiency. NIV, in contrast, is primarily a pressure-targeted modality, and it is pressure that is responsible for its success for its two main indications. These include acute respiratory failure in COPD exacerbations by counterbalancing intrinsic PEEP with external PEEP and providing pressure support to assist inhalation,19 and in acute cardiogenic pulmonary edema by applying CPAP or bilevel PAP to increase functional residual capacity, which improves oxygenation and lung compliance.20
Evidence for Efficacy
A number of studies have compared HFNC with NIV and SO. HFNC has been shown to be more comfortable and better tolerated than either.18 It provides better oxygenation as compared with SO21 , 22; however, it is not as good an oxygenator as NIV, presumably because mean airway pressure is less. Randomized clinical trials comparing the clinical efficacy of these various approaches with noninvasively supporting patients with acute hypoxemic respiratory failure are very few. Frat et al23 compared HFNC (50 L/min; Fio 2, 100%) with SO using an NRB mask (≥ 10 L/min), and with NIV using pressure support to achieve a tidal volume of 7 to 10 mL/kg ideal body weight. The major outcome variable, intubation rate, was 38%, 47%, and 50% in the three groups, respectively, which was not statistically significant. However, there was a significant drop in intubation rate in the HFNO group compared with the SO and NIV groups in the subgroup of patients with Pao 2/Fio 2 < 200. Mortality was also significantly less in the HFNO group than the others in the ICU and after 90 days (30%, 45%, and 49%, respectively).23 Other randomized studies have demonstrated that HFNC is noninferior to NIV in patients at risk for reintubation after cardiac surgery24 and after extubation following a bout of acute respiratory failure.25 It also reduced the reintubation rate compared with SO in lower risk respiratory failure patients after extubation.26 One recent study showed that the combination of HFNC alternating with NIV in postextubation patients reduced the reintubation rate more than with HFNC alone.27 Thus, HFNC offers a number of advantages over SO and NIV as well as some limitations, but should be a first-line consideration when patients with COVID-19 pneumonia are mildly to moderately hypoxemic.
The Controversy
As reflected by the varying recommendations in the guidelines offered by various eminent organizations, HFNC for the management of COVID-19 pneumonia has been very controversial (Table 1 ). Some guidelines caution against the routine use of HFNC or any noninvasive, potentially aerosol-generating approach28; others, such as the Surviving Sepsis Campaign/Society of Critical Care Medicine guideline,29 advocate it as a first-line approach. Some hospitals strongly discourage the use of noninvasive approaches, favoring early intubation, and others use noninvasive approaches quite commonly. The contention is that failure rates of noninvasive approaches in patients with COVID are high, and these are aerosol-generating procedures (AGPs) that place caregivers at increased risk of contracting COVID-19. The contrary view is that aerosol-mitigating interventions such as the use of negative-pressure rooms, high-energy particulate accumulator (HEPA) filters, and adequate personal protective equipment (PPE) are sufficient to protect staff. In addition, the noninvasive approaches will avoid unneeded intubations that are well-known generators of considerable amounts of aerosol, thus protecting staff. Avoidance of intubation might improve patient outcomes and preserve much-needed critical care ventilators that have been in short supply in “hot spot” areas. There are a number of studies that have examined aerosol dispersion during use of various AGPs that, regarding HFNC, have been reassuring. These show a smaller distance of dispersion for HFNC than for a number of other AGPs,30, 31, 32 but because most have been performed with human mannequins with smoke or some other substitute particulate, they have not universally allayed the concerns of some practitioners. A recent study suggests that a droplet (surgical) mask placed over the nasal interface can greatly reduce dispersion of aerosol.33 Another recent study of healthy volunteers showed no increase in aerosol over background in a simulated hospital room with HFNC up to maximal flow rates of 60 L/min, compared with use of a standard nasal cannula or an NRB mask.34
Table 1.
Recommendations of International Societies Regarding Use of High-Flow Nasal Cannula and Noninvasive Ventilation in COVID-19 Pandemic
| Organization/Country | HFNC |
NIV |
||
|---|---|---|---|---|
| Recommendation | Comment | Recommendation | Comment | |
| Asociación Argentina de Medicina Respiratoria, Argentina | PRO | Nasal prongs tight to minimize aerosol | PRO | Short trial (1 h) |
| Australian National COVID-19 Clinical Evidence Taskforce, Australia | None | None | CONTRA | Consider only with concomitant COPD with type 2 respiratory failure or CPE |
| Australian and New Zealand Intensive Care Society (ANZICS), Australia and New Zealand | Suggest | None | Not routine | None |
| Austrian ICU therapy guideline for the treatment of patients with SARS-CoV-2 infection, Austria | No mention | None | CONTRA | Consider short trial only if HFNC is not feasible |
| Associação Brasileira de Fisioterapia Cardiorrespiratória e Fisioterapia em Terapia Intensiva, Brazil | No mention | None | PRO (conditional) | In certain situations a short trial (30 min) |
| Canadian Critical Care Society, Canada | None | None | PRO (conditional) | In certain situations a short trial (30 min) |
| Sociedad Chilena de Kinesiología Respiratoria, Chile | None | None | PRO (conditional) | Short trial only if HFNC is not feasible. Helmet suggested |
| Chinese National Health Commission, China | None | None | PRO | Short trial (1 h) |
| German recommendations for critically ill patients with COVID-19, Germany | Restrictive | None | Restrictive | Only in patients with P/F > 200; helmet suggested |
| Irish Thoracic Society, Ireland | PRO | HFNC 30 L/min in negative-pressure room | PRO | Helmet suggested |
| Italian Thoracic Society and Italian Respiratory Society, Italy | None | None | PRO | None |
| Société Libanaise de Pneumologie, Lebanese; Society of Critical Care Medicine, Lebanese; Society of Anesthesiologists, Lebanon | CONTRA | Favor early intubation | CONTRA | None |
| Pakistan Chest Society, Pakistan | Conditional | If in negative-pressure room | CONTRA | None |
| Sociedade Portuguesa de Pneumologia, Portugal | No mention | None | Conditional | Short trial (1 h) Facial mask suggested |
| Sociedad Española de Neumología y Cirugía Torácica, Spain | PRO | Maintain > 2-m distance | PRO | None |
| Swiss Academy of Medical Sciences, Switzerland | None | None | CONTRA | Eventually only in the ICU |
| National Health Care System guidelines, UK | CONTRA | No benefit but some risk | PRO | CPAP for mild hypoxia and NIV for acute or chronic respiratory failure |
| American College of Chest Physicians, USA | None | None | Careful use | The recommendations are only for home-based ventilated patients |
| World Health Organization interim guidance, January 2020 | Selected | Not for COPD, CPE, hemodynamic instability | Selected use | None |
| US Department of Defense COVID management guidelines | PRO | None | CONTRA | Early intubation over NIV if HFNC fails |
| US Surviving Sepsis Campaign/SCCM guidelines | Suggesta | HFNC next modality in those not tolerating supplemental O2 | None | Suggest if HFNC unavailable or patient is not tolerating it |
CONTRA = against; COVID-19 = coronavirus disease 2019; CPE = cardiogenic pulmonary edema; HFNC = high-flow nasal cannula; NIV = noninvasive ventilation; P/F = Pao2/Fio2 ratio; PRO = for; SARS-CoV-2 = severe acute respiratory syndrome coronavirus type 2; SCCM = Society of Critical Care Medicine.
HFNC not considered an aerosol-generating procedure.
Indications and Application for HFNC in Patients With COVID-19
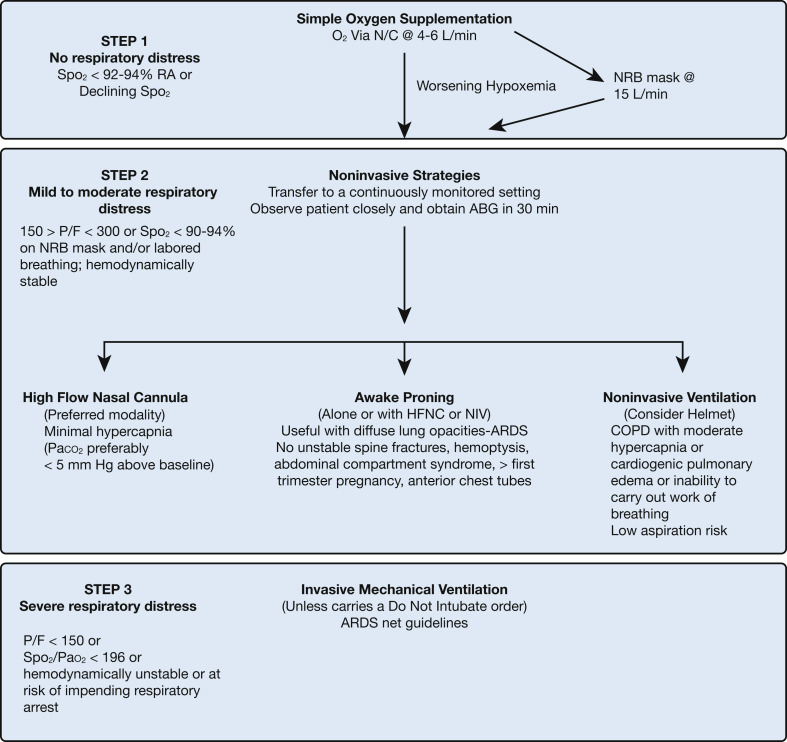
Figure 1 and Tables 2 and 3 summarize the indications, contraindications, and technique for performing HFNC, NIV, and awake proning. HFNC is a better tolerated and more efficacious alternative to the NRB mask when standard nasal prongs are deemed insufficient. This would have been the preferred initial choice at many centers in the United States. HFNC is likely to be better tolerated than NIV with an orofacial mask or the helmet and may also facilitate the use of proning. To our knowledge there are not, at present, any head-to-head trials comparing HFNC with the helmet, but we have seen some patients fail to oxygenate adequately with HFNC and go on to improve with the helmet, presumably because of the helmet’s greater positive airway pressure. On the other hand, we have seen HFNC succeed when the helmet fails, mainly because of patients’ better tolerance of HFNC. Expense is greater for HFNC over nasal cannula but not inordinately so ($85.75 including interface, circuit, and water bag for HFNC vs $0.33 for nasal cannula at a hospital in New York City).
Figure 1.
Algorithmic approach to respiratory failure in coronavirus disease 2019. ABG = arterial blood gas; HFNC = high-flow nasal cannula; N/C = nasal cannula; NIV = noninvasive ventilation; NRB = nonrebreather; P/F = Pao2/Fio2 ratio; RA = room air; Spo2 = arterial oxygen saturation as determined by pulse oximetry.
Table 2.
Physiologic Effects, Indications, and Recommended Precautions With High-Flow Nasal Cannulas, Noninvasive Ventilation, and Awake Proning
| Clinical Variables | HFNC | Awake Proning | NIV ± Helmet |
|---|---|---|---|
| Physiologic effects |
|
|
|
| Indications |
For incipient respiratory failure:
|
|
For incipient respiratory failure:
|
| Precautions |
|
|
|
PAP = positive airway pressure; PEEP = positive end-expiratory pressure; ROX = ratio of oxygen saturation as measured by pulse oximetry/Fio2 to respiratory rate; RR = respiratory rate; WOB = work of breathing. See Table 1 legend for expansion of other abbreviations.
Table 3.
How I Do It: Technique and Monitoring of High-Flow Nasal Cannula, Noninvasive Ventilation, and Awake Proning
| HFNC | Awake Proning | NIV ± Helmet | |
|---|---|---|---|
| Technique |
|
|
|
| Monitoring |
|
|
|
HFNC Failure
The greatest danger when using HFNC, especially with patients with COVID-19, is to fail to monitor closely enough, leading to an unanticipated need for intubation with increased risk to the patient of respiratory arrest and increased risk of aerosol exposure to the intubating team. Thus, it is important to have patients at risk for progression in a closely monitored setting such as an ICU or intermediate care unit. Indicators of impending failure include increasing tachypnea and tachycardia, failure to adequately support oxygenation despite a high flow rate and Fio 2, a climbing Paco 2 in a struggling patient, development of dyssynchronous breathing, alteration in mental status, and hemodynamic instability. In some patients who are not too unstable, a trial of NIV may be worthwhile, but it is important to intubate before a crisis occurs. Recently, the ROX index has been suggested as a way of predicting impending failure of HFNC. This consists of calculating (oxygen saturation [Sao 2]/Fio 2)/respiratory rate (RR), thus incorporating an index of gas exchange with another of breathing effort. Roca et al35 reported that an ROX score > 4.88 at 12 h predicted success of HFNC with an area under the curve of 0.75. An ROX score ≤ 3.85 at 12 h predicted failure with nearly 100% specificity.
Summary
HFNC is very simple and safe to apply and is a favorite of respiratory therapists for that reason. Relevant to caregivers, patients tend to leave the HFNC prongs in place more than is the case with mask oxygen or NIV, reducing the number of needed visits into the room. The major precautions that should be exercised in its application are related to putting an HFNC on unstable or severely hypoxemic patients and not monitoring them adequately. This may culminate in severe hypoxemia and an emergency intubation. This is catastrophic because it takes minutes for the code and intubation teams to apply appropriate PPE, and intubation is a high-risk AGP, not to mention the greater risk of morbidity and mortality to the patient.
Noninvasive Positive-Pressure Ventilation With Helmet
Description of Technique
NIV is a well-established technique that has gained popularity in the last 30 years.36 Usually NIV is delivered by critical care ventilators for severely hypoxic patients. These ventilators allow the option of setting Fio 2 through a blender, permit visualization of waveform displays, and allow separate inspiratory and expiratory circuits. By placing a fixed exhalation valve, filtering in a closed system reduces aerosol dispersion, features not always available in dedicated NIV platforms that have a single circuit and a fixed exhalation valve.37 Filters can be attached to the exhalation valve with some NIV platforms, which may reduce contamination of the environment.
Spontaneous modes are generally used with NIV to enhance synchrony and comfort. Therefore, deep sedation cannot be used for safety reasons. This limits the use of NIV only in mildly to moderately hypoxemic patients, because volume-targeted ventilation is not feasible.
In clinical practice, pressure support ventilation, together with the addition of external PEEP, is virtually the only mode used.
At present, most ICU ventilators have the so-called NIV option, which is able to better compensate for the unavoidable leaks with NIV.38
Concerning the interfaces recommended, most studies have used full or total face masks, whereas for pandemics the European Respiratory Society/European Society of Intensive Care Medicine has suggested the use of the helmet for the safety of health-care personnel from exhaled air dispersion as described below. During the COVID-19 outbreaks, the Italian societies strongly recommended use of the helmet.39 , 40 When applying this interface, the specific settings used are different than with an oronasal (full face) mask. The “usual” inspiratory and expiratory pressures used to deliver NIV through an oronasal mask are increased by 50% and the fastest pressurization rate is applied.41 Examples would be PEEP of 8 to 12 cm H2O and pressure support of 12 to 20 cm H2O.
Safety and Precautions
As illustrated in Table 1, some international societies do not recommend the use of NIV for COVID-19 treatment. These suggestions are driven by concerns about dispersion and the stability of COVID-19 in clinical spaces. World Health Organization guidelines for the management of respiratory failure in COVID-19 do advocate, however, the use of CPAP or NIV, provided that appropriate PPE is worn.28
On the basis of a recent review of the literature on maximum exhaled air dispersion via different oxygen administration and ventilatory support strategies, we have concluded that CPAP via an oronasal mask and NIV via a helmet equipped with an inflatable neck cushion are the ventilatory support methods that allow minimum room air contamination; less than with every other oxygen delivery system.42
These studies were conducted with a human simulator device in a negative-pressure room with at least six air exchanges per hour (minimum air changes per hour recommended by the World Health Organization is 12). In medical wards not equipped with negative-pressure rooms, higher exhaled air dispersion and contamination are likely, so the use of a HEPA filter is recommended. If negative-pressure rooms are not available, rooms with natural ventilation with airflow of at least 160 L/s per patient is recommended.
Indications
The most recent European Respiratory Society/American Thoracic Society guidelines made no recommendation for or against the use of NIV during a pandemic, due to insufficient evidence, but they do state that during pandemics a trial of NIV could be considered in carefully selected patients at experienced centers in a protected environment.43 However, further research is needed before this could be recommended.
Discouraging NIV in the COVID-19 pandemic may increase the need for intubation and lead to increased morbidity and mortality and decreased ventilator availability, especially in those geographical areas hit hard by the pandemic.
Observational studies showed that NIV or CPAP may stabilize the clinical course of a patient with mild to moderate acute respiratory failure due to COVID-19, provided the patient does not demonstrate a high inspiratory drive or exert excessive inspiratory efforts.5 , 8 Caveats would include careful patient selection so as not to delay intubation where appropriate.
In an observational study of 459 ICU patients from 50 countries, Bellani et al44 demonstrated that NIV was used in real life in about 15% of patients with ARDS, irrespective of severity of disease. However, NIV was associated with higher ICU mortality in patients with a Pao 2/Fio 2 ratio less than 150 mm Hg, suggesting that the “potential” target for a cautious NIV trial may be above this Pao 2/Fio 2 ratio.
Observing patients for 1 to 2 hours after instituting NIV is important. An increasing respiratory rate and recruitment of accessory muscles use would indicate high work of breathing, suggesting the need for intubation.45 In conclusion, a cautious NIV trial may be indicated in a subset of patients with mild to moderate acute respiratory failure, using interfaces that minimize droplet dispersion (ie, the helmet) in negative-pressure rooms or even in a space with sufficient airflow, and protecting the personnel with personal protective equipment.
Awake Proning
Background
First described in the 1970s in both intubated and spontaneously breathing patients,46 the use of prone positioning has become a mainstay tool to ameliorate physiology and survival in hypoxemic respiratory failure requiring mechanical ventilation associated with ARDS. Although the frequent beneficial effects on hypoxemia are touted, the survival benefit in mechanically ventilated patients is independent of the improvement in arterial oxygen saturation.47 As the availability of both invasive and noninvasive adjuncts for mechanical ventilation may not meet demand in a pandemic, and the avoidance of invasive mechanical ventilation may itself be beneficial, the concept of proning in spontaneously breathing patients has been reexplored in small studies, attaining more urgency with the COVID-19 pandemic.
Evidence
Although there have been few studies and case reports in the nonintubated population with hypoxemic respiratory failure, and the total number of patients included is relatively small, the results of these studies have been uniformly positive. In the pre-COVID era, Scaravilli et al48 reported retrospective data confirming improvement in oxygenation during prone positioning in patients receiving oxygen or noninvasive ventilation modalities with moderate to severe hypoxemic respiratory failure. The improvement was reversed on resupination. Ding et al49 reported a series of 20 nonintubated patients with moderate to severe ARDS treated with HFNC or NIV who underwent a mean of approximately two proning sessions per day for an average of 2 hours each. Most of the patients experienced improved oxygenation, and intubation was avoided in 11 of the patients.
In the nascent COVID-19 era, Caputo et al50 have reported their ED experience using awake self-proning for 50 consecutive patients with COVID-19 with Spo 2 < 93% on supplemental oxygen, excluding patients requiring NIV. The median Spo 2 on supplemental oxygen therapy improved from 84% to 94% after 5 min of self-proning; seven of the patients did require endotracheal intubation within 60 min of the intervention. In a case series of 24 patients, Elharrar et al51 found that 63% of patients tolerated 3 hours or more of proning and 25% had a > 20% increase in Pao 2 but returned toward baseline on resupination. In another case series of 15 patients receiving NIV in the prone position for a median of two cycles with a total duration of 3 hours, Sartini et al52 found improvements in Pao 2:Fio 2 ratio and Spo 2 that were sustained 60 min after pronation in 80% of the patients. All patients experienced a decrease in respiratory rate and most felt an improvement in comfort. In a retrospective study by Xu et al,53 10 patients with diagnosed COVID-19 infection and P/F < 300 were given early awake proning for more than 16 hours per day combined with HFNC. None of these patients required invasive mechanical ventilation and all survived. The authors proposed the concept of using early prone positioning with HFNC as a concept to, “reduce the proportion of severe COVID-19 conversion to critical illness.”
Guidelines
There are no formal guidelines for proning nonintubated patients. Patients are frequently advised to remain prone for as long as tolerated. However, protocolization may improve compliance and provide a time frame that may be helpful. Suggestions include having the patient vary their position every 2 hours among prone, left and right lateral decubitus, and supine positions. Provision of an informational flyer to the patient may be of use in promoting compliance.
In our experience, lack of compliance may be encountered in obese patients and those with a history of back pain. For these patients it may be helpful to start with shorter intervals of time and to provide a team to help with positioning. Multiple mattress adjuncts have been proposed that may help adaptation.
Precautions
Caution and vigilance must be exercised in all proned patients. Optimal timing in this position remains unknown. It may be tempting and useful in individual circumstances to aid compliance with the use of mild sedation or anxiolysis, but this cannot be advocated without close monitoring of vital signs and oxygenation. Oxygenation adjuncts may become displaced during the practice of proning, with life-threatening results. Although the development of pressure ulcers is unlikely in the awake proned patient, provision of appropriate padding to pressure points such as shoulders and knees should be considered. The judicious use of pillows positioned under the pelvis may be useful. Finally, the improvement in oxygenation may be transient or lead to a false sense of security, delaying a potentially life-saving conversion to invasive mechanical ventilation.
To exemplify the clinical application of these modalities, we present an illustrative case in e-Appendix 1.
Conclusions
Patients with COVID-19 frequently develop pulmonary involvement resulting in hypoxemic respiratory failure. The prior dictum of progressing from nasal cannula to nonrebreather face mask and then to invasive mechanical ventilation is applicable to the majority of these patients. However, there may be approximately 20% to 25% of patients with COVID-19 in whom modalities such as high-flow nasal cannula therapy, noninvasive ventilation, and awake proning may stabilize their respiratory status and obviate the need for intubation. Similarly, a fraction of recently extubated patients, who are demonstrating respiratory distress or hypoxemia, may be stabilized without needing reintubation with these modalities. Helmet masks have been used commonly in Italy during the COVID pandemic with good results. There are very few data to prove that high-flow nasal cannula and noninvasive ventilation (especially with a helmet mask) result in dispersion of viral particles. However, it is imperative that when these devices are used, negative-pressure rooms or HEPA filters along with proper PPE including N-95 masks be used to protect health-care providers.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. N. is on the advisory board for Breathe and has received honoraria from Philips Respironics for medical lectures. N. S. H. is consultant for and has received research grants to his institution from Fisher & Paykel. He is also a consultant and has served on scientific advisory boards for Philips Respironics. None declared (S. R., C. C.).
Other contributions: The authors acknowledge the assistance of Linda Kirschenbaum, DO, in helping to prepare the algorithm and Patrice Balistreri, AS, for secretarial assistance.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Supplementary Data
References
- 1.Hui D.S., Chow B.K., Chu L., et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J.W., Li Y. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7(12):758. doi: 10.1016/S1473-3099(07)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W., Elankumaran S., Marr L.C. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J R Soc Interface. 2011;8(61):1176–1184. doi: 10.1098/rsif.2010.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonnet A., Chetboun M., Poissy J., et al. LICORN and the Lille COVID-19 and Obesity Study Group High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-Co-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):P475–P481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhataraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong B.C., Lee N., Li Y., et al. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin Infect Dis. 2010;51(10):1176–1183. doi: 10.1086/656743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L., Chiumello D., Caironi P., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 15.Cheung J.C., Ho L.T., Cheng J.V., et al. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020;8(4):e19. doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nourdine K., Combes P., Carton M.J., Beuret P., Cannamela A., Ducreux J.C. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999;25(6):567–573. doi: 10.1007/s001340050904. [DOI] [PubMed] [Google Scholar]
- 18.Spoletini G., Alotaibi M., Blasi F., Hill N.S. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1):253–261. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 19.Appendini L., Patessio A., Zanaboni S., et al. Physiologic effects of positive end-expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;149(5):1069–1076. doi: 10.1164/ajrccm.149.5.8173743. [DOI] [PubMed] [Google Scholar]
- 20.Mehta S., Jay G.D., Woolard R.H., et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25(4):620–628. doi: 10.1097/00003246-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Roca O., Hernández G., Díaz-Lobato S., et al. Spanish Multidisciplinary Group of High Flow Supportive Therapy in Adults (HiSpaFlow) Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20(1):109. doi: 10.1186/s13054-016-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roca O., Riera J., Torres F., Masclans J.R. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408–413. [PubMed] [Google Scholar]
- 23.Frat J.P., Thille A.W., Mercat A., et al. FLORALI Study Group; REVA Network High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 24.Stéphan F., Barrucand B., Petit P., et al. BiPOP Study Group High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 25.Hernández G., Vaquero C., Colinas L., et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 26.Hernández G., Vaquero C., González P., et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354–1356. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 27.Thille A.W., Muller G., Gacouin A., et al. HIGH-WEAN Study Group and REVA Research Network Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322(15):1465–1475. doi: 10.1001/jama.2019.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance. 28 January, 2020. https://apps.who.int/iris/handle/10665/330893
- 29.Alhazzani W., Hylander Møller M., Arabi Y.M., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui D.S., Chow B.K., Lo T., et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4):1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 31.Hui D.S., Hall S.D., Chan M.T., et al. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest. 2007;132(2):540–546. doi: 10.1378/chest.07-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui D.S., Hall S.D., Chan M.T., et al. Noninvasive positive-pressure ventilation: an experimental model to assess air and particle dispersion. Chest. 2006;130(3):730–740. doi: 10.1378/chest.130.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard S., Atwood C.W., Jr, Walsh B.K., et al. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask: implications for the high-flow nasal cannula. Chest. 2020;158(3):1046–1049. doi: 10.1016/j.chest.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwashyna T.J., Boehman A., Capecelatro J., et al. Aerosol production across oxygen delivery devices in spontaneously breathing human subjects. medRxiv. [Preprint] April 20, 2020. https://www.medrxiv.org/content/10.1101/2020.04.15.20066688v1
- 35.Roca O., Caralt B., Messika J., et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 36.Meduri G.U., Conoscenti C.C., Menashe P. Noninvasive mechanical ventilation in acute respiratory failure: happy 30-year anniversary! Chest. 2020;157(2):255–257. doi: 10.1016/j.chest.2019.09.035. [DOI] [PubMed] [Google Scholar]
- 37.Crimi C., Noto A., Princi P., Esquinas A., Nava S. A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36(2):362–369. doi: 10.1183/09031936.00123509. [DOI] [PubMed] [Google Scholar]
- 38.Vignaux L., Piquilloud L. Varying leaks: a challenge for modern ventilators? Respir Care. 2013;58(12):2194–2195. doi: 10.4187/respcare.02933. [DOI] [PubMed] [Google Scholar]
- 39.Vitacca M., Nava S., Santus P., Harari S. Early consensus management for non-ICU acute respiratory failure SARS-CoV-2 emergency in Italy: from ward to trenches. Eur Respir J. 2020;55(5):2000632. doi: 10.1183/13993003.00632-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Italian Thoracic Society (AIPO); Italian Respiratory Society (SIP). Managing the Respiratory Care of Patients With COVID-19. March 8, 2020. https://ers.app.box.com/s/j09ysr2kdhmkcu1ulm8y8dxnosm6yi0h. Accessed July 27, 2020.
- 41.Vargas F., Thille A., Lyazidi A., Campo F.R., Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37(6):1921–1928. doi: 10.1097/CCM.0b013e31819fff93. [DOI] [PubMed] [Google Scholar]
- 42.Ferioli M., Cisternino C., Leo V., Pisani L., Palange P., Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir J. 2020;29(155):200068. doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochwerg B., Brochard L., Elliott M.W., et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 44.Bellani G., Laffey J.G., Pham T., et al. LUNG SAFE Investigators. ESICM Trials Group Noninvasive ventilation of patients with acute respiratory distress syndrome: insights from the LUNG SAFE Study. Am J Respir Crit Care Med. 2017;195(1):67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 45.Brochard L., Slutsky A., Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 46.Douglas W.W., Rehder K., Beynen F.M., Sessler A.D., Marsh H.M. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis. 1977;115(4):559–566. doi: 10.1164/arrd.1977.115.4.559. [DOI] [PubMed] [Google Scholar]
- 47.Albert R.K., Keniston A., Baboi L., Ayzac L., Guérin C., Proseva Investigators Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189(4):494–496. doi: 10.1164/rccm.201311-2056LE. [DOI] [PubMed] [Google Scholar]
- 48.Scaravilli V., Grasselli G., Castagna L., et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Ding L., Wang L., Ma W., He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multicenter prospective cohort study. Crit Care. 2020;24(1):28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caputo N.D., Strayer R.J., Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elharrar X., Trigui Y., Dols A.-M., et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sartini C., Tresoldi M., Scarpellini P., et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323(22):2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Q., Wang T., Qin X., Jie Y., Zha L., Lu W. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care. 2020;24(1):250. doi: 10.1186/s13054-020-02991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.