Abstract
Furin, a cleavage enzyme, is increasingly recognized in the pathogenesis of metabolic syndrome. Its cleavage action is an essential activation step for the endothelial pathogenicity of several viruses including SARS-CoV-2. This Furin-mediated endothelial tropism seems to underlie the multi-organ system involvement of COVID-19; which is a feature that was not recognized in the older versions of coronaviridae. Obese and diabetic patients, males, and the elderly, have increased serum levels of Furin, with its increased cellular activity; this might explain why these subgroups are at an increased risk of COVID-19 related complications and deaths. In contrast, smoking decreases cellular levels of Furin, this finding may be at the origin of the decreased severity of COVID-19 in smokers. Chinese herbal derived luteolin is suggested to be putative Furin inhibitor, with previous success against Dengue Fever. Additionally, Furin intracellular levels are largely dependent on concentration of intracellular ions, notably sodium, potassium, and magnesium. Consequently, the use of ion channel inhibitors, such as Calcium Channel blockers or Potassium Channel blockers, can prevent cellular transfection early in the course of the illness. Nicotine patches and Colchicine have also been suggested as potential therapies due to Furin mediated inhibition of COVID-19.
Keywords: COVID-19, Polybasic cleavage site, Furin, Endothelial pathogenicity, Chinese herbals, Ion channel inhibitors
List of abbreviations
- ACE
angiotensin-converting enzyme
- CoV
Coronaviridae
- COVID-19
Coronavirus disease 2019
- CCB
calcium channel blocker
- DM
Diabetes mellitus
- FDA
Food and drug administration
- HIV
Human immunodeficiency virus
- LDL
Low-density lipoprotein
- MERS
Middle East Respiratory syndrome
- PCSK
pro-protein convertase subtilizing/kexin
- SARS
Severe acute respiratory distress syndrome
The Coronaviridae family of viruses incorporates an assortment of pathogens that are linked to a zoonotic origin. This is one of the fundamental elements causing former outbreaks of the severe acute respiratory syndrome (SARS- CoV) and Middle East respiratory syndrome (MERS-CoV) (Phan et al., 2018), as well as the current pandemic severe acute respiratory syndrome coronavirus (Payne, 2017) (SARS-CoV-2) or Coronavirus Disease 2019 (COVID-19).
However, COVID-19 has shown exponential dissemination in the community, faster than MERS and SARS (Petrosillo et al., 2020). This is supported by confirmed metrics on the total number of COVID-19 cases (as of June 2nd 2020) to be 6.06 million (Velavan and Meyer, 2020), compared to SARS-CoV-1 and MERS that were 8439 and 2519 (Aguanno et al., 2018) respectively. Furthermore, the clinical picture of COVID-19, which is due to multi-organ system damage on account of microvascular injury, seems to be slightly different than that of SARS-CoV-1 and MERS, mainly targeting the lungs (AbdelMassih et al., 2020a, 2020b; 2020c, 2020d).
1. Furin and COVID-19 infection
FURIN is an enzyme that belongs to the PCSK (pro-protein convertase subtilizing/kexin) family, a type 1 membrane-bound protease that processes latent precursor proteins into their biologically active products. Furin is also utilized by several pathogens, where cleavage by Furin is needed for their functionality. This includes bacterial toxins, viral envelopes such as those of Human Immunodeficiency Virus (HIV), Ebola, and Marburg virus, as well as the spike protein of the SARS-Cov-2. Targeting host cell Furin is therefore a potential novel therapeutic option against these Furin-dependent infections. Of note, viruses develop high pathogenicity once they become reactive with Furin and other PC (Solovyeva et al., 2017).
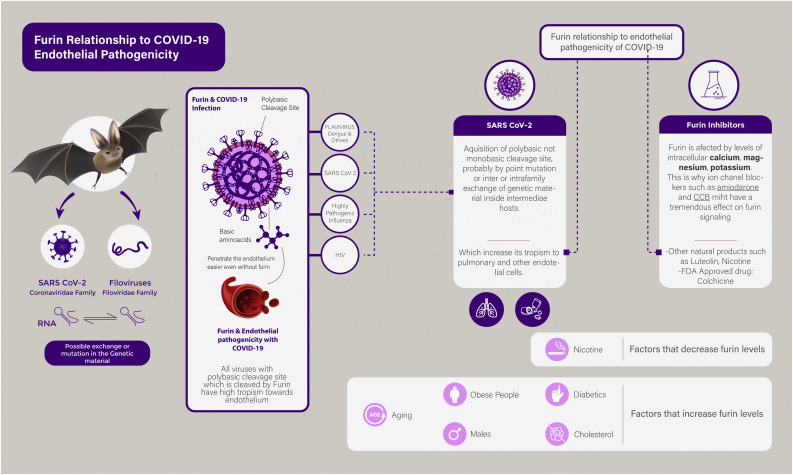
A notable feature of SARS-CoV-2 is a polybasic cleavage site at the junction of S1 and S2, the two subunits of the spike. This allows effective cleavage by Furin, and has a role in determining viral infectivity and host range. These efficient polybasic cleavage sites have not been observed in related lineages of beta-coronaviruses. The acquisition of such polybasic cleavage site might have resulted from a point mutation, or from exchange of genetic material with another bat virus such as one of the filoviruses that share this common feature and a common intermediate host, namely bats (Hoffmann et al., 2020).
The subtilisin-like proteases, such as Furin, require polybasic cleavage sites rather than monobasic cleavage sites to activate respective viruses. A good example for this is, the difference in pathogenicity between highly pathogenic influenza strains, and those with lesser pathogenicity (Braun and Sauter, 2019).
2. Furin upregulation in relation to metabolic syndrome, aging and androgen levels
Several demographic factors as well as disease states have been linked to increased COVID-19 complications, notably metabolic syndrome including diabetes and obesity (Abbas and Kamel, 2020; Belančić et al., 2020; Mediouni et al., 2020; Rahmati-Ahmadabad and Hosseini, 2020). Aging and male gender have been also linked to augmented profile of COVID-19 complications. Could there be a relationship between Furin and COVID-19 severity in such risk factors (Lithander et al., 2020; Rahmati-Ahmadabad and Hosseini, 2020; Wambier et al., 2020).
Although Furin is a membrane protein, earlier work indicates the existence of a secreted form of Furin. It has been previously shown that cells transfected with a recombinant Furin gene secrete a soluble truncated Furin protein, with similar enzymatic activity as the membrane-bound protein. Furthermore, it is established that Furin is partially shed from most cells.
2.1. Furin and diabetes
Plasma Furin levels were associated with future DM (Diabetes Mellitus) and obesity, independently of all traditional DM risk factors. Regarding potential mechanisms, as Furin is responsible for the maturation of the insulin pro-receptor; one could speculate that more Furin in the circulation reflects a compensatory mechanism to increase the synthesis of active insulin receptors (Fernandez et al., 2018; He et al., 2020). Another possible mechanism of action of Furin in DM development may be via pancreatic beta cells, as Furin has been demonstrated to control the proliferation and differentiation of pancreatic beta cell lines, as well as involvement in the maturation of insulin secretory granules (Kayo et al., 1997).
2.2. Furin and cholesterol
Furthermore, circulating Furin cleavage of PCSK9 (proprotein convertases subtilisin/kexin 9) implies a regulatory role on LDL (low-density lipoproteins) receptors and cholesterol levels. There appears to be a sort of cholesterol dependent priming of SARS-Cov-2 viral entry, where cellular loading with cholesterol using apolipoprotein E, showed enhancement of endocytic entry of SARS-Cov-2. Twice the normal entry points for SARS-Cov-2 were found in the setting of increased cholesterol. Cholesterol is further implicated in trafficking of ACE2 (Angiotensin Converting Enzyme 2), the known receptor and entry point of the SARS-Cov-2 virus; cholesterol increased receptor binding domains. A cholesterol dependent theory for COVID-19 mortality is suggested; as those with increased cholesterol, i.e. obese, diabetic, and elderly, are at increased risk owing to Furin priming, and increased viral entry points (Wei et al., 2020).
2.3. Furin and obesity
Kappert et al. underlined the importance of intracellular Furin in the pathogenesis of obesity, and related cardiometabolic syndrome. Furin has been found to play a role in redirecting lipid deposition in white adipose tissue. Moreover, Furin was found in abundance in mononuclear cells leading to increased interaction and cell signaling between adipocytes and mononuclear inflammatory cells. The latter paracrine mechanism induces vascular remodeling, and atherosclerosis through lipid deposition and chronic inflammation (Kappert et al., 2013).
The increased cellular and serum levels of Furin in diabetes, hypercholesterolemia, and obesity might explain the increased profile of COVID-19 complication in such disease states.
2.4. Furin and androgen
The only proven relationship between PCs including furin and androgen, has been proven in the context of androgen dependent prostatic cancer. Couture et al. found that proprotein convertase activity is upregulated in prostate cancer. This might be mediated through increased androgen levels, or through increased activity of androgen receptors that act as protein chaperones to several types of proprotein convertases. This linear relationship between PCs and androgen might explain why COVID-19 disease burden is higher in males compared to females (Couture et al., 2012; Wadman, 2020).
2.5. Furin and aging
There is little data about Furin upregulation in aging. To our knowledge the only study to date to outline this relationship was conducted by Grobelna and colleagues. Grobelna et al. proved that Furin is implicated in vascular aging; and proved as well that the serum levels of Furin are positively correlated with age dependent atherosclerosis (Grobelna et al., 2019).
The above relationship might explain the increased deaths from COVID-19 in the elderly.
In view of the above, serum Furin levels might serve as an independent predictor of COVID-19 complications in at risk groups.
2.6. Furin levels in smokers
Interestingly, Ranta and colleagues found an interesting relationship between smoking and shed serum Furin levels. Their study explored whether serum Furin levels can be used as a biomarker for septicemia, and results showed that serum Furin levels were reduced in smokers compared to non-smokers (Ranta et al., 2015). The latter finding might explain why smokers had lesser COVID-19 complications (Israel et al., 2020).
3. Furin and endothelial pathogenicity and tropism
Furin is highly expressed in vascular endothelial cells. Mayer and colleagues succeeded in revealing the exact endothelial location of Furin. They demonstrated by electron microscopy that Furin is located over the plasma membrane of endothelial cells of the continuous, fenestrated, and discontinuous capillaries. This location goes in agreement with several previous studies relating Furin to the endothelial permeability of many molecules, as well as some organisms (Mayer et al., 2004).
Moreover, Furin-processed proteins, such as cadherins, vascular endothelial growth factor, factor IX, and von Willebrand factor, are known to be involved in vascular permeability. These facts constitute strong evidence that Furin plays a major role in viral tropism to the endothelium. As discussed earlier, several viruses harbor a polybasic cleavage site that allows easier and canonical binding to Furin, allowing them to exploit Furin to regulate cell entry and infectivity (Schlokat et al., 1996). These include; Flaviviruses including Dengue fever virus, HIV, highly pathogenic strains of influenza viruses, and SARS-CoV-2 (Braun and Sauter, 2019). The aforementioned viruses have a selective tropism to the endothelium. Their pathogenesis relies either on transfection of endothelial cells, or impairment of endothelial function through indirect inflammatory mechanisms without endothelial penetration. It is unclear if the observed endothelial pathogenicity is the result of Furin mediated canonical damage, or due to other incompletely understood mechanisms (Racaniello, 2020) (Table 1 summarizes the mechanisms of each of the mentioned viruses in inducing endothelial damage and the resultant clinical manifestations).
Table 1.
Summary of endothelial pathogenicity of viruses activated canonically by Furin.
| Virus | Endothelium Tropism | Clinical evidence of endothelial dysfunction | |
|---|---|---|---|
| SARS-CoV-2 | Direct: Transfection of endothelial cells by ACE2 receptors that are highly expressed in endothelial cells Indirect
|
|
|
| HIV | Direct:
|
Accelerated atherosclerosis starting by increased intima-media thickness. Subsequent increase in coronary artery disease, peripheral vascular disease |
|
| Influenza | Direct: Apoptosis of infected endothelial cells and significant drop in TEER indicated increased permeability to ions and macromolecules. Indirect: -Influenza induced degradation of tight junction protein claudin-5 by matrix metalloproteinase -Increases macrophage entry into arterial wall |
Acute Lung injury owing to pulmonary microvascular leakage and apoptosis Exacerbation of existing cardiovascular disease with increased risk for CV mortality Risk of myocardial infarction, pericarditis and effusions, heart failure, myocarditis, and cardiac conduction system affection Deceased mortality and incidence of any cardiovascular event in vaccinated persons Increased pro-inflammatory and pro-thrombotic cytokines, increased plasma viscosity, hemo-concentration, hypoxia, and demand ischemia Increase platelet aggregation, clotting time, DIC, FDP, soluble fibrin, tissue factor |
|
| Hemorrhagic Fevers | Filovirus: Marburg virus |
Direct Viral replication in endothelial cells associated with virus induced-cytokine release lead to endothelial damage Indirect: MBG virus's primary target cells are monocytes and macrophage. MBG infects mono\macrophages leading to their activation resulting in release of several cytokines, one of which is TNF alpha. TNF alpha leads to an increase in Para endothelial permeability by formation of inter-endothelial gaps. This vascular instability results in fulminant shock and defective hemostasis |
DIC Hemorrhage (mucosal/sub mucosal/internal) and third space loss leading to hypovolemic and distributive shock CNS: Migraines, Strokes |
| Dengue Fever Viruses | Direct: Direct Transfection of endothelial cells has been rarely demonstrated from autopsies Indirect:
|
||
Abbreviations: ACE2: Angiotensin-converting enzyme 2, CNS: Central nervous system, CV: Cardiovascular, DIC: Disseminated intravascular coagulopathy, eNOS: Endothelial Nitric Oxide Synthase, FDP: Fibrin degradation products, gp120: Envelope glycoprotein 120, HIV: Human immunodeficiency virus, IL: Interleukin, MBG: Marburg virus, Nef: Negative regulatory factor, Tat: transactivator of transcription, TEER: Trans epithelial/Trans endothelial Electrical Resistance, TNF: Tumor necrosis factor.
An important theory that can explain the endothelial pathogenicity of those viruses is the “vascular zip code” theory. Endothelial penetrability of drugs and other molecules have been the focus of many onco-pharmacology studies. The high vascularity of tumors makes targeting the endothelium an important selective strategy for treatment. Such studies have revealed that coupling drugs or nanoparticles with basic amino-acid residues such as lysine or arginine, renders those compounds highly tropic to the endothelium. SARS-CoV-2, filoviruses, HIV, and highly pathogenic strains of influenza, harbor a polybasic amino-acid sequence that not only make them readily activated by Furin, but might explain their ability to transfect the endothelial cells (Enbäck and Laakkonen, 2007; Gilbert et al., 2008; Han et al., 2006; Ruoslahti, 2004).
4. Furin inhibition; exploiting an old strategy against COVID-19
4.1. Naturally occurring substances acting as selective furin inhibitors
Furin inhibition has been tried against viruses with polybasic Furin cleavage sites with debatable results. Luteolin, a molecule derived from traditional Chinese herbal products, has been found to decrease Dengue Fever severity and viral load through selective inhibition of Furin (Peng et al., 2017).
Further evidence to the role of Furin inhibitors as a potential therapeutic target, is in their use in the treatment of HIV. Hallenberger et al. confirmed the role of an old generation of Furin inhibitors, peptidyl-chloromethyl ketones, in interruption of formation of infectious viral particles. A major setback is the high toxicity of these compounds (Hallenberger et al., 1992).
Gagnon et al. concluded that the use of a nanomolar particle stabilized by Aza-Beta-amino-acid could effectively inhibit H5N1 influenza (Gagnon et al., 2014).
As mentioned previously, COVID-19 related deaths and complications are paradoxically reduced in smokers, smoking might operate through reducing endothelial penetration of COVID-19 by inhibition of Furin (Ranta et al., 2015). Israel et al. proved in his large observational study that COVID-19 complications were lesser insmokers (Israel et al., 2020); a finding that was also confirmed by Gonzalez-Rubio and colleagues, who concluded that cytokine release is tamed by Nicotine (Gonzalez-Rubio et al., 2020). The latter findings could lead to the therapeutic use of Nicotine patches after confirming this role through clinical trials.
4.2. The FDA (Food and drug administration) approved colchicine
Colchicine is a drug that is used in the management of inflammatory complications of Gout, as well as auto-inflammatory syndromes such as familial Mediterranean fever. Colchicine acts by targeting several inflammatory pathways, and has been found to target specifically endothelial inflammation and vascular degeneration through Furin. (Xu, 2016). Montealegre-Gómez confirmed colchicine drug potential in the setting ofCOVID-19, in a small cohort of patients in Bogota, Colombia. This role should be consolidated by larger scale clinical trials (Montealegre-Gómez et al., 2020).
4.3. Ion-channel inhibitors
Furin is a cellular protein. There is clear evidence that Furin interacts with intracellular ions. Magnesium, the second most occurring intracellular ion after Potassium, has been shown to activate Furin (Izidoro et al., 2010).
Molloy and colleagues demonstrated that Furin is a calcium dependent enzyme. The levels of intracellular calcium are critical for the activation of Furin (Molloy et al., 1992). Another evidence of the relationship between Furin activity and calcium has been elucidated by Yamada et al. Yamada et al. underlined the role of Furin inhibitors in prevention of excessive neuronal damage resulting from calcium influx following hypoxic insult (Yamada et al., 2018). In this aspect, blocking calcium channels can be an effective strategy for Furin-activated organisms. Li et al. proved, in 2019, that Calcium Channel Blocker (CCB) can decrease the severity of severe fever and thrombocytopenia syndrome; a tick-borne hemorrhagic fever. Zhang et al. underlined the beneficial role of CCB in patients with COVID-19 (Li et al., 2019).
There is no clear relationship between intracellular potassium levels and Furin activity; however amiodarone had been shown to block viral entry of some flaviruses. This role has been confirmed against COVID-19 by Gehring et al. (2014).
This antiviral role of ion channel blockers is speculated to be more effective with low viral loads, and at the start of infection, when virus entry into target cells is the dominant step (Fig. 1 ).
Fig. 1.
Summarizes the Furin mediated endothelial tropism of SARS-CoV-2 and the ensuing therapeutic targets.
5. Conclusion
The acquisition of polybasic cleavage site by the new SARS-CoV-2 seems to be an important feature for endothelial penetrability and pathogenicity of COVID-19. This might occur through its canonical activation by Furin, and through endothelial affinity to basic amino acid residues. Additionally, the interaction of SARS-CoV-2 with Furin offers an explanation to the augmented manifestations of COVID-19 in obese and diabetic patients, as well as in males and elderly people.
Screening serum levels of Furin early in the course of COVID-19 complications might serve as an important strategy to anticipate COVID-19 poor outcomes, and preventing them.
Finally, and importantly, this relationship opens up new therapeutic strategies in the treatment of COVID-19, such as the use of Chinese herbals, ion channel inhibitors, nicotine patches, and Colchicine, for prevention of endothelial involvement and multi-organ failure in affected patients.
6. Recommendations
In view of the above, we strongly recommend the screening of baseline serum Furin levels early in the course of COVID-19 infections, and correlating such levels with COVID-19 complications and mortality; we recommend the launching of clinical trials involving the use of the aforementioned Furin inhibitors in COVID-19 patients.
CRediT authorship contribution statement
Antoine AbdelMassih and Raghda Fouda : Conceptualization Writing review&editing.
All other authors: Datacuration, Writing-review&editing.Visualization,Investigation,Writing-original draft.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgment
As a first author I wanted to thank my students and colleagues who made this work possible. I wanted also to thank the families of my students, interns and residents, their efforts in wonderfully raising them and constructing their critical thinking has made them the way they are.
References
- Abbas Ahmed M., Kamel Mark Mohsen. Dietary habits in adults during quarantine in the context of COVID-19 pandemic. Obes. Med. 2020;19(April):100254. doi: 10.1016/j.obmed.2020.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdelMassih Antoine, et al. Cardiovascular Endocrinology & Metabolism Publish Ah; 2020. Obese Communities Among the Best Predictors of COVID-19-Related Deaths.https://journals.lww.com/10.1097/XCE.0000000000000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmassih Antoine F., et al. Possible molecular and paracrine involvement underlying the pathogenesis of COVID-19 cardiovascular complications. Cardiovasc. Endocrinol. Metabol. Publish Ah. 2020 doi: 10.1097/XCE.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdelMassih Antoine., et al. Unleashing the mysterious link between COVID-19 and a famous childhood vasculitis: Kawasaki disease. Egypt Pediatr. Assoc. Gaz. 2020;68:21. doi: 10.1186/s43054-020-00029-9. [DOI] [Google Scholar]
- AbdelMassih Antoine Fakhry, et al. Is it infection or rather vascular Inflammation? Game-changer insights and recommendations from patterns of multiorgan involvement and affected subgroups in COVID-19. Cardiovasc. Endocrinol. Metabol. Publish Ah. 2020 doi: 10.1097/XCE.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguanno Ryan, et al. MERS: progress on the global response, remaining challenges and the way forward. Antivir. Res. 2018;159:35–44. doi: 10.1016/j.antiviral.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belančić Andrej, Kresović Andrea, Rački Valentino. Potential pathophysiological mechanisms leading to increased COVID-19 susceptibility and severity in obesity. Obes. Med. 2020;19(May):100259. doi: 10.1016/j.obmed.2020.100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Elisabeth, Sauter Daniel. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl. Immunol. 2019;8(8) doi: 10.1002/cti2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture Frédéric, et al. 2012. Role of Proprotein Convertases in Prostate Cancer Progression. Neoplasia (United States) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enbäck J., Laakkonen P. Tumour-homing peptides: tools for targeting, imaging and destruction. Biochem. Soc. Trans. 2007:780–783. doi: 10.1042/BST0350780. [DOI] [PubMed] [Google Scholar]
- Fernandez C., et al. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J. Intern. Med. 2018;284(4):377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon Hugo, et al. Optimization of furin inhibitors to protect against the activation of influenza hemagglutinin H5 and shiga toxin. J. Med. Chem. 2014;57(1):29–41. doi: 10.1021/jm400633d. [DOI] [PubMed] [Google Scholar]
- Gehring Gerrit, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J. Antimicrob. Chemother. 2014;69(8):2123–2131. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert Mark, Mousa Shaymaa, Mousa Shaker A. Current status of anti-vascular endothelial growth factor (VEGF) strategies and future directions. Drugs Future. 2008;33(6):515–525. [Google Scholar]
- Gonzalez-Rubio Jesus, et al. Cytokine release syndrome (CRS) and nicotine in COVID-19 patients: trying to calm the storm. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobelna Malwina K., Strauss Ewa, Zbigniew Krasiński. “The role of proprotein convertase subtilisin-kexin type 9 (PCSK9) in the vascular aging process – is there a link? Kardiochirurgia i Torakochirurgia Polska. 2019;16(3):128–132. doi: 10.5114/kitp.2019.88602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenberger Sabine, et al. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein Gpl60. Nature. 1992;360(6402):358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- Han Yu, et al. Specific phage-displayed peptides binding to tumor vasculature. Int. J. Pept. Res. Therapeut. 2006;12(4):365–371. [Google Scholar]
- He Yan, et al. Deficient serum furin predicts risk of abdominal obesity: findings from a prospective cohort of Chinese adults. Postgrad. Med. 2020 doi: 10.1136/postgradmedj-2019-137422. [DOI] [PubMed] [Google Scholar]
- Hoffmann Markus, Kleine-Weber Hannah, Pöhlmann Stefan. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78(4):779–784. doi: 10.1016/j.molcel.2020.04.022. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Ariel, et al. Smoking and the risk of COVID-19 in a large observational population study. medRxiv. 2020 [Google Scholar]
- Izidoro Mario A., et al. Effects of magnesium ions on recombinant human furin: selective activation of hydrolytic activity upon substrates derived from virus envelope glycoprotein. Biol. Chem. 2010;391(9):1105–1112. doi: 10.1515/BC.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappert Kai, et al. Proprotein convertase subtilisin/kexin type 3 promotes adipose tissue-driven macrophage chemotaxis and is increased in obesity. PLoS One. 2013;8(8):1–12. doi: 10.1371/journal.pone.0070542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayo Tsuyoshi, et al. Proprotein-processing endoprotease furin controls growth of pancreatic β-cells. Diabetes. 1997;46(8):1296–1304. doi: 10.2337/diab.46.8.1296. [DOI] [PubMed] [Google Scholar]
- Li Hao, et al. calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 2019;29(9):739–753. doi: 10.1038/s41422-019-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithander Fiona E., et al. COVID-19 in older people: a rapid clinical review. Age Ageing. 2020;(May) doi: 10.1093/ageing/afaa093. 501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer Gaétan, Guy Boileau, Bendayan Moïse. Sorting of furin in polarized epithelial and endothelial cell: expression beyond the golgi apparatus. J. Histochem. Cytochem. 2004;52(5):567–579. doi: 10.1177/002215540405200502. [DOI] [PubMed] [Google Scholar]
- Mediouni Mohamed, Madiouni Riadh, Elżbieta Kaczor-Urbanowicz Karolina. COVID-19: how the quarantine could lead to the depreobesity. Obes. Med. 2020;19(April) doi: 10.1016/j.obmed.2020.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy S.S., et al. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 1992;267(23):16396–16402. [PubMed] [Google Scholar]
- Montealegre-Gómez Giovanni, et al. Colchicine: a potential therapeutic tool against COVID-19. Experience of 5 patients. Reumatol. Clínica. 2020 doi: 10.1016/j.reuma.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne Susan. 2017. “Family Coronaviridae.” in Viruses. [Google Scholar]
- Peng Minhua, et al. Luteolin restricts Dengue virus replication through inhibition of the proprotein convertase furin. Antivir. Res. 2017;143:176–185. doi: 10.1016/j.antiviral.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Petrosillo N., et al. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan My V.T., et al. Identification and characterization of Coronaviridae genomes from Vietnamese bats and rats based on conserved protein domains. Virus Evol. 2018;4(2):1–12. doi: 10.1093/ve/vey035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello Vincent. Furin cleavage site in the SARS-CoV-2 coronavirus glycoprotein. Virol. Blog. 2020 about viruses and viral disease (February): 1–34. [Google Scholar]
- Rahmati-Ahmadabad Saleh, Hosseini Fahimeh. Exercise against SARS-CoV-2 (COVID-19): does workout intensity matter? (A mini review of some indirect evidence related to obesity) Obes. Med. 2020;19(April):100245. doi: 10.1016/j.obmed.2020.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta N., et al. The plasma level of proprotein convertase furin in patients with suspected infection in the emergency room: a prospective cohort study. Scand. J. Immunol. 2015;48(3):173–177. doi: 10.1111/sji.12386. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem. Soc. Trans. 2004:397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- Schlokat Uwe, et al. Production of highly homogeneous and structurally intact recombinant von Willebrand factor multimers by furin-mediated propeptide removal in vitro. Biotechnol. Appl. Biochem. 1996;24(3):257–267. [PubMed] [Google Scholar]
- Solovyeva N.I., et al. Furin as proprotein convertase and its role in normal and pathological biological processes. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2017;11(2):87–100. [Google Scholar]
- Velavan Thirumalaisamy P., Meyer Christian G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020 doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadman Meredith. Sex hormones signal why virus hits men harder. Science. 2020 doi: 10.1126/science.368.6495.1038. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- Wambier, Gustavo Carlos, et al. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev. Res. 2020 doi: 10.1002/ddr.21688. April): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Congwen, et al. 2020. Cholesterol metabolism--impact for SARS-CoV-2 infection prognosis, entry, and antiviral therapies.2020.04.16.20068528 medRxiv. [Google Scholar]
- Xu Weiming. Proprotein convertase subtilisin/kexin type 9 (PCSK9): a promising therapeutic target for cardiovascular diseases. Enzyme Eng. 2016;5(3) [Google Scholar]
- Yamada Mariko, et al. Furin inhibitor protects against neuronal cell death induced by activated NMDA receptors. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-23567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]