To the Editor:
The first patient with confirmed COVID-19 in Portugal was seen in our emergency department on March 2, 2020.1 Our hospital is located in one of the most hard-hit areas in the country and admitted more patients with COVID-19 than any other. However, the first case and subsequent beginning of the pandemic were recorded in Portugal with an average delay of 1 month compared to other neighboring western European countries. This delay allowed health authorities to initiate a series of public health measures and individual medical departments to delineate strategies to deal with patients with and without COVID-19. Our department is a high-volume hepatology center, as attested by 11,500 adult outpatient clinics/year, 3,000 of which are new referrals, and nearly 500 inpatient admissions/year; thus, even a slowdown in clinical services was likely to have a substantial impact on outcomes.
We read with great interest the EASL-ESCMID position paper on the care of patients with liver disease during the COVID-19 pandemic first published in early April.2 In particular, we share Boettler et al.’s concerns regarding cirrhotic patients. Our Gastroenterology and Hepatology department established strategies to prioritize the care of these patients in times of limited healthcare resources. By the 13th of March, based on the limited literature available by then and on the experience with previous pandemics, these measures were outlined in a well-defined protocol, aiming to prevent SARS-CoV-2 infection, guarantee the best treatment to avoid hepatic decompensation, reduce loss to follow-up and avoid delayed medical referrals. The protocol was implemented in our practice by 18th of March and was in course until the end of state emergency on 2nd of May. Interestingly, some of the measures that were taken are also reflected in the aforementioned position paper.2 However, and because our protocol was approved before the publication of this position paper, some of them were not included. In addition, it only focused on patients with cirrhosis. Herein, we briefly describe the strategies implemented by our department and the obtained outcomes.
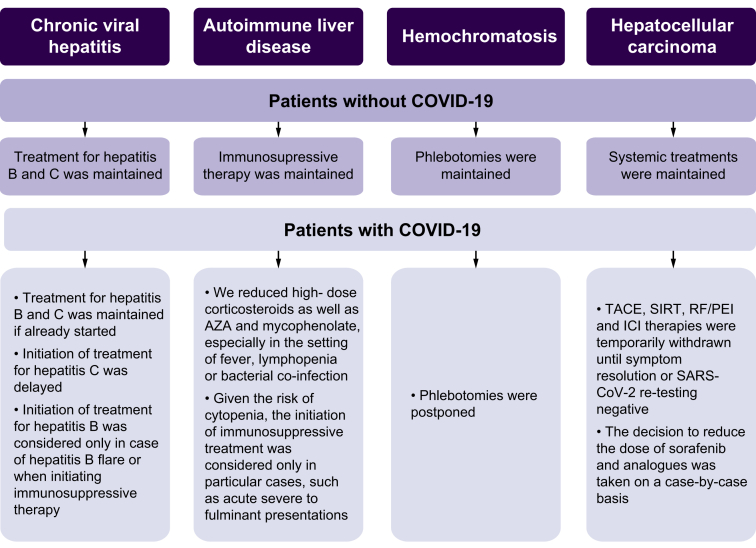
All outpatient clinics for patients with compensated cirrhosis were made by phone, ensuring that essential medications were available. Directed therapies for different etiologies were maintained. However, in case of SARS-CoV-2 infection, therapeutic adjustments were made (Fig. 1).
Fig. 1.
Therapeutic management according to the etiology of liver cirrhosis and associated complications.
AZA, azathioprine; ICI, immune checkpoint inhibitor; RF/PEI, radiofrequency ablation/percutaneous ethanol injection; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolization.
Non-invasive methods were used for the screening and surveillance of esophageal varices, namely through platelet count. Upper endoscopy was reserved for patients at high risk of bleeding, particularly if there was a history of previous bleed or signs of significant portal hypertension. In cirrhotic patients with COVID-19, endoscopy was performed only in life-threatening conditions. All patients proposed for endoscopic procedures were previously tested for SARS-CoV-2, through an RT-PCR nasopharyngeal swab test, and health professionals always used protective equipment during procedures. For primary prophylaxis beta-blockers were preferred instead of endoscopic band ligation, unless large varices or bleeding stigmata were discovered in an emergent endoscopy. Prophylaxis of spontaneous bacterial peritonitis and hepatic encephalopathy were maintained.3 Hepatocellular carcinoma (HCC) screening by ultrasonography has been delayed. Computed tomography and magnetic resonance imaging were performed with no delay if malignancy was suspected. Liver biopsy was reserved for marked elevation in aminotransferase levels of unknown etiology and suspicious liver nodules. Liver biopsies were postponed in patients with COVID-19.
A weekly hepato-biliary multidisciplinary cancer group meeting was maintained using a local web-platform. In case of HCC, systemic treatments were maintained according to guidelines.4 However, general precautions were in place, such as systematic screening for symptoms and fever before treatments. In patients with COVID-19, locoregional and immune checkpoint inhibitor therapies were temporarily withdrawn until symptom resolution or when SARS-CoV-2 re-testing was negative.
We created a “COVID-19 free” area in our day-hospital for patients who are going for therapeutic paracentesis or phlebotomy. Waiting rooms have been remodeled to allow sufficient distance between individuals and procedures were scheduled to reduce waiting times.
Lastly, a set of measures to prevent SARS-CoV-2 infection during hospitalizations were implemented, namely forbidding visits and strengthening cleaning services. In addition, all patients who were admitted had been tested for SARS-CoV-2, through an RT-PCR nasopharyngeal swab test, allowing the creation of COVID-19 and COVID-19-free wards. Patients with cirrhosis who tested positive for SARS-CoV-2 were admitted for inpatient care if another poor prognostic factor was present, such as cardiovascular diseases, Child B/C or HCC and they were managed in COVID-19 units by multidisciplinary teams. In-ward patients infected with SARS-CoV-2 were treated with 5-day hydroxychloroquine (400 mg bid on day 1 followed by 200 mg bid on day 2-5).
During the state of emergency, there were 37 hospital admissions due to decompensated cirrhosis (portosystemic encephalopathy [55%], ascites [13%] and variceal bleeding [10%]); compared to the same period last year, there was no significant increase in the rate of hospital admissions. In addition, 3 patients were admitted for locoregional therapy for HCC, which corresponds to 20% of the expected elective admissions. Only 2 elective endoscopic band ligations and 1 liver biopsy were performed, an overwhelming reduction on the number of elective procedures compared to the same period last year. We achieved a rate of almost 95% of medical appointments by telemedicine and therapeutic compliance in 90% of cases (assessed both by questioning and by the uptake of medicines in hospital pharmacy). In-person visits were restricted to 10 patients with decompensated cirrhosis. None of our patients were lost to follow-up. During the lockdown period, our hospital admitted 756 patients with COVID-19, 6 (0.8%) of whom had cirrhosis. Two of these cirrhotic patients had developed nosocomial COVID-19 infection.
This protocol, mostly mirroring the EASL position paper, shows that the level of care for cirrhotic patients can be maintained during the pandemic. Although laborious, it allowed a high level of patient adherence without an increase in frequency of cirrhotic complications. The impact of the strategies implemented by our Department during the lockdown period should be re-evaluated in the near future, so they may inform anticipatory changes in resource allocation during future pandemics.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
Isabel Garrido drafted the manuscript. Isabel Garrido, Rodrigo Liberal, Rui Gaspar and Guilherme Macedo have critically revised and finalized the manuscript. All authors have approved the final version of the manuscript. Guarantor of the article: Isabel Garrido.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100146.
Supplementary data
References
- 1.Direção-Geral da Saúde Comunicado da Diretora-Geral da Saúde com informação atualizada a 02/03/2020 | 17:28 - casos de infeção por novo Coronavírus (COVID-19) https://www.dgs.pt/a-direccao-geral-da-saude/comunicados-e-despachos-do-director-geral.aspx Available at:
- 2.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. [DOI] [PMC free article] [PubMed]
- 3.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.