Abstract
Rationale & Objective
Hemodialysis patients are at increased risk for coronavirus disease 2019 (COVID-19) transmission due in part to difficulty maintaining physical distancing. Our hemodialysis unit experienced a COVID-19 outbreak despite following symptom-based screening guidelines. We describe the course of the COVID-19 outbreak and the infection control measures taken for mitigation.
Study Design
Retrospective cohort study.
Setting & Participants
237 maintenance hemodialysis patients and 93 hemodialysis staff at a single hemodialysis center in Toronto, Canada.
Exposure
Universal screening of patients and staff for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Outcomes
The primary outcome was detection of SARS-CoV-2 in nasopharyngeal samples from patients and staff using reverse transcriptase–polymerase chain reaction (RT-PCR).
Analytical Approach
Descriptive statistics were used for clinical characteristics and the primary outcome.
Results
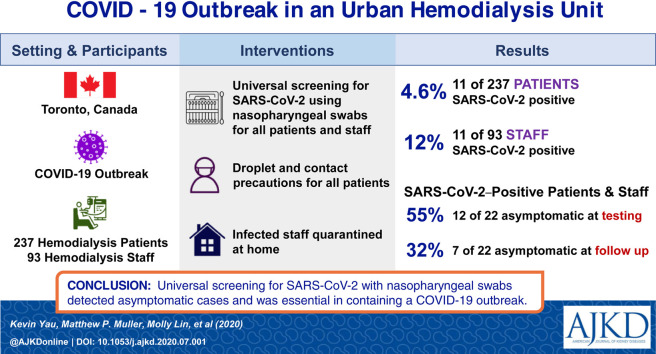
11 of 237 (4.6%) hemodialysis patients and 11 of 93 (12%) staff members had a positive RT-PCR test result for SARS-CoV-2. Among individuals testing positive, 12 of 22 (55%) were asymptomatic at time of testing and 7 of 22 (32%) were asymptomatic for the duration of follow-up. One patient was hospitalized at the time of SARS-CoV-2 infection and 4 additional patients with positive test results were subsequently hospitalized. 2 (18%) patients required admission to the intensive care unit. After 30 days’ follow-up, no patients had died or required mechanical ventilation. No hemodialysis staff required hospitalization. Universal droplet and contact precautions were implemented during the outbreak. Hemodialysis staff with SARS-CoV-2 infection were placed on home quarantine regardless of symptom status. Patients with SARS-CoV-2 infection, including asymptomatic individuals, were treated with droplet and contact precautions until confirmation of negative SARS-CoV-2 RT-PCR test results. Analysis of the outbreak identified 2 index cases with subsequent nosocomial transmission within the dialysis unit and in shared shuttle buses to the hemodialysis unit.
Limitations
Single-center study.
Conclusions
Universal SARS-CoV-2 testing and universal droplet and contact precautions in the setting of an outbreak appeared to be effective in preventing further transmission.
Index Words: Coronavirus 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), hemodialysis, end-stage kidney disease (ESKD), kidney failure, outbreak, infection prevention, nosocomial transmission, screening, nasopharyngeal swabs, asymptomatic infection, dialysis clinic
Graphical abstract
Plain-Language Summary.
The coronavirus disease 2019 (COVID-19) pandemic has presented unique challenges to patients receiving hemodialysis. Despite having risk factors for severe infection, dialysis patients must visit health care facilities thrice weekly, where physical distancing is challenging. Despite protocols in place to identify and isolate symptomatic individuals, we report a COVID-19 outbreak in a hemodialysis unit in Toronto, Ontario, that prompted the screening of all hemodialysis patients and most staff for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection detected in nasopharyngeal swab specimens, regardless of symptoms. 11 of 237 (4.6%) hemodialysis patients and 11 of 93 (12%) staff tested positive for COVID-19. Notably, 55% of those with positive test results were asymptomatic at the time of testing. This study demonstrates the importance of universal testing in stopping the spread of COVID-19 during an outbreak.
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has prompted widespread restrictions on ambulatory in-person health care encounters. However, patients with kidney failure who receive maintenance hemodialysis must continue to receive life-sustaining treatment, typically 3 times per week.1 Hemodialysis attendance, including travel to and from the center, entails close interaction with individuals who may be infected with SARS-CoV-2.2 Concerns regarding viral acquisition are heightened because hemodialysis recipients have multiple risk factors for severe COVID-19.3 The US Centers for Disease Control and Prevention and the American Society of Nephrology have issued interim guidance to prevent COVID-19 in outpatient hemodialysis units, including screening protocols to identify symptomatic patients or health care workers.4 However, a recent outbreak at a skilled nursing facility has led to increasing recognition of the role of asymptomatic individuals in disease transmission.5 We report the dynamics and course of a recent COVID-19 outbreak affecting patients and staff at an urban hemodialysis unit.
Methods
St. Michael’s Hospital is an academic medical center in Toronto, Canada, at which 240 patients receive maintenance hemodialysis. The hemodialysis unit is divided into 2 large rooms on the same floor down the hall from each other. Each room is further subdivided into 3 clusters of 4 to 8 dialysis stations referred to as “pods.” Hemodialysis staff are assigned to work with patients in a specific pod, although they may assist patients in other pods. Hemodialysis patients typically dialyze 3 times a week on a morning, afternoon, evening, or overnight shift.
Before the outbreak, physical distancing was implemented in the waiting room and 2 layers of prescreening for symptoms were conducted before dialysis: the first by telephone on the day before the scheduled dialysis session and the second following the patient’s arrival in the dialysis unit waiting area. Dialysis prescreening involved recording tympanic temperature and a standard questionnaire screening for clinical symptoms. The questionnaire consisted of the following 3 questions: (1) “Do you have any of the following symptoms: fever, new or worsening cough, new sore throat, new runny nose, or new shortness of breath?” (2) “Have you had close unprotected contact with someone who has tested positive for COVID-19 in the last 14 days?” (3) “Have you traveled outside of the country in the last 14 days?” Patients with a fever or with a positive answer in the screening questionnaire were sequestered in a designated room for acquisition of a COVID-19 nasopharyngeal swab specimen and hemodialysis was performed under droplet and contact precautions. Universal masking for staff in patient care areas was implemented on March 26, 2020.
This study was approved by the Unity Health Research Ethics Board. Patient and staff consent were waived due to infection control measures with the exception of the 2 COVID-19–infected patients admitted to the intensive care unit, from whom informed consent was obtained. The reporting of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.6
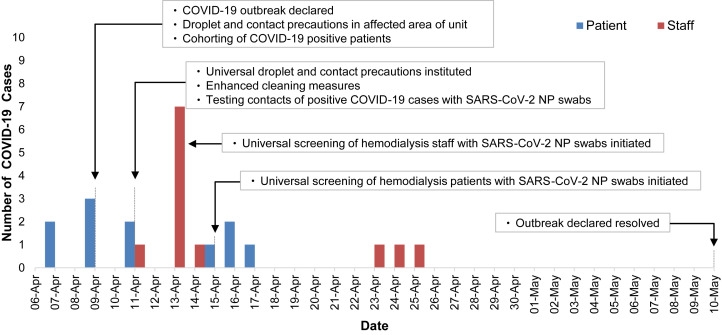
Two patients had COVID-19 diagnosed on April 7, 2020. The detection of 3 additional cases on April 9, 2020, led to the declaration of an outbreak. Investigation efforts were undertaken by an outbreak management team that was led primarily by the hospital Infection Prevention and Control (IPAC) team in collaboration with public health authorities and the hemodialysis unit. Between April 11, 2020, and April 22, 2020, all remaining patients and staff who interacted with hemodialysis patients were tested for SARS-CoV-2 using nasopharyngeal swabs.
SARS-CoV-2 nasopharyngeal swab specimens were collected by personnel who had received instruction in the proper technique. Nasopharyngeal swab collection was performed by physicians, nurse practitioners, and staff from the hospital’s COVID-19 Assessment Centre under droplet and contact precautions in the hemodialysis unit with curtains drawn around the dialysis station at which the patient was being swabbed. SARS-CoV-2 testing was performed with the NucliSENS easyMAG extractor/ABI QuantStudio5 platform using the Altona RealStar SARS-CoV-2 RT-PCR [reverse transcriptase–polymerase chain reaction] Kit 1.0. Hemodialysis unit staff who were sent for screening included physicians, nurse practitioners, nurses, dialysis support assistants, medical imaging technologists, allied health professionals, porters, and environmental services staff. Universal droplet and contact precautions including gloves, face shields, surgical masks, and isolation gowns were initiated on April 9, 2020, on the dialysis shift with known affected patients and expanded to the entire dialysis unit on April 10, 2020.
Contact tracing was a joint undertaking led by IPAC and the hospital’s occupational health department, with IPAC taking the primary lead for patient contact tracing, and occupational health, for staff contact tracing. Public health authorities conducted contact tracing for family members and community contacts. This included symptom screening of contacts and ongoing monitoring for 14 days postexposure. All symptomatic contacts were referred for testing but asymptomatic household contacts were not routinely tested as per public health protocols at the time.
For hemodialysis patients testing positive for SARS-CoV-2, information regarding symptoms, date of symptom onset, dialysis shift, location within the dialysis unit, and recent contacts was recorded. Patients frequently use a government-funded shuttle bus for transportation to and from the hemodialysis unit. These buses typically carry 5 to 10 individuals per vehicle. Since January 30, 2020, the service reported taking additional measures to reduce the risk for transmission of COVID-19, including additional cleaning, touch points being wiped down, education of employees regarding the procedure for handling individuals with COVID-19, and adjustment of patients allowed per trip to maintain adequate physical distancing. Given the risk for COVID-19 transmission in this venue, patient use of this shared shuttle bus service was documented.
Hemodialysis staff testing positive for SARS-CoV-2 had information recorded regarding dates worked, symptoms, date of symptom onset, duration of symptoms, locations worked while symptomatic, locations worked during 48 hours to 2 weeks before symptom onset, personal protective equipment use, and recent contacts, including patient interactions.
Results
Universal SARS-CoV-2 Testing, Clinical Characteristics, and Outcomes
Among 237 (99%) hemodialysis patients who agreed to testing, 11 (4.6%) tested positive for SARS-CoV-2, while 11 of 93 (12%) staff tested were found to be positive (Fig 1 ). At the time of testing, 6 (55%) patients and 6 (55%) staff positive for SARS-CoV-2 infection were asymptomatic. Three (27%) patients and 4 (36%) staff remained asymptomatic for the entire duration of follow-up (Table 1 ). Among the 11 patients with COVID-19, median age was 66 (interquartile range, 63-72) years, 6 (55%) were men, and 7 (64%) were dialyzed on the same shift (Table 2 ). One patient was an inpatient at the time of SARS-CoV-2 infection and 4 additional patients with COVID-19 were hospitalized; 2 (18%) patients required admission to the intensive care unit. No hemodialysis staff with SARS-CoV-2 infection required hospital admission. At a median of 30 days’ follow-up, no patients required mechanical ventilation or had died.
Figure 1.
Epidemic curve of the coronavirus disease 2019 (COVID-19) outbreak in the St. Michael’s Hospital hemodialysis unit. Abbreviations: NP, nasopharyngeal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Clinical Symptoms and Outcomes Among Hemodialysis Patients and Staff With COVID-19
| Hemodialysis Patients (n = 11) | Hemodialysis Staff (n = 11) | |
|---|---|---|
| Symptoms at time of positive NP swab | ||
| Fever | 1 (9%) | 2 (18%) |
| Cough | 3 (27%) | 2 (18%) |
| Shortness of breath | 0 (0%) | 1 (9%) |
| Rhinorrhea | 0 (0%) | 2 (18%) |
| Sore throat | 0 (0%) | 5 (45%) |
| Altered level of consciousness | 1 (9%) | 0 (0%) |
| Asymptomatic | 6 (55%) | 6 (55%) |
| Clinical outcomes | ||
| Follow-up period, da | 30 (26-38) | 28 (16-30) |
| Hospitalization | 5 (45%) | 0 (0%) |
| Supplementary oxygen | 5 (45%) | 0 (0%) |
| Mechanical ventilation | 0 (0%) | 0 (0%) |
| Intensive care unit admission | 2 (9%) | 0 (0%) |
| Death | 0 (0%) | 0 (0%) |
| Asymptomatic throughout illness | 3 (27%) | 4 (36%) |
Abbreviations: COVID-19, coronavirus disease 2019; NP, nasopharyngeal.
Median (range).
Table 2.
Baseline Clinical Characteristics of Hemodialysis Patients With COVID-19
| Characteristic | Values |
|---|---|
| Age, y | 66 [63-72] |
| Female sex | 5 (45%) |
| White race | 5 (46%) |
| History of hypertension | 11 (100%) |
| History of heart failure | 7 (64%) |
| History of myocardial infarction | 3 (27%) |
| History of diabetes | 10 (91%) |
| History of obstructive lung disease | 2 (18%) |
| Medication use | |
| ACE inhibitor | 2 (18%) |
| ARB | 4 (36%) |
| ACE inhibitor or ARB | 6 (55%) |
| Laboratory values at time of diagnosis | |
| Hemoglobin, g/dL (n = 9) | 9.5 (6.6-11.6) |
| WBC count, ×103/μL (n = 9) | 4.72 (3.1-21.8) |
| Patients with WBC count < 400 ×103/μL | 3/9 (33%) |
| Lymphocyte count, ×103/μL (n = 9) | 0.54 (0.05-1.38) |
| Patients with lymphocyte count < 103/μL | 8/9 (89%) |
| Ferritin, ng/mL (n = 4) | 1,461.5 (604-1,500) |
| Patients with ferritin level > 900 ng/mL | 3/4 (75%) |
| D-Dimer, μg/mL (n = 4) | 1.642 (0.746-3.895) |
| Patients with D-dimer level > 0.5 μg/mL | 4/4 (100%) |
| CRP, mg/dL (n = 4) | 17.6 (6.4-26.1) |
| Patients with CRP level > 5 mg/dL | 4/4 (100%) |
| Imaging features of bronchopneumonia | 5/6 (83%) |
Note: n = 11. Values for continuous variables given as median [interquartile range] or median (range); for categorical variables, as count (percentage) or n/N (percentage).
Abbreviation: ACE, angiotensin-converting enzyme; ARB, angiotenin receptor blocker; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; WBC, white blood cells.
Outbreak Analysis
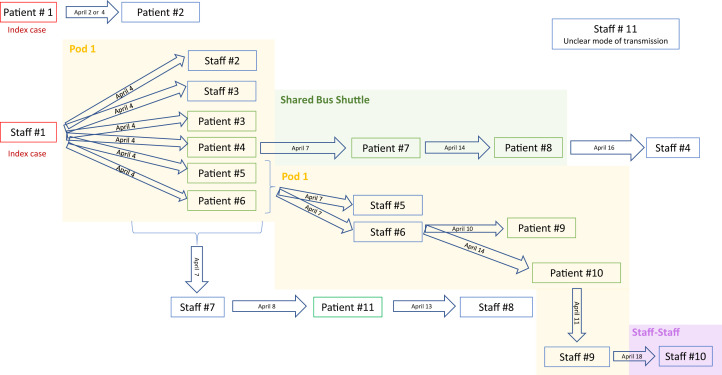
Analysis of the outbreak through contact tracing suggests that the 2 index cases acquired infection outside the dialysis unit. Our first suspected index case was a patient who resided at a skilled nursing facility that was experiencing a COVID-19 outbreak. The second suspected index case was an asymptomatic hemodialysis staff member who likely acquired SARS-CoV-2 infection through community transmission. Subsequent nosocomial transmission likely occurred within the hemodialysis unit and on the shuttle bus service during transit to and from dialysis (Fig 2 ). Nosocomial transmission within the hemodialysis unit is supported by the temporal and positional clustering of 6 hemodialysis staff and 6 patient cases in a specific pod. Of the 11 patients infected with SARS-CoV-2, 5 (45%) were using the shuttle bus service. Based on analysis of the outbreak, 2 patients likely contracted COVID-19 while sharing the shuttle bus with other infected patients. These 2 patients took the same shuttle bus service to the same hemodialysis shift as other infected patients but dialyzed in different parts of the hemodialysis unit, suggesting that they acquired COVID-19 outside the hemodialysis unit.
Figure 2.
Infection control authorities concluded that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission during an outbreak at the St. Michael’s Hospital hemodialysis unit was likely to have originated from 2 index cases. Patient 1 acquired the virus through an outbreak at a skilled nursing facility and hemodialysis staff 1 likely acquired the virus in the community. Subsequent transmission likely occurred from patient-to-patient interactions or indirectly through staff. Later transmission likely occurred through a shared shuttle bus service to and from dialysis despite implementation of universal droplet and contact precautions within the hemodialysis unit. Our hemodialysis unit is divided into a 2 separate rooms, each of which is further subdivided into 3 clusters of dialysis chairs referred to as pods. Dates on the arrows reflect the day of hypothesized transmission.
Response to the Outbreak
Following declaration of the outbreak, additional infection control measures were implemented. Droplet and contact precautions were mandated for all patient contact until outbreak resolution. SARS-CoV-2–positive patients were cohorted in a dedicated waiting room that was subjected to thorough cleaning after the patient’s departure. The number of environmental services staff was escalated to increase the frequency of unit cleaning. “Safety coaches” were deployed to the hemodialysis unit to provide feedback to staff regarding proper use of personal protective equipment. All inpatients were dialyzed in their hospital room regardless of COVID-19 status. Porters were required to use a face shield and mask when transporting dialysis patients, and universal masking of patients was implemented. Patient movement between dialysis shifts was restricted and extra dialysis sessions (eg, a Saturday session for a patient who normally dialyzes on Monday, Wednesday, and Friday) were put on hold to limit a given patient’s exposure to additional cohorts of patients.
Patients with confirmed SARS-CoV-2 infection, including asymptomatic individuals, were dialyzed in a dedicated room separate from the main hemodialysis unit for the duration of their infection and maintained on droplet and contact precautions. Repeat testing was performed following symptom resolution and a minimum of 14 days from symptom onset. Two negative SARS-CoV-2 nasopharyngeal swab test results within a 24-hour period were required before the patient being allowed to return to his or her regular station in the dialysis unit. Among the 6 patients with a persistently positive SARS-CoV-2 nasopharyngeal swab result, 5 (83%) were hospitalized (Table 3 ).
Table 3.
Hemodialysis Patients With COVID-19 Who Had Repeat SARS-CoV-2 Nasopharyngeal Swab Tests
| Repeat NP Swab |
||
|---|---|---|
| Positive (n = 6) | Negative (n = 5) | |
| Time to re-swab, da | 20 (18-25) | 17 (14-19) |
| Hospitalized | 5 (83%) | 0 (0%) |
| Asymptomatic during entire follow-up | 0 (0%) | 3 (60%) |
Note: n = 11.
Abbreviations: COVID-19, coronavirus disease 2019; NP, nasopharyngeal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Median (range).
Hemodialysis staff with confirmed SARS-CoV-2 infection, including those who were asymptomatic, were asked to self-isolate at home. Five hemodialysis staff were allowed to return to work following symptom resolution and documentation of 2 negative results of SARS-CoV-2 nasopharyngeal swab tests performed 14 days from symptom onset. The return-to-work policy for hemodialysis staff was revised by IPAC on May 7, 2020, to no longer require repeat SARS-CoV-2 testing. Following this change in policy, the remaining 6 hemodialysis staff with SARS-CoV-2 infection were allowed to return to work 14 days from symptom onset assuming that symptoms had resolved, without demonstration of a negative SARS-CoV-2 nasopharyngeal swab test result.
Outbreak Resolution
The outbreak was declared resolved on May 10, 2020, by IPAC on the basis of no new cases being detected in the hemodialysis unit over a 14-day period. Because the outbreak was declared over, only individuals who reported symptoms during the predialysis screening process were tested. No additional patient or staff cases have been identified as of June 19, 2020. Although droplet and contact precautions were rescinded in the hemodialysis unit, masks remain mandatory throughout the hospital and face shields must be worn by all hemodialysis staff in the course of patient care.
Discussion
This report highlights the unique susceptibility of hemodialysis patients and providers to infection with SARS-CoV-2. Despite implementation of recommended symptom-based screening measures nearly 1 month before the outbreak, nosocomial transmission occurred in a crowded health care environment. Universal screening during this outbreak showed that 4.6% of hemodialysis patients and 12% of hemodialysis staff had positive SARS-CoV-2 nasopharyngeal swab test results.
Studies of COVID-19 in hemodialysis patients have been limited. A hemodialysis center in China that used a computed tomography–based screening algorithm for SARS-CoV-2 reported a prevalence of 17% among patients and 12% among staff.7 A large dialysis center in the United Kingdom reported that 19.6% of patients developed COVID-19 over a 6-week period, with clustering of cases on specific dialysis shifts and high rates of nursing staff illness.8 A study of 1,027 hemodialysis patients in China identified 9.6% of patients with SARS-CoV-2 infection, of whom 51% were asymptomatic for the duration of infection. In this report, 48% of cases had negative RT-PCR test results and were identified only by positive immunoglobulin M (IgM) or IgG antibodies directed against the nucleocapsid and spike protein of SARS-CoV-2.9
Hemodialysis units are high-risk venues for mass transmission of SARS-CoV-2 infection across a vulnerable population. In this study, a shared shuttle bus service was identified as an additional site for transmission of COVID-19 outside the hemodialysis unit. Despite 12% of staff testing positive, a greater number of patients did not become infected, likely secondary to the implementation of universal droplet and contact precautions on April 11, 2020, one day following the date of symptom onset reported by the first 2 staff members with COVID-19 diagnosed.
Following the resolution of the COVID-19 outbreak, universal screening with SARS-CoV-2 nasopharyngeal swab tests was not repeated and only individuals who reported symptoms during the predialysis screening process were tested. Ongoing testing of symptomatic patients and staff has not identified any additional cases despite ongoing community transmission of COVID-19. Given the limited sensitivity of SARS-CoV-2 nasopharyngeal swab tests, false-negative test results may have underestimated the true disease burden during the outbreak.10 This stresses the importance of mandating universal droplet and contact precautions until outbreak resolution to prevent transmission from individuals with false-negative test results.
Although we observed that 5 of the 6 patients with positive RT-PCR results for SARS-CoV-2 at least 14 days after an initial positive nasopharyngeal swab result were hospitalized, the clinical significance of these persistently positive SARS-CoV-2 nasopharyngeal swab results remains uncertain at this time. Out of an abundance of caution, our infection control policy for patients has been to obtain 2 negative SARS-CoV-2 nasopharyngeal swab test results within 24 hours of each other following clinical resolution and a minimum of 14 days from date of diagnosis before discontinuing droplet and contact precautions. For hemodialysis staff, a time- and test-based strategy requiring 2 negative SARS-CoV-2 nasopharyngeal swab results was initially used to determine eligibility for return to work. A change to only a time-based strategy to allow for return to work was made due to concerns that persistently positive SARS-CoV-2 nasopharyngeal swab test results could lead to hemodialysis staffing shortages. Given that hemodialysis staff wore masks and face shields for all patient interactions, the change in policy was thought to pose a minimal risk for further transmission.
Rigorous pretreatment screening procedures are needed to promptly identify symptomatic patients with COVID-19. However, during this outbreak, 55% of cases were asymptomatic at the time of testing, highlighting the need for comprehensive contact tracing and unit-wide screening of hemodialysis patients and staff when clusters of new COVID-19 cases are discovered. In conclusion, universal screening for SARS-CoV-2 infection, implementation of universal droplet and contact precautions until outbreak resolution, isolation of infected patients during dialysis, and home quarantine for infected staff were essential in containing our COVID-19 outbreak.
Article Information
Authors’ Full Names and Academic Degrees
Kevin Yau, MD, Matthew P. Muller, MD, PhD, Molly Lin, MD, Naureen Siddiqui, MHSc, Sanja Neskovic, BA, HIM, Gagan Shokar, BScN, Ramzi Fattouh, PhD, Larissa M. Matukas, MD, MSc, William Beaubien-Souligny, MD, Alison Thomas, MN, Jordan J. Weinstein, MD, Jeffrey Zaltzman, MD, MSc, and Ron Wald, MDCM, MPH.
Authors’ Contributions
Research idea and study design: KY, MPM, RW; data acquisition: NS, SN, GS, AT; data analysis/interpretation: KY, MPM, ML, NS, RF, LMM, WB-S, AT, JJW, JZ, RW, supervision or mentorship: RW. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank all hemodialysis staff at St. Michael’s Hospital for their dedication to patient care during the COVID-19 pandemic.
Peer Review
Received May 25, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 2, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Kliger A.S., Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020;15(5):707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong F., Tang H., Liu L. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;3(7):1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate COVID-19 [published online ahead of print April 2020]. N Engl J Med. https://doi.org/10.1056/NEJMcp2009249 [DOI] [PubMed]
- 4.Ikizler T.A. COVID-19 and dialysis units: what do we know now and what should we do? Am J Kidney Dis. 2020;76(1):1–3. doi: 10.1053/j.ajkd.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. N Engl J Med. 2020;382(22):2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H. Maintenance hemodialysis and coronavirus disease 2019 (COVID-19): saving lives with caution, care, and courage. Kidney Med. 2020;2(3):365–366. doi: 10.1016/j.xkme.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett R.W., Blakey S., Nitsch D. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31(8):1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang H., Tian J., Dong J. Serologic detection of SARS-CoV-2 infections in HD centers population. Am J Kidney Dis. 2020;76(4):490–499.e1. doi: 10.1053/j.ajkd.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection — challenges and implications [published online ahead of print June 2020]. N Engl J Med. https://doi.org/10.1056/NEJMp2015897 [DOI] [PubMed]