Highlights
-
•
Large proportions of inpatients with COVID-19 had mental health problems.
-
•
Sex and levels of inflammatory markers were related to mental health symptoms.
-
•
Self-perceived illness severity mediated effect of disease duration on mental health.
Keywords: COVID-19, Patients, Hospitalization, Inflammatory markers, Mental health
Abstract
Objective
To evaluate the mental health status of hospitalized patients with coronavirus disease 2019 (COVID-19) and to explore the related factors.
Method
This was a cross-sectional survey among COVID-19 inpatients in two isolation wards of a designated hospital in Wuhan, China, from March 7, 2020, to March 24, 2020. Participants’ demographic data, clinical data and levels of circulating inflammatory markers were collated. Mental health symptoms were evaluated with questionnaires, which included the Insomnia Severity Index (ISI) scale, the 9-item Patient Health Questionnaire (PHQ-9), the 7-item Generalized Anxiety Disorder (GAD-7) scale, and questions about patients’ self-perceived illness severity. Multivariate linear regression analysis was performed to explore factors that associated with mental symptoms, and a structural equation model (SEM) was used to assess the possible relationships between those factors and the patients’ mental health.
Results
Among the 85 participants, 45.9% had symptoms of depression (PHQ-9 ≥ 5), 38.8% had anxiety (GAD-7 ≥ 5), and 54.1% had insomnia (ISI ≥ 8). According to multivariate regression analysis, female sex, a higher level of interleukin (IL)-1β and greater self-perceived illness severity were all significantly associated with a higher PHQ-9 score, higher GAD-7 score and higher ISI score. In addition, the disease duration and the neutrophil to lymphocyte ratio (NLR) were positively related to patients’ self-perceived illness severity. The results of the SEM analyses suggested that sex (β = 0.313, P < 0.001), self-perceived illness severity (β = 0.411, P < 0.001) and levels of inflammatory markers (β = 0.358, P = 0.002) had direct effects on patients’ mental health. The disease duration (β = 0.163, P = 0.003) and levels of inflammatory markers (β = 0.101, P = 0.016) also indirectly affected patients’ mental health, with self-perceived illness severity acting as a mediator.
Conclusion
A majority of COVID-19 infected inpatients reported experiencing mental health disturbances. Female sex, disease duration, levels of inflammatory markers and self-perceived illness severity are factors that could be used to predict the severity of patients’ mental symptoms.
1. Introduction
In December 2019, an outbreak of a novel coronavirus disease 2019 (COVID-19) occurred in Wuhan, China (Zhu et al., 2020) and subsequently spread domestically and internationally. As of 11 May 2020, COVID-19 had spread to 215 countries, areas or territories, and more than 4 million confirmed cases of COVID-19 had been reported (World Health Organization, 2020). As a global pandemic, COVID-19 is becoming a major threat to public health worldwide. Although most patients had mild symptoms, approximately 15%-20% of the COVID-19 infected patients were severe cases, some of whom develop severe pneumonia and organ failure, which can lead to death (Gong et al., 2020, Sohrabi et al., 2020, Verity et al., 2020). Because of the disease’s high level of transmissibility, COVID-19 patients have to stay in isolated units. In Wuhan, those with mild symptoms received treatment in temporary quarantined hospital facilities (Fangcang Hospital), while patients with more severe symptoms were sent to the designated hospitals for more aggressive therapy. While being treated in isolation, patients may experience both physical and psychological discomfort (Xiang et al., 2020), which could result in mental health problems. According to previous studies, a majority of the infected inpatients experienced various mental disturbances during the epidemics of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). A range of mental problems, including insomnia, anxiety, depression, posttraumatic stress symptoms and even suicidality, have been reported (Kim et al., 2018, Li and Zhang, 2003, Sheng et al., 2005). Furthermore, many patients’ psychological symptoms remained after discharge and lasted for a long time (Hong et al., 2009, Mak et al., 2009). These studies remind us that the mental health of patients with COVID-19 should not be ignored. However, to date, studies about the mental health of the COVID-19 patients have been rare. To address this gap, we conducted a survey to evaluate the mental health status of inpatients with COVID-19 in a designated hospital by assessing their mental symptoms, including depression, anxiety, and insomnia. Factors that associated with mental health symptoms were also explored. We hope that our findings will call attention to the mental health of patients with COVID-19.
2. Method
2.1. Participants
We conducted a questionnaire survey among 85 inpatients in two isolation wards of Tongji Hospital, Wuhan (a designated hospital for adult patients with severe COVID-19) from March 7, 2020, to March 24, 2020. All the recruited participants were definitively diagnosed with COVID-19. Patients with any of the following conditions were not included: unstable vital signs, mechanical ventilation, SP02 < 95% while on oxygen therapy, impaired consciousness, dementia, or severe psychotic disorders.
2.2. Study design
The study was a cross-sectional and observational survey. Participants completed a questionnaire prior to their discharge. According to the patients’ preferences, an online questionnaire was provided to 48 participants, while the other 37 were provided with a paper questionnaire with the same content.
Before commencing the study, ethical clearance was sought from Xiamen Xianyue Hospital. All surveyed participants agreed to participate and provided verbal informed consent before their enrollment. Participants were allowed to end the survey at any time. All information identifying the participants was kept confidential. After this survey, patients who reported mental health problems received help and were further evaluated by psychiatrists if so desired.
2.3. Data collection
2.3.1. Demographic and clinical data
Data including sex, age, marital status, educational level, duration of disease, length of hospital stay and use of oxygen therapy were extracted from the electronic medical record.
2.3.2. Questionnaires
There were four parts to the questionnaires: the Chinese version of the Insomnia Severity Index (ISI) scale, the Chinese version of the 9-item Patient Health Questionnaire (PHQ-9), the Chinese version of the7-item Generalized Anxiety Disorder-7 (GAD-7) scale, and a 4-point scale used to assess patients’ self-perceived illness severity.
PHQ-9
The PHQ-9 is a 9-item depression questionnaire that is designed to identify probable cases of depression and to assess symptom severity in the past two weeks. The depression scale has been validated and has good reliability (Cronbach’s α = 0.86–0.89) in different medical settings. The total score ranges from 0 to 27, and a higher score indicates more severe depression symptoms. The scores were categorized as follows: absence of depression (0–4), mild depression (5–9), moderate depression (10–14), and severe depression (15–27) (Kroenke et al., 2001).
GAD-7
The GAD-7 scale is a 7-item self-reported anxiety questionnaire with high reliability (Cronbach’s α = 0.89) and validity in primary care patients and the general population. Although originally designed to detect generalized anxiety disorder (GAD), the GAD-7 has also been shown to be a good screening tool for other common anxiety disorders. All the items are rated on a 4-point scale, and the total score ranges from 0 to 21 and is interpreted as follows: absence of anxiety (0–4), mild anxiety (5–9), moderate anxiety (10–14), and severe anxiety (15–21) (Lowe et al., 2008).
ISI
The ISI is a valid self-reported instrument that was designed to diagnose insomnia and measure the symptom severity in the previous 2 weeks. The reliability of the scale is high, with a Cronbach’s α = 0.90–0.91. A 5-point Likert scale is used to rate the 7- items, yielding a total score ranging from 0 to 28. A higher score suggests more severe insomnia, and the total score is categorized as follows: absence of insomnia (0–7), mild insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28) (Morin et al., 2011).
Self-perceived illness severity
Self-perceived illness severity was assessed by asking the participants the question “What do you think the severity of your disease is currently?” The answer options were less severe, normal, severe, and very severe. The score ranges from 1 to 4.
2.3.3. Inflammatory markers
The values of circulating inflammatory markers were recorded; these included cytokines (interleukin (IL)-1β, IL-6, IL-8, IL-10, tumor necrosis factor-α (TNF-α)), high-sensitivity C-reactive protein (hsCRP), and blood cell counts (white blood cells, neutrophils, lymphocytes). Due to the limitations of assay sensitivity, concentrations of IL-1β lower than 5 pg/mL, concentrations of IL-6 lower than 1.5 pg/mL, and concentrations of IL-10 lower than 5 pg/mL were undetectable. The neutrophil to lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count. All recorded inflammatory markers were measured within one week of the date on which the questionnaire was completed.
2.4. Statistical analysis
Continuous data following a normal distribution were present as the mean ± standard deviation (SD), otherwise, they were expressed as the median with interquartile range (IQR); The median value between zero and the assay sensitivity threshold was used for samples with undetectable values, and the impact of the imputed value was minimized by the use of the rank-sum test. Participants were divided into different subgroups on the basis of their PHQ-9 scores, GAD-7 scores, and ISI scores. Differences between subgroups were analyzed, Student’s t tests or Wilcoxon rank-sum tests were used for continuous data. Meanwhile, χ2 tests or Fisher’s exact tests were chosen for categorical data, as appropriate. The standard Cronbach’s α coefficient was used to evaluate the internal reliability of the PHQ-9, GAD-7, and ISI scores. To explore the potential factors associated with mental health, Spearman’s correlation analysis and multivariable linear regressions were performed. A structural equation model (SEM) was used to further characterize the possible relationships among the study variables. To test the model, six goodness-of-fit indices were used: the chi-square to degree of freedom ratio (χ2/df), comparative fit index (CFI), Tucker-Lewis index (TLI), standardized root mean square residual (SRMR), and root mean square error of approximation (RMSEA). All data were analyzed with IBM SPSS Statistics version 21.0 and Mplus 7.4. A 2-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics
A total of 85 patients were enrolled in this study, of whom 56.5% completed the survey online and 44.5% completed the questionnaire on paper (Table 1 ). Overall, females accounted for 49.5%. The average age of the participants was 48.8 years. A total of 55.3% had a high school or higher educational level. Most participants were married (85.9%). The average disease duration was 32.4 days, and the median length of hospital stay was 5.7 days. 49.4% of the participants received oxygen therapy on the day of the assessment. Participants were divided into two subgroups according to their PHQ-9 scores, GAD-7 scores and ISI scores. A total of 45.9% of the participants had depression symptoms (PHQ-9 ≥ 5), and 54.1% did not have depression symptoms (PHQ-9 < 5). Patients with depression symptoms had a longer disease duration (37.4 ± 18.7 vs 28.3 ± 19.7, p = 0.033) than those without depression symptoms. There was no significant difference between the two groups in other aspects. When patients were categorized by the GAD-7 score, 38.8% were in the group with anxiety symptoms (GAD-7 ≥ 5), and 54.1% were in the group without anxiety symptoms (GAD-7 < 5). Compared to the group without anxiety symptoms, the group with anxiety had a higher percentage of females (63.6% vs 40.4%, p = 0.046), a longer disease duration (37.9 ± 18.9 vs 28.9 ± 19.5, p = 0.039) and a longer hospital stay (median 7.0 vs 5.7, p = 0.019). A total of 54.1% of the participants had insomnia symptoms (ISI ≥ 8), and 45.9% had no insomnia symptoms (ISI < 8). The group with insomnia symptoms had a higher percentage of females (60.9% vs 35.9% p = 0.030) and a longer hospital stay (median 7.0 vs 5.7, p = 0.040) than the group without insomnia.
Table 1.
Demographic and clinical characteristics of total cohort and different subgroups.
| Total sample | PHQ-9 ≥ 5N = 39 | PHQ-9 < 5N = 46 | P value | GAD-7 ≥ 5N = 33 | GAD-7 < 5N = 52 | P value | ISI ≥ 8N = 46 | ISI < 8N = 39 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Assessment type(%) | ||||||||||
| online | 56.5% | 64.1% | 50.0% | 0.272 | 60.6% | 53.8 | 0.655 | 45.9% | 54.1% | 0.197 |
| paper | 44.5% | 35.9% | 50.0% | 39.4% | 46.2% | 37.0% | 51.3% | |||
| Female(%) | 49.4% | 59.0% | 41.3% | 0.130 | 63.6% | 40.4% | 0.046 | 60.9% | 35.9% | 0.030 |
| Age(year) | 48.8 ± 14.3 | 48.5 ± 14.1 | 49.2 ± 14.7 | 0.810 | 49.3 ± 15.2 | 48.6 ± 13.9 | 0.838 | 49.61 ± 14.7 | 48.0 ± 14.0 | 0.609 |
| Marital status(%) | ||||||||||
| single | 8.2% | 5.1% | 10.0% | 0.240 | 6.1% | 9.6% | 0.921 | 2.2% | 15.4% | 0.145 |
| married | 85.9% | 87.2% | 84.8% | 87.9% | 84.6% | 91.3% | 79.5% | |||
| divorced | 2.4% | 2.6% | 4.3% | 3.0% | 3.8% | 4.3% | 2.6% | |||
| widowed | 3.5% | 5.1% | 0.0% | 3.0% | 1.9% | 2.2% | 2.6% | |||
| Educational level(%) | ||||||||||
| ≥High school | 55.3% | 61.1% | 50.5% | 0.382 | 63.3% | 50.0% | 0.266 | 54.3% | 56.4% | 0.849 |
| <High school | 44.7% | 38.9% | 50.0% | 36.7% | 50.0% | 45.7% | 43.6% | |||
| Disease duration (day) | 32.4 ± 19.6 | 37.4 ± 18.7 | 28.3 ± 19.7 | 0.033 | 37.9 ± 18.9 | 28.9 ± 19.5 | 0.039 | 35.4 ± 18.2 | 29.0 ± 21.0 | 0.141 |
| Hospital stay(day) | 5.7(4.0,8.5) | 7.0(4.0,10.0) | 5.6(3.7,7.2) | 0.139 | 7.0(4.6,10.0) | 5.7(3.7,7.0) | 0.019 | 7.0(4.0,10.9) | 5.6 (4.0,7.0) | 0.040 |
| Oxygen therapy(%) | 49.4% | 61.5% | 39.1% | 0.051 | 60.6% | 42.3% | 0.122 | 52.2% | 46.2% | 0.665 |
3.2. Questionnaire results
The internal reliability of all scales for the current study was excellent: Cronbach’s α for the PHQ-9 scale was 0.914, that for the GAD-7 scale was 0.907, and that for the ISI was 0.955. A summary of the severity categories for depression, anxiety, and insomnia is provided in Table 2 . High proportions of the participants had moderate or severe symptoms of depression (24.7%), anxiety (16.5%), and insomnia (21.1%). In the total cohort, the mean ISI, PHQ-9 and GAD-7 scores were 9.1 points, 6.1 points, and 4.7 points, respectively (Table 3 ). Patients with mental health symptoms (depression, anxiety, or insomnia) had greater self-perceived illness severity than those without mental health symptoms.
Table 2.
Severity categories of depression, anxiety, and insomnia.
| Severity | PHQ-9 depression symptom |
GAD-7 Anxiety symptom |
ISI Insomnia symptom |
|||
|---|---|---|---|---|---|---|
| n | Percent | n | Percent | n | Percent | |
| Normal | 46 | 54.1% | 52 | 61.2% | 39 | 45.9% |
| Mild | 18 | 21.2% | 19 | 22.4% | 28 | 32.9% |
| Moderate | 13 | 15.3% | 10 | 11.8% | 15 | 17.6% |
| Severe | 8 | 9.4% | 4 | 4.7% | 3 | 3.5% |
Abbreviations: PHQ-9: 9-item Patient Health Questionnaire; GAD-7: 7-item Generalized Anxiety Disorder; ISI:Insomnia Severity Index.
Table 3.
Questionnaire results of total cohort and different subgroups.
| Total sample | PHQ-9 ≥ 5N = 39 | PHQ-9 < 5N = 46 | P value | GAD-7 ≥ 5N = 33 | GAD-7 < 5N = 52 | P value | ISI ≥ 8N = 46 | ISI < 8N = 39 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| PHQ-9 score | 6.1 ± 5.9 | 11.2 ± 5.5 | 1.6 ± 1.5 | <0.001 | 11.1 ± 5.5 | 2.9 ± 3.8 | <0.001 | 9.3 ± 6.3 | 2.3 ± 2.6 | <0.001 |
| GAD-7 score | 4.7 ± 4.6 | 8.4 ± 4.8 | 1.5 ± 1.9 | <0.001 | 9.8 ± 4.0 | 1.4 ± 1.4 | <0.001 | 7.5 ± 5.0 | 1.4 ± 1.8 | <0.001 |
| ISI score | 9.1 ± 6.8 | 13.3 ± 6.3 | 5.5 ± 4.9 | <0.001 | 14.7 ± 5.5 | 5.5 ± 4.9 | <0.001 | 14.1 ± 5.0 | 3.1 ± 2.5 | <0.001 |
| Self-perceived illness severity | ||||||||||
| Less severe (1 score) | 50.6% | 30.8% | 67.4% | 0.001 | 37.0% | 66.7% | 0.049 | 24.2% | 67.3% | 0.001 |
| Normal (2 score) | 30.6% | 43.6% | 19.6% | 39.1% | 20.5% | 42.4% | 23.1% | |||
| Severe (3 score) | 12.9% | 12.8% | 13.0% | 17.4% | 7.7% | 21.2% | 7.7% | |||
| Very severe (4 score) | 5.9% | 12.8% | 0.0% | 6.5% | 5.1% | 12.1% | 1.9% |
3.3. Inflammatory marker results
Inflammatory markers data of 70/85 patients were available, and the results are shown in Table 4 . Patients with depression symptoms had higher level of IL-1β, higher mean count of neutrophil and lower mean count of lymphocyte than those without depression symptoms. However, all the differences were not statistical significant. When compared to patients without anxiety symptoms, those with anxiety symptoms had a higher level of IL-1β (2.5 (2.5, 6.2) vs. 2.5 (2.5, 2.5), p = 0.045), a higher NLR (2.1 (1.5, 3.2) vs. 1.7 (1.3, 2.2), p = 0.049), and a lower mean lymphocyte count (1.6 ± 0.4 vs. 1.9 ± 0.7, p = 0.015). Patients with insomnia symptoms had a lower level of IL-10 (2.5 (2.5, 2.5) vs. 2.5 (2.5, 5.8), p = 0.039) and lower lymphocyte count (1.6 ± 0.4 vs. 2.0 ± 0.7, p = 0.010) than patients without insomnia symptoms.
Table 4.
Levels of inflammatory markers in different subgroups.
| Missing Data | PHQ-9 ≥ 5 | PHQ-9 < 5 | P value | GAD-7 ≥ 5 | GAD-7 < 5 | P value | ISI ≥ 8 | ISI < 8 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cytokines | ||||||||||
| IL-1β(pg/mL) | 15 | 2.5(2.5,6.0) | 2.5(2.5,2.5) | 0.080 | 2.5(2.5,6.2) | 2.5(2.5,2.5) | 0.045 | 2.5(2.5,5.6) | 2.5(2.5,2.5) | 0.274 |
| IL-6 (pg/mL) | 15 | 2.2(0.8,5.5) | 2.1(0.8,3.8) | 0.894 | 2.3(0.8,5.6) | 2.0(0.8,3.6) | 0.678 | 1.9(0.8,4.2) | 2.4(1.5,3.8) | 0.333 |
| IL-8 (pg/mL) | 15 | 5.7(0.8,10.9) | 8.8(6.0,12.9) | 0.660 | 7.3(5.4,11.4) | 9.2(6.1,12.0) | 0.413 | 7.6(5.2,11.6) | 9.3(6.5,11.6) | 0.444 |
| IL-10 (pg/mL) | 15 | 2.5(2.5,2.5) | 2.5(2.5,4.9) | 0.442 | 2.5(2.5,2.5) | 2.5(2.5,4.0) | 0.581 | 2.5(2.5,2.5) | 2.5(2.5,5.8) | 0.039 |
| TNF-α(pg/mL) | 15 | 7.3(6.1,9.3) | 7.4(5.5,9.7) | 0.891 | 7.2(6.3,8.6) | 7.8(5.4,9.9) | 0.458 | 7.0(5.8,8.7) | 8.0(5.4,10.2) | 0.180 |
| Hs-CRP (mg/L) | 15 | 1.0(0.5,2.6) | 1.2(0.6,2.7) | 0.417 | 0.9(0.3,2.0) | 0.8(0.6,2.8) | 0.119 | 1.0(0.3,2.0) | 1.4(0.6,2.8) | 0.085 |
| Blood cell counts | ||||||||||
| White blood cell (*10^9/L) | 15 | 6.5 ± 2.6 | 6.0 ± 1.7 | 0.357 | 6.5 ± 2.71 | 6.1 ± 1.7 | 0.427 | 6.2 ± 2.4 | 6.3 ± 1.8 | 0.816 |
| Neutrophil (*10^9/L) | 15 | 4.1 ± 2.4 | 3.4 ± 1.1 | 0.073 | 4.2 ± 2.6 | 3.4 ± 1.2 | 0.146 | 3.8 ± 2.2 | 3.5 ± 1.3 | 0.450 |
| Lymphocyte (*10^9/L) | 15 | 1.7 ± 0.4 | 1.9 ± 0.7 | 0.099 | 1.6 ± 0.4 | 1.9 ± 0.7 | 0.015 | 1.6 ± 0.4 | 2.0 ± 0.7 | 0.010 |
| NLR | 15 | 2.0 (1.4,2.9) | 1.7(1.2,2.3) | 0.141 | 2.1(1.5,3.2) | 1.7(1.3,2.2) | 0.049 | 2.1(1.4,2.8) | 1.6(1.2,2.2) | 0.070 |
Abbreviations: IL:interleukin; TNF-α: tumor necrosis factor-α; hsCRP: high-sensitivity C-reactive protein; NLR: neutrophil to lymphocyte ratio
3.4. Correlation analysis
Prior to establishing multivariate regression models, correlations among the study variables were explored. As seen in Table 5 , the PHQ-9 score for depression was significantly related to the disease duration (r = 0.25, p < 0.05), the level of IL-1β (r = 0.50, p < 0.001), NLR (r = 0.36, p < 0.01), self-perceived illness severity (r = 0.46, p < 0.01), ISI score (r = 0.70, p < 0.01), and GAD-7 score (r = 0.80, p < 0.01). The GAD-7 score for anxiety was positively correlated with the length of hospital stay (r = 0.22, p < 0.05), level of IL-1β (r = 0.46, p < 0.001), NLR (r = 0.30, p < 0.05), self-perceived illness severity (r = 0.44, p < 0.01), and ISI score (r = 0.75, p < 0.01). Patients’ self-perceived illness severity was also associated with the disease duration, length of hospital stay, level of IL-1β and NLR. In addition, the association between the level of IL-1β and the NLR was significant (r = 0.55, p < 0.01).
Table 5.
Correlations between study variables.
| Hospital stay | IL-1β | NLR | self-perceived illness severity | ISI | GAD-7 | PHQ-9 | |
|---|---|---|---|---|---|---|---|
| Disease duration | 0.07 | −0.05 | 0.11 | 0.37** | 0.13 | 0.17 | 0.25* |
| Hospital stay | – | 0.12 | 0.10 | 0.27* | 0.19 | 0.22* | 0.13 |
| IL-1β | – | 0.55** | 0.32* | 0.32** | 0.46*** | 0.50*** | |
| NLR | – | 0.43*** | 0.22 | 0.30* | 0.36** | ||
| self-perceived illness severity | – | 0.29** | 0.44** | 0.46** | |||
| ISI | – | 0.75** | 0.70** | ||||
| GAD-7 | – | 0.80** |
Notes: * p < 0.05 ,** p < 0.01,***p < 0.001.
3.5. Regression model
All multivariate regression models for self-perceived illness severity, ISI score, GAD-7 score, and PHQ-9 score are presented in Table 6 . We explored the factors related to self-perceived illness severity and the ISI score by using multivariate linear regression and explored the factor related to the GAD-7 and PHQ-9 scores with hierarchical regression. In the self-perceived illness severity regression model, a longer hospital stay (β = 0.35, P < 0.01) and higher NLR (β = 0.27, P < 0.05) were independently associated with greater self-perceived illness severity after adjusting for oxygen therapy, sex, disease duration, and IL-1β, and the model accounted for 20% of the variance. The ISI model accounted for 20% of the variance. Female sex (β = 0.24, P < 0.05), a higher level of IL-1β (β = 0.25, P < 0.05) and greater self-perceived illness severity (β = 0.28, P < 0.05) were related to a higher ISI score. The overall GAD-7 model accounted for 46% of the variance. The first step accounted for 30% of the variance, with sex (β = 0.34, P < 0.05) and IL-1β (β = 0.35, P < 0.05) identified as significant factors. The second step accounted for 16% of the variance, with self-perceived illness severity emerging as an independent predictor. In the PHQ-9 model, disease duration was a significant factor in the first step but did not remain significant after adjusting for self-perceived illness severity in the second step. The overall model accounted for 54% of the variance, and the ultimate result showed that sex (β = 0.31, P < 0.01), IL-1β (β = 0.41, P < 0.001) and self-perceived illness severity (β = 0.39, P < 0.001) were related to the PHQ-9 score.
Table 6.
Regression models of Self-perceived illness severity, ISI score, GAD-7 score, and PHQ-9 score.
| Self-perceived illness severity |
ISI |
GAD-7 |
PHQ-9 |
||||
|---|---|---|---|---|---|---|---|
| Variable | β | Variable | β | Variable | β | Variable | β |
| R2 = 0.20** | R2 = 0.47** | Step1:R2 = 0.30** | Step1:R2 = 0.42*** | ||||
| Sex# | −0.17 | Sex# | 0.24* | Sex# | 0.25* | Sex# | 0.26* |
| Oxygen therapy | 0.01 | IL-1β | 0.25* | Hospital stay | 0.18 | Disease duration | 0.31** |
| Disease duration | 0.32** | Self-perceived illness severity | 0.28* | IL-1β | 0.43** | IL-1β | 0.49*** |
| Hospital stay | 0.20 | NLR | 0.07 | NLR | 0.09 | ||
| IL-1β | 0.17 | Step2:R2 = 0.46** | Step2:R2 = 0.54*** | ||||
| NLR | 0.27* | Sex# | 0.34*** | Sex# | 0.33** | ||
| IL-1β | 0.35** | Disease duration | 0.17 | ||||
| Hospital stay | 0.05 | IL-1β | 0.42*** | ||||
| Self-perceivedillness severity | 0.51*** | Self-perceived illness severity | 0.41*** | ||||
| NLR | −0.08 | NLR | −0.02 | ||||
Notes:* p < 0.05 ,** p < 0.01,***p < 0.001; #:male sex = 1, female sex = 2.
3.6. Structural equation model (SEM)
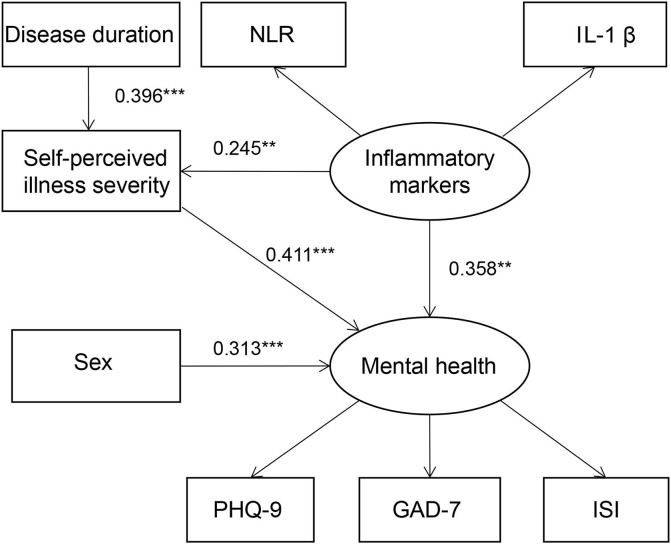
The SEM was constructed to describe possible relationships between the study variables and mental health. The model is presented in Fig. 1 , and the detailed path coefficients are provided in Table 7 . The indices for the degree of fit of the model were ideal: χ2/df was 0.956, CFI was 0.999, TLI was 1.005, SRMR was 0.040, and RMSEA was 0.001. All the path coefficients were significant. The model shows that sex (β = 0.313, P < 0.001), self-perceived illness severity (β = 0.411, P < 0.001) and inflammatory markers (β = 0.358, P = 0.002) had direct effects on mental health. In addition, the disease duration (β = 0.163, P = 0.003) and inflammatory markers (β = 0.101, P = 0.016) indirectly affected mental health with self-perceived illness severity as a mediator.
Fig. 1.
The SEM shows the relationships between sex, disease duration, self-perceived illness severity, inflammatory markers and mental health. Abbreviations: ISI: Insomnia Severity Index; PHQ-9: 9-item Patient Health Questionnaire; GAD-7: 7-item Generalized Anxiety Disorder. Note: *p < 0.05, **p < 0.01, ***p < 0.001.
Table 7.
Direct and indirect effects in SEM.
| Direct or indirect effect of pathway | Standardized path coefficient | S.E | P value |
|---|---|---|---|
| Sex → mental health | 0.313 | 0.082 | <0.001 |
| Self-perceived illness severity → mental health | 0.411 | 0.093 | <0.001 |
| Inflammatory markers → mental health | 0.358 | 0.116 | 0.002 |
| Disease duration → Self-perceived illness severity | 0.396 | 0.088 | <0.001 |
| Inflammatory markers → Self-perceived illness severity | 0.245 | 0.098 | 0.012 |
| Disease duration → Self-perceived illness severity → mental health | 0.163 | 0.055 | 0.003 |
| Inflammatory markers → Self-perceived illness severity → mental health | 0.101 | 0.042 | 0.016 |
4. Discussion
To our knowledge, this is the first study to evaluate the mental health status and explore the factors related to mental health symptoms among inpatients with COVID-19. As a novel and life-threatening disease, COVID-19 can cause substantial panic and stress in patients, especially those who are being treated in the isolation ward (Xiang et al., 2020). On the one hand, uncertainty regarding prognosis and the experience of witnessing adverse events during hospitalization may intensify inpatients’ fear. On the other hand, isolation may make patients feel lonely and bored, and make them oversensitive with regard to taking precautions and avoiding disease transmission (Purssell et al., 2020). A previous study found that patients who were quarantined had higher levels of anxiety, depression and perceptions of stigma compared with those who were not (Abad et al., 2010). Furthermore, physical discomfort and adverse side effects of treatment would also worsen mental distress. In our study, we found that high proportions of inpatients with COVID-19 experienced depression (45.9%), anxiety (38.8%) and insomnia (54.1%). Although it is common to have an emotional response to extraordinary stress, dysregulated emotions can result in several psychological disorders (Paulus et al., 2018), which might aggravate patients’ suffering and impair their functioning at work and in daily life. Moreover, excessive stress-induced psychological reactivity may impact patients’ physical health and disease outcomes (Turner et al., 2020, Yaribeygi et al., 2017). Studies about the mental health of inpatients with COVID-19 are limited. Therefore, we reviewed previous papers about the mental health outcomes in SARS patients. Sheng et al reported that approximately 35%-40% of the SARS patients in the acute phase had psychological symptoms (Sheng et al., 2005), which is similar to the result of our study on COVID-19 patients. In previous surveys, a majority of SARS survivors still suffered from psychological disturbances after discharge. Approximately 30%-35% of survivors had anxiety and depression 1–3 months after discharge (Cheng et al., 2004, Kwek et al., 2006). In a 4-year follow-up study, the incidence of posttraumatic stress disorder (PTSD) was 44% in SARS survivors (Hong et al., 2009). Because COVID-19 and SARS share some similarities, it is necessary to identify COVID-19 patients with severe psychological symptoms in the early stage and timely provide intervention.
Factors related to the mental health of inpatients with COVID-19 were explored in our study. Regression analysis showed that female sex was a robust risk factor for insomnia, anxiety and depression. The SEM also illustrated that sex had a direct and moderate effect on mental health. This finding is in accordance with those of previous studies which demonstrated that females were more likely to suffer from mood disorders, such as depression, anxiety, and posttraumatic stress disorder. (Hammen, 2018, Songtachalert et al., 2018). This sex difference in mental diseases is likely due to sex steroid hormones and genetics (Jaggar et al., 2020).
Another interesting finding was that the levels of inflammatory markers might be correlated with mental problems in COVID-19 patients. Our results demonstrated that patients with mental symptoms had higher levels of IL-1β, higher NLRs, lower levels of IL-10 and lower lymphocyte counts than those without mental symptoms. Moreover, correlation analysis revealed that both the level of IL-1β and the NLR were related to the severity of mental symptoms. This finding is not just a coincidence. Previous studies have found increased concentrations of pro-inflammatory cytokines (such as IL-6, IL12, and TNFα)) and reduced concentrations of anti-inflammatory cytokines (such as IL-4 and IL10) in patients with depression and GAD (Hou et al., 2017, Osimo et al., 2020). Evidence has shown that sleep disturbance is associated with elevated levels of inflammatory biomarkers (Nowakowski et al., 2018). Furthermore, deficiencies in the T cell system in patients with major depressive disorder have also been reported (Grosse et al., 2016). A growing body of evidence suggests that psychological disorders are associated with inflammation; however, the mechanisms underlying the association are unclear. Two different perspectives have been proposed: one view is that psychological disorders might cause inflammation, and the other is that inflammation could cause psychological disorders. In this study, we suggest that the evidence supports the latter viewpoint. First, evidence has shown that peripheral pro-inflammatory cytokines may affect the brain (Johnson et al., 2008), and it is well known that COVID-19 can result in elevated levels of serum inflammatory markers by activating the immune response (Azkur et al., 2020). Second, given that the duration of the participants’ mental symptoms was not long, it was unlikely that enough peripheral markers could have been generated, even if inflammation can be caused by mental disease. Thus, it is possible that inflammation could contribute to mental health symptoms. Based on this hypothesis, the putative relationship between inflammatory markers and mental health was established in the SEM. In the model, mental health and inflammatory markers were set as latent variables, while the scores on the three mental health assessments (PHQ-9 score, GAD-7 score and ISI score) and two inflammatory markers (IL-1β and the NLR) were included as observed variables. As shown in the SEM, inflammatory markers had significant effect on the mental health.
Our study also found that patients’ self-perceived illness severity was significantly related to the severity of mental health symptoms. The possible explanations might be that patients’ worries about their disease would added to their psychological burden. In addition, previous research also suggested that patients’ illness perception was associated with their mental distress and mental health outcome (Aitken et al., 2016). Regression analysis showed that the disease duration and levels of inflammatory markers were two factors related to self-perceived illness severity. Patients who had a longer disease duration might have a more pessimistic attitude regarding their illness. It was reported that COVID-19 patients with elevated inflammatory markers might have more severe symptoms (Wang et al., 2020). The results of the SEM suggested that self-perceived illness severity served as a mediator of the effect of the disease duration and inflammatory markers on mental health.
There were some limitations of our study. First, due to the restriction on contact with COVID-19 patients, we only conducted the survey in two isolation wards, and the sample size was small. A multicentre study with a larger sample size is needed to further verify our results. Second, as this study was a cross-sectional survey, we could not observe the patients’ mental health symptoms dynamically, and it was difficult to explore the causal relationships among the variables. Third, not all the participants had their inflammatory markers measured, and those missing data might have resulted in information bias. Fourth, this study only evaluated some of the factors associated with mental health, and further studies about other potential factors are needed.
In conclusion, the results demonstrated that large proportions of COVID-19 patients experienced mental disturbances during hospitalization. It is necessary to pay close attention to these patients’ mental health and provide timely interventions. Related factors, including female sex, the disease duration, the levels of peripheral inflammatory markers and self-perceived illness severity, may be useful to identify vulnerable patients who need psychiatric care and attention.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abad C., Fearday A., Safdar N. Adverse effects of isolation in hospitalised patients: a systematic review. J. Hosp. Infect. 2010;76:97–102. doi: 10.1016/j.jhin.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken L.M., Macfarlane B., Chaboyer W., Schuetz M., Joyce C., Barnett A.G. Physical Function and Mental Health in Trauma Intensive Care Patients: A 2-Year Cohort Study. Crit. Care Med. 2016;44:734–746. doi: 10.1097/CCM.0000000000001481. [DOI] [PubMed] [Google Scholar]
- Azkur A.K., Akdis M., Azkur D., Sokolowska M., Veen W., Brüggen M.-C., O’Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.v75.710.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.K., Wong C.W., Tsang J., Wong K.C. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS) Psychol. Med. 2004;34:1187–1195. doi: 10.1017/s0033291704002272. [DOI] [PubMed] [Google Scholar]
- Gong, J., Ou, J., Qiu, X., Jie, Y., Chen, Y., Yuan, L., Cao, J., Tan, M., Xu, W., Zheng, F., Shi, Y., Hu, B., 2020. A Tool to Early Predict Severe Corona Virus Disease 2019 (COVID-19) : A Multicenter Study using the Risk Nomogram in Wuhan and Guangdong, China. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed]
- Grosse L., Hoogenboezem T., Ambrée O., Bellingrath S., Jörgens S., de Wit H.J., Wijkhuijs A.M., Arolt V., Drexhage H.A. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav. Immun. 2016;54:38–44. doi: 10.1016/j.bbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Hammen C. Risk factors for depression: an autobiographical review. Annu. Rev. Clin. Psychol. 2018;14:1–28. doi: 10.1146/annurev-clinpsy-050817-084811. [DOI] [PubMed] [Google Scholar]
- Hong X., Currier G.W., Zhao X., Jiang Y., Zhou W., Wei J. Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: a 4-year follow-up study. Gen. Hosp. Psychiat. 2009;31:546–554. doi: 10.1016/j.genhosppsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R., Garner M., Holmes C., Osmond C., Teeling J., Lau L., Baldwin D.S. Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case-controlled study. Brain Behav. Immun. 2017;62:212–218. doi: 10.1016/j.bbi.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar M., Rea K., Spichak S., Dinan T.G., Cryan J.F. You've got male: Sex and the microbiota-gut-brain axis across the lifespan. Front. Neuroendocrinol. 2020;56 doi: 10.1016/j.yfrne.2019.100815. [DOI] [PubMed] [Google Scholar]
- Johnson R.W., Kelley K.W., O'Connor J.C., Dantzer R., Freund G.G. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Yoo S., Lee B., Lee S.H., Shin H. Psychiatric findings in suspected and confirmed middle east respiratory syndrome patients quarantined in hospital: a retrospective chart analysis. Psychiat. Invest. 2018;15:355–360. doi: 10.30773/pi.2017.10.25.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwek S.K., Chew W.M., Ong K.C., Ng A.W., Lee L.S., Kaw G., Leow M.K. Quality of life and psychological status in survivors of severe acute respiratory syndrome at 3 months postdischarge. J. Psychosom. Res. 2006;60:513–519. doi: 10.1016/j.jpsychores.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.Q., Zhang Y.H. Clinical features of 77 patients with severe acute respiratory syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2003;15:404–407. [PubMed] [Google Scholar]
- Lowe B., Decker O., Muller S., Brahler E., Schellberg D., Herzog W., Herzberg P.Y. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med. Care. 2008;46:266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- Mak I.W.C., Chu C.M., Pan P.C., Yiu M.G.C., Chan V.L. Long-term psychiatric morbidities among SARS survivors. Gen. Hosp. Psychiat. 2009;31:318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C.M., Belleville G., Belanger L., Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski S., Matthews K.A., von Känel R., Hall M.H., Thurston R.C. Sleep characteristics and inflammatory biomarkers among midlife women. Sleep. 2018;41 doi: 10.1093/sleep/zsy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Pillinger T., Rodriguez I.M., Khandaker G.M., Pariante C.M., Howes O.D. and Immunity; Brain, Behavior: 2020. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus D.J., Gallagher M.W., Bartlett B.A., Tran J., Vujanovic A.A. The unique and interactive effects of anxiety sensitivity and emotion dysregulation in relation to posttraumatic stress, depressive, and anxiety symptoms among trauma-exposed firefighters. Compr. Psychiatry. 2018;84:54–61. doi: 10.1016/j.comppsych.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Purssell E., Gould D., Chudleigh J. Impact of isolation on hospitalised patients who are infectious: systematic review with meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng B., Cheng S.K., Lau K.K., Li H.L., Chan E.L. The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. Eur. Psychiatry. 2005;20:236–242. doi: 10.1016/j.eurpsy.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songtachalert T., Roomruangwong C., Carvalho A.F., Bourin M., Maes M. Anxiety disorders: sex differences in serotonin and tryptophan metabolism. Curr. Top. Med. Chem. 2018;18:1704–1715. doi: 10.2174/1568026618666181115093136. [DOI] [PubMed] [Google Scholar]
- Turner A.I., Smyth N., Hall S.J., Torres S.J., Hussein M., Jayasinghe S.U., Ball K., Clow A.J. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrino. 2020;114 doi: 10.1016/j.psyneuen.2020.104599. [DOI] [PubMed] [Google Scholar]
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunubá Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Jiang, M., Chen, X., Montaner, L.J., 2020. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. [DOI] [PMC free article] [PubMed]
- Xiang Y.T., Yang Y., Li W., Zhang L., Zhang Q., Cheung T., Ng C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet. Psychiat. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaribeygi H., Panahi Y., Sahraei H., Johnston T.P., Sahebkar A. The impact of stress on body function: A review. Excli J. 2017;16:1057–1072. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2020. World Health Organization. Novel Coronavirus(2019-nCoV): Situation Report - 112