Abstract
Objective
To explore the clinical and radiological characteristics of COVID-19 patients with progressive and non-progressive CT manifestations.
Methods
160 patients with COVID-19 were retrospectively included from Wenzhou and Wuhan, China. CT features including lesion position, attenuation, form and total scores (0–4) at the segment level were evaluated. Other images signs were also assessed. 65 patients were classified as progressive (Group 1) and 95 as non-progressive CT (Group 2) groups according to score changes between the initial and second CT.
Results
Symptoms onset-initial CT interval time in group 1 [5 (2, 7) days] were significantly shorter than that in group 2 [10 (8, 14) days] (P < 0.001). Group 2 had higher radiological scores, with more lobes and segments affected, and other CT signs (P < 0.05). In group 1, radiological scores, the number of lobes and segments affected as well as lesions in both peripheral and central distribution, mixed ground grass opacity and consolidation density, and patchy form increased in the second CT (P < 0.05). More reticular pattern, subpleural linear opacity and bronchial dilatation were also found (P < 0.05).
Conclusion
Typically radiological characteristics of progressive CT patients could potentially help to predict changes and increase understanding of the natural history of COVID-19.
Keywords: COVID-19, CT, Pneumonia, Progression
1. Introduction
Since December 2019, a series of acute respiratory illness of unknown cause has emerged in the world [1]. The outbreaks were due to the infection of a novel betacoronavirus, namely coronavirus disease 2019 (COVID-19) [2,3]. Virus nucleic acid detection using a real-time reverse transcriptase polymerase chain reaction (RT-PCR) test is considered as the reference for the diagnosis of COVID-19 [4]. However, it plays a very limited role in the evaluation and prediction of dynamic changes.
Chest computed tomography (CT) is considered superior to chest radiography in the diagnosis and management of COVID-19 [5]. To present, we have thoroughly investigated the contributions of CT to the diagnosis of COVID-19 [[6], [7], [8]]. Common findings are bilateral involvement, subpleural distribution, along with ground glass opacity (GGO) and consolidation. Following initial diagnosis, it is crucial for physicians to know whether the CT manifestations tend to progress, remain unchanged or regress in the next few days. Temporal changes of CT manifestations during the course of COVID-19 have also been reported with slight findings in the early stage, i.e., less than 5 days from onset, peak findings in the mediate stage, i.e., about 5–13 days from onset, and recovered findings in the late stage, i.e., more than 14 days from onset [[9], [10], [11]]. However, whether the time course is the only factor which determines the progressive or non-progressive CT changes at the initial diagnosis remains unknown. In addition, the detailed CT temporal changes are yet to be discovered.
Thus, the purpose of our study is to compare the clinical and radiological characteristic of COVID-19 patients with progressive and non-progressive CT manifestations, in order to predict patients at high risk of progression and investigate the natural history of CT findings in COVID-19 infection.
2. Materials and methods
2.1. Population
This retrospective multi-center cohort study was approved by the Institutional Review Board of each participating site (KY2020-26). Written informed consent from patients was waived. From January 17 to February 25, 2020, 246 consecutive patients diagnosed with COVID-19 by RT-PCR in 3 hospitals in Wenzhou, Zhejiang Province, China, and in Wuhan, Hubei Province, China, were enrolled in our study. Only patients who had undergone CT examinations twice or more were included in the current study. 160 patients were ultimately selected. Baseline generalized clinical information, symptoms, history, and laboratory data of all patients were collected. Pneumonia was diagnosed according to the IDSA/ATS guidelines [12]. In addition, pneumonia severity was evaluated using the CURB-65 score [13]. Severe cases were determined according to the Chinese National Health Commission's COVID-19 guidance [14].
2.2. Radiological assessment
Both initial CT and the subsequent review CT were evaluated. Two types of multidetector CT scanners (Somatom Perspective, Siemens, Germany and Optima CT 540, GE, America) were used. Images with thickness of 1.0–1.5 mm and lung kernel for reconstruction were analyzed. All patients were classified into two groups with group 1 being the progressive CT group defined by the progressive of total CT scores regardless of any regression at the segment level; and group 2 being the non-progressive CT group defined by unchanged and regressive of total CT scores. All imaging features and signs were co-determined by two senior radiologists (with over 5 and 10 years of experience of cardiothoracic radiology respectively). Radiologic assessment in the current study was also as same as that in our previous study [15]. Briefly, one lesion on each pulmonary segment was assessed for location, i.e., periphery, center, and both periphery and center, score, i.e., 0–4 according to the extent of lesion, attenuation, i.e., GGO, consolidation, and mixed GGO, and form i.e., patchy and oval. Specifically, periphery and center location were distinguished by the outer 1/3 of lung. Scores were based on the size of pneumonia lesion as grade 1 (diameter, <1 cm), grade 2 (diameter, 1–3 cm), grade 3 (diameter, 3 cm to 50% of the segment), or grade 4 (over 50% of the segment) [16]. Other signs associated with pneumonia were also evaluated, including air bronchogram, centrilobular nodules, tree-in-bud, reticular pattern, subpleural linear opacity, bronchial dilatation, cystic change, lymphadenopathy and pleural effusion [17].
2.3. Statistical analysis
All statistical analysis was performed on SPSS 22.0 (IBM, USA) and GraphPad (version 8.0.2, GraphPad Software, USA). The Kolmogorov–Smirnov test was used to evaluate the normalized distribution of all data. Normally distributed data were expressed as mean ± standard deviation. Non-normally distributed data were expressed as median (25th percentile, 75th percentile). Binary data and ordinal data were expressed as N (number) and % (percentage). The Independent Sample t-Test, Fisher Exact Test, Chi-square Test and Mann–Whitney U Test were used to compare the differences between clinical and radiological data between the two groups as appropriate. The Wilcoxon Test was used to compare the initial and second CT scores. A P value less than 0.05 was considered as statistically significant.
3. Results
Of all 160 patients, 86 were male and 74 were female; 128 (80%) patients had an exposure history of having stayed in Wuhan. According to dynamic changes of radiologic scores, 65 patients were classified into the progressive CT group (group 1), and the other 95 patients into the non-progressive CT group.
3.1. Comparison of clinical and laboratory data
In the multiple comparison of clinical and laboratory data between the two groups, our results demonstrated that platelet count in group 2 was significantly higher than that in Group 1 (P = 0.004). In Group 1, 12 patients had decreased platelets (66−123 × 109/L) and 2 patients had increased platelets (422−443 × 109/L), while in Group 2, 11 patients had decreased platelet (92−123 × 109/L) and 12 patients had increased platelet (352−570 × 109/L). More patients had symptoms of a cough in group 1 than in group 2 (P = 0.018). No significant differences of other clinical and laboratory data were found between the two groups. Detailed information is presented in Table 1, Table 2, Table 3 .
Table 1.
Demographics, baseline characteristics of 160 patients.
| General data | Overall (N = 160) | Group 1 (N = 65) | Group 2 (N = 95) | P value |
|---|---|---|---|---|
| Age | 51.6 ± 13.8 | 51.2 ± 12.9 | 51.8 ± 14.5 | 0.990 |
| BMI | 23.9 ± 3.4 | 24.5 ± 3.3 | 23.5 ± 3.4 | 0.103 |
| Exposure history | ||||
| Stay in Wuhan | 128 (80.0%) | 49 (75.4%) | 79 (83.2%) | 0.318 |
| Stay in Hubei Province except Wuhan | 0 (0%) | 0 (0%) | 0 (0%) | |
| Contact with people from Hubei Province | 23 (14.4%) | 14 (21.5%) | 9 (9.5%) | |
| No relation with Hubei Province | 9 (5.6%) | 2 (3.1%) | 7 (7.4%) | |
| Severe cases | 33 (20.6%) | 13 (20.0%) | 20 (21.1%) | 0.872 |
| Chronic disease | ||||
| cardio-cerebrovascular disease | 48 (30.0%) | 18 (27.7%) | 30 (31.6%) | 0.570 |
| digestive system diseases | 16 (10.0%) | 5 (7.7%) | 11 (11.6%) | 0.422 |
| endocrine diseases | 17 (10.6%) | 8 (12.3%) | 9 (9.5%) | 0.569 |
| malignant tumor | 3 (1.9%) | 2 (3.1%) | 1 (1.1%) | 0.355 |
| neural system diseases | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| respiratory system diseases | 6 (3.8%) | 1 (1.5%) | 5 (5.3%) | 0.225 |
| Others | 7 (4.4%) | 3 (4.6%) | 4 (4.2%) | 0.902 |
| Temperature (°C) | 38.5 (37.6,38.9) | 38.5 (37.55,38.9) | 38.4 (37.6,38.9) | 0.943 |
| SaO% (range ≥95%) | 0.98 (0.96175,0.99) | 0.98 (0.97,0.99) | 0.98 (0.9515,0.9875) | 0.099 |
| Heart rate (beats per minute) | 88 (80,99) | 88 (79,99.5) | 88 (80,99) | 0.540 |
| Systolic pressure (mmHg) | 130 (120,140) | 130 (120,140) | 130 (120,140) | 0.741 |
| Diastolic pressure (mmHg) | 80 (74,88) | 80 (75,87.5) | 80 (72,88) | 0.773 |
BMI: body mass index; SaO: oxygen saturation.
Table 2.
Laboratory test of 160 patients.
| Blood routine | Overall (N = 160) | Group 1 (N = 65) | Group 2 (N = 95) | P value |
|---|---|---|---|---|
| Leucocyte (×109 / L) (range 3.5–9.5) | 4.75 (3.5475,6.545) | 4.6 (3.4,6.265) | 5.2 (3.69,6.9) | 0.354 |
| Neutrophil (×109 / L) (range 1.8–6.3) | 3.1 (2.185,4.6975) | 2.89 (2.135,4.15) | 3.3 (2.25,5.01) | 0.345 |
| Lymphocyte (×109 / L) (range 1.1–3.2) | 0.96 (0.72,1.4) | 0.84 (0.7,1.35) | 1 (0.73,1.4) | 0.163 |
| Platelet count (×109 / L) (range 125–350) | 178 (144,236.5) | 160 (131.5,203.5) | 201 (150,247) | 0.004 |
| Blood coagulation | ||||
| Active partial thrombin time (s, range 22–36) | 32.0 ± 5.6 | 32.9 ± 6.7 | 31.5 ± 0.7 | 0.069 |
| Prothrombin time (s, range 10–13.5) | 12.05 (11.375,12.9) | 12.1 (11.1,12.9) | 12 (11.5,12.9) | 0.396 |
| D-dimer (mg/L; normal range <0.55) | 0.68 (0.256,2.32) | 0.5635 (0.22,2.455) | 0.72 (0.29,1.92) | 0.232 |
| Blood biochemistry | ||||
| Glucose (mmol/L, range 3.9–6.1) | 6 (5.2,7.39) | 5.885 (5.0475,7.6075) | 6.15 (5.2,7.39) | 0.581 |
| Albumin (g/L, range 35–57) | 38.1 ± 5.2 | 38.4 ± 5.2 | 37.8 ± 5.3 | 0.222 |
| ALT (IU/L, range 0–64) | 26 (17,42.75) | 22 (16,40.5) | 28 (17,45) | 0.250 |
| AST (IU/L, range 8–40) | 27 (20.25,41.75) | 25 (19,36.5) | 28 (22,44) | 0.107 |
| Total bilirubin (μmol/L, range 4.7–24) | 8.8 (6.3,11.6) | 9.25 (6.05,12.7) | 8.5 (6.4,11.5) | 0.603 |
| Urea nitrogen (mmol/L, range 2.6–7.5) | 3.9 (3.1,4.85) | 3.9 (3.1,5.075) | 3.9 (3.05,4.85) | 0.942 |
| Creatinine (μmol/L, range 41–73) | 67 (54,77.15) | 64.25 (52.7,80.125) | 68 (55,75.5) | 0.676 |
| LDH (U/L, range 12–250) | 240 (186.25,312) | 229 (185.25,312) | 249 (190,312) | 0.852 |
| CK (mmol/L, range 40–200) | 78 (51,137.5) | 81 (45,137) | 75.5 (53.75,138.25) | 0.897 |
| K (mmol/L) | 3.67 (3.3625,4.1) | 3.7 (3.35,4.12) | 3.6 (3.37,4.1) | 0.866 |
| Na (mmol/L) | 139.2 (137,142) | 139 (136.2,141.5) | 140 (137.6,142) | 0.130 |
| Infection-associated | ||||
| C-reactive protein (mg/L, 0.0–6.0) | 20.33 (7.2,50) | 17.26 (3.25,67.65) | 21.77 (9.01,49.4475) | 0.190 |
ALT: alanine aminotransferase; AST: aspartate transaminase; LDH: lactate dehydrogenase; CK: creatine kinase; Na: sodium; K: potassium.
Table 3.
Signs, symptoms and CURB-65 scores of 160 patients.
| General data | Overall (N = 160) | Group 1 (N = 65) | Group 2 (N = 95) | P value |
|---|---|---|---|---|
| Signs and symptoms at admission | ||||
| Fever | 142 (88.8%) | 57 (87.7%) | 85 (89.5%) | 0.727 |
| Cough | 117 (73.1%) | 41 (63.1%) | 76 (80%) | 0.018 |
| Expectoration | 54 (33.8%) | 19 (29.2%) | 35 (36.8%) | 0.319 |
| Dyspnea | 22 (13.8%) | 6 (9.2%) | 16 (16.8%) | 0.171 |
| Muscle pain | 15 (9.4%) | 7 (10.8%) | 8 (8.4%) | 0.618 |
| Unconsciousness | 2 (1.3%) | 2 (3.1%) | 0 (0%) | 0.086 |
| Headache | 14 (8.8%) | 5 (7.7%) | 9 (9.5%) | 0.696 |
| Sore throat | 13 (8.1%) | 4 (6.2%) | 9 (9.5%) | 0.452 |
| Snotty | 7 (4.4%) | 2 (3.1%) | 5 (5.3%) | 0.508 |
| Chest pain | 6 (3.8%) | 4 (6.2%) | 2 (2.1%) | 0.187 |
| Chest tightness | 47 (29.4%) | 18 (27.7%) | 29 (30.5%) | 0.700 |
| Chill | 25 (15.7%) | 12 (18.5%) | 13 (13.7%) | 0.415 |
| Diarrhea | 17 (10.6%) | 4 (6.2%) | 13 (13.7%) | 0.130 |
| Nausea and vomiting | 7 (4.4%) | 2 (3.1%) | 5 (5.3%) | 0.508 |
| CURB-65 score | 0/1/2/3/4 (120/27/10/2/1) | 0/1/2/3/4 (50/10/4/0/1) | 0/1/2/3/4 (70/17/6/2/0) | 0.655 |
3.2. Comparison of the initial CT
Symptoms onset-baseline CT interval time in Group 1 were significantly shorter than that in Group 2 (P < 0.001). More lobes and segments involved were found in group 2 (P < 0.001). Similarly, group 2 had higher radiological scores (P < 0.001). Significantly more lesions in peripheral and both peripheral and central distribution, with mixed and consolidation attenuation and in patchy form were found in group 2 in comparison with those in group 1 (P < 0.05). More oval lesions were found in group 1 than in group 2 (P = 0.001). In group 2, there were also significantly higher proportion of air bronchogram, reticular pattern, subpleural linear opacity and bronchial dilatation (P < 0.05). Details are shown in Table 4 .
Table 4.
Initial CT data of 160 patients.
| Characteristics | Overall (N = 160) | Group 1 (N = 65) | Group 2 (N = 95) | P value |
|---|---|---|---|---|
| Onset-CT interval (day) | 8 (5,11) | 5 (2,7) | 10 (8,14) | <0.001 |
| Lobes involved | 5 (2.25,5) | 4 (1,5) | 5 (4,5) | <0.001 |
| Segments involved | 12 (5,16) | 7 (1,15) | 14 (9,17) | <0.001 |
| Pulmonary infiltration (number of segments involved) | ||||
| Location | ||||
| Peripheral | 7 (2,11) | 4 (1,10) | 8 (4,11) | <0.001 |
| Both | 1 (0,5) | 0 (0,3) | 2 (0,9) | 0.002 |
| Central | 0 (0,1) | 0 (0,1) | 0 (0,0) | 0.486 |
| Density | ||||
| GGO | 2 (0, 5) | 1 (0, 4.5) | 2 (0, 5) | 0.210 |
| Mixed | 5 (1,10) | 2 (0,6.5) | 7 (2,11) | <0.001 |
| Consolidation | 0 (0,1) | 0 (0,1) | 0 (0,2) | 0.051 |
| Form | ||||
| Patchy | 12 (4,16) | 5 (1,13) | 13 (8,17) | <0.001 |
| Oval | 0 (0,0) | 0 (0,1) | 0 (0,0) | 0.001 |
| Radiological score | 26 (9.25,42.75) | 14 (3,32.5) | 31 (19,45) | <0.001 |
| Other Radiological pattern | ||||
| Air bronchogram | 116 (72.5%) | 41 (63.1%) | 75 (78.9%) | 0.023 |
| Centrilobular nodules | 3 (1.9%) | 0 (0%) | 3 (3.2%) | 0.149 |
| Tree-in-bud | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| Reticular pattern | 104 (65.0%) | 33 (50.8%) | 71 (74.7%) | 0.002 |
| Subpleural linear opacity | 53 (33.1%) | 12 (18.5%) | 41 (43.2%) | 0.001 |
| Bronchial dilatation | 36 (22.5%) | 5 (7.7%) | 31 (32.6%) | <0.001 |
| Cystic change | 9 (5.6%) | 3 (4.6%) | 6 (6.3%) | 0.648 |
| Lymphadenopathy | 5 (3.1%) | 3 (4.6%) | 2 (2.1%) | 0.372 |
| Pleural effusion | 19 (11.9%) | 5 (7.7%) | 14 (14.7%) | 0.177 |
GGO: ground glass opacity.
3.3. Comparison of the second CT
Symptoms onset-second CT interval time in group 1 were significantly shorter than that in group 2 (P < 0.001). No differences of lobes, segments involved or radiological scores were found (P = 0.340, 0.915 and 0.158, respectively). Significantly more lesions in peripheral distribution were found in group 2 than those in group 1 (P = 0.003). More oval lesions were found in group 1 than those in group 2 (P = 0.006). A higher proportion of air bronchogram was found in group 1 (P = 0.015) and a higher proportion of subpleural linear opacity and bronchial dilatation was found in group 2 (P = 0.004 and 0.028, respectively). Details are shown in Table 5 .
Table 5.
Second CT data of 160 patients.
| Characteristics | Overall (N = 160) | Group 1 (N = 65) | Group 2 (N = 95) | P value |
|---|---|---|---|---|
| Onset-second CT interval (day) | 14 (10,17) | 11 (8,14) | 16 (13,19) | <0.001 |
| Lobes involved | 5 (3, 5) | 5 (3,5) | 5 (4,5) | 0.340 |
| Segments involved | 13 (7.25, 17) | 13 (6.5,18) | 13 (8,17) | 0.915 |
| Pulmonary infiltration (number of segments involved) | ||||
| Location | ||||
| Peripheral | 7 (3,10) | 6 (2,8.5) | 7 (4,12) | 0.003 |
| Both | 2 (0,7) | 2 (0,7) | 2 (0,6) | 0.491 |
| Central | 0 (0,1) | 0 (0,1) | 0 (0,0) | 0.101 |
| Density | ||||
| GGO | 2 (0, 5) | 2 (0, 6) | 1 (0, 5) | 0.897 |
| Mixed | 7 (2,11) | 5 (2,10) | 7 (3,11) | 0.179 |
| Consolidation | 0 (0,2) | 0 (0,2) | 0 (0,2) | 0.843 |
| Form | ||||
| Patchy | 13 (6,17) | 12 (3,17) | 13 (7,17) | 0.250 |
| Oval | 0 (0,0) | 0 (0,1) | 0 (0,0) | 0.006 |
| Radiological score | 27 (13, 43) | 33 (11.5,51) | 25 (15,38) | 0.158 |
| Other radiological pattern | ||||
| Air bronchogram | 100 (62.5%) | 48 (73.8%) | 52 (54.7%) | 0.015 |
| Centrilobular nodules | 3 (1.9%) | 1 (1.5%) | 2 (2.1%) | 0.796 |
| Tree-in-bud | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| Reticular pattern | 120 (75.0%) | 44 (67.7%) | 76 (80.0%) | 0.078 |
| Subpleural linear opacity | 79 (49.4%) | 23 (35.4%) | 56 (58.9%) | 0.004 |
| Bronchial dilatation | 58 (36.3%) | 17 (26.2%) | 41 (43.2%) | 0.028 |
| Cystic change | 13 (8.1%) | 6 (9.2%) | 7 (7.4%) | 0.673 |
| Lymphadenopathy | 4 (2.5%) | 2 (3.1%) | 2 (2.1%) | 0.700 |
| Pleural effusion | 23 (14.4%) | 12 (18.5%) | 11 (11.6%) | 0.224 |
GGO: ground glass opacity.
3.4. Comparison between the initial and second CT
No differences of the initial and second CT interval time were found between the two groups (P = 0.595). Significant differences between the initial and second CT score were found in group 1 and group 2 (P < 0.001).
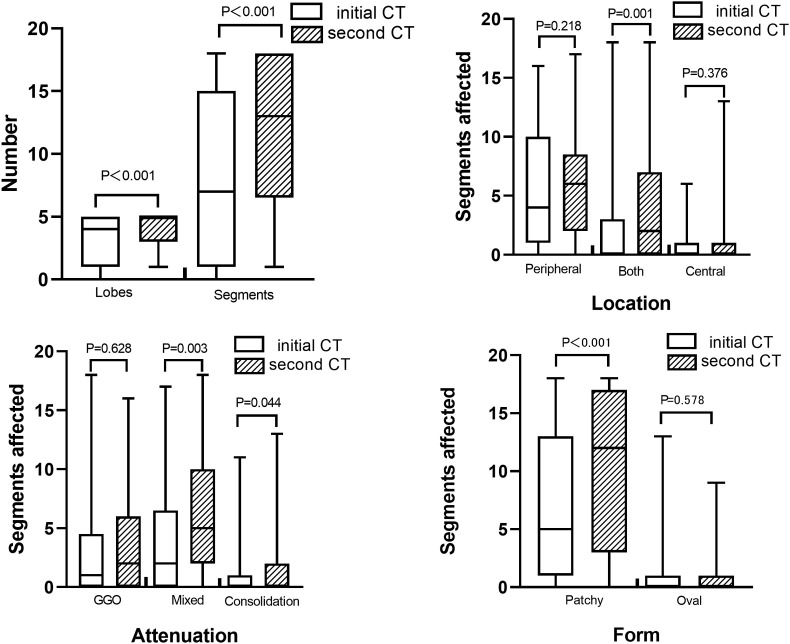
In group 1, more lobes and segments affected as well as more lesions in both distribution, mixed GGO and consolidation, and patchy form were found in the second CT (P < 0.05). In addition, more patients had reticular pattern, subpleural linear opacity and bronchial dilatation in the second CT (P = 0.012, 0.008 and 0.001, respectively). Other CT signs had no statistically significant differences between the initial and second CT (P = 0.127–0.317,1.000) (Fig. 1 ).
Fig. 1.
Comparison between the initial and second CT findings in the progressive CT group. In the second CT, A, More lobes and segments affected were found; B, More lesions in both peripheral and central distribution were found; C, More lesions in mixed GGO and consolidation attenuation were found; D, More lesions in patchy form were found.
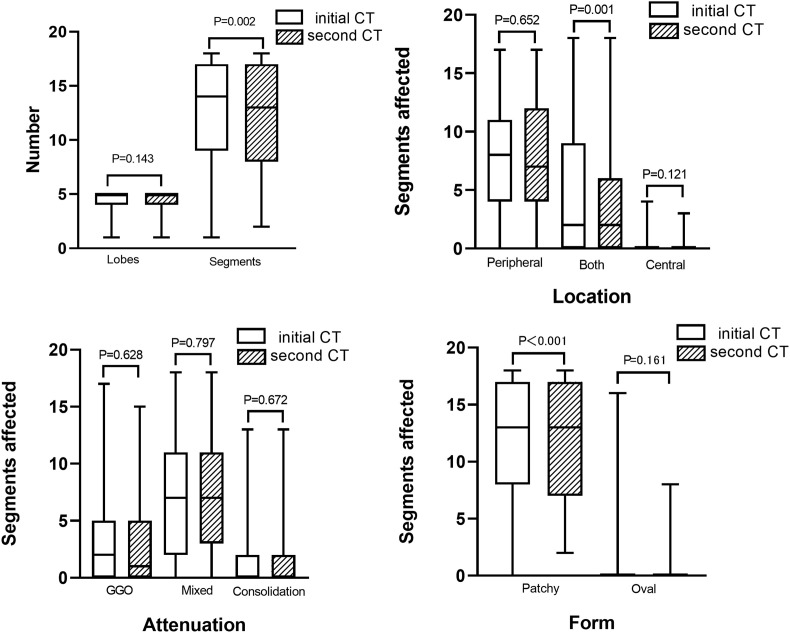
In group 2, less segments affected along with less lesions in both distribution and patchy form were found in the second CT (P < 0.05). Less patients had air bronchogram and bronchial dilatation (P < 0.001 and P = 0.012, respectively), but more patients had subpleural linear opacity (P = 0.004) in the second CT. Other CT signs had no statistically significant differences between the initial and second CT (P = 0.257–1.000) (Fig. 2 ). Examples of patients with progressive and non-progressive CT manifestations were shown in Fig. 3, Fig. 4 , respectively.
Fig. 2.
Comparison between the initial and second CT findings in the non-progressive CT group. In the second CT, A, Less segments affected were found; B, Less lesions in both peripheral and central distribution were found; C, No differences of lesion attenuation were found; D, Less lesions in patchy form were found.
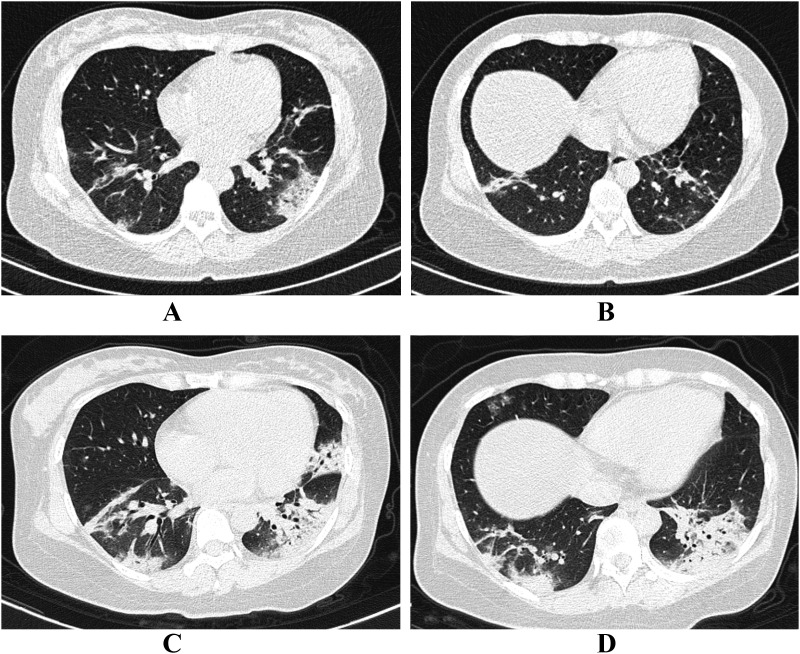
Fig. 3.
Progressive CT manifestation in a 36-year old female. A and B, Initial CT with one day from onset showed patchy consolidation predominately located in the peripheral area. Air bronchogram could be detected. C and D, The second CT was performed 3 days later. Patchy consolidation lesions progressed rapidly with extensive infiltration of the two lower lobes and lingual segment of left lung. Air bronchogram was extremely distinct. Platelet of this patients was 163 × 109/L.
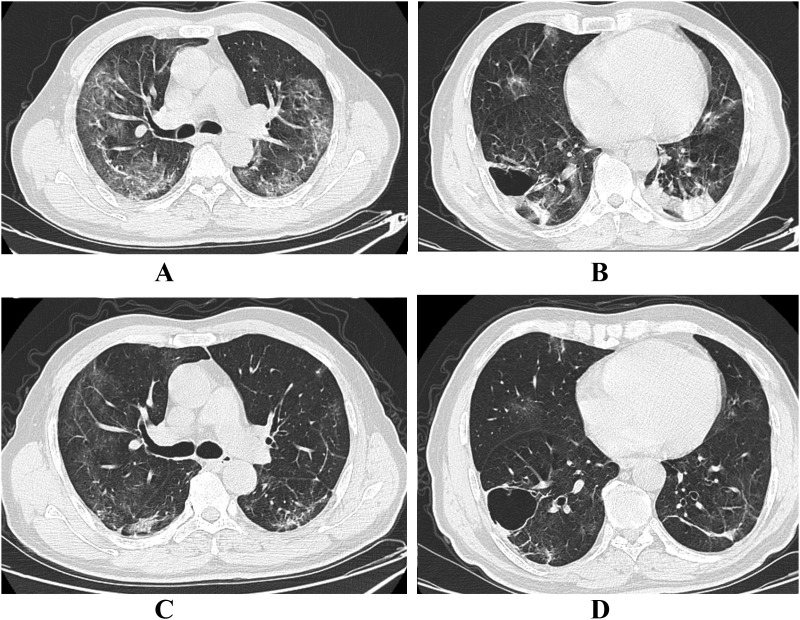
Fig. 4.
Regressive CT manifestation in a 62-year old male. A and B, Initial CT with 11 days from onset showed extensive GGO and mixed lesion in the subpleural area of upper lobes. Consolidation lesions were seen in the lower lobes. Bullae was occasionally detected in the right lower lobe. C and D, Eight days later, the patient underwent the second CT where extensive GGO, mixed and consolidation lesions were absorbed.
4. Discussion
The most important finding of our study is that patients with progressive CT manifestations had a shorter period of the disease, and the characteristic findings of progressive pattern on CT were lesions in both peripheral and central distribution, mixed GGO and consolidation attenuation and patchy form, along with CT signs of reticular pattern, subplueral linear opacity and bronchial dilatation.
The temporal changes of CT manifestations in COVID-19 patients have been investigated in several studies. In a smaller single-center study of 21 patients, Pan et al. showed that the involvement of lung on CT reached peak at approximately 10 days from the symptoms onset and then the lesions gradually decreased after 14 days [9]. One multi-center study by Bernheim et al. has reported the relationship between the symptoms onset-CT interval time and CT manifestations [10]. In this study, 0–2, 3–5 and 6–12 days of symptoms onset-CT interval time were defined as early, intermediate and late stage, respectively. 58 patients at the intermediate and late stages had more frequent CT findings than 36 patients at the early stage. Similarly, in a study of 90 patients, Wang et al. demonstrated that the peak stage of CT scores and areas affected was at the duration between 6 and 11 days from symptoms onset, and then the inflammation kept at the high level for a period of time [11].
In line with previous studies, the results of our study confirmed that for patients with the initial CT, symptoms onset-CT interval time was a specifically strong factor determining the progression of CT findings. Although platelet count in the progressive CT group was significantly lower, the number of patients with abnormal lower platelet in the two groups were similar (12/65 vs 11/95) and a majority of patients in group 1 (78.5%) had a normal platelet count. It has been reported that 36.2% of COVID-19 patients had lower platelet count, but the influence of this on the severity and outcomes of COVID-19 remains unknown [3]. Furthermore, in the current study, the typical patterns of CT from 5 to 11 days of symptoms onset-CT interval time indicated the rapid progression from symptoms onset to the peak stage.
Since chest CT is accepted as a useful tool for physicians to comprehend the disease severity in COVID-19, our results of dynamic CT changes had great clinical significance [18]. Das et al. concluded that higher chest CT scores correlated with poor prognosis and high mortality in patients with MERS (Middle East respiratory syndrome) [19]. Both Chung et al. and Huang et al. reported that higher CT severity score and bilateral multiple lobular and segmental consolidation were found in patients admitted to intensive care units [1,8]. Pan et al. revealed that diffuse lung lesions and widely increased density of both lungs on chest CT would lead to the serious impairment of lung function [20]. Thus, in the period of peak stage, i.e., symptoms onset-CT interval time of 5–11 days, it was important to review chest CT in order to be aware of the CT findings and patients' conditions and provide personalized therapies, because no other clinical and laboratory factors at baseline could predict the emergence and extent of CT progression. It was noteworthy that after the peak stage, CT manifestations would gradually decrease during the period of more than 6 days when CT reviews seemed to be unnecessary.
Regarding the CT manifestations, our previous study showed that GGO was the most common CT findings of COVID-19 at the early stage [21]. Ai et al. showed in a study of 1014 patients that GGO and consolidation with bilateral distribution were the main CT findings in COVID-19 patients [22]. Our current results have further demonstrated that progressive CT patients were characterized by much higher proportion of mixed GGO and consolidation lesions with diffuse distribution beyond peripheral area. These results were inconsistent with the findings of Wang et al., which indicated that the extensive lung involvement required CT reviews to understand it [11]. Besides, reticular pattern, subpleural linear opacity and bronchial dilatation were discovered to appear in the peak stage. The reticular pattern pathologically reflected the interstitial infiltration of inflammation which was the characteristics of viral pneumonia [23]. In our study, baseline CT of non-progressive CT patients also had much more reticular pattern than that of progressive CT patients did, indicating that non-progressive CT patients were already at the later stage in the duration of COVID-19 infection. We inferred that subpleural linear opacity tended to be the lung fibrosis caused by the sequela of inflammation, whereas bronchial dilatation was stimulated by the small airway inflammation [17]. The hypothesis was evidenced by the fact that non-progressive CT patients had more subpleural linear opacity following the inflammation absorption and less bronchial dilatation due to the relief of inflammation in the second CT.
Our study has some limitations. First, only the first two CT examinations were included for analysis. Lengthy observation and study could be focused on the pattern of lesion absorption on CT and its relationship with clinical outcomes. Second, the number of severe patients included in the current study was relatively small. The main reason was that the proportion of severe COVID-19 patients was relatively small with ICU incidence of about 5% and mortality of approximately 1% according to the latest report [3]. But monitoring the dynamic CT changes and its association with clinical information was also important for the personalized therapies and shorter hospital stay in non-severe patients. Finally, future studies should concentrate on the CT changes associated with the transmission from non-severe to severe conditions.
5. Conclusion
Progressive CT patients with COVID-19 had shorter time course of disease and typical CT manifestations. It could potentially help to predict the changes of diseases, have a profound knowledge about the natural history of CT findings and guide the time point of CT reviews for these patients.
Ethic statement
This study was approved by the Ethics Committee of Shanghai Jiaotong University Medical School Affiliated Ruijin Hospital.
Conflict of interest
None.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of Beijing You'an Hospital affiliated to Capital Medical University.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W-j, Ni Z-y, Hu Y., Liang W-h, Ou C-q, He J-x. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus disease 2019 (COVID-19) A perspective from China. Radiology 2020: 200490. [DOI] [PMC free article] [PubMed]
- 6.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H. Emerging coronavirus 2019-nCoV pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan F., Ye T., Sun P., Gui S., Liang B., Li L. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19) relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Dong C., Hu Y., Li C., Ren Q., Zhang X. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia A longitudinal study. Radiology. 2020:200843. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 doi: 10.1086/511159. S27e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., GI T. Defining community-acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377e82. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Health Commission of People's Republic of China . 2020. Pneumonia treatment program for coronavirus disease 2019 (Trail Version 7), 2.19. [Google Scholar]
- 15.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong K.T., Antonio G.E., Hui D.S., Lee N., Yuen E.H., Wu A. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228:395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 17.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 18.Kim H. Outbreak of novel coronavirus (COVID-19): what is the role of radiologists? Eur Radiol. 2020;30:3266–3267. doi: 10.1007/s00330-020-06748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das K.M., Lee E.Y., Enani M.A., AlJawder S.E., Singh R., Bashir S. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol. 2015;204:736–742. doi: 10.2214/AJR.14.13671. [DOI] [PubMed] [Google Scholar]
- 20.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Z., Lu Y., Cao Q., Qin L., Pan Z., Yan F. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22959. [DOI] [PubMed] [Google Scholar]
- 22.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China A report of 1014 cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo H.J., Lim S., Choe J., Choi S.H., Sung H., Do K.H. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38:719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]