Abstract
The vestibular organs of reptiles, birds, and mammals possess type I and type II sensory hair cells, which have distinct morphologies, physiology, and innervation. Little is known about how vestibular hair cells adopt a type I or type II identity or acquire proper innervation. One distinguishing marker is the transcription factor Sox2, which is expressed in all developing hair cells but persists only in type II hair cells in maturity. We examined Sox2 expression and formation of afferent nerve terminals in mouse utricles between postnatal day 0 (P0) and P17. Between P3 and P14, many hair cells lost Sox2 immunoreactivity and the density of calyceal afferent nerve terminals (specific to type I hair cells) increased in all regions of the utricle. At early time points, many calyces enclosed Sox2-labeled hair cells, while some Sox2-negative hair cells within the striola had not yet developed a calyx. These observations indicate that calyx maturation is not temporally correlated with loss of Sox2 expression in type I hair cells. To determine which type(s) of hair cells are formed postnatally, we fate-mapped neonatal supporting cells by injecting Plp-CreERT2:Rosa26tdTomato mice with tamoxifen at P2 and P3. At P9, tdTomato-positive hair cells were immature and not classifiable by type. At P30, tdTomato-positive hair cells increased 1.8-fold compared to P9, and 91% of tdTomato-labeled hair cells were type II. Our findings show that most neonatally-derived hair cells become type II, and many type I hair cells (formed before P2) downregulate Sox2 and acquire calyces between P0 and P14.
Keywords: Vestibular, hair cell, afferent, calyx, type I, type II, Sox2, RRID AB_10015251, RRID AB_177520, RRID AB_531793, RRID AB_2721321, RRID AB_2286684
Graphical Abstract
Introduction
Mammals have five sensory epithelia specialized to detect head movements: three cristae ampullari, which sense angular head movements, and two maculae (the utricle and saccule), which detect linear head movements. Within each epithelium, vestibular hair cells serve as mechanoreceptors for head movements. Mammals have two types of vestibular hair cells – known as type I and II – that are distinguished by morphological, molecular, and physiological features, as well as by the type of afferent innervation they receive (reviewed in Eatock and Songer, 2011; Burns and Stone, 2017; Eatock, 2018). Type I hair cells are flask-shaped and have relatively long stereociliary bundles. In contrast, type II hair cells are more cylindrical and have thicker necks, shorter stereocilia, and nuclei that are positioned lumenal to those of type I hair cells. Type II hair cells also have extensive cytoplasmic processes that extend from their basal surfaces (Pujol et al., 2014). The two hair cell types can also be distinguished by protein markers. For example, although hair cell progenitors and immature hair cells express the transcription factor Sox2, only type II hair cells continue to express Sox2 in maturity (Hume et al., 2007; Oesterle et al., 2008). Furthermore, type I and II hair cells also have distinct physiological properties; type I hair cells have low-voltage activated K+ currents, which allow them to respond faster than type II hair cells (Rennie et al., 1996; Rüsch and Eatock, 1996). The two hair cell types also differ with respect to afferent innervation; type I hair cells are almost completely enclosed by large calyceal nerve terminals, while type II hair cells are contacted by small bouton terminals.
The developmental processes by which type I and type II hair cells acquire their distinct properties and innervation are not well understood (reviewed in Burns and Stone, 2017). In mouse utricles, vestibular hair cells are first born between embryonic day (E) 10 and 12 (Ruben, 1967). Differentiating hair cells are detected initially around E10.5, as reflected by expression of the transcription factor Atoh1 (Shailam et al., 1999; McInturff et al., 2018), and cells with hair cell morphology emerge around E13 (Anniko et al., 1979, 1983; Mbiene et al., 1988). Hair cells continue to be produced over the next 7–8 days of development and into the first two weeks of postnatal life (Ruben, 1967; Sans and Chat, 1982; Rüsch et al., 1998; Denman-Johnson and Forge, 1999; Kirkegaard and Nyengaard, 2005; Hume et al., 2007; Raft et al., 2007; Burns et al., 2012). In fact, approximately half of the hair cells in the mouse utricle emerge between postnatal day (P0) and P12 (Burns et al., 2012). Of these, about one third are derived from cell divisions that occur between P0 and P2, while the remaining hair cells presumably differentiate from hair cell precursors that exited the cell cycle prior to P0. Postnatally, hair cells are most frequently added to the lateral region of the macula or near the striola (Burns et al., 2012; Bucks et al., 2017). While there is evidence of sporadic synaptic boutons of vestibular afferent nerves at E15 and partial calyces at E18 in mice, most hair cells do not appear to be innervated by vestibular afferents until birth or a few days afterwards (Anniko et al., 1983; Nordemar, 1983; Anniko, 1985; Mbiene et al., 1988; Rüsch et al., 1998). No detailed analysis of calyx development has been conducted in mouse utricles.
The present study characterized the development of type I and II hair cells and calyceal afferent terminals in utricles of postnatal mice. Using a combination of immunofluorescence and transmission electron microscopy (TEM), we examined the spatial and temporal patterns of Sox2 down-regulation and calyx formation in type I hair cells. Between P0-P14, we observed a ~25-fold increase in the density of well-formed calyces throughout the utricle, and a corresponding increase in Sox2-negative (presumptive type I) hair cells. During early postnatal stages, calyces enclosed either Sox2-positive or Sox2-negative hair cells. By P14, however, calyces enclosed only Sox2-negative hair cells. Next, we fate-mapped Plp-CreERT2-expressing hair cell progenitors between P2 and P30 and found that the majority of the fate-mapped hair cells are type II and located in extrastriolar regions. Finally, we found evidence of spontaneous hair cell death and phagocytic clearance by supporting cells at P9 and P30. Our observations indicate that the majority of hair cells generated in the neonatal utricle become type II. Furthermore, calyceal afferents are formed in all regions of the utricle during the first postnatal week and are often observed prior to the loss of Sox2 expression in their type I hair cell targets.
Materials and methods
Mouse models
Characterization of hair cell differentiation and the development of calyx nerve terminals were conducted using utricles from C57Bl/6J mice. Timed-pregnant C57Bl/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Fate-mapping studies were performed using Plp-CreERT2 (stock #5975; (Doerflinger et al., 2003) and Rosa26CAG-loxP-stop-loxP-tdTomato (Rosa26tdTomato) (also called Ai14, stock #7908, (Madisen et al., 2010) mice purchased from The Jackson Laboratory (Bar Harbor, ME); when mated, progeny were on a mixed background dominated by C57Bl/6J. Genotyping was performed by Transnetyx, Inc. (Cordova, TN). Both genders were used in all studies. All procedures were conducted in accordance with approved animal protocols from the Institutional Animal Care and Use Committees at Southern Illinois University School of Medicine (Springfield, IL), Washington University (St. Louis, MO), and the University of Washington (Seattle, WA).
Tamoxifen treatment
Plp-CreERT2:Rosa26tdTomato mice were injected with tamoxifen [3 mg/40 g, intraperitoneal injection (IP); Sigma-Aldrich (St. Louis, MO)] at P2 and P3 (~20–24hr apart). Samples were collected one week post-tamoxifen injection (at P9) or one month post-tamoxifen (at P30). Controls were age-matched Plp-CreERT2:Rosa26tdTomato mice that did not receive tamoxifen injection and were housed separately from the tamoxifen-treated experimental mice.
Immunofluorescent staining
The development of hair cell phenotype and the formation of calyx nerve terminals were examined in utricles of neonatal C57Bl/6J mice. Temporal bones were harvested at P0, P3, P5, P7, P14, and P17 and placed in chilled HEPES-buffered culture medium (Medium-199, Thermo Fisher, Waltham, MA). Small openings were made at the cochlear apex and along an exposed semicircular canal, and temporal bones were fixed for 60 min by immersion in 4% paraformaldehyde (PFA). Following thorough rinsing in PBS, the temporal bones were decalcified overnight in 0.1M EDTA at 4° C. Utricles were then isolated and processed for immunofluorescent staining, either as whole mounts or as 20 μm frozen sections. Hair cells and/or neurons were labeled with the following antibodies (details provided in Table 1): anti-ßIII-tubulin (RRID AB_2721321), anti-myosin VIIa (RRID AB_10015251), anti-neurofilament (160 kD, RRID AB_531793, or 200 kD, RRID AB_177520), and anti-Sox2 (RRID AB_2286684). Specimens were incubated in primary antibodies overnight at room temperature. The next day, they were rinsed 5x in PBS and incubated for 2 hours in secondary antibodies (anti-mouse,-rabbit, -chick, and -goat IgG, conjugated with Alexa488, 555 or 647; Thermo Fisher, Waltham, MA). Utricles were then stained for 30 minutes with DAPI; 1 μg/ml in 1X PBS; Sigma-Aldrich, St. Louis MO). Both whole mounts and labeled frozen sections were coverslipped in glycerol:PBS (9:1).
Table 1.
Antibodies used in the study
| Antigen | Source and type of antibody | Immunogen | Specificity Controls | Dilution |
|---|---|---|---|---|
| ßIII-tubulin | BioLegend Cat# 801212 Mouse monoclonal clone TUJ-1 RRID: AB_2721321 | Rat brain microtubules | Validated by immunoblot (Moody etal., 1996). Shows expected pattern of mouse hair cell labeling, as defined (e.g., Stone and Rubel,2000). | 1:200 |
| Myosin VIIa | Proteus Biosci. Cat# 25-6790 Rabbit polyclonal RRID: AB_10015251 | Amino acids 877-1075 of human myosin VIIa | Validated by immunoblot (Hasson etal., 1995). Shows expected pattern of mouse hair cell labeling, as defined (e.g., Hasson et al., 1997). | 1:500 |
| Neurofilament (200 kD) | Millipore Cat# AB5539 Chicken polyclonal RRID: AB_177520 | Purified bovine heavyweight neurofilament | Validated by immunoblot (manufacturer). Shows expected pattern of labeling of mouse inner ear nerve fibers, as defined by Del Rio et al. (2013). | 1:1000 |
| Neurofilament (~160 kD) | Develop. Studies Hybridoma Bank Mouse monoclonal Cat #2H3 RRID: AB_531793 | Embryonic rat spinal cord membrane preparations | Shows expected pattern of labeling of mouse inner ear nerve fibers, as defined by Del Rio et al. (2013). | 2 μg/ml |
| Sox2 | Santa Cruz Bioch. Cat# 17320 Goat polyclonal RRID: AB_2286684 | Peptides from human Sox2 Cterminus | Validated by immunoblot (manufacturer). Shows expected pattern of mouse hair cell labeling, as defined by Oesterle et al. (2008). | 1:100 |
In fate-mapping studies using Plp-CreERT2:Rosa26tdTomato mice, temporal bones were removed and post-fixed in electron microscopy grade 4% PFA (Polysciences, Inc., Warrington, PA) overnight at room temperature. After fixation, temporal bones were stored in 10 mM phosphate buffered saline [PBS; Sigma-Aldrich (St. Louis, MO)] until whole utricles were dissected from temporal bones and placed in 96-well plates for free-floating immunofluorescent labeling. Utricles were immunofluorescently labeled using standard methods, as described in Bucks et al. (2017), using the same antibodies described above. Some samples were counter-labeled with DAPI.
Transmission electron microscopy
Mice at P2, P4, P6, and P8 were euthanized by CO2 gas overdose. The temporal bone was exposed, and a small hole was made above the utricle. Fixative (4% glutaraldehyde in 0.1M cacodylate buffer) was dripped into the hole using a Pasteur pipette. The temporal bone was dissected and placed in fresh fixative for 2 hours at room temperature followed by overnight at 4°C. Utricles were dissected and treated with 2% osmium in 0.1M cacodylate buffer for 1 hour then rinsed in the same buffer. Utricles were treated with 1% uranyl acetate overnight at 4°C then rinsed in 0.1M cacodylate buffer. Utricles were embedded in plastic (Eponate cat #18010, Ted Pella Inc., Redding, CA). Some blocks were oriented to obtain cross-sections through the macula, perpendicular to the lumenal-stromal axis. Other blocks were oriented to obtain horizontal sections, parallel to the lumenal-stromal axis. All blocks were oriented such that most sections sampled striolar and extrastriolar regions, but the exact location of each section was not known. Semi-thin (2 μm) sections were mounted on microscope slides and coverslipped with DPX mounting medium (cat #13412, Electron Microscopy Sciences, Hatfield, PA). Once a full section through striolar and extrastriolar regions was obtained, a series of ultra-thin (80–90 nm) sections was collected on mesh and/or Formvar-coated grids and examined on a JEOL 1230 transmission electron microscope with an AMT XR80 digital camera (Woburn, MA), located in the University of Washington’s Vision Core Lab. One or two mice per age were examined.
Imaging and quantitative analysis of temporal hair cell and calyx development
Quantification of hair cells and calyx nerve terminals was conducted using confocal images obtained from standardized regions of whole-mount utricles. A Zeiss LSM700 confocal microscope (Carl Zeiss, Germany) was used to acquire images from the lateral extrastriolar, medial extrastriolar, and striolar regions of individual specimens. Image stacks were reconstructed and visualized using Volocity software (PerkinElmer, Akron, OH), and cell quantification was conducted from 50×50 μm regions of z-slices. Hair cells were identified by their strong cytoplasmic immunoreactivity for myosin VIIa and were further classified as Sox2-negative only if their nuclei displayed no detectable Sox2 immunoreactivity above background. Supporting cells lacked myosin VIIa immunoreactivity, and their nuclei were located very close to the basal lamina. Calyces were counted only if they appeared to fully enclose the basal to mid-regions of a target hair cell at the level of the cell nucleus. Hair cells were classified as mature type I hair cells if they were Sox2-negative and completely enclosed by calyces. In contrast, mature type II hair cells were Sox2-positive and lacked any sign of neural enclosure (calyx) (Rüsch et al., 1998; Eatock and Songer, 2011; Pujol et al., 2014; Bucks et al., 2017). For qualitative analyses, 5–8 mice per time point were used. For quantitative analyses, 4–5 mice per time point were used.
Imaging and quantitative analysis for fate-mapping studies
Quantification of tdTomato-positive hair cells in Plp-CreERT2:Rosa26tdTomato mice was performed on confocal images of whole-mount specimens. Using an Olympus FV1000 confocal microscope (Olympus, Center Valley, PA), we acquired images through the macula of each utricle. To determine numbers of tdTomato-positive cells that were myosin VIIa-positive, we imaged the entire utricle with a 60x oil objective and counted cells offline using the CellCounter plugin in Fiji (http://fiji.sc/). For cell counting, we relied on the criteria described in the prior section to classify hair cells as type I or II, and we also considered a few other features. Hair cells were considered type II if they had nuclei that were ovoid or irregular in shape, bigger than type I hair cell nuclei, and (for the extrastriolar regions) located apical to type I hair cell nuclei. Basolateral cytoplasmic processes were also definitive identifiers of type II hair cells (Pujol et al., 2014). Some hair cells in early neonatal utricles could not be unambiguously identified as either type I or type II. To determine numbers of tdTomato-positive hair cells that had Sox2-positive nuclei and cells that had neurofilament-positive calyces, we acquired 6 images of the macula with a 60x oil objective at 2x zoom, which comprised 36% of the macular area. Data were processed in Excel (Microsoft, Redmond, WA), and final figures were generated using Photoshop (Adobe, San Jose, CA). In tamoxifen-treated mice, we examined 5 mice at P9 and 5 mice at P30. In control mice without tamoxifen treatment, we examined 4 mice at P9 and 3 mice at P30. One utricle per animal was assessed.
Imaging and quantitative analysis of phagosomes
Utricles from Plp-CreERT2:Rosa26tdTomato mice at 7 or 28 days post-Tam (equivalent to P9 or P30) were fixed with 4% PFA overnight and incubated with Alexa Flour-conjugated phalloidin (1:100; Invitrogen, Carlsbad, CA; #A12379 or #A22287) in 1X PBS for 30 minutes, then counterstained with DAPI. Whole utricles were imaged at 20x using an Olympus FV1000 confocal microscope, and all phalloidin-labeled phagosomes in the supporting cell nuclear layer were counted. We examined utricles from 5 mice at P9 and at P30. One utricle per animal was examined.
Statistical Analysis
All statistics were performed using unpaired Student’s t-tests or two-way ANOVAs with GraphPad Prism version 6.00 (La Jolla, CA) or Microsoft Excel (Redmond, WA). Animal numbers for each experiment are provided in Materials and Methods and in Figure Legends.
Results
Development of hair cell type and calyceal innervation
In initial studies, we characterized hair cell differentiation and calyx formation in the mouse utricle during the first 2.5 weeks of postnatal development. The utricle is a otolithic organ whose sensory epithelium (macula) is subdivided into three zones or regions: the striola, the lateral extrastriola, and the medial extrastriola (Fig. 1a; reviewed in Eatock and Songer, 2011). In mice, type I and II hair cells are distributed throughout all utricular zones, with near-equal numbers of each cell type in the extrastriola and a slightly higher proportion of type I hair cells in the striola (Desai et al., 2005). Utricles were immunolabeled for myosin VIIa (to distinguish hair cells from supporting cells; Hasson et al., 1997), ßIII-tubulin/neurofilament (to label neurons and calyces), and Sox2 (to distinguish between mature type I and type II hair cells; Hume et al., 2007; Oesterle et al., 2008). Quantification of hair cell density was conducted from standardized regions located in the lateral extrastriolar, striolar, and medial extrastriolar portions of utricles (Fig. 1a). Resulting data indicated that hair cell density in the striolar and medial extrastriolar regions was approximately constant between P0-P14, while hair cell density in the lateral region increased between P0-P5 (Fig. 1b).
Figure 1.
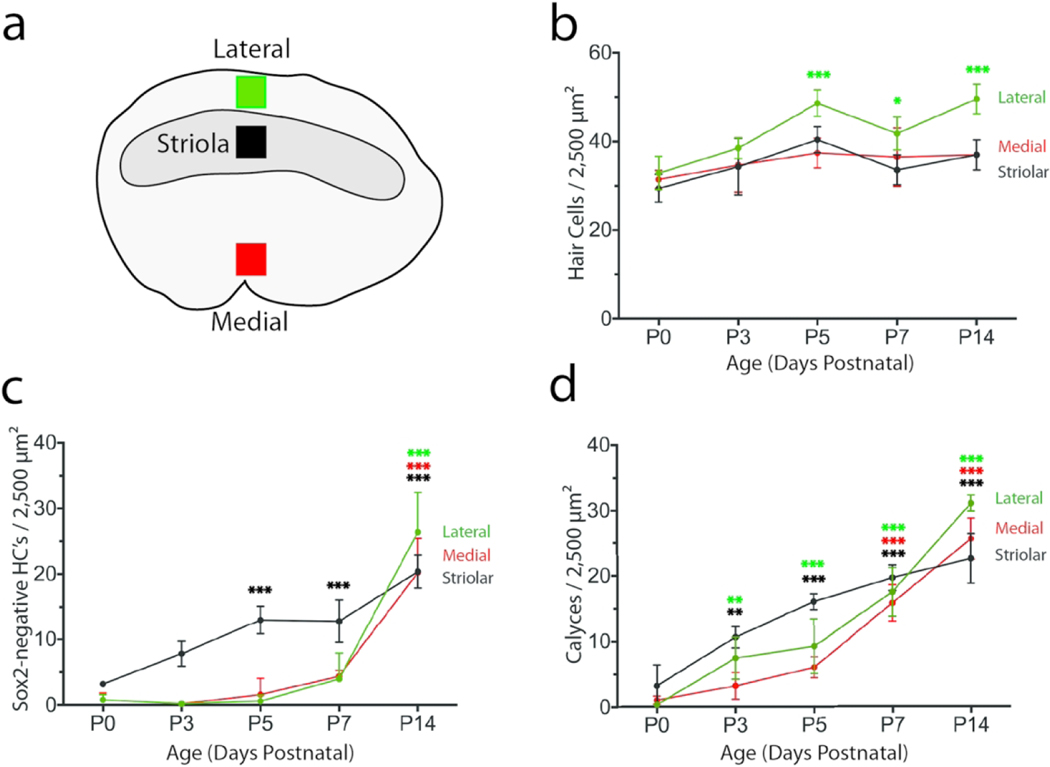
Quantitative data on utricular hair cell density, Sox2 expression, and calyx formation during neonatal development. Specimens were fixed at P0, P3, P5, P7, and P14 (n = 4–5 mice/time point) and processed for immunolabeling of hair cells, neural calyces, and Sox2 expression. All data were obtained from 50 × 50 μm regions within the lateral extrastriolar, striolar, and medial extrastriolar regions of each utricle. (a) Locations of the sampled regions, which were situated along an axis approximately midway between the anterior and posterior poles of the utricle. (b) Hair cell density as a function of postnatal age. A small developmental increase in hair cell density was noted in the lateral region. (c): Density of Sox2-negative hair cells (presumptive Type I hair cells) as a function of age. At P0, Sox2-negative cells were mainly confined to the striolar region. Although the numbers of Sox2-negative hair cells in the extrastriolar regions showed a slight increase after P5, a statistically significant loss of Sox2 expression in extrastriolar hair cells (relative to P0) was not observed until P14. (d): Density of calyx nerve terminals as a function of age. A few calyces were observed in the striola at P0. Extrastriolar calyces were first noted at P3. Calyx numbers in all regions continued to increase through P14. The increase in calyx numbers, along with the concomitant loss of Sox2 expression in hair cells, likely reflects the maturation of Type I hair cells. Data are expressed as mean ± SD. Statistical tests used two-way ANOVA followed by a Tukey’s post hoc test to determine significance between P0 and all subsequent time points (*p < 0.05, **p < 0.01, ***p < 0.001)
During mouse inner ear development, all sensory progenitors and early hair cell precursors are Sox2-positive (Woods et al., 2004; Kiernan et al., 2005; Hume et al., 2007). However, as the hair cell subtypes differentiate, Sox2 expression is lost from type I hair cells, while the nuclei of mature type II hair cells remain Sox2-positive (Hume et al., 2007; Oesterle et al., 2008). To characterize how Sox2 expression changes in vestibular hair cells as they acquire type I and II-specific features, we immunolabeled mouse utricles between P0-P17 with anti-Sox2 antibodies. At P0, a small number of hair cells lacked Sox2 immunoreactivity and those Sox2-negative cells were confined to the striola (Fig. 1c; Fig. 2a–c). Additional striolar hair cells had lost Sox2 expression at P5, but no significant loss of Sox2 expression in extrastriolar hair cells was evident until P14 (Fig. 1c; Fig. 2d–f). By P14, approximately half of the hair cells in both the striolar and extrastriolar regions were Sox2-negative (Fig. 1c; Fig. 2g–i). Although the intensity of Sox2 immunoreactivity in many hair cells became increasingly weak between P3-P14, the data plotted in Fig. 1c reflect hair cells that appeared to completely lack Sox2 expression. This pattern likely reflects the maturation of type I hair cells, and it suggests that type I hair cell maturation occurs earlier in the striola relative to the extrastriola.
Figure 2.
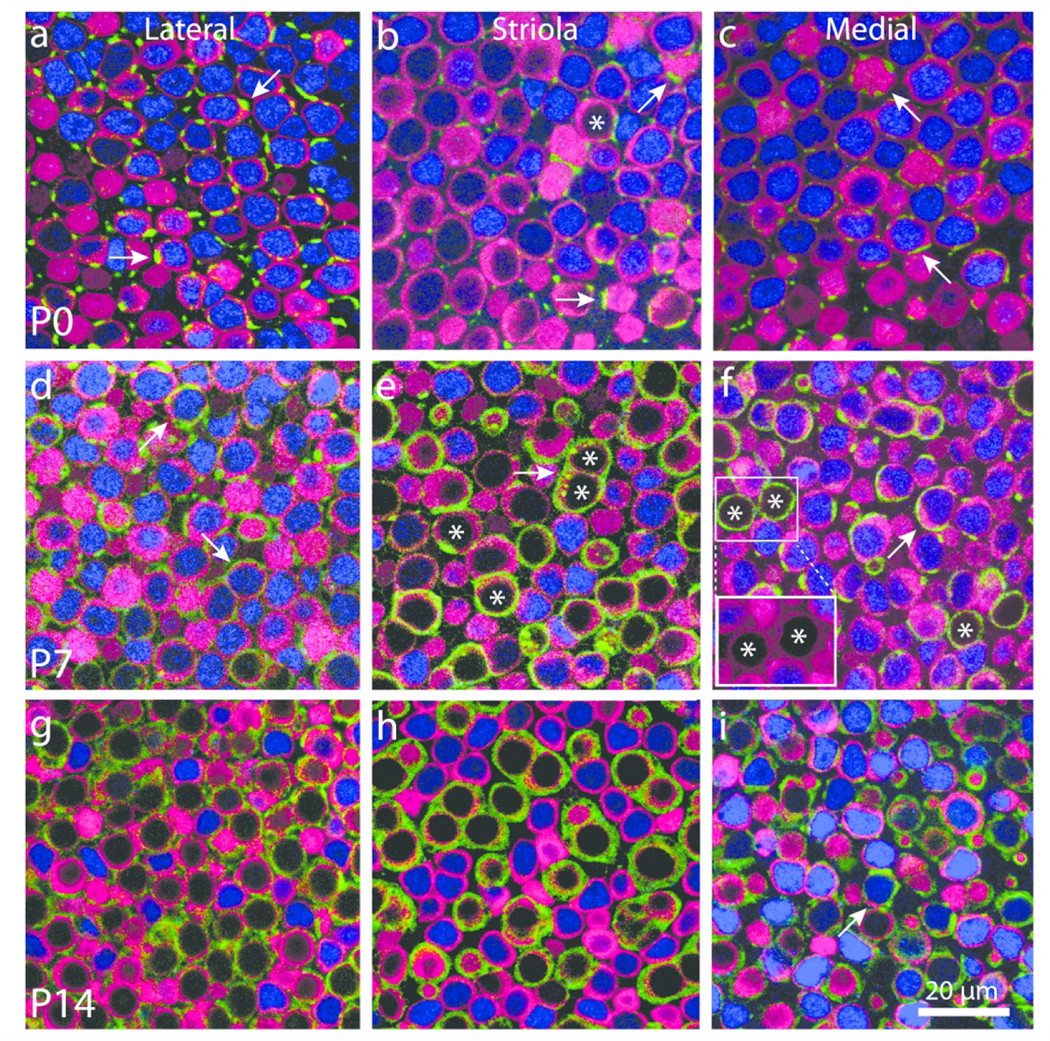
Calyceal development is not strictly correlated with down-regulation of Sox2 expression. Confocal sections through the sensory epithelia of whole-mount utricles at P0, P7, and P14 immunolabeled for myosin VIIa (hair cells, magenta), β-III tubulin/neurofilament (neurons, green), and Sox2 (blue). Images from the lateral extrastriolar, striolar, and medial extrastriolar region are shown for each age. (a–c) At P0, hair cells that lacked Sox2 expression were present only in the striola (asterisk in panel b). Many hair cells were contacted by thin neuronal processes (green, arrows in a–c), but calyces were very rare. (d–f) Hair cells with Sox2-negative nuclei (examples labeled with asterisk) became more common in the striola and were also observed in the extrastriolar regions. Mature and developing calyces were observed in all regions of the sensory epithelium (arrows in d,f). Such calyces often enclosed hair cells with Sox2-positive nuclei (arrows in d,f). Immunolabeling for hair cells and calyces often overlapped. However, reducing the intensity of the neuronal color channel (green) revealed that all calyces enclosed myosin VIIa-labeled hair cells (red, insert, f). (g–i) Hair cells and calyces were nearly mature in all regions at P14. Most calyx terminals enclosed hair cells that were Sox2-negative, although a few calyces still enclosed Sox2-expressing cells (arrow in i)
We next focused on the development of calyx nerve terminals during this same neonatal period. At early time points, we observed a number of hair cells that were contacted by processes that were immunoreactive for βIII-tubulin/neurofilament and extended along a portion of hair cell bodies, but did not completely enclose those cells (e.g., Fig. 3, top two rows; Fig. 4, bottom row). Such structures likely represent the early stages of developing calyx nerve terminals; this assertion is supported by observations with TEM in Fig. 5 (discussed below). For purposes of quantification (Fig. 1d), calyx terminals were counted only if they completely surrounded individual hair cells with a continuous band of β-III tubulin/neurofilament immunoreactivity that extended though the level of the cell nucleus. Such structures were rare at P0, but they became increasingly common during the first two postnatal weeks (Fig. 1d; Figs. 2 and 3), reflecting maturation of these hair cells toward a type I identity. The temporal pattern of calyx terminal formation differed between the striolar and extrastriolar regions (Fig. 1d) and was not reliably correlated with the loss of Sox2 expression in target hair cells. A subpopulation of Sox2-negative hair cells was observed in the striolar region prior to P5, but those cells did not possess calyces (Fig. 3 2nd row). After P5, however, the formation of calyces in the striola occurred roughly in parallel with the loss of Sox2 expression. In contrast, calyx formation in the lateral and medial extrastriolar regions often occurred prior to the loss of Sox2 expression, and immature calyces in both of these regions frequently enclosed Sox2-positive hair cells (Figs. 2 and 4). Together, these observations suggest that innervation of type I hair cells does not require downregulation of Sox2.
Figure 3.
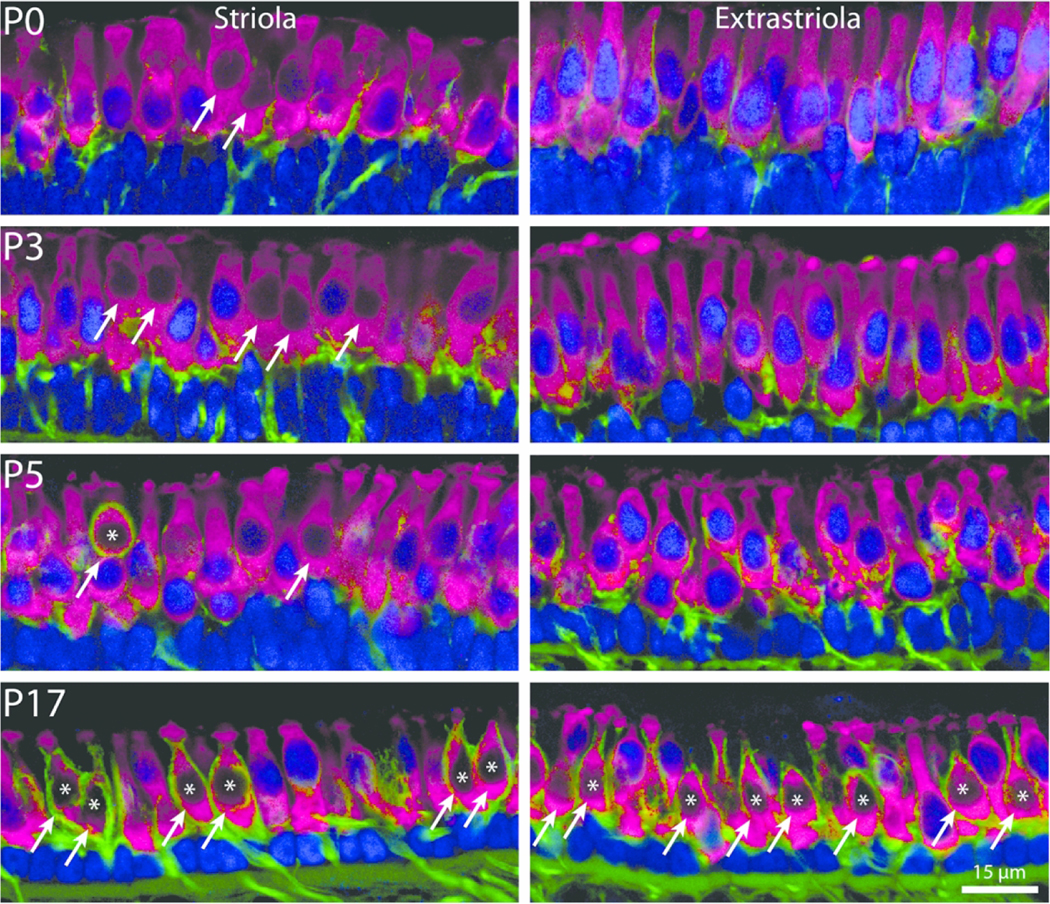
Regional changes in Sox2 expression and calyceal development. Cryosections of striolar and extrastriolar regions of the mouse utricle at P0, P3, P5, and P17 that were immunolabled for myosin VIIa (hair cells, magenta), β-III tubulin/neurofilament (neurons, green), and Sox2 (blue). Hair cells that lack Sox2-labeled nuclei (arrows) were present in the striola at all developmental stages. In contrast, very few Sox2-negative hair cells were observed in the extrastriolar regions until after P5. Hair cells enclosed by mature calyx nerve terminals (labeled with asterisk) were observed in the striola beginning at P5 and became common in all regions of the utricle at later ages
Figure 4.
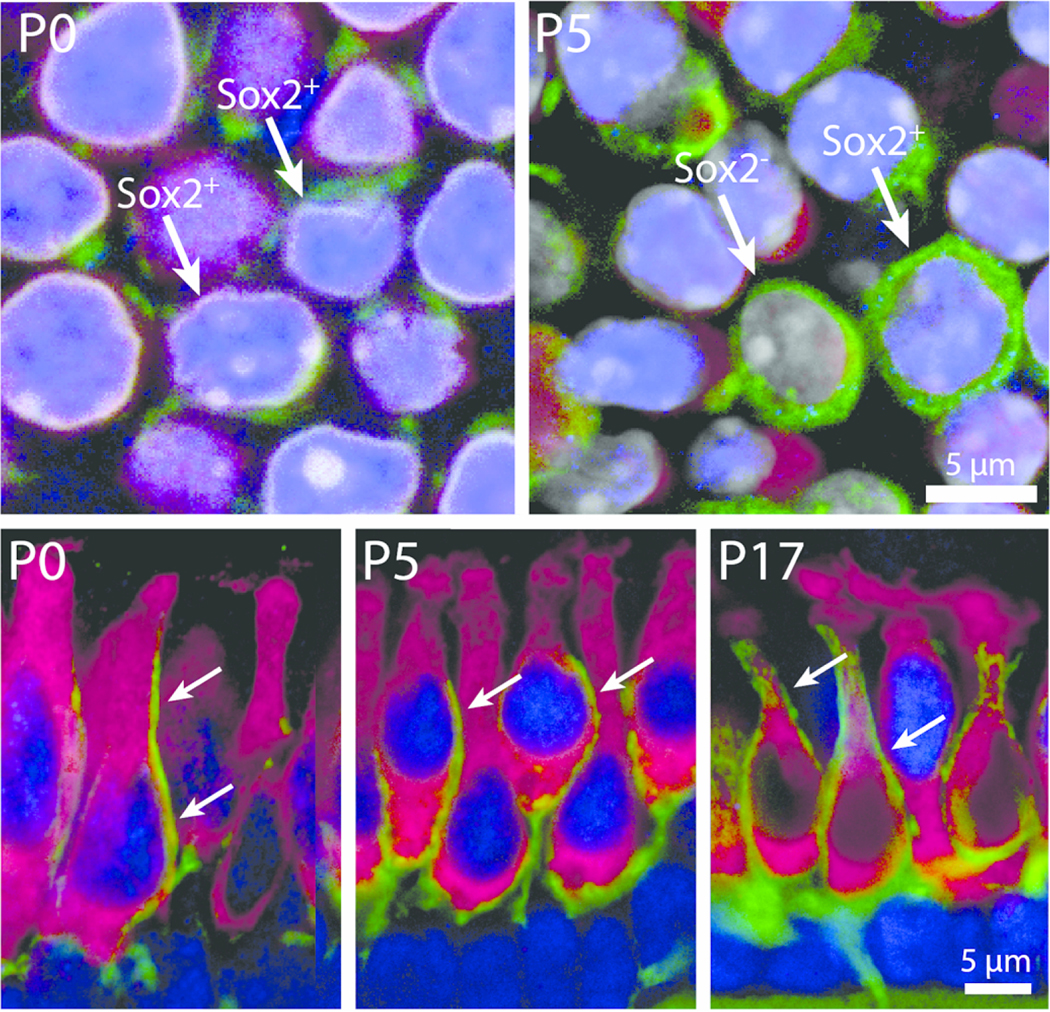
Postnatal development of calyceal innervation. High magnification images showing the developmental maturation of Type I hair cells and their associated calyx nerve terminals in whole-mount and sectioned utricles. Top row : Z-slice images from the extrastriolar region of the utricle showing hair cells (magenta, myosin VIIa) and developing afferent terminals (green, βIII tubulin/neurofilament). At P0, very few extrastriolar hair cells were enveloped by calyx terminals (green) and nearly all possessed Sox2-positive nuclei (blue, arrows). At P5, however, complete calyces were occasionally observed, and they enclosed either a Sox2-positive hair cell or a Sox2-negative hair cell (arrows). Bottom row : The development of calyx nerve terminals as revealed in cross-sections of frozen specimens. At early postnatal stages (P0 and P5), numerous hair cells in all regions of the sensory epithelium were contacted by long neuronal processes that extended along the apical-basal axis of the hair cell but did not fully enclose the cell body (arrows, P0 and P5). Such processes frequently contacted Sox2-expressing hair cells (blue nuclei). By P17, mature calyx terminals were common in all regions and primarily enclosed Sox2-negative hair cells (P17, arrows)
Figure 5.
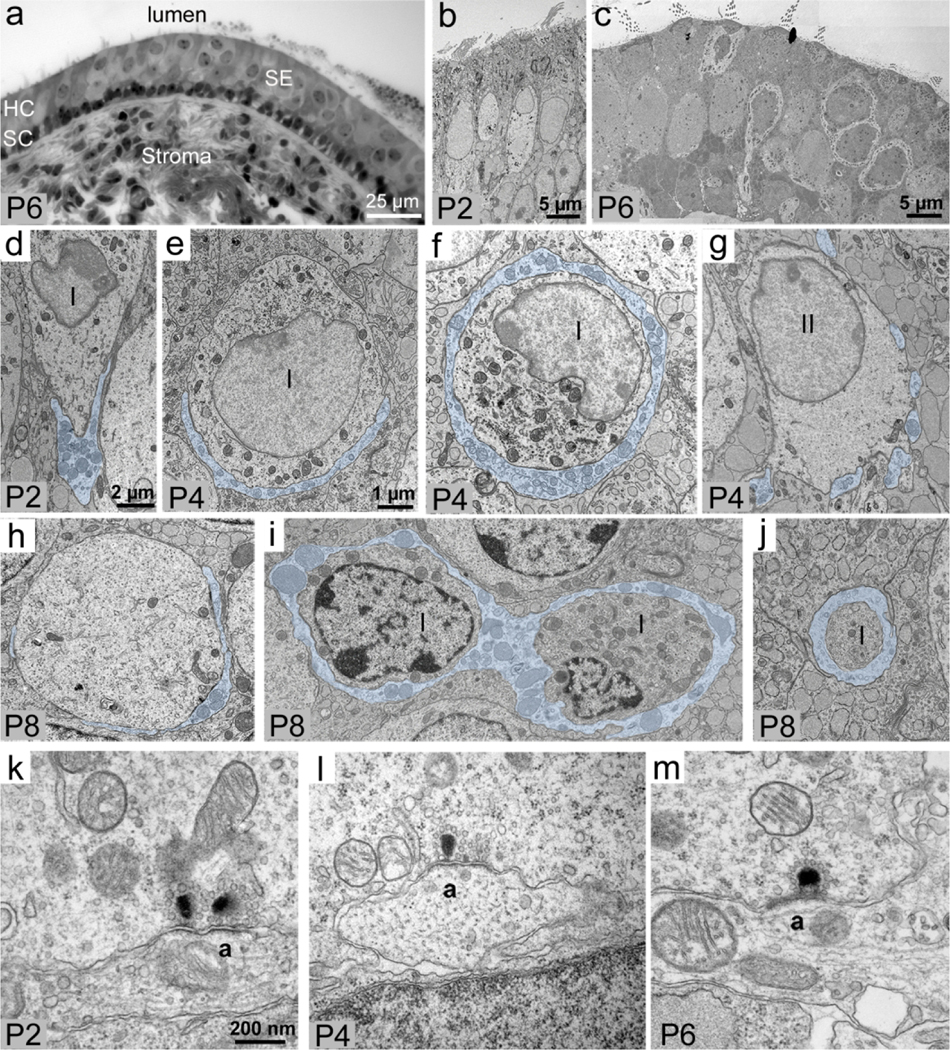
Ultrastructural development of calyces. (a) Semi-thin cross-section of the macula at P6, showing the histology of the utricular sensory epithelium (SE) and connective tissue stroma. The cells with light nuclei near the lumen are hair cells (HC), and the dark nuclei lined up at the base of the sensory epithelium are supporting cells (SC). (b): Low-magnification ultra-thin cross-section of the utricular macula at P2, showing immature columnar hair cells (arrow). (c) Low-magnification ultra-thin cross-section of the utricular macula at P6, showing differentiating hair cells, including Type I hair cells with a well-developed calyx (arrows). (d—g) High-magnification cross-sections (perpendicular to the lumen). (d,e) Two presumably immature Type I hair cells with a cup-like partial calyx at the base (transparently filled in blue) at P2 (d) and P4 (e). (f) A presumed differentiating Type I hair cell with a calyx (blue fill) that fully wraps the perinuclear region at P4. (g) An example of a presumed Type II hair cell with multiple small bouton afferents (blue fill) at P4. (h–j) Horizontal sections (parallel to the lumen) through presumed differentiating Type I hair cells at P8, with calyx afferents highlighted in blue fill. (h) A hair cell with a partial calyx (blue fill). (i) Two presumed hair cells fully wrapped by a common calyx (blue fill) at P8. (J) The neck of a hair cell wrapped by a calyx (blue fill). (k) A double ribbon synapse of a presumed Type I hair cell upon a simple calyx afferent (a) at P4. (l) A ribbon synapse of a presumed Type II hair cell upon a bouton afferent (a) at P4. (m) A ribbon synapse of a presumed Type I hair cell upon a complex calyx afferent (a), like the one shown in panel I. For (k–m), arrowheads point to the presynaptic ribbon. Scale bar in e pertains to (e–j). Scale bar in (k) pertains to (k–m)
Using TEM, we further examined afferent nerve calyces in cross-sections between P2 and P8 (Fig. 5a–c), since this was a dynamic period of calyx formation (Fig. 1d). At P2, we detected only hair cells with partial calyces that were located at the base of the cell (Fig. 5d). These hair cells were undifferentiated, lacking characteristics specific for either type I or type II hair cells. At P4, we detected some hair cells with a partial calyx (Fig. 5e) and hair cells with a calyx that completely surrounded the perinuclear region (Fig. 5f), suggesting they were approaching maturity. We also saw hair cells with multiple bouton afferent terminals characteristic of type II hair cells (Fig. 5g). At P8, there were hair cells with a partial calyx (Fig. 5h), indicating some calyces were still developing, and we also identified hair cells with a full calyx (Fig. 5i,j; a multi-calyx afferent is shown in panel i). Although we sampled only a small portion of the utricle, our observations are in general agreement with our findings in whole utricles using confocal microscopy (Figs. 1–4).
At P2, we detected immature (double-ribbon) synapses between a type I-like hair cell and an afferent calyx (Fig. 5k). At P6, ribbon synapses between type I hair cells and calyces were more mature-appearing (Fig. 5m). We also saw ribbon synapses between a type II-like hair cell and an afferent bouton (Fig. 5l).
Most hair cells that differentiate after P2 become type II’s
Progenitors in the developing mouse utricle continue to proliferate until early in the first postnatal week (Ruben, 1967; Burns et al., 2012). Consistent with this finding, we observed occasional mitotic figures of hair cell progenitors in the lateral extrastriola of utricles that were fixed at P0 (Fig. 6) but not at later postnatal stages. These cells retained intensive Sox2 immunoreactivity as they divided.
Figure 6.
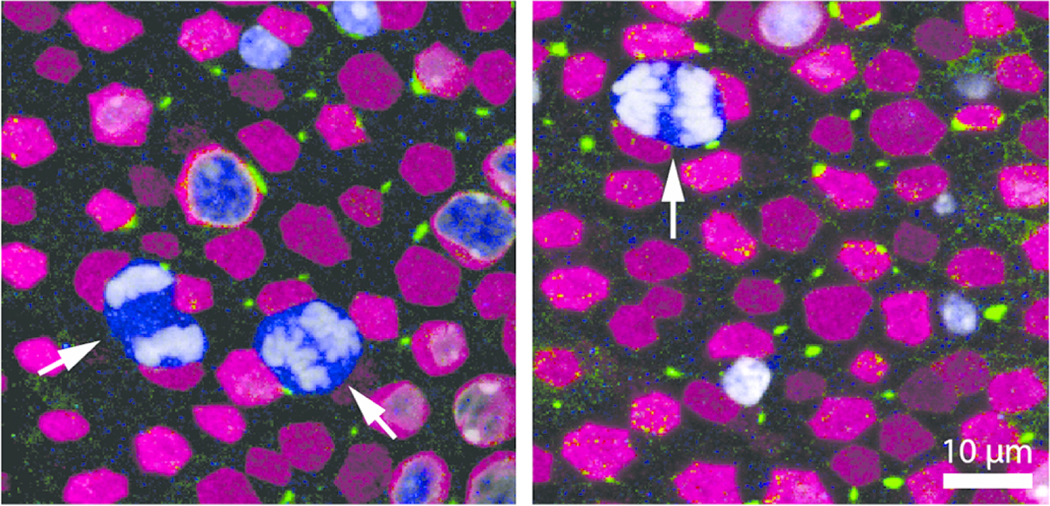
Dividing cells in the sensory region of the mouse utricle at P0. Confocal micrographs of whole-mount utricles at P0, immunolabled for myosin VIIa (hair cells, magenta), β-III tubulin/neurofilament (neurons, green), and Sox2 (blue), and also stained with DAPI (white). Arrows point to three Sox2-positive cells with mitotic figures located in the lateral extrastriola
To determine which types of vestibular hair cells are added to the utricle during the postnatal period, we fate-mapped hair cell progenitors (either prosensory cells or supporting cells) with Plp-CreERT2:Rosa26tdTomato mice that were injected with tamoxifen at P2 and P3, using methods similar to Burns et al. (2012). At P9 (one week after the first tamoxifen injection), we immunolabeled utricles for myosin VIIa and the nuclear marker DAPI, and quantified supporting cells and hair cells that expressed the red fluorescent reporter tdTomato, using criteria described in the Materials and Methods. At P9, 17% of the tdTomato-positive cells were myosin VIIa-positive (hair cells) and 83% were myosin VIIa-negative (presumptive supporting cells) (Fig. 7a1,a2, c–e). Both hair cells and supporting cells had tdTomato labeling in their cytoplasm and nucleus. Some tdTomato-positive cells were also seen in the stroma (connective tissue underlying the sensory epithelium) (Fig. 7b). There were 1564 ± 193 supporting cells (mean ± SD) per utricle labeled with tdTomato, which comprised ~76% of the total supporting cell population (Fig. 7a1, a2, e). Analysis of the striolar and extrastriolar regions showed regional differences in the tdTomato-positive supporting cells. Specifically, ~93% of supporting cells in the extrastriolar regions were labeled, and ~53% of supporting cells in the striola were labeled. This observation is similar to that reported previously for Plp-CreERT2:Rosa26tdTomato mice after receiving tamoxifen during either the neonatal period (Burns et al., 2012) or in adulthood (Bucks et al., 2017).
Figure 7.
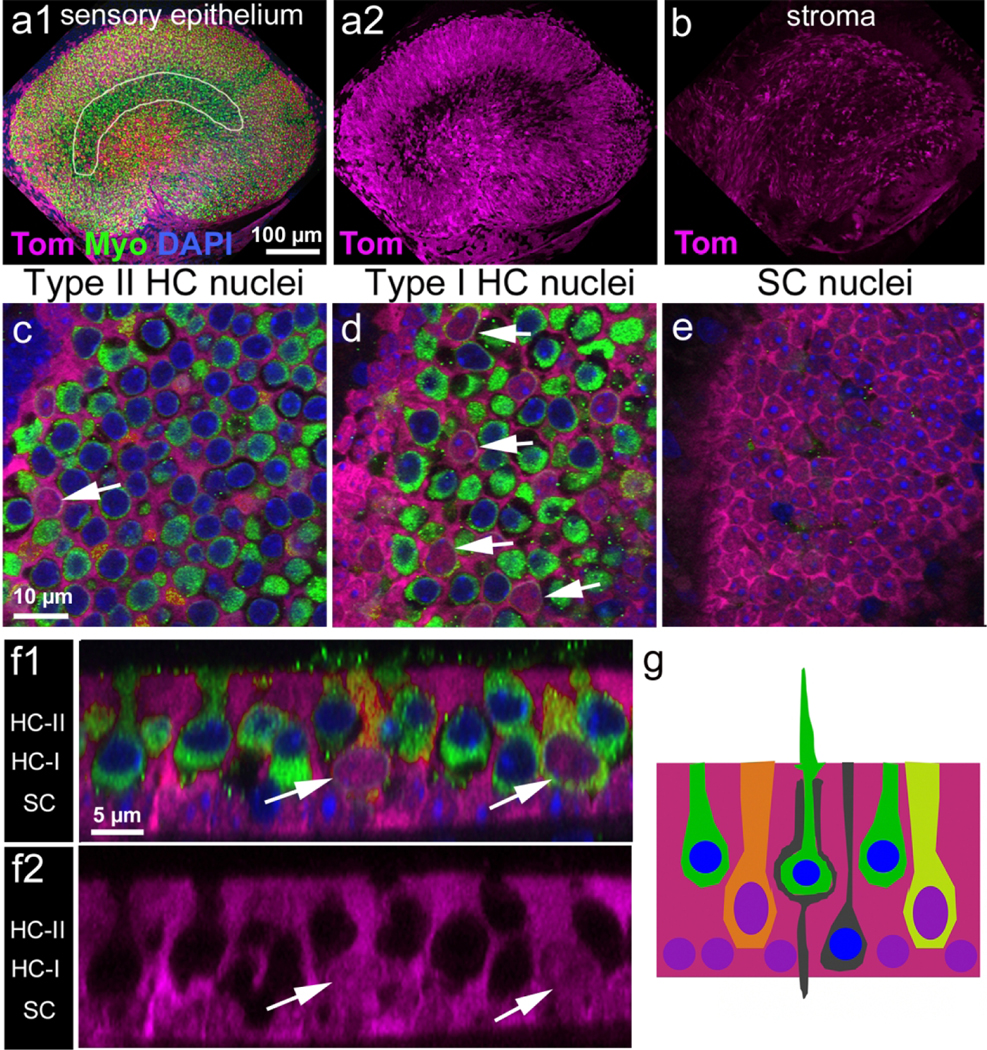
Hair cells added between P2 and P9 remain immature. (a1) Low magnification image of the lumenal surface of the utricle sensory epithelium from a Plp-CreER T2 :Rosa26 tdTomato mouse injected with tamoxifen at P2/P3 and killed at P9. The utricle was labeled with tdTomato (Tom, magenta) and antibodies to myosin VIIa (Myo, green). The approximate position of the striola is outlined in white. (a2) Same field as panel a with tdTomato labeling only. (b) tdTomato labeling in the stroma under the sensory epithelium. (c–e) High-magnification slice images through the sensory epithelium in the positions where Type II hair cell nuclei, Type I hair cell nuclei, and supporting cell nuclei would reside in mature utricles are shown in (c), (d), and (e), respectively. Most tdTomato-labeled hair cells (magenta, arrows) had nuclei located mid-way through the epithelium, where Type I hair cells should normally reside, and they lacked features unique to either Type I hair cells or II hair cells. The extrastriolar region is shown. (f1) Optical cross-sections showing tdTomato-labeled hair cells (arrows) with thick necks and relatively large nuclei. The extrastriolar region is shown. (f2) Same image as shown in (f1), with tdTomato only. In (f1) and (f2), the positions where Type II hair cell nuclei (HC-II), Type I hair cell nuclei (HC-I), and supporting cell nuclei (SC) would reside in mature epithelia are labeled on the left. (g) Diagram showing the types of cells that were tdTomato-positive in utricles at P9. Magenta = tdTomato labeling (in cytoplasm and nuclei); green = myosin VIIa labeling (in cytoplasm); blue = DAPI labeling (in nucleus). All tdTomato-labeled cells had purple nuclei (red plus blue). TdTomato-labeled hair cells had orange or yellow-green cytoplasm (magenta plus green). Scale bar in (a1) pertains to (a1), (a2), and (b); scale bar in (c) pertains to (c–e); scale bar in (f1) pertains to (f1) and (f2)
At P9, we observed that 399 ± 96 hair cells per utricle were co-labeled with myosin VIIa and tdTomato (Fig. 7c, d, f1, f2; Fig. 9a). All tdTomato/myosin VIIa double-positive hair cells had oval nuclei and thick necks, similar to type II hair cells. However, their nuclei were located just above the supporting cell nuclear layer, which is the location of type I hair cell nuclei in the mature utricle, particularly in the extrastriola (Fig. 7g). At the time of injection (P2/P3), it is likely that the majority of these labeled cells were Sox2-positive and lacked a calyx (Hume et al., 2007; see Figs. 1–4). These findings indicate that the tdTomato-positive hair cells were still in the process of maturing, which is consistent with the notion that hair cell differentiation extends into the postnatal period (Ruben, 1967; Burns et al., 2012; see Figs. 1–4). In age-matched controls that were not injected with tamoxifen and housed separately, we observed only 15.8 ± 18.8 tdTomato-positive hair cells (data not shown).
Figure 9.
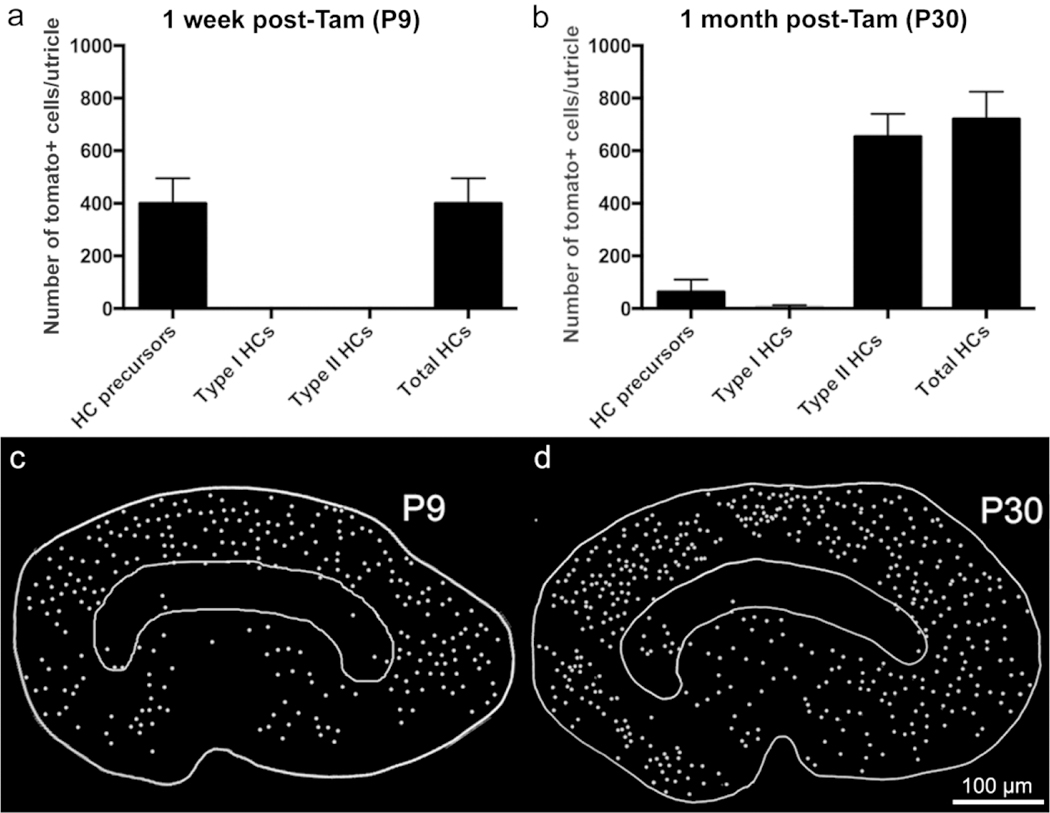
Quantification and mapping of tdTomato-labeled hair cells at P9 and P30 (7 and 28 days post-tamoxifen). (a,b) Graphs showing numbers of tdTomato-positive cells in different cell categories (hair cell or HC) precursors, Type I hair cells, and Type II hair cells from Plp-CreER T2 :Rosa26 tdTomato mice injected with tamoxifen at P2/P3 and killed at 1 week post-tamoxifen (equivalent to P9; shown in panel [a]) and at 1 month post-tamoxifen (equivalent to P30; shown in panel [b]). Error bars = +1 SD. N = 5 mice for P9 and 5 mice for P30. (c,d) Maps of tdTomato-positive hair cells from a single exemplary utricle at each age. The approximate position of the striola is outlined, the lateral side is toward the top, and the anterior side is toward the right
Additional samples were collected one month after tamoxifen injection (at P30) and labeled with an antibody to myosin VIIa and with DAPI. Many tdTomato-positive hair cells were detected (Fig. 8a, b, d, e, h). These specimens contained a statistically significant 1.8-fold increase in the number of tdTomato-positive hair cells per utricle that were derived from Plp-CreERT2-expressing supporting cells or progenitor cells (721 ± 104 at P30) compared to utricles collected at P9 (399 ± 96)(Fig. 9b, p<0.001 determined by Student’s t-test). The majority of the tdTomato-labeled hair cells (654 ± 87, ~91% of labeled cells) had type II-specific characteristics: nuclei located in the apical-most nuclear layer (Fig. 8a, d–h), at least one basolateral process (Fig. 8d, e), and a relatively thick neck. Most type I hair cells, which have more basally located nuclei and lack basolateral processes, were tdTomato-negative (Fig. 8b, d–h). However, we observed 5 ± 8 tdTomato-positive hair cells per utricle with type I-specific features, which comprised 1% of tdTomato-labeled hair cells (Fig. 9b). We also found 62 ± 45 tdTomato-positive hair cells per utricle that expressed myosin VIIa but lacked features of type I or II hair cells (Fig. 8h; Fig. 9b). These cells, which comprised ~8% of labeled hair cells, resembled the presumably immature hair cell precursors seen at P9 (Fig. 7) in all respects. The morphology of labeled cell types observed at this age is depicted in Fig. 8i. In age-matched controls that were not injected with tamoxifen and housed separately, we observed only 5.0 ± 3.61 tdTomato-positive hair cells (data not shown).
Figure 8.
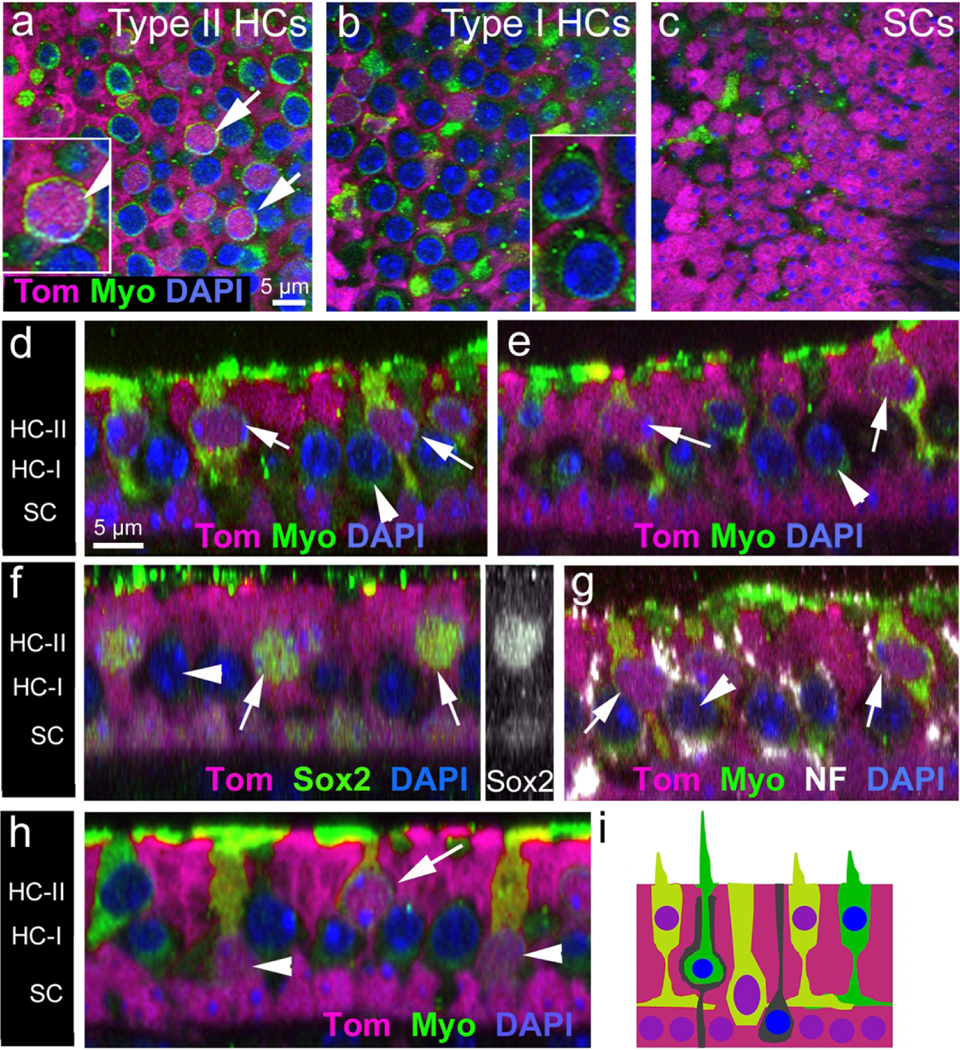
Hair cells added between P2 and P30 differentiate as Type II hair cells, and some remain immature. (a—c) Confocal sections (XY plane) through the Type II hair cell nuclear layer (a), Type I hair cell nuclear layer (b), and supporting cell nuclear layer (c) in the extrastriolar region of utricles from Plp-CreER T2 :Rosa26 tdTomato mice that received tamoxifen at P2/P3 and were killed at P30. Utricles were labeled with tdTomato (Tom, magenta), antibodies to myosin VIIa (Myo, green), and DAPI (blue). Most tdTomato-labeled hair cells (arrows in panel [a] and shown at higher magnification in the inset in [a]) had nuclei in the Type II hair cell nuclear layer and lacked a tdTomato-negative space around them, where the calyx would reside. By contrast, tdTomato-negative hair cells (arrowheads in [b]) resembled Type I hair cells because they had a tdTomato-negative calyx around them. The hair cells indicated by arrowheads in panel (b) are shown in the inset in (b), with arrowheads pointing to the tdTomato-negative calyx for each hair cell. (d,e) Optical cross-sections from two extrastriolar regions show tdTomato-positive hair cells (arrows) that have a thick neck, a basolateral process, and a nucleus in the apical-most layer (nearest the lumen), characteristic of Type II hair cells. By contrast, Type I hair cells, which were tdTomato-negative (arrowheads), had more basally located nuclei and had tdTomato-negative spaces around them, where the calyx would be. The position of Type II hair cells nuclei (HC-II), Type I hair cell nuclei (HC-I), and supporting cell nuclei (SC) are indicated on the left. (F–h) Cross-sections through the extrastriola. (f) All tdTomato-positive hair cells (arrows) had Sox2 labeling (green) in their nuclei. Arrowhead points to a Sox2-negative (presumed Type I) hair cell. The inset on the right side of panel f shows Sox2 labeling (white) only. (g) All tdTomato-positive hair cells (arrows) lacked a calyx, as indicated by the absence of neurofilament (NF) labeling (white) around their periphery. By contrast, unlabeled Type I hair cells (arrowhead) had NF labeling around the cell body periphery, characteristic of a calyx. (h) Some tdTomato-positive hair cells (arrowheads) had a basal nucleus and a thick neck, suggesting it had not differentiated as Type I or II yet. Other labeled hair cells (arrow) were Type II-like. (i) Schematic diagram showing the types of cells that were tdTomato-positive in utricles at P30. Magenta = tdTomato labeling (in cytoplasm and nucleus); green = myosin VIIa labeling (in cytoplasm); blue = DAPI labeling (in nucleus). All tdTomato-labeled cells had purple nuclei (red plus blue). TdTomato-labeled hair cells had orange or yellow-green cytoplasm (red plus green). Scale bar in a pertains to (a–c); scale bar in (d) pertains to (d–h)
To further investigate if the hair cells added between P2 and P30 from the Plp-CreERT2-expressing population possessed a type II phenotype, we immunolabeled utricles for markers specific to type I or type II hair cells. All tdTomato-labeled hair cells at P30 had nuclear Sox2, which is expressed by mature type II, but not type I, hair cells (Hume et al., 2007; Oesterle et al., 2008; Fig. 8f; Table 2). In addition, fewer than 1% of tdTomato-positive hair cells were surrounded by neurofilament-labeled calyces, typical of type I hair cells (e.g., Desai et al., 2005; Fig. 8g; Table 2). These three different analyses – cellular morphology, Sox2 labeling, and neurofilament labeling-strongly suggest that nearly all hair cells formed between P2 and P30 are type II.
Table 2. Density of tdTomato-positive hair cells labeled with different markers in Plp-CreERT2:Rosa26tdTomato mice at P30 (28 days post-tamoxifen).
Density = number of hair cells per 10,000 μm2. Data are represented as mean ± 1 standard deviation. N = mouse number.
| P30 | |||||
|---|---|---|---|---|---|
| All Tom+ HCs | Type I HCs | Type II HCs | % Type II HCs | N | |
| Myosin VIla labeling | 36.56 ±3.45 | 0.05 ±0.06 | 33.36 ±3.88 | 100% | 5 |
| All Tom+ HCs | Sox2-negative HCs | Sox2-positive HCs | % Sox2-positive HCs | N | |
| Sox2 labeling | 47.32 ±10.73 | 0 | 47.32 ±10.73 | 100% | 3 |
| All Tom+ HCs | With NF+ calyx | Without NF+ calyx | % with NF+ calyx | N | |
| NF labeling | 36.17 ±1.34 | 1.41 ±1.31 | 35.66 ±0.02 | 4% | 3 |
We next generated maps of tdTomato-positive hair cells in representative utricles at P9 and P30. At P9, the majority of the tdTomato-positive hair cells, which were immature (Fig. 7), were located in the lateral and medial extrastriolar regions, with a small number of cells found in the striola (Fig. 9c). At P30, differentiated, tdTomato-positive type II hair cells were distributed in a similar pattern across the utricle (Fig. 9d). Although we did not quantify these results, they suggest there are not significant movements of hair cells from their primarily peripheral places of genesis.
Clearance of hair cells during development
We demonstrated previously that supporting cells in adult (>P42) mouse utricles phagocytose hair cells as part of the turnover process that occurs under normal physiological conditions (Bucks et al., 2017). Therefore, we investigated the presence of phagosomes in Plp-CreERT2:Rosa26tdTomato mice at P9 and P30 using phalloidin to label filamentous actin, which is abundant in phagosomes. We found that phagosomes were present at both developmental time points and resembled those seen in adult mice (Fig. 10a1, a2, b, d1, d2, e). Such phagosomes were concentrated in the central region of the utricle (Fig. 10b,e), similar to their location in adult mice (Bucks et al., 2017). Furthermore, all phagosomes were basket-like or ring-like, and some had spike-like structures protruding from the basket or ring, which pierced a hair cell (Fig. 10c1–c3, f1–f3). We counted 24.2 ± 9.63 phagosomes per utricle at P9 and 25.6 ± 7.07 phagosomes per utricle at P30, which is similar to numbers observed in adult Plp-CreERT2:Rosa26tdTomato mice (27.8 ± 4.3; Bucks et al., 2017). Between 0 and 2 phagosomes per utricle were associated with a spike. To determine whether the phagosomes were generated by supporting cells, we injected tamoxifen into Plp-CreERT2:Rosa26tdTomato mice at P2 and P3 and examined them at P9. Many basket-like phagosomes were tdTomato-positive (Fig. 10g1, g2), indicating that supporting cells generate at least some phagosomes in young utricles, similar to mature utricles (Bucks et al., 2017).
Figure 10.
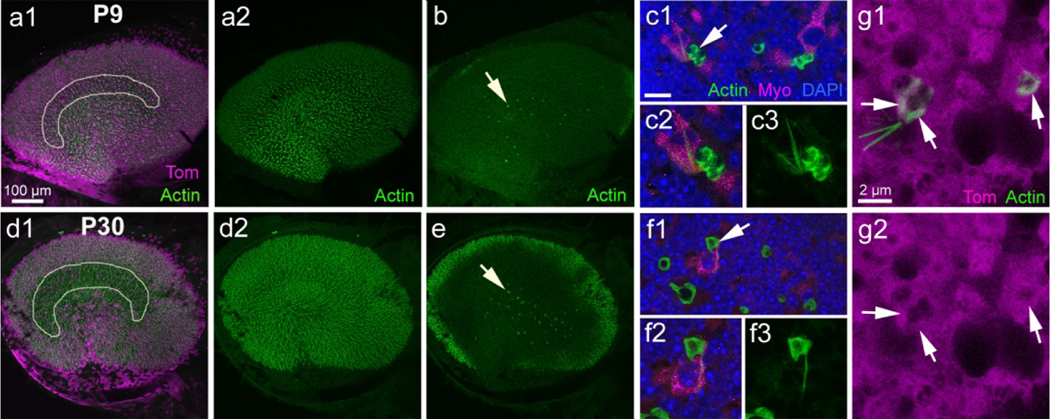
Supporting cell-derived phagosomes do not change with age. a1,d1: Low magnification images of utricles from Plp-CreER T2 :Rosa26 tdTomato mice injected with tamoxifen at P2/P3 and killed at P9 (a1) or at P30 (d1). The utricle was labeled with tdTomato (Tom, magenta) and phalloidin (actin, green). The focus is at the level of the hair cell stereocilia. The white outline indicates the presumed location of the striola. (a2,d2) Same image as in a1 and d1, but showing actin labeling only. (b,e) Same field as panels a1 and d1, but showing actin labeling in the supporting cell nuclear layer. Arrows point to actin-labeled phagosomes. (c1,f1) High magnification view of the supporting cell nuclear layer showing actin-labeled phagosomes (green) associated with myosin VIIa (Myo)-labeled hair cells (magenta) at P9 (c1) and P30 (f1). Nuclei are labeled with DAPI (blue). The arrow points to a phagosome with an associated spike. (c2,f2) Higher magnification view of phagosomes indicated by arrows in (c1) and (f1). (c3,f3) Same view as c2 and f2, but showing actin labeling only. (g1) Image from a Plp-CreER T2 :Rosa26 tdTomato mouse utricle at P9 focused on two phagosomes in the supporting cell nuclear layer that are labeled for both actin and tdTomato. (g2) Same image as panel (g1) but showing tdTomato (Tom) only. Arrows point to the location of actin labeling in both images. Scale bar in (a1) pertains to (a1), (a2), (b), (d1), (d2), and (e). Scale bar in (c1) is 5 μm for (c1) and (f1) and 3 μm for (c2), (c3), (f2), and (f3). Scale bar in (g1) pertains to (g1) and (g2)
Discussion
Hair cells in the utricles of mice begin to form at ~E13 and continue to develop and differentiate throughout the first 2–3 weeks of postnatal life (Ruben, 1967; Sans and Chat, 1982; Rüsch et al., 1998; Denman-Johnson and Forge, 1999; Kirkegaard and Nyengaard, 2005; Burns et al., 2012). In this study, we combined fate-mapping of hair cell progenitors with immunofluorescent and TEM analysis of hair cell differentiation and innervation to characterize the acquisition of hair cell type and the spatio-temporal patterns of hair cell and neural differentiation during postnatal development. Our analysis focused on the first two postnatal weeks, since a prior study showed that hair cell numbers, hair cell density, and the surface area of the sensory epithelium appear mature by P14 (Burns et al., 2012). As discussed below, our findings provide a more thorough understanding of the development of type I and II hair cells and their distinct patterns of innervation.
Developmental changes in hair cell density and type
Our data indicate that hair cell densities in the striolar and medial extrastriolar regions of the mouse utricle remain approximately constant between P0-P14, while the hair cell density in the lateral extrastriolar region undergoes a moderate increase. The relatively constant density of striolar hair cells between P0-P14 is consistent with the observations that the cells in this region are the first to be generated during embryogenesis (Sans and Chat, 1982) and that striolar hair cells begin to differentiate during the prenatal stages of utricular development (Denman-Johnson and Forge, 1999). In contrast, hair cells in the extrastriolar regions are formed later in development (Denman-Johnson and Forge, 1999; Burns et al., 2012), and the increase in hair cell density within the lateral extrastriolar region is consistent with the notion that many lateral hair cells are added after birth (Burns et al., 2012; our findings here). It should be noted, however, that our study focused on developmental changes in hair cell density, which will be affected both by the absolute number of hair cells in a region as well as overall expansion of the sensory epithelium. Considerable postnatal growth of the sensory epithelium occurs in the lateral region and the medial ‘cusp’ (Burns et al., 2012); thus, the relatively small changes in hair cell density in that region are consistent with combined hair cell addition and epithelial growth.
Hair cells in the vestibular organs of reptiles, birds, and mammals can be sub-classified as either type I or type II, based on their shape, membrane conductances, and innervation (reviewed in Eatock and Songer, 2011; Burns and Stone, 2017; Eatock, 2018). The mechanisms that regulate the development of these two hair cell subtypes are poorly understood. All hair cells originate from Sox2-expressing precursors, and immature hair cells in both the cochlea and vestibular organs transiently express Sox2. During development, Sox2 expression is lost from cochlear hair cells and from type I vestibular hair cells (Hume et al., 2007; Kempfle et al., 2016), but a distinguishing characteristic of type II hair cells is that they continue to express Sox2 after full maturation (Oesterle et al., 2008). Our data indicate that, by P0, some striolar hair cells have already lost Sox2 expression, but that significant loss of Sox2 from extrastriolar hair cells does not occur until P14. These observations are consistent with the notion that striolar type I hair cells begin to differentiate prior to birth, but in the lateral and medial extrastriolar regions, type I hair cell differentiation is not complete until the second postnatal week (Nordemar, 1983). The mechanism that triggers the loss of Sox2 expression, as well as the role of Sox2 in the developmental fate choice between type I versus type II hair cells is not clear. Our data from fate-mapping studies (discussed below) suggest that the identity of type I hair cells is specified prior to P2, but our immunolabeling data indicate that complete differentiation of type I hair cells (including loss of Sox2) does not occur until the second postnatal week.
Loss of Sox2 does not strictly coincide with calyx formation on differentiating type I hair cells
As just described, Sox2 expression is lost from differentiating type I hair cells in a central-to-peripheral gradient, but the differentiation of calyx afferent terminals on type I hair cells occurs in a more uniform spatial pattern. Furthermore, cell-by-cell analysis of Sox2 expression and calyx differentiate suggests there is little temporal or spatial correlation between the loss of Sox2 expression and the formation of calyx terminals in target type I hair cells. At P0, an appreciable number of striolar hair cells (~5 hair cells per 2,500 μm2) were Sox2-negative, even though very few mature calyces were present. Immature calyces were observed at P0 and primarily contacted Sox2-expressing hair cells. By P5, fully-formed calyces surrounded both Sox2-expressing and Sox2-negative hair cells. By P17, nearly all calyces enclosed Sox2-negative hair cells. Together, these observations indicate there is no clear causal relationship between the loss of Sox2 expression in type I hair cells and process of calyx formation. The significance of this finding is not clear, but it would appear to rule out both 1) an ‘instructive’ role for the calyx-bearing afferent in determining the phenotype of its target hair cell and 2) the requirement for loss of Sox2 for completion of calyx formation. Future studies are needed to determine the signals that guide developing calyx (or dimorphic) afferents to immature type I hair cells.
Identity and positioning of neonatally-added hair cells
The study of Burns et al. (2012) demonstrated that ~50% of hair cells in the mouse utricle are added after birth, but it was not clear whether both type I and II hair cells are formed during this neonatal period. To address this question, we labeled hair cell progenitors using Plp-CreERT2:Rosa26tdTomato mice starting at P2 and fate-mapped them over time. The numbers of labeled hair cells nearly doubled between P9 and P30, likely due to differentiation of hair cell precursors, since previous studies showed that supporting cells are quiescent after P2 (Burns et al., 2012; Gnedeva et al., 2017). Using markers of hair cells and calyces, we determined that the vast majority of hair cells derived from Plp-CreERT2-expressing cells between P2 and P30 were type II and were added primarily to the extrastriolar zones. These findings are consistent with a concurrent study (McInturff et al., 2018) that fate-mapped either progenitor cells or hair cells in young mice using Plp-CreERT2 and Atoh1CreERT2 lines, respectively. Comparison of our findings with other published work (Bucks et al., 2017) indicate that the rate at which hair cell progenitors (supporting cells) produce new type II hair cells slows considerably after mice reach sexual maturity but is significantly upregulated after hair cell destruction. These studies, conducted in the adult utricle, also fate-mapped supporting cells using Plp-CreERT2 mice over a 28-day period. Here, we showed that, between P2 and P30, 721 type II hair cells were added, which is significantly more than the number added between P42 and P60 (16 type II hair cells) and during the 28 day-period after hair cell damage at P42 (100 type II hair cells). In all contexts, all new hair cells were type II and were added primarily to extrastriolar zones, indicating that after birth, production of new type II hair cells is regionally restricted by unknown factors.
It is notable that the formation of type I hair cells becomes highly restricted by the early postnatal period, and after that time, type I hair cells are no longer formed as part of normal development, hair cell turnover (Bucks et al., 2017), or regeneration (Forge et al., 1993, 1998; Kawamoto et al., 2009; Golub et al., 2012). Although we fate-mapped the majority of neonatal hair cell progenitors in the utricle, it is possible that new type I hair cells could emerge from Plp-CreERT2-negative cells, which were not tracked in this transgenic model. Additional study is needed to determine if the lack of type I hair cell production in postnatal mice is caused by the loss of a type I-specific progenitor cell over time, changes in signaling within the developing organ, or another reason. Such information could potentially be used to promote regeneration of type I hair cells in mature mammals, which may enhance recovery of vestibular function after hair cell loss.
A small number of tdTomato-labeled hair cells at P30 still had a precursor-like morphology, indicating that their differentiation was delayed or protracted, or that they had been generated recently, perhaps as part of the normal slow rate of turnover of type II hair cells that occurs in mature mice (Bucks et al., 2017). In normal mice older than 6 weeks, type I and II hair cells are removed from the utricle by supporting cells, which act like resident phagocytes, and type II hair cells (but not type I hair cells) are replaced by supporting cells (Bucks et al., 2017). Similar hair cell turnover appears to occur in bats (Kirkegaard and Jørgensen, 2000; Kirkegaard and Jørgensen, 2001) and guinea pigs (Forge et al., 1993, 1998; Lambert et al., 1997). Here, we found evidence that, at P9 and P30, utricular supporting cells are also actively phagocytosing hair cells in similar numbers and using similar a mechanism as what occurs in adult mice. The purpose of this early hair cell clearance is unclear, although it is possible that supporting cells clear supernumerary hair cells or hair cells that develop abnormal features or innervation.
Taken together data from our immunofluorescent staining, TEM, and fate-mapping experiments suggest that type I hair cells are specified before P2 and the majority of hair cells that are added to the utricle after birth become type II’s. However, the morphology and innervation of type I and type II hair cells do not mature until P14-P17. In contrast to the neighboring cochlea, this elongated period of development correlates with the increased plasticity of the adult utricle, where both turnover (Bucks et al., 2017) and spontaneous hair cell regeneration (Forge et al., 1993, 1998; Kawamoto et al., 2009; Golub et al., 2012; Bucks et al., 2017) can occur.
Acknowledgements
This work was funded by the National Institutes of Health (R01DC013771 to JSS, R01DC014441 to BCC, and R01DC006283 to MEW), the Office of the Assistant Secretary of Defense for Health Affairs (W81XWH-15-1-0475 to BCC), and a Virginia Merrill Bloedel Traveling Scholar Award to RP. For technical assistance, we thank Irina Omelchenko, Jialin Shang, and Glen MacDonald (University of Washington), Xiao-Chun Jin (Washington University), and Kaley Graves and Michelle Randle (Southern Illinois University School of Medicine). The Core Vision Lab (supported by P30 EY01730) provided technical assistance with preparing TEM sections and access to its JEOL TEM microscope; we are grateful to the help we received from Dale Cunningham and Ed Parker at this facility.
Footnotes
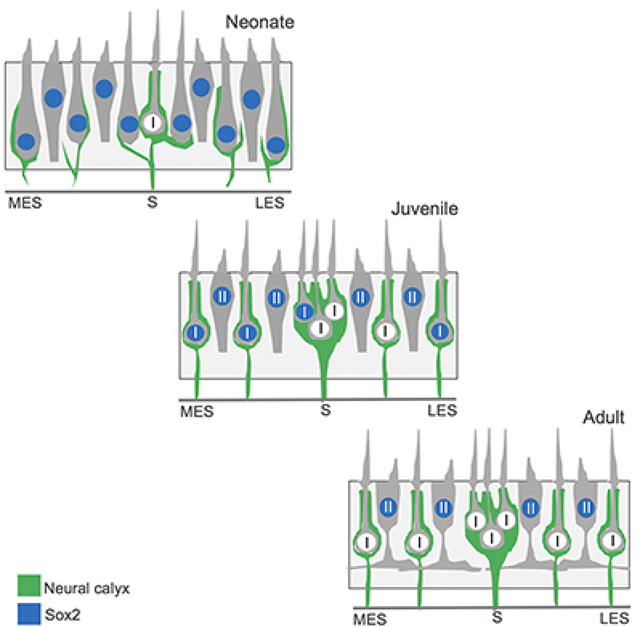
This study examined postnatal development of hair cells and primary afferent nerves in a mouse vestibular organ. This schematic illustrates our finding that the timing of afferent nerve calyx formation is not strictly correlated with loss of Sox2 expression from the afferent target cells, type I hair cells.
References
- Anniko M. (1985). Formation and Maturation of the Vestibular Ganglion. J Otorhinolaryngol Relat Spec 47:57–65. [DOI] [PubMed] [Google Scholar]
- Anniko M, Nordemar H, Sobin A. (1983). Principles in embryonic development and differentiation of vestibular hair cells. Otolaryngol Neck Surg 91:540–549. [DOI] [PubMed] [Google Scholar]
- Anniko M, Nordemar H, Wersäll J. (1979). Genesis and maturation of vestibular hair cells. Adv Otorhinolaryngol 25:7–11. [DOI] [PubMed] [Google Scholar]
- Bucks SA, Cox BC, Vlosich BA, Manning JP, Nguyen TB, Stone JS. (2017). Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice. Elife 6:e18128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, On D, Baker W, Collado MS, Corwin JT. (2012). Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones. J Assoc Res Otolaryngol 13:609–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Stone JS. (2017). Development and regeneration of vestibular hair cells in mammals. Semin Cell Dev Biol 65:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman-Johnson K, Forge A. (1999). Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J Neurocytol 28:821–35. [DOI] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. (2005). Comparative Morphology of Rodent Vestibular Periphery. I. Saccular and Utricular Maculae. J Neurophysiol 93:251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger N, Macklin W, Popko B. (2003). Inducible site-specific recombination in myelinating cells. Genesis 35:63–72. [DOI] [PubMed] [Google Scholar]
- Eatock RA. (2018). Specializations for Fast Signaling in the Amniote Vestibular Inner Ear. Integr Comp Biol 58:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE. (2011). Vestibular Hair Cells and Afferents: Two Channels for Head Motion Signals. Annu Rev Neurosci 34:501–534. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin J, Nevill G. (1993). Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259:1616–1619. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Nevill G. (1998). Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol 397:69–88. [PubMed] [Google Scholar]
- Gnedeva K, Jacobo A, Salvi JD, Petelski AA, Hudspeth AJ. (2017). Elastic force restricts growth of the murine utricle. Elife 6:e25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub J, Tong L, Ngyuen T, Hume C, Palmiter RD, Rubel EW, Stone JS. (2012). Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J Neurosci 32:15093–15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao YD, Yee AG, Mooseker MS, Corey DP. (1997). Unconventional myosins in inner-ear sensory epithelia. J Cell Biol 137:1287–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. (1995). Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci U S A 92:9815–9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume C, Bratt D, Oesterle E. (2007). Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns 7:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. (2009). Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res 247:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempfle JS, Turban JL, Edge ASB. (2016). Sox2 in the differentiation of cochlear progenitor cells. Sci Rep 6:23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KKH, Tang ASP, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KSE. (2005). Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434:1031–1035. [DOI] [PubMed] [Google Scholar]
- Kirkegaard M, Jørgensen JM. (2000). Continuous hair cell turnover in the inner ear vestibular organs of a mammal, the Daubenton’s bat (Myotis daubentonii). Naturwissenschaften 87:83–6. [DOI] [PubMed] [Google Scholar]
- Kirkegaard M, Jrgensen JM. (2001). The inner ear macular sensory epithelia of the Daubenton’s bat. J Comp Neurol 438:433–444. [DOI] [PubMed] [Google Scholar]
- Kirkegaard M, Nyengaard JR. (2005). Stereological study of postnatal development in the mouse utricular macula. J Comp Neurol 492:132–144. [DOI] [PubMed] [Google Scholar]
- Lambert PR, Gu R, Corwin JT. (1997). Analysis of small hair bundles in the utricles of mature guinea pigs. Am J Otol 18:637–43. [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbiene JP, Favre D, Sans A. (1984). The pattern of ciliary development in fetal mouse vestibular receptors. A qualitative and quantitative SEM study. Anat Embryol (Berl) 170:229–38. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Favre D, Sans A. (1988). Early innervation and differentiation of hair cells in the vestibular epithelia of mouse embryos: SEM and TEM study. Anat Embryol (Berl) 177:331–40. [DOI] [PubMed] [Google Scholar]
- McInturff S, Burns JC, Kelley MW. (2018). Characterization of spatial and temporal development of Type I and Type II hair cells in the mouse utricle using new cell-type-specific markers. Biol Open 7:bio038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA, Miller V, Spanos A, Frankfurter A. (1996). Developmental expression of a neuron-specific beta-tubulin in frog (Xenopus laevis): a marker for growing axons during the embryonic period. J Comp Neurol 364:219–30. [DOI] [PubMed] [Google Scholar]
- Nordemar H. (1983). Postnatal development of the vestibular sensory epithelium in the mouse. Acta Otolaryngol 96:447–56. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. (2008). Sox2 and Jagged1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol 9:65–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R, Pickett SB, Nguyen TB, Stone JS. (2014). Large basolateral processes on type II hair cells are novel processing units in mammalian vestibular organs. J Comp Neurol 522:3141–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie KJ, Ricci AJ, Correia MJ. (1996). Electrical filtering in gerbil isolated type I semicircular canal hair cells. J Neurophysiol 75:2117–2123. [DOI] [PubMed] [Google Scholar]
- Del Rio T, Nishitani AM, Yu W-M, Goodrich LV. (2013). In vivo analysis of Lrig genes reveals redundant and independent functions in the inner ear. PLoS Genet 9:e1003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben RJ. (1967). Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol Suppl 220:1–44. [PubMed] [Google Scholar]
- Rüsch A, Eatock RA. (1996). A delayed rectifier conductance in type I hair cells of the mouse utricle. J Neurophysiol 76:995–1004. [DOI] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. (1998). Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18:7487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans A, Chat M. (1982). Analysis of temporal and spatial patterns of rat vestibular hair cell differentiation by tritiated thymidine radioautography. J Comp Neurol 206:1–8. [DOI] [PubMed] [Google Scholar]
- Shailam R, Lanford PJ, Dolinsky CM, Norton CR, Gridley T, Kelley MW. (1999). Expression of proneural and neurogenic genes in the embryonic mammalian vestibular system. J Neurocytol 28:809–19. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. (2000). Temporal, spatial, and morphologic features of hair cell regeneration in the avian basilar papilla. J Comp Neurol 417:1–16. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. (2004). Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci 7:1310–8. [DOI] [PubMed] [Google Scholar]


