Abstract
Objective:
This systematic review and meta-analysis investigated risk factors for pneumothorax following CT-guided percutaneous transthoracic lung biopsy.
Methods:
A systematic search of nine literature databases between inception to September 2019 for eligible studies was performed.
Results:
36 articles were included with 23,104 patients. The overall pooled incidence for pneumothorax was 25.9% and chest drain insertion was 6.9%. Pneumothorax risk was significantly reduced in the lateral decubitus position where the biopsied lung was dependent compared to a prone or supine position [odds ratio (OR):3.15]. In contrast, pneumothorax rates were significantly increased in the lateral decubitus position where the biopsied lung was non-dependent compared to supine (OR:2.28) or prone position (OR:3.20). Other risk factors for pneumothorax included puncture site up compared to down through a purpose-built biopsy window in the CT table (OR:4.79), larger calibre guide/needles (≤18G vs >18G: OR 1.55), fissure crossed (OR:3.75), bulla crossed (OR:6.13), multiple pleural punctures (>1 vs 1: OR:2.43), multiple non-coaxial tissue sample (>1 vs 1: OR 1.99), emphysematous lungs (OR:3.33), smaller lesions (<4 cm vs 4 cm: OR:2.09), lesions without pleural contact (OR:1.73) and deeper lesions (≥3 cm vs <3cm: OR:2.38).
Conclusion:
This meta-analysis quantifies factors that alter pneumothorax rates, particularly with patient positioning, when planning and performing a CT-guided lung biopsy to reduce pneumothorax rates.
Advances in knowledge:
Positioning patients in lateral decubitus with the biopsied lung dependent, puncture site down with a biopsy window in the CT table, using smaller calibre needles and using coaxial technique if multiple samples are needed are associated with a reduced incidence of pneumothorax.
Introduction
CT-guided percutaneous transthoracic lung biopsy (CT-PTLB) is a well-established procedure to obtain tissue from pulmonary lesions.1,2 Previous studies identify that pneumothorax is the most frequent complication.3 This can lead to complications associated with chest tube insertion and increased length of hospitalization.3 Several societies have published guidelines which include the risks of CT-PTLB as outlined in Table 1.
Table 1.
Selected reference guidelines for pneumothorax rates and pneumothorax rates requiring chest drains for CT-PTLB
| Society/Guideline | Pneumothorax rate | Pneumothorax rate requiring drain/intervention |
|---|---|---|
| SIR with the ACR | 45% | 20% |
| BTS | 20.5% | 3.1% |
| Cardiovascular and interventional CIRSE | 18.8–25.3% | 4.3–5.1% |
ACR, American College of Radiology; BTS, British Thoracic Society; PTLB, percutaneous transthoracic lung biopsy; SIR, Society of Interventional Radiology.
As per British Thoracic Society Society guidelines, operators performing radiologically guided lung biopsy should strive to achieve the lowest possible complication rate to minimize morbidity and mortality.1 Multiple studies have concluded that pneumothorax is dependent on factors that can and cannot be modified by the clinician.3–6 Modifiable factors include patient position, needle biopsy type and technique factors. Non-modifiable factors include patient age and presence of emphysema. The impact of these factors on pneumothorax rates varies in reported literature.1,7,8 Post-biopsy manoeuvres such as tract sealants and patient positioning also impact complication rates. These have been recently reviewed.9
This systematic review and meta-analysis aims to collate the best available evidence into single effect estimates that can be utilized by clinicians. These, in turn, can be used to inform consent, optimize patient selection, technique, inform guidelines and direct future research
Methods
Search strategy
The search strategy was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis checklist and recommendations made by the Cochrane Collaboration.10,11 The study was registered with PROSPERO (ID: CRD42018084414) We performed an electronic search of nine literature databases including Medline, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, ACP Journal Club, Database of Abstracts of Reviews and Effectiveness, Google Scholar and NHS Economic Evaluation Database for articles published between inception to September 2019. Search terms included the terms pneumothorax, biopsy, lung, computed tomography, complications, risks and injuries inclusive of relevant truncations, MeSH terms and keywords. Results were limited to studies of the English Language and humans. Duplicates were removed. Reference lists of included studies were screened to identify additional potentially relevant studies.
Study selection
The literature search and abstracts were reviewed for eligibility independently by two investigators (ARH and IL) and disagreements or omissions were resolved by senior investigators (MVC and YRH). Articles were eligible for full-text review if the (1) lung biopsies were performed using CT-guidance, (2) reported pneumothorax and/or chest drain insertion rates with and without at least one risk factor, (3) had ethics approval, and (4) have a study sample >200 patients. Abstracts, editorials, case reports, case series, systematic reviews, meta-analyses and unpublished articles were excluded. Studies that examined post-biopsy manoeuvres such as tract sealants and rollover were excluded.
Data extraction
The data were extracted independently by two investigators (ARH and IL). Any discrepancies were resolved by consensus amongst all authors. Data extraction focused on the rate of pneumothorax and chest drain insertion following lung biopsy and associated risk factors. Pneumothorax detection protocols via plain film and/or CT were noted. Non-modifiable patient and lesion factors identified and included age, gender, smoking history, emphysema presence, lesion size, depth and lobe location. Modifiable factors were stratified into four categories: operator factors, biopsy needle factors, biopsy technique and patient position during biopsy. Operator factors included experience (attending vs resident) and whether sample adequacy was reviewed by cytologist immediately. Biopsy needle factors included fine needle aspiration (FNA) vs core biopsy, non-coaxial vs coaxial and largest guide/biopsy needle gauge (guide if coaxial technique used or biopsy needle if non-coaxial needle used) (≤18G vs >18G). Biopsy technique factors included needle entry location on chest wall (anterior, lateral, posterior), needle angle, whether a bulla or fissure was crossed, number of pleural punctures and number of tissues samples. Patient factors included position during biopsy, puncture site location, breath-hold, and sedation method.
Data analysis
The random effects model was used to pool the odds ratios for each risk factor. Heterogeneity was tested using the I2 statistic. Statistical analysis was performed using Review Manager (v. 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Bias was assessed using the Newcastle-Ottawa Scale for non-randomized studies.12
Results
Included studies
4011 unique articles were identified through 9 electronic databases searches, of which 72 met the criteria for full-text review as potentially relevant studies (Figure 1). 36 studies met the inclusion criteria.4,13–47 There were 31 retrospective cohort studies, 3 prospective cohort studies and 2 randomized controlled trials (Table 2). These studies enrolled a total of 23,104 participants. Table 2 provides information of the study characteristics including study design, total biopsies, pneumothorax incidence, and follow-up protocol to diagnose a pneumothorax. No studies distinguished how the individual patient’s pneumothorax were diagnosed. There were 6, 13, and 17 studies from the North America, Asia, and Europe/Middle East region respectively. The criteria for chest drain insertion was described in 22 studies but not described in 14 studies. The most common criteria used by the studies included patient being symptomatic (17 studies), an expanding pneumothorax (6 studies), and ≥30% hemithorax (4 studies) (Table 2). The median Newcastle-Ottawa risk of bias score was 7 out of 9 (range: 6–8). Significant and non-significant risk factors for pneumothorax and chest drain insertion are summarized in Table 3.
Figure 1.
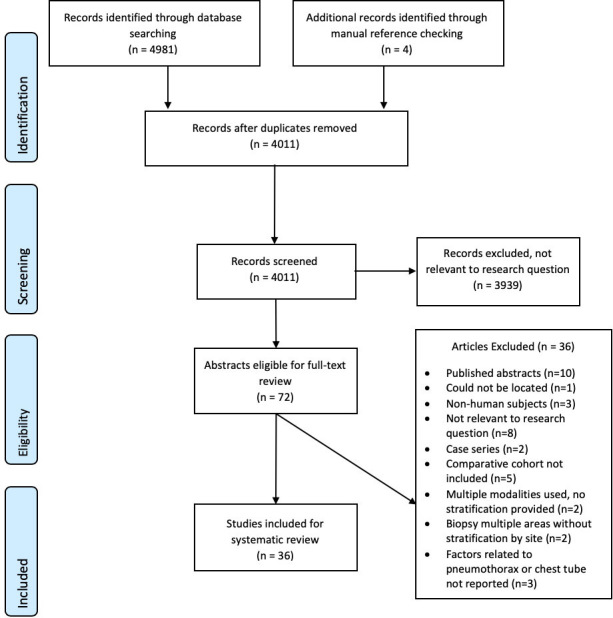
Flowchart describing selection of studies included in systematic review and meta-analysis of modifiable factors associated with pneumothorax and pneumothorax requiring chest tube following CT-PTLB. PTLB,percutaneous transthoracic lung biopsy.
Table 2.
Study characteristics, pneumothorax rates and chest tube insertion rates of included studies
| Study | Study design | Study Country | Biopsy # | PTX % | PTX + CD % | Follow-up protocol | Technique | Needle Gauge | Criteria for Chest Drain insertion | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 Drumm | R | Ireland | 373 | 18.7% | 4.8% | CT immediately +CXR 3 h post-biopsy | Coaxial NR |
19G guide 20G biopsy needle |
Symptomatic OR Hypoxia |
8 |
| 2019 Taslakian | R | USA | 338 | 34% | 8.9% | CT immediately +CXR 1–2 h post-biopsy | Coaxial Automated / FNA (some) |
19G guide, 20G biopsy needle 21G FNA needle |
Lung surface retraction >2 cm, expanding OR symptomatic | 7 |
| 2019 Yan | R | China | 1452 | 22.5% | 4.3% | CT immediately | Non-axial (before 06/2017) Coaxial (after 06/2017) |
18G biopsy needle | Symptomatic OR ratio of lung compression >50% | 7 |
| 2018 Ozturk | R | Turkey | 822 | 15.4% | 3.5% | CT immediately +CXR 2 h post-biopsy | Non-coaxial Semi-automated |
18G biopsy needle | Symptomatic OR Expanding | 8 |
| 2018 Zhang | R | China | 677 | 40.2% | 2.8% | CT immediately +CXR 4 h post-biopsy | Coaxial Automated |
17G guide, 18G biopsy needle |
NR | 7 |
| 2018 Kuriyama | R | Japan | 325 | 49.2% | NR | CT immediately | Coaxial Automated |
19G guide, 22G or 20G biopsy needle | NR | 7 |
| 2017 Uzun | R | Turkey | 442 | 19.0% | 2.9% | CT immediately +CXR 6 h & 24 h post-biopsy | Non-coaxial FNA |
20G or 22G aspiration needle | Symptomatic OR ≥30% hemithorax |
8 |
| 2017 Ashraf | RCT | Denmark/Norway | 301 | 40% | 10% | CT immediately +CXR 1–2 h post-biopsy | Coaxial Automated |
16G guide, 18G biopsy needle |
NR | - |
| 2016 Vagn-Hansen | R | Denmark | 520 | 30% | 15% | CXR 2 h post-biopsy | Non-coaxial | 18G biopsy needle | NR | 6 |
| 2016 Sangha | R | Canada | 254 | 52.4% | 2.8% | CT immediately +CXR post-biopsy | Non-Coaxial FNA |
22G aspiration needle | Symptomatic OR ≥25% hemithorax |
8 |
| Coaxial Automated |
19G guide, 20G biopsy needle |
|||||||||
| 2016 Ocak | R | Belgium | 204 | 24.5% | 10.3% | CT immediately +CXR 2 h & 24 h post-biopsy | Non-Coaxial FNA |
22G aspiration needle | Symptomatic, Hypoxia, Tension OR pleura completely separated from base to apex and >2 cm between lung to chest wall at hilum level | 8 |
| Coaxial Manual |
13G guide, 14G biopsy needle |
|||||||||
| 2016 Nour-Eldin | R | Germany | 650 | 25.1% | 4.3% | CT immediately +CXR 4–6 h | Coaxial Automated |
17G guide 18G biopsy needle |
≥4 cm retraction OR 2–4 cm retraction and progressive after immediate manual evacuation of air |
8 |
| Non-Coaxial Automated |
18G biopsy needle | |||||||||
| 2016 Lee | R | South Korea | 591 | 16.9% | 4.1% | CXR 2hrs + 12hrs post-biopsy | Non-coaxial Automated |
18G biopsy needle | Symptomatic OR “Large” pneumothorax |
7 |
| 2015 Anzidel | R | Italy | 342 | 45.3% | 11.4% | CT immediately +CXR 4 h | Non-coaxial FNA |
14–21G aspiration needle | 86.7% of PTX with >4 cm retraction | 8 |
| 2015 Wei | R | China | 1106 | 18.7% | 0.3% | CT immediately +CXR 4 h post-biopsy | Non-coaxial automated | 23G biopsy needle | ≥4 cm retraction | 8 |
| 2015 Schulze | R | Germany | 664 | 21.7% | 6% | CT immediately +CXR 3–4 h & 24 h post-biopsy | Coaxial Semi-automatic | 17G guide, 18G biopsy needle |
NR | 6 |
| 2015 Kuban | R | USA | 4262 | 30.2% | 15% | CT immediately,+CXR 3 h post-biopsy | Coaxial Automatic |
18G/19G guide 20/22G biopsy needle |
Symptomatic, expanding, OR ≥30% hemithorax |
6 |
| 2015 Kim | R | South Korea | 1227 | 21.4% | 2.9% | CT immediately & CXR 3 h post-biopsy | Coaxial | 17G guide, 18G biopsy needle | Symptomatic, Hypoxia OR ≥35% hemithorax | 6 |
| 2014 De Filippo | R | Italy | 538 | 28.6% | 0% | CT immediately | Non-coaxial | 22G cutting | NR | 6 |
| 2014 Wang | R | China | 343 | 17.5% | 1.5% | CT immediately +CXR 12 h post-biopsy | Non-coaxial Automated |
18G biopsy needle | NR | 6 |
| 2014 Lim | R | Taiwan | 381 | 29.9% | 1.8% | CT immediately +CXR 4 h post-biopsy | Coaxial | 19G guide, 20G biopsy needle | Symptomatic AND >2 cm retraction | 7 |
| 2014 Lee | R | South Korea | 1153 | 17% | 6.9% | CT immediately | Coaxial Semi-automated |
17G guide, 18G biopsy needle | NR | 6 |
| 2013 Yazar | R | Iran | 316 | 9.2% | 1.6% | CT immediately +CXR 2–4 h post-biopsy | Non-coaxial | 22G biopsy needle | NR | 6 |
| 2013 Malone | R | USA | 242 | 30.6% | 13.2% | CT immediately +CXR 1 h & 3 h post-biopsy | Coaxial Automated |
17G/19G guide, 18G/20G biopsy needle |
NR | 8 |
| 2013 Lim | R | South Korea | 430 | 19.8% | NR | CT immediately | Coaxial Semi-automated |
18G guide, 20G biopsy needle |
NR | 8 |
| 2010 Priola | P | Italy | 321 | 26.8% | 4.0% | CT immediately +CXR 3 h post-biopsy | FNA | 21G or 20G aspiration needle | Symptomatic OR “Large” PTX | 7 |
| Coaxial Automated |
17G guide 18G biopsy needle |
|||||||||
| 2010 Hiraki | R | Japan | 1098 | 42.4% | 11.9% | CT immediately +CXR 3 h post-biopsy | Coaxial | 19G guide, 20G biopsy needle | Symptomatic OR ≥30% hemithorax | 8 |
| 2010 Fassina | P | Italy | 305 | 4.3% | 0% | CT immediately | Non-coaxial FNA |
20–22G aspiration needle | NR | 8 |
| 2006 Kinoshita | R | Japan | 236 | 23.7% | 8.4% | CT immediately +CXR 2 h & 12 h post-biopsy | Non-coaxial Automated |
20G biopsy needle | “Severe” Symptoms | 7 |
| 2006 Karam | R | Iran | 505 | 6.7% | 0% | CT immediately | Non-coaxial | 20G biopsy needle | NR | 6 |
| 2005 Topal | R | Turkey | 284 | 35% | 5.6% | CT immediately +CXR 2 h post-biopsy | Non-coaxial Semi-automated |
18G, 20G, 22G biopsy needle | Symptomatic OR expanding on CXR 4 h post-biopsy | 6 |
| 2004 Covey | P | USA | 453 | 23.3% | 6.3% | CT immediately +CXR 2 h post-biopsy | Non-coaxial | 22G, 20G biopsy needle | Symptomatic, circumferential OR expanding between CXR | 7 |
| 2004 Yeow | R | Taiwan | 660 | 23% | 1% | CT immediately +4 h post-CXR | Coaxial | Guide + 1G larger than 16–20G biopsy needle | ≥30% hemithorax | 7 |
| 2003 Geraghty | R | USA | 846 | 26.7% | 8.7% | CT immediately + CXR 2 h post-biopsy |
Coaxial automated |
18G, 19G guide, 20/21G biopsy needle | Expanding PTX | 6 |
| 2000 Laurent | R | France | 223 | 17.9% | 2.2% | CT immediately +CXR4-6 h post-biopsy | Coaxial FNA |
19G or 18G guide, 22G or 20G biopsy needle |
Symptomatic | 8 |
| Coaxial Automated |
18.5G guide, 19.5G biopsy needle |
|||||||||
| 1997 Santambrogio | RCT | Italy | 220 | 23.6% | 5.5% | CT immediately | Non-coaxial FNA |
21G or 23G aspiration needle | NR | - |
| TOTAL | 23,104 | |||||||||
| MEAN | 25.9% | 6.9% |
-, not reported; %, incidence; #, number; CD, Chest drain; FNA, fine needle aspiration; NOS, Newcastle Ottawa Scale; NOS, Newcastle-Ottawa Scale;NR, Not reported; P, Prospective Study; PTX, Pneumothorax; R, Retrospective study; RCT, Randomized Controlled Trial; UK, UnitedKingdom; USA, United States of America.
Table 3.
Significant and non-significant risk factors for pneumothorax and chest drain insertion
| Pneumothorax | Pneumothorax requiring chest drain insertion | |
|---|---|---|
| Significant risk factors | Larger guide/Needle gauges (≤18G) Bullae crossed Fissure crossed Emphysema No pleural contact Puncture site up (vs site down with aperture in CT Gantry table) Prone or supine (vs lateral decubitus position with biopsied lung-dependent) Lateral decubitus position with biopsied lung non-dependent (vs prone or supine) Multiple non-coaxial tissue Samples Smaller lesions Deeper lesions >1 Pleural puncture Interactive breath-hold |
Larger guide/Needle gauges (≤18G) Bullae crossed Fissure crossed Emphysema No pleural contact Puncture site up (vs site down with aperture in CT Gantry table) |
| Non-significant risk factors | Training level Immediate evaluation by cytologist FNA vs core Non-coaxial vs coaxial Number of samples from coaxial Biopsy approach Needle entry angle Sedation Age Sex Smoking status Lobe |
Training Level Immediate evaluation by cytologist FNA vs core Non-coaxial vs coaxial Needle entry angle Number of pleural punctures Number of samples from coaxial Patient position Puncture site location Interactive breath-hold Sedation Sex Lobe |
FNA, fine needle aspiration.
Overall pneumothorax and chest drain incidence
The pooled overall pneumothorax incidence was 25.9% (range: 4.3–52.4%) (Table 2). The highest pneumothorax rates were in patients where a bulla was crossed (59.2%), fissure was crossed (52.8%) and patients positioned in lateral decubitus during biopsy (43%) (Table 4).
Table 4.
Pooled pneumothorax incidence and odds ratio based on risk factors
| Study # | Pneumothorax rate | Pooled odds ratio (95% CI) | I2 | |||
|---|---|---|---|---|---|---|
| Group A | Group B | |||||
| OPERATOR FACTORS | ||||||
| Attending (A) vs Resident (B) | 2 | 23.6% | 16.4% | 1.07 (0.75–1.55) | 0% | |
| Immediate evaluation by cytologist: Yes (A) vs No (B) | 1 | 26.4% | 20.9% | 1.35 (0.72–2.53) | - | |
| BIOSPY NEEDLE TYPE | ||||||
| FNA (A) vs Automated core (B) | 5 | 33.2% | 30.0% | 1.20 (1.04–1.39)a | 0% | |
| FNA (A) vs Manual core (B) | 1 | 18.6% | 30.4% | 0.52 (0.27–1.01) | - | |
| Non-coaxial (A) vs Coaxial (B) | 1 | 23.2% | 27.0% | 0.81 (0.57–1.16) | - | |
| Larger guide/Needle gauge:≤18G (A) vs >18G (B) | 4 | 35.3% | 25.1% | 1.55 (1.19–2.01)** | 61% | |
| BIOPSY TECHNIQUE | ||||||
| Chest wall needle entry location: | ||||||
| Anterior (A) vs Posterior (B) | 2 | 39.4% | 26.8% | 1.83 (1.51–2.21) *** | 14% | |
| Anterior (A) vs Lateral (B) | 2 | 39.3% | 27.7% | 1.10 (0.33–3.65) | 90%a | |
| Posterior (A) vs Lateral (B) | 2 | 26.8% | 27.7% | 0.53 (0.12–2.34) | 94%a | |
| Needle angle: | ||||||
| ≤30 (A) vs >30(B) degrees | 4 | 26.4% | 21.7% | 1.03 (0.70–1.52) | 23% | |
| ≤45 (A) vs >45(B) degrees | 2 | 27.9% | 39.0% | 0.79 (0.21–2.88) | 86%a | |
| Perpendicular (A) vs Oblique (B) | 1 | 10.3% | 4.7% | 2.34 (0.69–7.99) | - | |
| Bulla crossed: Yes (A) vs No (B) | 1 | 59.2% | 19.1% | 6.13 (3.73–10.06)*** | - | |
| Fissure crossed: Yes (A) vs No (B) | 5 | 52.8% | 24.6% | 3.75 (2.57–5.46) *** | 46% | |
| Pleural punctures: >1 (A) vs 1 (B) | 6 | 30.1% | 19.0% | 2.43 (1.39–4.25)** | 78%a | |
| Coaxial tissue Ssmples: ≤2 (A) vs >2 (B) | 3 | 21.2% | 19.9% | 1.09 (0.76–1.56) | 60% | |
| Coaxial tissue samples: >1 (A) vs 1 (B) | 1 | 27.8% | 26.6% | 1.06 (0.64–1.76) | - | |
| Non-coaxial tissue samples: >1 (A) vs 1 (B) | 1 | 29.7% | 17.5% | 1.99 (1.18–3.34)** | - | |
| PATIENT FACTORS | ||||||
| Patient position during biopsy: | ||||||
| Supine (A) vs Prone (B) | 10 | 24.5% | 21.6% | 1.22 (0.45–1.76) | 89%a | |
| Lateral (A) vs Supine (B) | 7 | 43.0% | 20.4% | 2.28 (1.21–4.32)a | 85%a | |
| Lateral (A) vs Prone (B) | 7 | 43.0% | 17.4% | 3.20 (2.54–4.05) *** | 20% | |
| Puncture site up (A) vs Site down +with aperture in CT Gantry table (B) | 1 | 41.6% | 12.9% | 4.79 (2.53–9.09) *** | - | |
| Prone or supine (A) vs Lateral decubitus position with biopsied lung down (B) | 1 | 27.2% | 10.6% | 3.15 (1.79–5.55) *** | - | |
| Interactive breath-hold: Yes (A) vs No (B) | 1 | 46.9% | 33.9% | 1.72 (1.08–2.75)a | - | |
| Conscious sedation (A) vs Local anesthesia (B) | 1 | 19.1% | 24.9% | 0.72 (0.42–1.21) | - | |
| NON-MODIFIABLE FACTORS | ||||||
| >50 years (A) vs ≤50 years (B) | 6 | 26.7% | 10.4% | 1.3 (0.91–1.86) | 59%a | |
| Male (A) vs Female (B) | 14 | 28.3% | 25.7% | 1.17 (0.98–1.41) | 77%a | |
| Ex/current smoker (A) vs Non-smoker (B) | 4 | 25.4% | 28.5% | 0.89 (0.66–1.20) | 43% | |
| Emphysema (A) vs No emphysema (B) | 11 | 41.1% | 24.3% | 3.33 (2.15–5.16)*** | 93%a | |
| Lesion size | ||||||
| ≤1 cm (A) vs >1 cm (B) | 4 | 32.7% | 23.9% | 1.57 (0.75–3.28) | 76%a | |
| ≤2 cm (A) vs >2 cm (B) | 4 | 39.9% | 24.1% | 1.98 (1.55–2.51)*** | 61% | |
| ≤3 cm (A) vs >3 cm (B) | 4 | 32.7% | 19.1% | 1.72 (1.49–1.99)*** | 0% | |
| ≤4 cm (A) vs >4 cm (B) | 4 | 28.8% | 16.2% | 2.09 (1.33–3.31)** | 79%a | |
| ≤5 cm (A) vs >5 cm (B) | 2 | 24.5% | 7.4% | 2.94 (1.54–5.62)** | 48% | |
| Lesion depth | ||||||
| >1 cm (A) vs ≤1 cm (B) | 2 | 23.0% | 11.8% | 2.36 (0.70–7.95) | 96%a | |
| >2 cm (A) vs ≤2 cm (B) | 6 | 36.1% | 18.0% | 2.16 (1.31–3.57)** | 94%a | |
| >3 cm (A) vs ≤3 cm (B) | 1 | 34.9% | 12.2% | 2.38 (1.60–3.53)*** | - | |
| >5 cm (A) vs ≤5 cm (B) | 1 | 14.7% | 2.0% | 8.47 (3.44–20.9)*** | - | |
| Upper/Middle lobe (A) vs Lower lobe (B) | 15 | 26.7% | 25.6% | 0.95 (0.75–1.20) | 87%a | |
| No pleural contact (A) vs Pleural contact (B) | 3 | 40.0% | 28.8% | 1.73 (1.17–2.55)** | 79%a | |
CI, confidence interval.
p-value < 0.05; **p-value < 0.01, ***p-value < 0.001, 95% CI - 95% CI.
The pooled incidence of pneumothorax requiring chest tube was 6.9% (Range: 0–15%) (Table 2). The highest chest drain insertion rates were in patients where a fissure was crossed (27.9%), had emphysema (27.2%), and the anterior biopsy approach (20.4%) (Table 5).
Table 5.
Pooled chest drain insertion incidence and odds ratios based on risk factors
| Study # | Pneumothorax requiring chest drain | Odds ratio (95% CI) | I2 | |||
| Group A | Group B | |||||
| OPERATOR FACTORS | ||||||
| Training level: Attending (A) vs Resident (B) | 2 | 7.6% | 4.5% | 0.74 (0.30–1.87) | 35% | |
| Immediate evaluation by cytologist: Yes (A) vs No (B) | 1 | 6.4% | 4.5% | 1.43 (0.44–4.64) | - | |
| BIOSPY NEEDLE FACTORS | ||||||
| FNA (A) vs Automated core (B) | 3 | 12.5% | 13.3% | 0.99 (0.77–1.27) | 0% | |
| FNA (A) vs Manual core (B) | 1 | 10.8% | 9.8% | 1.11 (0.45–2.75) | - | |
| Non-coaxial (A) vs Coaxial (B) | 1 | 3.6% | 5.0% | 0.71 (0.33–1.52) | - | |
| Larger guide/Needle gauge: £18G (A) vs > 18G (B) | 3 | 16.1% | 11.7% | 1.39 (1.18–1.62)*** | 0% | |
| BIOPSY TECHNIQUE | ||||||
| Chest wall needle entry location: | ||||||
| Anterior (A) vs Posterior (B) | 1 | 20.4% | 11.6% | 1.94 (1.62–2.32)*** | - | |
| Anterior (A) vs Lateral (B) | 1 | 20.4% | 13.6% | 1.64 (1.24–2.15)*** | - | |
| Posterior (A) vs Lateral (B) | 1 | 11.6% | 13.6% | 0.84 (0.64–1.11) | - | |
| Needle angle: | ||||||
| £30 (A) vs > 30(B) degrees | 1 | 4.8% | 7.8% | 0.59 (0.17–1.98) | - | |
| £45 (A) vs > 45(B) degrees | 1 | 5.2% | 4.4% | 1.18 (0.58–2.38) | - | |
| Bulla crossed: Yes (A) vs No (B) | 1 | 18.3% | 1.9% | 11.04 (5.32–22.90)*** | - | |
| Fissure crossed: Yes (A) vs No (B) | 5 | 27.9% | 10.7% | 3.54 (2.32–5.40)*** | 39% | |
| Pleural punctures:>1 (A) vs 1 (B) | 2 | 7.8% | 4.5% | 1.25 (0.07–21.80) | 89%* | |
| Coaxial tissue samples: £2 (A) vs > 2 (B) | 1 | 2.0% | 3.3% | 0.61 (0.27–1.41) | - | |
| PATIENT FACTORS | ||||||
| Supine (A) vs Prone (B) | 4 | 5.4% | 3.3% | 1.54 (0.75–3.16) | 74%* | |
| Puncture site up (A) vs Site down + with aperture in CT Gantry table (B) | 1 | 18.0% | 2.7% | 7.84 (2.53–24.29)*** | - | |
| Prone or supine (A) vs Lateral decubitus position with biopsied lung down (B) | 1 | 5.4% | 4.2% | 1.30 (0.50–3.37) | - | |
| Interactive breath-hold: Yes (A) vs No (B) | 1 | 10% | 9.4% | 1.08 (0.50–2.33) | - | |
| Conscious sedation (A) vs Local anesthesia (B) | 1 | 7.8% | 6.8% | 1.16 (0.52–2.59) | - | |
| NON-MODIFIABLE FACTORS | ||||||
| Male (A) vs Female (B) | 5 | 11.0% | 9.5% | 1.11 (0.48–2.55) | 88%* | |
| Emphysema (A) vs No emphysema (B) | 4 | 27.2% | 8.8% | 6.44 (4.27–9.72)*** | 58% | |
| Upper/Middle lobe (A) vs Lower lobe (B) | 5 | 11% | 7.0% | 1.37 (0.90–2.10) | 70%* | |
| No pleural contact (A) vs Pleural contact (B) | 2 | 4.7% | 2.4% | 1.97 (1.21–3.21)** | 0% | |
CI, confidence interval.
p-value < 0.05; **p-value < 0.01, ***p-value < 0.001, 95% CI - 95% CI.
The pneumothorax rates were 30.4%, 25.4%, and 22.4% in the North America, Asia and Europe/Middle East regions respectively. The chest drain insertion rates were 12.7, 4.4 and 4.9% in the North America, Asia, Europe/Middle East regions respectively.
Significant risk factors for pneumothorax and/or chest drain insertion
Lateral decubitus position with biopsied lung non-dependent (vs supine and prone)
Lateral patient position with the biopsied lung non-dependent significantly increased the risk of a pneumothorax compared to supine position (43% vs 20.4%, OR 2.28, 95% CI 1.21–4.32) and prone position (43% vs 17.4%, OR 3.20, 95% CI 2.48–5.79) (Table 4). No studies assessed the association with chest drain insertion rates.
Prone or supine (vs lateral decubitus position with biopsied lung-dependent)
The study by Drumm and colleagues16 compared patients who were biopsied in prone or supine vs those who placed in lateral decubitus position with the biopsied lung down (dependent) and the biopsy needle entered either via the anterior or posterior chest wall.16 They demonstrated a significant decrease in pneumothorax rates in patients placed in the lateral decubitus position with the biopsied lung=dependent (12.9% vs 41.6%, OR 3.20 95% CI 2.54–4.05). However, there was no significant difference in chest drain insertion rates (4.2% vs 5.4%).
Puncture site up (vs site down biopsy via aperture in CT Gantry table)
Kinoshita and colleagues22 had an aperture in the CT gantry table and biopsied lung lesions from below the patient.22 They demonstrated patients who were placed with puncture site down during biopsy significantly reduced pneumothorax rates (12.9% vs 41.6%, OR 4.79 95% CI 2.53–9.09). Chest drain insertion rates also significantly reduced in those with the puncture site down (2.7% vs 18.0%, OR 7.84, 95% CI 2.53–24.29).
Larger guide/Needle gauges ≤18G
A total of four studies compared pneumothorax rates based on gauge size, however three studies used coaxial technique so the gauges refers to the coaxial guide gauge,18,23,30 whilst one study used non-coaxial technique so the gauge refers to the biopsy needle gauge.39 Pooled outcomes demonstrated larger gauges (≤18G) had significantly higher pneumothorax rates compared to smaller gauges (>18G) (35.3% vs 25.1%, OR 1.55, 95% CI 1.19–2.01) (Table 4). Similarly, chest drain insertion rates were also significantly higher in larger gauges (≤18G) (16.1% vs 11.7%, OR 1.39, 95%CI1.18–1.62) (Table 5).
Bullae crossed
Only one study compared pneumothorax and chest drain insertion rates if a bulla was crossed.21 If a bulla was crossed, it resulted in the highest pneumothorax rates (59.2% vs 19.1%, OR 6.13 95% CI 3.73–10.06) and chest drain insertions (18.3% vs 1.9%, OR 11.04 95% CI 5.32–22.90) (Tables 4 and 5).
Fissure crossed
Biopsies where the needle crossed a fissure had a significant increase in the risk of pneumothorax (52.8% vs 24.6%, OR 3.75 95% CI 2.57–5.46) (Table 4). Moreover, the highest pooled chest drain insertion rate was found when a fissure was crossed (27.9%). The risk of chest drain insertion increased more than threefold (OR 3.54, 95% CI 2.32–5.40) (Table 5).
Needle entry through the anterior chest wall (compared to posterior or lateral entry)
Two studies have compared pneumothorax rates based on location of needle entry on the chest wall.4,23 One study stated they placed all their patients in a supine position following biopsy regardless of needle entry,23 whilst the other study did not comment on post-biopsy management of patients.4
Pooled outcomes demonstrated needle entry through the anterior chest wall significantly increased the risk of a pneumothorax compared to the needle access through the posterior chest wall (39.4% vs 26.8%, OR 1.83, 95% CI 1.51–2.21) (Table 4). There were no significant differences in pneumothorax rates between the anterior vs lateral needle entry, and the posterior vs lateral needle entry.
Similarly, needle entry through the anterior chest wall significantly increased the risk of a chest drain compared to entry via the posterior chest wall (20.4% vs 11.6%, OR: 1.94, 95% CI 1.62–2.32) and lateral approach (20.4% vs 13.6%, OR: 1.64, 95% CI 1.24–2.15) (Table 5). There was no significant difference in chest drain insertion rates between the posterior vs lateral needle access.
Multiple pleural punctures
Multiple pleural punctures (>1) more than tripled the risk of a pneumothorax rate compared to one pleural puncture (30.1% vs 19.0%, OR:2.43, 95% CI1.39–4.25). However, chest drain insertion rates did not significantly increase (7.8% vs 4.5%, OR 1.25, 95% CI 0.07–21.80) with more than one pleural puncture.
Multiple non-coaxial tissue samples
Multiple non-coaxial tissue samples (>1) significantly increased the risk of pneumothorax compared to one tissue sample (29.7% vs 17.5%, OR 1.99, 95% CI 1.18–3.34). No studies investigated rates of chest drain insertion.
Emphysema
Emphysema was significantly associated with an increased pneumothorax incidence (41.1% vs 24.3%, pooled from 11 studies OR: 3.33, 95% CI 2.15–5.16). The risk of chest drain insertion was even greater in patients with emphysema compared to those without emphysema (27.2% vs 8.8%, pooled from four studies OR: 6.44, 95% CI 4.27–9.72).
The most common definition of emphysema was radiologic findings along the needle path and categorized as "present" or "absent".19,21,23,27 Some studies only stated the presence of emphysema and did not specify the location,37,47 whilst others defined emphysema was present if it was in the same lobe of the biopsy.26 De-Filippo and colleagues only considered patients with severe emphysema (>50%) positive for emphysema and graded patients with mild (<25%) and moderate (25–50%) emphysema into no emphysema.15 One study defined emphysema as ≤900 Hounsfield unit along needle path and stratified emphysema into: not present, surrounding the lesion but distant from the needle tract, and along the needle tract.43
Smaller lesions
Pneumothorax incidence was significantly higher in tumors ≤ 2 cm (39.9%) compared to >2 cm (24.1%) (pooled OR: 1.98, 95% CI 1.55–2.51) in four studies that examined this.23,38,43,47 Similarly, this correlation remained present with the 3 cm threshold (32.7% vs 19.1%; OR 1.72, 95% CI 1.49–1.99), 4 cm threshold (28.8% vs 16.2%; OR 2.09, 95% CI 1.33–3.31) and 5 cm threshold (24.5% vs 7.4%; OR 2.94, 95% CI 1.52–5.62) (Table 4). No studies assessed association between lesion size and chest drain insertion rate.
Deeper lesions
Pneumothorax incidence was significantly higher in tumors > 2 cm deep (36.1%) compared to more superficial tumors (18.0%) (pooled OR: 2.16, 95% CI 1.31–3.57) in six studies that examined this.23,27,39,43,46,47 Similarly, this correlation remained present with the 3 cm threshold (34.9% vs 12.2%; OR 2.38, 95% CI 1.60–3.53) and 5 cm threshold (14.7% vs 2.0%; OR 8.47, 95% CI 3.44–20.9) (Table 5). No studies assessed the association between lesion depth and chest drain insertion rate.
No pleural contact
Tumors without pleural contact had a significantly higher risk of pneumothorax compared to tumors with pleural contact (40% vs 28.8%, OR 1.73, 95% CI 1.17–2.55); pooled from three studies.19,21,46 Similarly, chest tube insertion rates were significantly higher in tumors without pleural contact (4.7% vs 2.4%, OR 1.97, 95% CI 1.21–3.21; pooled from two studies.19,21
Fine needle biopsies
Fine needle biopsies had an increased pneumothorax rate compared to automated core biopsies (33.2% vs 30.0%, OR 1.20 95% CI 1.04–1.39) (Table 4). A significant difference was not demonstrated for drain insertion rates (12.5% vs 13.3%, OR 0.99 95% CI 0.77–1.27) (Table 5). No significant difference was demonstrated between FNA and semi-automated core pneumothorax or drain insertion rates.
Interactive breath-hold
Interactive breath-hold significantly increased pneumothorax rate compared to no interactive breath-hold (46.9% vs 33.9%, OR 1.72 95% CI 1.08–2.75) (Table 4). There was no effect on drain insertion rates (10% vs 9.4%, OR 1.08 95% CI 0.50–2.33) (Table 5).
Heterogeneity analysis
The risk factors that were significantly associated with pneumothorax which had significant heterogeneity included pleural punctures (I2:78%), lateral patient position compared to supine patient position (I2:85%), emphysema (I2:93%), lesion size 4 cm threshold (I2:79%), lesion depth 2 cm threshold (I2:94%), and pleural contact (I2:79%) (Table 4). There was no significant heterogeneity between the remaining risk factors significantly associated with pneumothorax or those associated with chest drain insertion (Table 5).
Discussion
Pneumothorax is the most common complication that occurs during or immediately after a percutaneous CT-guided lung biopsy. This meta-analysis included 23,104 cases with an incidence of 25.9% and chest drain insertion rate of 6.9%. This present meta-analysis demonstrates substantially higher pneumothorax rates when larger guide/needle gauges are used (≤18G), a bulla is crossed, a fissure is crossed, puncture site up (vs site down with biopsy via an aperture in CT Gantry table), emphysematous lungs, lesions with no pleural contact, a lateral decubitus position (with the biopsied lung ante-dependent) compared to supine and prone position, prone or supine position (vs lateral decubitus position with biopsied lung down), multiple pleural punctures, multiple tissue samples from non-coaxial technique, FNA biopsies compared to automated core, breath-hold during biopsy, smaller lesions and deeper lesions. The higher pneumothorax rates from FNA sampling may be due to sampling technique contributing to more pleural agitation and/or injury to normal lung.
The risk of a chest drain insertion was significantly associated when larger guide/needle gauges are used (≤18G), a bulla is crossed, a fissure is crossed, puncture site up, emphysematous lungs, lesions with no pleural contact.
Whilst many of these factors have long been factored into proceduralists’ consideration for performing CT-PTLB, this meta-analysis highlights interesting techniques that may have not been considered such as modifying the CT table with a biopsy window to enable the operator to perform the procedure with the patient positioned biopsy side-down as demonstrated by Kinoshita and colleagues.22
A logistically simpler technique described by Drumm and colleagues16 where patients were placed in a lateral decubitus position with the target lesion in the dependent lung is promising.16 This position significantly reduced pneumothorax rates, increased technical success rate and reduced haemoptysis incidence. It is hypothesized that the reduction in pneumothorax rates with the biopsy-side-down/lateral decubitus dependent lung position is due to the weight of the lung that compresses the alveoli and increases pleural apposition, which helps to seal the biopsy tract.16,48 However, both these techniques were only examined in one study, and additional studies are warranted to confirm these findings.
The importance of having the biopsied lung-dependent is further supported by the finding in this meta-analysis that patients in the lateral decubitus position where the biopsied lung was ante-dependent had significantly higher pneumothorax rates than the supine and prone position. The study by Wang and colleagues found significantly higher pneumothorax rates in the lateral decubitus position (44%) compared to supine (15.2%) and prone (12.8%) position (p > 0.05). They hypothesized that the lateral decubitus position with the biopsied lung up (ante-dependent) separates the parietal and visceral pleura more than the supine and prone positions, and therefore air is more likely to enter the pleural cavity as the needle is taken out.
Therefore, in terms of descending order of preferred patient position based on pneumothorax risk: lateral decubitus position with biopsy the dependent lung, prone position, supine position, and finally lateral decubitus position with biopsy of the ante-dependent lung. However, it is important to also consider how patient position influences other complications, particularly an air embolism which is a rare but very serious complication. Fortunately, the lateral decubitus position has the lowest published rates of air embolism, followed by supine position and the highest rates are seen in the prone position.49 We hypothesize this may be due to increased partial pressure of dependent lung, reducing the potential difference between the needle tip and outside air.
Although this meta-analysis demonstrated a significantly higher pneumothorax rates when the biopsy needle entered the anterior chest wall compared to the posterior chest wall, we believe this finding to be confounded by patient position post-biopsy. Two studies examined this issue: one of which clearly stated that all patients were placed in a supine position following biopsy regardless of needle entry,23 whilst the other did not comment and thus we presumable also placed patients in the supine position post-biopsy.4 A recent meta-analysis has demonstrated the “roll-over” of patient post-biopsy to the needle entry location side-down significantly reduced pneumothorax rates.9 Hence, the patients where the biopsy needle entered via the posterior wall essentially had the “roll-over” manoeuvre post-biopsy as patients were all placed in the supine position. We hypothesize the “roll-over” effect accounts for the lower rates of pneumothorax in the posterior approach. Therefore, we suggest placing patient in a prone position following an anterior needle entry to reduce the risk of a pneumothorax.
Smaller lesions were also associated with higher pneumothorax rates and in principle, biopsy of normal lung would increase the risk of complication without any diagnostic benefit. At our institution (Concord Repatriation General Hospital, NSW, Australia) for small lesions the tip of the coaxial needle would be close to the lesion and the throw of the needle should terminate just beyond the lesion. To achieve this, the biopsy throw would commence within the coaxial needle. Confirmatory evidence for this approach is unlikely to be achievable by trials.
It is also important to highlight factors that are commonly assumed to be correlated with pneumothoraxes did not reach a statistically significant association such as needle angle at skin penetration, training level (attending vs resident), conscious sedation vs local anaesthesia and upper/middle lobe vs lower lobe. It is possible that these factors do not affect pneumothorax rates, however only a small number of published studies assessing these are available in the literature. This highlights the need for larger, more comprehensive studies to assess these factors to confirm which factors influence pneumothorax rates.
These findings must be considered in the context of their limitations. Firstly, a majority were retrospective studies. Retrospective studies have an increased risk of bias as many of the modifiable factors assessed such as needle size, number of pleural punctures and positioning were not randomized. Secondly, many risk factors were only compared in one study, such as non-coaxial vs coaxial biopsies, multiple vs one tissue sample from non-coaxial technique. Future studies are warranted to validate these risk factors for pneumothorax and drain placement. Third, another contributing factor between the large variability of pneumothorax rates may be associated by different post-biopsy techniques utilized by studies such as some studies rolling their patients over to puncture site down, whilst others do not. However, a recent meta-analysis demonstrated rapid rollover to puncture site down compared to no rollover did not significantly reduce pneumothorax rates but reduced chest drain insertion rates.9 Another potential confounder is the methodology of diagnosing pneumothorax with CT being more sensitive. Finally, there was a large variation in incidence of chest drains between studies (lowest 0.3%43 vs highest 15%).23,41This indicates variability amongst institutional protocols as to when a chest drain is required for a pneumothorax. These differences contribute to the heterogeneity between studies. As such, a random effects model was used to pool studies in order to minimize the influence of individual differences in response to an effect.
Conclusion
This meta-analysis quantifies risks associated with CT-PTLB pneumothorax and drain insertion rates, particularly with inherent patient risk factors, positioning and equipment type. The pooled overall pneumothorax incidence was 25.9% and chest tube insertion rate was 6.9%. Positioning patients in lateral decubitus with the biopsied lung-dependent, puncture site down with a biopsy window in the CT table, using smaller calibre guide/biopsy needles and using coaxial technique if multiple samples are needed are associated with a reduced incidence of pneumothorax.
Contributor Information
Ya Ruth Huo, Email: ruth.huo@gmail.com.
Michael Vinchill Chan, Email: michaelvchan@gmail.com.
Al-Rahim Habib, Email: arhabib9@gmail.com.
Isaac Lui, Email: dr.isaac.lui@gmail.com.
Lloyd Ridley, Email: Lloyd.Ridley@health.nsw.gov.au.
REFERENCES
- 1. Manhire A, et al. Guidelines for radiologically guided lung biopsy. Thorax 2003; 58): : 920–36 p.. doi: 10.1136/thorax.58.11.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winokur RS, et al. Percutaneous lung biopsy: technique, efficacy, and complications. in Seminars in interventional radiology: Thieme Medical Publishers; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiener RS, Wiener DC, Gould MK. Risks of transthoracic needle biopsy: how high? Clin Pulm Med 2013; 20: 29–35. doi: 10.1097/CPM.0b013e31827a30c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anzidei M, Sacconi B, Fraioli F, Saba L, Lucatelli P, Napoli A, et al. Development of a prediction model and risk score for procedure-related complications in patients undergoing percutaneous computed tomography-guided lung biopsy. Eur J Cardiothorac Surg 2015; 48: e1–6. doi: 10.1093/ejcts/ezv172 [DOI] [PubMed] [Google Scholar]
- 5. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJM, Vliegenthart R, Oudkerk M, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017; 27: 138–48. doi: 10.1007/s00330-016-4357-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T, et al. Duration of pneumothorax as a complication of CT-guided lung biopsy. Australas Radiol 2006; 50: 435–41. doi: 10.1111/j.1440-1673.2006.01619.x [DOI] [PubMed] [Google Scholar]
- 7. Cardinal JS, Gunderman RB, Tarver RD. Informing patients about risks and benefits of radiology examinations: a review article. J Am Coll Radiol 2011; 8: 402–8. doi: 10.1016/j.jacr.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 8. Murad MH, Montori VM, Ioannidis JPA, Jaeschke R, Devereaux PJ, Prasad K, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users' guides to the medical literature. JAMA 2014; 312: 171–9. doi: 10.1001/jama.2014.5559 [DOI] [PubMed] [Google Scholar]
- 9. Huo YR, Chan MV, Habib A-R, Lui I, Ridley L. Post-Biopsy manoeuvres to reduce pneumothorax incidence in CT-guided transthoracic lung biopsies: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2019; 42: 1062–72. doi: 10.1007/s00270-019-02196-8 [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG, .PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–9. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 11. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343(oct18 2): d5928: d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells G, Wells GA, Shea B, O'Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011;.
- 13. Ashraf H, Krag-Andersen S, Naqibullah M, Minddal V, Nørgaard A, Naur TMH, et al. Computer tomography guided lung biopsy using interactive breath-hold control: a randomized study. Ann Transl Med 2017; 5: 253. doi: 10.21037/atm.2017.05.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT, et al. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol 2004; 15: 479–83. doi: 10.1097/01.RVI.0000124951.24134.50 [DOI] [PubMed] [Google Scholar]
- 15. De Filippo M, Saba L, Silva M, Zagaria R, Concari G, Nizzoli R, et al. Ct-Guided biopsy of pulmonary nodules: is pulmonary hemorrhage a complication or an advantage? Diagn Interv Radiol 2014; 20: 421–5. doi: 10.5152/dir.2014.14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drumm O, Joyce EA, de Blacam C, Gleeson T, Kavanagh J, McCarthy E, et al. Ct-Guided lung biopsy: effect of Biopsy-side down position on pneumothorax and chest tube placement. Radiology 2019; 292: 190–6. doi: 10.1148/radiol.2019182321 [DOI] [PubMed] [Google Scholar]
- 17. Fassina A, Corradin M, Zardo D, Cappellesso R, Corbetti F, Fassan M, et al. Role and accuracy of rapid on-site evaluation of CT-guided fine needle aspiration cytology of lung nodules. Cytopathology 2011; 22: 306–12. doi: 10.1111/j.1365-2303.2010.00802.x [DOI] [PubMed] [Google Scholar]
- 18. Geraghty PR, Kee ST, McFarlane G, Razavi MK, Sze DY, Dake MD, et al. Ct-Guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003; 229: 475–81. doi: 10.1148/radiol.2291020499 [DOI] [PubMed] [Google Scholar]
- 19. Hiraki T, Mimura H, Gobara H, Shibamoto K, Inoue D, Matsui Y, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol 2010; 194: 809–14. doi: 10.2214/AJR.09.3224 [DOI] [PubMed] [Google Scholar]
- 20. Karam MB, et al. Ct-Guided percutaneous fine-needle aspiration biopsy of pulmonary lesions. 2006;.
- 21. Kim JI, Park CM, Lee SM, Goo JM. Rapid needle-out patient-rollover approach after cone beam CT-guided lung biopsy: effect on pneumothorax rate in 1,191 consecutive patients. Eur Radiol 2015; 25: 1845–53. doi: 10.1007/s00330-015-3601-y [DOI] [PubMed] [Google Scholar]
- 22. Kinoshita F, Kato T, Sugiura K, Nishimura M, Kinoshita T, Hashimoto M, et al. Ct-Guided transthoracic needle biopsy using a puncture site-down positioning technique. AJR Am J Roentgenol 2006; 187: 926–32. doi: 10.2214/AJR.05.0226 [DOI] [PubMed] [Google Scholar]
- 23. Kuban JD, Tam AL, Huang SY, Ensor JE, Philip AS, Chen GJ, et al. The effect of needle gauge on the risk of pneumothorax and chest tube placement after percutaneous computed tomographic (CT)-guided lung biopsy. Cardiovasc Intervent Radiol 2015; 38: 1595–602. doi: 10.1007/s00270-015-1097-0 [DOI] [PubMed] [Google Scholar]
- 24. Kuriyama T, Masago K, Okada Y, Katakami N. Computed tomography-guided lung biopsy: association between biopsy needle angle and pneumothorax development. Mol Clin Oncol 2018; 8: 336–41. doi: 10.3892/mco.2017.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laurent F, Latrabe V, Vergier B, Montaudon M, Vernejoux JM, Dubrez J, et al. Ct-Guided transthoracic needle biopsy of pulmonary nodules smaller than 20 MM: results with an automated 20-gauge coaxial cutting needle. Clin Radiol 2000; 55: 281–7. doi: 10.1053/crad.1999.0368 [DOI] [PubMed] [Google Scholar]
- 26. Lee H-Y, Lee IJ. Assessment of independent risk factors of developing pneumothorax during percutaneous core needle lung biopsy: focus on lesion depth. Iranian Journal of Radiology 2016; 13. doi: 10.5812/iranjradiol.30929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM, et al. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology 2014; 271: 291–300. doi: 10.1148/radiol.13131265 [DOI] [PubMed] [Google Scholar]
- 28. Lim C, Lee KY, Kim YK, Ko JM, Han DH. Ct-Guided core biopsy of malignant lung lesions: how many needle passes are needed? J Med Imaging Radiat Oncol 2013; 57: 652–6. doi: 10.1111/1754-9485.12054 [DOI] [PubMed] [Google Scholar]
- 29. Lim C-S, Tan L-E, Wang J-Y, Lee C-H, Chang H-C, Lan C-C, et al. Risk factors of pneumothorax after CT-guided coaxial cutting needle lung biopsy through aerated versus nonaerated lung. J Vasc Interv Radiol 2014; 25: 1209–17. doi: 10.1016/j.jvir.2014.03.031 [DOI] [PubMed] [Google Scholar]
- 30. Malone LJ, Stanfill RM, Wang H, Fahey KM, Bertino RE. Effect of intraparenchymal blood patch on rates of pneumothorax and pneumothorax requiring chest tube placement after percutaneous lung biopsy. AJR Am J Roentgenol 2013; 200: 1238–43. doi: 10.2214/AJR.12.8980 [DOI] [PubMed] [Google Scholar]
- 31. Nour-Eldin N-EA, Alsubhi M, Emam A, Lehnert T, Beeres M, Jacobi V, et al. Pneumothorax complicating coaxial and non-coaxial CT-guided lung biopsy: comparative analysis of determining risk factors and management of pneumothorax in a retrospective review of 650 patients. Cardiovasc Intervent Radiol 2016; 39: 261–70. doi: 10.1007/s00270-015-1167-3 [DOI] [PubMed] [Google Scholar]
- 32. Ocak S, Duplaquet F, Jamart J, Pirard L, Weynand B, Delos M, et al. Diagnostic accuracy and safety of CT-guided percutaneous transthoracic needle biopsies: 14-gauge versus 22-gauge needles. J Vasc Interv Radiol 2016; 27: 674–81. doi: 10.1016/j.jvir.2016.01.134 [DOI] [PubMed] [Google Scholar]
- 33. Ozturk K, Soylu E, Gokalp G, Topal U, et al. Risk factors of pneumothorax and chest tube placement after computed tomography-guided core needle biopsy of lung lesions: a single-centre experience with 822 biopsies. Polish Journal of Radiology 2018; 83: 407: e407–14. doi: 10.5114/pjr.2018.79205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Priola AM, Priola SM, Cataldi A, Di Franco M, Pazè F, Marci V, et al. Diagnostic accuracy and complication rate of CT-guided fine needle aspiration biopsy of lung lesions: a study based on the experience of the cytopathologist. Acta Radiol 2010; 51: 527–33. doi: 10.3109/02841851003691979 [DOI] [PubMed] [Google Scholar]
- 35. Sangha BS, Hague CJ, Jessup J, O'Connor R, Mayo JR, et al. Transthoracic computed tomography–guided lung nodule biopsy: comparison of core needle and fine needle aspiration techniques. Can Assoc Radiol J 2016; 67: 284–9. doi: 10.1016/j.carj.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 36. Santambrogio L, Nosotti M, Bellaviti N, Pavoni G, Radice F, Caputo V, et al. Ct-Guided fine-needle aspiration cytology of solitary pulmonary nodules: a prospective, randomized study of immediate cytologic evaluation. Chest 1997; 112: 423–5. doi: 10.1378/chest.112.2.423 [DOI] [PubMed] [Google Scholar]
- 37. Schulze R, Seebacher G, Enderes B, Kugler G, Fischer J, Graeter T et al. Complications in CT-guided, semi-automatic coaxial core biopsy of potentially malignant pulmonary lesions : RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. 187: © Georg Thieme Verlag KG; 2015. 697–702. doi: 10.1055/s-0034-1399648 [DOI] [PubMed] [Google Scholar]
- 38. Taslakian B, Koneru V, Babb JS, Sridhar D, et al. Transthoracic needle biopsy of pulmonary nodules: meteorological conditions and the risk of pneumothorax and chest tube placement. Journal of Clinical Medicine 2018; 8: 727. doi: 10.3390/jcm8050727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Topal U, Berkman YM. Effect of needle tract bleeding on occurrence of pneumothorax after transthoracic needle biopsy. Eur J Radiol 2005; 53: 495–9. doi: 10.1016/j.ejrad.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 40. Uzun Çağlar, Akkaya Z, Düşünceli Atman E, Üstüner E, Peker E, Gülpınar B, et al. Diagnostic accuracy and safety of CT-guided fine needle aspiration biopsy of pulmonary lesions with non-coaxial technique: a single center experience with 442 biopsies. Diagn Interv Radiol 2017; 23: 137–43. doi: 10.5152/dir.2016.16173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vagn-Hansen C, Roland Pedersen M, Rafaelsen SR. Diagnostic yield and complications of transthoracic computed tomography-guided biopsies. Dan Med J 2016; 63. [PubMed] [Google Scholar]
- 42. Wang Y, Li W, He X, Li G, Xu L. Computed tomography-guided core needle biopsy of lung lesions: diagnostic yield and correlation between factors and complications. Oncol Lett 2014; 7: 288–94. doi: 10.3892/ol.2013.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wei Y-H, Zhou F-X, Li Y, Zhou Y-F, Anish K, Xu L-Y, et al. Extrapleural locating method in computed tomography-guided needle biopsies of 1,106 lung lesions. Exp Ther Med 2015; 10: 1707–19. doi: 10.3892/etm.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan W, et al. Lobar location of lesions in computed tomography-guided lung biopsy is correlated with major pneumothorax: a STROBE-compliant retrospective study with 1452 cases. Medicine 2019; 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yazar E, Seçik F, Yıldız P. Does repeating CT-guided transthoracic fine needle aspiration increase diagnostic yield and complication rate? a single institution experience. Iran J Radiol 2013; 10: 56. doi: 10.5812/iranjradiol.10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeow K-M, Su I-H, Pan K-T, Tsay P-K, Lui K-W, Cheung Y-C, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004; 126: 748–54. doi: 10.1378/chest.126.3.748 [DOI] [PubMed] [Google Scholar]
- 47. Zhang HF, Liao MY, Zhu DY, Chen J, Wang YF, et al. Lung radiodensity along the needle passage is a quantitative predictor of pneumothorax after CT-guided percutaneous core needle biopsy. Clin Radiol 2018; 73: 319.e1–319.e7. doi: 10.1016/j.crad.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 48. Zidulka A, Braidy TF, Rizzi MC, Shiner RJ. Position may stop pneumothorax progression in dogs. Am Rev Respir Dis 1982; 126: 51–3. doi: 10.1164/arrd.1982.126.1.51 [DOI] [PubMed] [Google Scholar]
- 49. Rott G, Boecker F. Influenceable and avoidable risk factors for systemic air embolism due to percutaneous CT-guided lung biopsy: patient positioning and coaxial biopsy technique—case report, systematic literature review, and a technical note. Radiol Res Pract 2014; 2014: 1–8. doi: 10.1155/2014/349062 [DOI] [PMC free article] [PubMed] [Google Scholar]


