Abstract
Objectives:
To evaluate the feasibility and optimal restricted angle of the complete-directional-complete block (CDCB) technique in helical tomotherapy (HT) by including regional nodal irradiation (RNI) with the internal mammary node (IMN) in left-sided breast cancer.
Methods:
Ten left-sided breast cancer patients treated with 50 Gy in 25 fractions were compared with five-field intensity-modulated radiation therapy (5F-IMRT) and six types of HT plans. In the HT plans, complete block (CB), organ-based directional block (OBDB) and CDCB with different restricted angles were used.
Results:
The conformity index (CI) between the CDCB0,10,15,20 and 5F-IMRT groups was similar. Compared to CB, OBDB and 5F-IMRT, CDCB20 resulted in a decreased ipsilateral mean lung dose. The low-dose region (V5) of the ipsilateral lung in OBDB (84.0%) was the highest among all techniques (p < 0.001). The mean dose of the heart in CB was significantly reduced (by 11.5–22.4%) compared with other techniques. The V30 of the heart in CDCB20 (1.9%) was significantly lower than that of CB, OBDB and 5F-IMRT. Compared to the mean dose of the left anterior descending (LAD) artery of 5F-IMRT (27.0 Gy), CDCB0, CDCB10, CDCB15, CDCB20 and OBDB reduced the mean dose effectively by 31.7%, 38.3%, 39.6%, 42.0 and 56.2%, respectively. Considering the parameters of the organs-at-risk (OARs), CDCB10,15,20 had higher expectative values than the other techniques (p = 0.01).
Conclusions:
HT with the CDCB technique is feasible for treating left-sided breast cancer patients. The CDCB10-20 techniques not only achieved similar planning target volume coverage, homogeneity and dose conformity but also allowed better sparing of the heart and bilateral lungs.
Advances in knowledge:
For left-sided breast cancer patients whose RNI field includes the IMN, heart avoidance is an important issue. The CDCB technique achieved good PTV coverage, homogeneity and dose conformity and allowed better sparing of the mean dose of the lung, the LAD artery, and the heart and reduced the V30 of the heart.
Introduction
Thorsen et al1 demonstrated that internal mammary node irradiation (IMNI) results in a statistically significant increase in the 8-year overall survival (OS) of approximately 4% compared to patients without IMNI in patients with early-stage axillary node-positive breast cancer. Furthermore, 1 and 1.6% of the absolute benefits to the 10-year OS by regional node irradiation (RNI) with IMNI were reported in the MA.20 trial and in the European Organisation for Research and Treatment of Cancer (EORTC) trial, respectively.2–4
However, cardiotoxic effects, coronary atherosclerosis, pneumonitis and pulmonary fibrosis could potentially increase, especially in patients with left-sided breast irradiation.2,5 Conventionally, parallel-opposed tangential photon beams matching megavoltage photons and electron fields cause the anterior of the heart and the proximal left anterior descending (LAD) artery to receive high radiation doses.6 The rates of major coronary events were shown to increase linearly with the mean dose to the heart by 7.4% per gray (Gy).7 Additionally, a slight increase in pneumonitis and pulmonary fibrosis with RNI has recently been reported.2
The tangent angles techniques can have a limited dose to the ipsilateral lung and the contralateral breast, but it is difficult to generate a concave dose distribution conforming to the breast target.8Intensity-modulated radiation therapy (IMRT) techniques have demonstrated advantages in target conformity and homogeneity for breast irradiation while sparing the anterior heart and ipsilateral lung tissue from high doses of radiation, but they also involve the organs exposed in the low-dose region.9
The purpose of this study was to develop an optimal treatment plan by using the complete-directional-complete block (CDCB) technique with different angle restrictions to minimize the risk of cardiac, LAD artery and lung irradiation for patients with left-sided breast cancer who were treated with whole-breast irradiation and RNI.
Methods and materials
Patient selection and structure delineation
Ten patients with locally advanced left-sided breast cancer who received radiotherapy were enrolled, and CT images were obtained. Each patient received treatment planning via inverse planning by different techniques in the same CT images. Prospective data were collected after receiving approval from the Institutional Review Board of the Far Eastern Memorial Hospital (FEMH-IRB- 104105-E). The staging characteristics for the selected patients are listed in Table 1.
Table 1.
The staging characteristics of the selected patients
| Patient | Stage | Without neoadjuvant treatment | Postneoadjuvant treatment | ER | PR | Her2/neu | Surgical technique | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pT | pN | M | ypT | ypN | M | ||||||
| 1 | IIIC | 2 | 3a | 0 | - | - | - | 0% | 0% | 3+/3+ | BCT |
| 2 | IIB | 2 | 1 | 0 | - | - | - | 95% | 95% | 2+/3+ | BCT |
| 3 | IIA | 2 | 0 (i+) | 0 | - | - | - | 1% | 1% | 0/3+ | BCT |
| 4 | IIIC | 2 | 3 | 0 | - | - | - | 90% | 90% | 2+/3+ | MRM |
| 5 | IIIA | 2a | 2a | 0 | - | - | - | 0% | 0% | 3+/3+ | MRM |
| 6 | IIIC | 2 | 3 | 0 | - | - | - | 0% | 0% | 0/3+ | MRM |
| 7 | IIA | 1b | 1 | 0 | - | - | - | 0% | 0% | 0/3+ | BCT |
| 8 | ypIIA | - | - | - | from 1c to 1a | from 1 to 1 |
0 | 90% | 90% | 1+/3+ | BCT |
| 9 | ypIIB | - | - | - | from 3 to 3 |
from 3a to 0 | 0 | 1% | 0% | 3+/3+ | MRM |
| 10 | ypIA | - | - | - | from 4b to 1b | from 2 to 0 (i+) |
0 | 0% | 0% | 3+/3+ | MRM |
BCT, breast-conserving surgery; MRM, modified radical mastectomy.
i+: ITCs only (malignant cell clusters no larger than 0.2 mm) in regional lymph nodes.
The patients were scanned with a CT scanner (GE, Discovery VCT PET/CT Imaging System) with 2.5 mm slice spacing, and then the image sets were transferred to a treatment planning system (Pinnacle3 Version7.6C) for targeting and organ delineation. Clinical target volumes (CTVs) included the whole breast/chest wall, the axillary (levels II and III) region, the supraclavicular fossa (SCF), and IMNs (in the first-to-third interspaces) and were obtained as per published guidelines.10 For the regional nodes, planning target volumes (PTVs) were expanded by 5 mm from the regional nodal CTV. For the breast/chest wall, an 8 mm isotropic margin was added to the CTV to account for setup uncertainty and respiratory motion. A virtual bolus 10 mm in thickness was used to cover the flash region of the PTV that extends into the surrounding air and was included in the body contour in the IMRT and tomotherapy plans. Organ-at-risk (OAR) volumes were contoured for each lung, the heart and the contralateral breast. The heart and LAD artery were contoured according to the validated University of Michigan cardiac atlas.11
All plans in this study were optimized with at least 95% of the PTV encompassed by the prescribed dose (Dp) of 50 Gy in 25 fractions. The maximum dose was less than 110% of the Dp, and dose volume points and penalties were adjusted throughout the optimization process to best meet OAR dose constraints without compromising the PTV coverage.
Planning designed by helical tomotherapy with complete-directional-complete block
The image sets with targets and OARs were transferred to a Tomotherapy Hi Art Planning system (v. 4.0.4. Tomotherapy, Inc., Madison, Wisconsin, USA). A field width of 2.5 cm, a pitch of 0.215 and a maximum modulation factor of 2.6 were used for all tomotherapy plans.
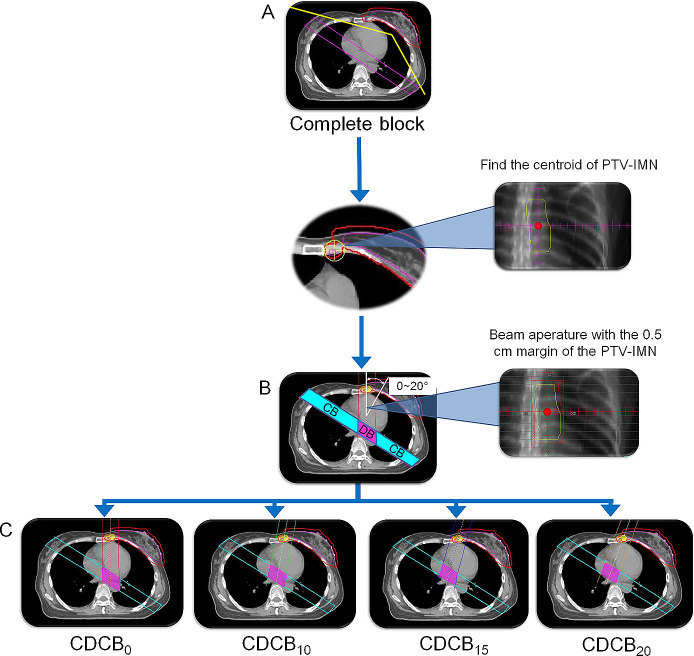
For the complete block (CB) helical tomotherapy (HT) plans (HT-CB), the designs of the directional and CB were reported in the previous study.8 For tomotherapy plans with CDCB, the CB was modified to improve in-dose conformality while sparing the lungs and heart from high doses of radiation as follows: (1) the CB was a rectangular structure with the ends connected 3 cm away from the margin of the PTV. It was designed to disable beamlets from entering or exiting through this structure; and (2) the directional-blocking area of the CDCB was determined by the intersection of where the CB and the beam aperture passed through the 0.5 cm margin of the IMN. Directional block was used to close the beamlets if the blocked structure was proximal to the target to limit the beamlet entrance direction. The CDCB restricts the beamlets to enter only within limited angles, and beam angles of 0, 10, 15 and 20 degrees were used as the restricted angles according to the geometric center of the IMN and were used to determine the optimal design of the CDCB (Figure 1). The heart, lungs and contralateral breast are referred to as the “organ-based directional block (OBDB)” to limit the primary beam from entering through these structures.
Figure 1.
The CDCB. The CB was a rectangular structure with the ends connected 3 cm away from the margin of the planning target volumes (PTV). The CB was designed to disable beamlets from entering or exiting through this structure. The directional-blocking area of the CDCB was determined by the intersection of where the CB and the beam aperture passed through the 0.5 cm margin of the PTV of the IMNs. Directional block was used to close the beamlets if the blocked structure was proximal to the target in order to limit the beamlet entrance direction. The CDCB restricts the beamlets to enter within limited angles, and beam angles of 0, 10, 15 and 20 degrees were used as the restricted angles according to the geometric center of the IMNs (in the first to third interspaces) and were used to determine the optimal design of the CDCB.
Five-field IMRT planning
Five-field IMRT (5F-IMRT) plans were generated for a Versa HD accelerator. The 5F-IMRT plans were executed on a Pinnacle treatment planning system (Pinnacle3 v. 9.8C) with a 0.2 cm calculation grid. Five fixed angles of 6 MV coplanar fields consisted of one anteroposterior beam, two medial beams (300 ~ 320 degrees) and two lateral beams (120 ~ 150 degrees), which were modulated using the direct machine parameter optimization technique available in the Pinnacle system. The maximum number of segments was set to 40, and the minimum segment MUs was set to 4.
Plan evaluation parameters
The uniformity index (UI) of the PTV was defined as the ratio of the minimum dose received in 5 and 95% of the PTV.12 The conformity index (CI) of the PTV was defined as CI= (TVPIV)2/(TV x PIV), where PIV is the prescription isodose volume, TVPIV is target volume covered by the PIV, and TV is the target volume.13Furthermore, V109% was calculated to assess dose homogeneity.
The normal tissue dose metrics were calculated for the plan comparisons. Additionally, the tomotherapy plan with OBDB that assigned OARs as “directionally” blocked structures was also compared with the CB, CDCB0,10,15,20 and 5F-IMRT plans.
Statistical methods
The treatment variables between groups were assessed by t-tests and chi-square tests. A generalized estimating equation (GEE) model was used to study the outcomes for dependent variables under different conditions. The statistical analyses were performed using the SPSS statistical package (v. 20.0, IBM Corporation, Armonk, NY, USA). P values had a significance level of 0.05. To determine whether the differences between all possible pairings of the planning techniques were statistically significant, we conducted Bonferroni tests that generated the presented p values.
Results
Planning target volume
The comparisons of the treatment plans are summarized in Tables 2 and 3 . There were no statistically significant differences in the D95% of the PTV between all seven planning techniques. However, the V95% of the CB plan (99.10±0.5%) was significantly higher than that of CDCB10-20 (p < 0.05). There were no significant differences in terms of PTV coverage as measured by the V95% among the CDCB0 and CDCB20, OBDB and 5F-IMRT plans. Additionally, the estimated p values for the compared treatment modalities.
Table 2.
Averages of the ten patients’ dose volume histogram parameters as a percentage of the conformity and uniformity indexes for the planning target volumes of left-sided breast cancer patients with irradiation of the whole breast/chest wall, the axillary (levels II and III) region, the supraclavicular field, and the internal mammary nodes by using different treatment techniques for comparison
| Technique | CB | CDCB0 | CDCB10 | CDCB15 | CDCB20 | OBDB | 5F-IMRT | p value | |
|---|---|---|---|---|---|---|---|---|---|
| PTV volume | Parameters | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | |
| 825.838 ± 267.38 ml (548.5–1844.0 ml) |
CI | 0.66 ± 0.06 | 0.69 ± 0.05 | 0.69 ± 0.05 | 0.69 ± 0.05 | 0.69 ± 0.05 | 0.73 ± 0.04 | 0.66 ± 0.07 | <0.001 |
| UI | 1.06 ± 0.01 | 1.07 ± 0.01 | 1.07 ± 0.01 | 1.07 ± 0.01 | 1.07 ± 0.01 | 1.06 ± 0.01 | 1.07 ± 0.01 | <0.001 | |
| D5% | 53.35 ± 0.40 | 53.68 ± 0.35 | 53.69 ± 0.35 | 53.89 ± 0.38 | 53.73 ± 0.29 | 53.17 ± 0.41 | 53.97 ± 0.26 | <0.001 | |
| D95% | 50.22 ± 0.20 | 50.19 ± 0.14 | 50.17 ± 0.19 | 50.26 ± 0.14 | 50.16 ± 0.21 | 50.17 ± 0.21 | 50.22 ± 0.44 | 0.091 | |
| V95% | 99.10 ± 0.50 | 98.82 ± 0.45 | 98.71 ± 0.48 | 98.57 ± 0.76 | 98.69 ± 0.64 | 98.80 ± 0.42 | 98.80 ± 0.55 | <0.001 | |
| V109% | 0.35 ± 0.40 | 0.54 ± 0.52 | 0.65 ± 0.56 | 1.37 ± 1.48 | 0.83 ± 0.69 | 0.23 ±0.51 | 1.07 ± 0.91 | <0.001 | |
CB, complete block; CDCB, complete-directional complete block; CI, conformity index; Dx%, the minimum doses delivered to x% of the planning target volume; 5F-IMRT, 5-field intensity-modulated radiotherapy; OBDB, organ-base-directional block; UI, uniformity index; Vx%, the volume of the PTV receiving x% of the prescribed dose.
Data are presented as the mean ± standard deviation.
Table 3.
The presented p values describe the statistical relevance of the differences in V95%, conformity index and uniformity index of the planning target volume between all possible pairs of the studied planning techniques
| Parameters | PTV | ||
|---|---|---|---|
| V95% | CI | UI | |
| A vs B | 0.097 | <0.001 | 0.017 |
| A vs C | 0.004 | 0.003 | 0.021 |
| A vs D | 0.004 | <0.001 | <0.001 |
| A vs E | 0.005 | 0.002 | 0.023 |
| A vs F | 1.000 | <0.001 | 1.000 |
| A vs G | 1.000 | 1.000 | 0.028 |
| B vs C | 1.000 | 1.000 | 1.000 |
| B vs D | 1.000 | 1.000 | 0.710 |
| B vs E | 1.000 | 1.000 | 1.000 |
| B vs F | 1.000 | 0.014 | 0.202 |
| B vs G | 1.000 | 1.000 | 1.000 |
| C vs D | 1.000 | 1.000 | 1.000 |
| C vs E | 1.000 | 1.000 | 1.000 |
| C vs F | 1.000 | 0.001 | 0.257 |
| C vs G | 1.000 | 1.000 | 1.000 |
| D vs E | 1.000 | 1.000 | 1.000 |
| D vs F | 1.000 | 0.001 | 0.030 |
| D vs G | 1.000 | 1.000 | 1.000 |
| E vs F | 1.000 | <0.001 | 0.100 |
| E vs G | 1.000 | 1.000 | 1.000 |
| F vs G | 1.000 | 0.003 | <0.001 |
A, CB; B, CDCB0; C, CDCB10; D, CDCB15; E, CDCB20; F, OBDB; G, 5F-IMRT.
Data are presented as the mean ± standard deviation.
A typical dose distribution for the plans is displayed in Figure 2A–G. There were no differences in PTV coverage with the 95% isodose for the HT techniques compared to the 5F-IMRT. However, the CB provided better coverage of V95%than 5F-IMRT (99.10 ± 0.50 vs 98.29 ± 0.54, p = 0.026). The V109% in CDCB15 and 5F-IMRT had the highest values. The OBDB plan had better conformity (0.73) than the other plans. The UIs in the CB and OBDB plans were worse than those of the other techniques. Additionally, the OBDB plan had the lowest D5%.
Figure 2.
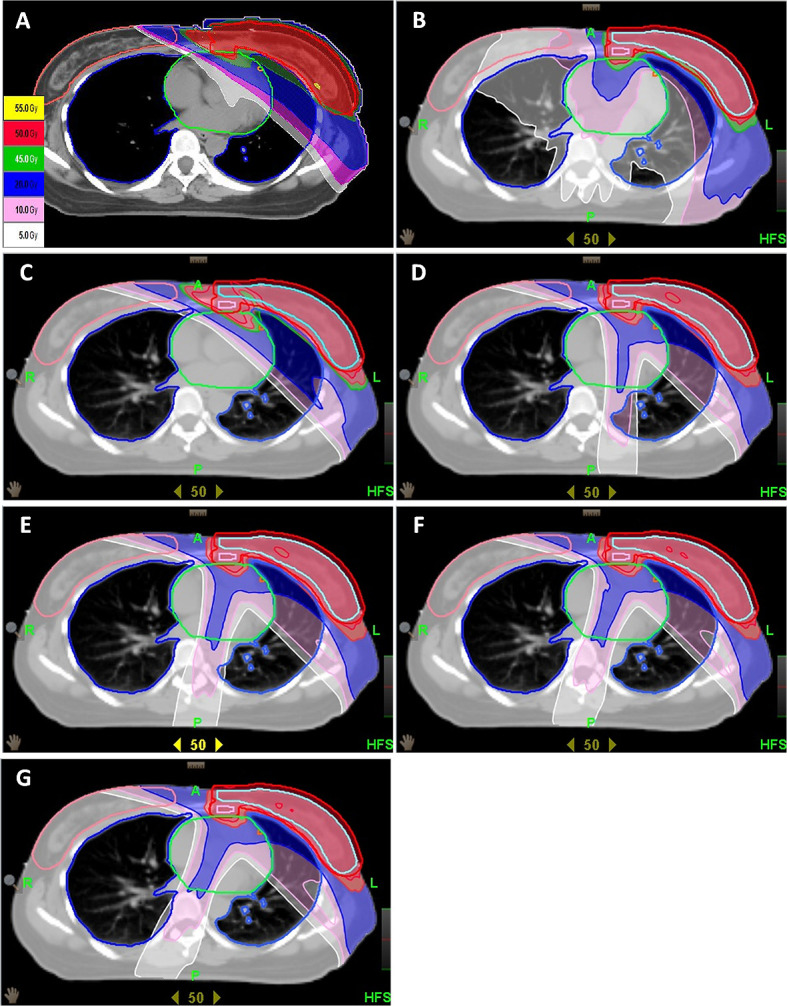
Axial dose distribution for the 5F-IMRT plan (A) and the HT plans: (B) OBDB, (C) CB, (D) CDCB0, (E) CDCB10, (F) CDCB15, and (G) CDCB20.
Normal tissue irradiation
The CDCB15 and CDCB20 plans had the lowest ipsilateral mean lung dose, V5, V10 and V20. For the contralateral lung, the CB and CDCB0 plans had the lowest mean dose. The CDCB0 and CDCB15 plans had the lowest V20 values in the contralateral lung. Interestingly, 5F-IMRT had the lowest contralateral mean breast dose. The OBDB plan had the highest heart mean doses (10.41 ± 1.72 Gy) and V5 (88.81±14.1%) compared to the other techniques. Compared to the other techniques, the CDCB20 and OBDB plans had lower mean doses in the LAD artery (Tables 4 and 5).
Table 4.
Comparison of dosimetric parameters in normal organs-at-risk for left-side-breast cancer patients with irradiation of the whole breast/chest wall, the axillary (levels II and III) region, the supraclavicular field, and the internal mammary nodes by using different treatment techniques
| Technique | CB | CDCB0 | CDCB10 | CDCB15 | CDCB20 | OBDB | 5F-IMRT | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Organs | Parameters | Mean ± SD | Mean ± SD | Mean ±S D | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Ipsilateral lung | Mean (Gy) | 15.82 ± 1.82 | 14.03 ± 1.38 | 13.14 ± 1.41 | 12.78 ± 1.43 | 12.77 ± 1.29 | 15.07 ± 2.29 | 15.69 ± 1.54 | <0.001 |
| V5 (%) | 56.15 ± 3.95 | 58.29 ± 4.66 | 54.82 ± 4.04 | 54.10 ± 3.90 | 54.03 ± 3.95 | 84.14 ± 8.56 | 60.64 ± 5.93 | <0.001 | |
| V10 (%) | 48.02 ± 4.43 | 45.48 ± 4.26 | 42.80 ± 4.12 | 41.51 ± 4.01 | 41.67 ± 3.51 | 50.73 ± 11.71 | 43.97 ± 3.73 | <0.001 | |
| V20 (%) | 34.31 ± 4.88 | 27.80 ± 4.50 | 25.80 ± 4.41 | 24.80 ± 4.46 | 24.93 ± 4.07 | 26.56 ± 5.93 | 30.56 ± 4.02 | <0.001 | |
| Contralateral lung | Mean (Gy) | 1.10 ± 0.24 | 1.14 ± 0.20 | 1.21 ± 0.24 | 1.41 ± 0.24 | 1.64 ± 0.21 | 6.04 ± 0.79 | 0.87 ± 0.11 | <0.001 |
| V20 (%) | 0.15 ± 0.18 | 0.07 ± 0.06 | 0.10 ± 0.08 | 0.08 ± 0.08 | 0.10 ± 0.09 | 0.84 ± 0.77 | 0.13 ± 0.10 | <0.05 | |
| Contralateral breast | Mean (Gy) | 2.81 ± 1.54 | 2.81 ± 1.23 | 2.53 ± 1.33 | 2.53 ± 1.35 | 2.52 ± 1.34 | 4.56 ± 0.76 | 1.75 ± 0.99 | <0.001 |
| Heart | Mean (Gy) | 5.09 ± 1.30 | 5.68 ± 1.27 | 6.11 ± 1.31 | 6.20 ± 1.43 | 6.23 ± 1.40 | 10.41 ± 1.72 | 6.11 ± 1.36 | <0.001 |
| V5 (%) | 19.98 ± 5.67 | 30.23 ± 6.29 | 33.15 ± 7.20 | 33.70 ± 7.74 | 33.94 ± 7.60 | 88.81 ± 14.14 | 34.56 ± 14.45 | <0.001 | |
| V25 (%) | 5.07 ± 2.20 | 3.41 ± 1.76 | 3.59 ± 1.77 | 5.33 ± 5.0 | 5.37 ± 5.36 | 5.45 ± 2.57 | 4.72 ± 1.73 | <0.001 | |
| V30 (%) | 3.58 ± 1.67 | 2.11 ± 1.27 | 2.12 ± 1.18 | 2.14 ± 1.20 | 1.93 ± 1.16 | 3.49 ± 1.74 | 3.56 ± 1.47 | <0.001 | |
| V40 (%) | 1.47 ± 0.88 | 0.63 ± 0.57 | 0.67 ± 0.49 | 0.70 ± 0.51 | 0.55 ± 0.46 | 1.16 ± 0.72 | 1.56 ± 1.03 | <0.001 | |
| LAD | Mean (Gy) | 22.85 ± 6.36 | 18.44 ± 5.95 | 16.67 ± 5.52 | 16.31 ± 5.58 | 15.66 ± 5.26 | 11.83 ± 3.24 | 27.00 ± 9.63 | <0.001 |
| Maximum (Gy) | 45.49 ± 5.41 | 43.73 ± 6.56 | 40.66 ± 9.29 | 40.08 ± 10.12 | 39.70 ± 10.33 | 34.93 ± 9.07 | 47.70 ± 3.61 | <0.001 | |
CB, complete block; CDCB, complete-directional-complete block; 5F-IMRT, 5-field intensity- modulated radiotherapy; LAD, left anterior descending oronary artery; OBDB, organ-base-directional block; Vx, the volume of the organ receiving x Gy.
Data are presented as the mean ± standard deviation.
Table 5.
The presented p values describe the statistical relevance of the differences in dose parameters in the ipsilateral lung, the heart and the left anterior descending artery between all possible pairs of the studied planning techniques
| Parameters | Ipsilateral lung | Heart | LAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dmean | V5 | V10 | V20 | Dmean | V5 | V25 | V30 | V40 | Dmean | Dmax | |
| A vs B | <0.001 | 0.105 | 0.035 | <0.001 | 0.048 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 |
| A vs C | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.027 |
| A vs D | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 | <0.001 | 0.003 | <0.001 | 0.027 |
| A vs E | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 | <0.001 | <0.001 | <0.001 | 0.014 |
| A vs F | 1.000 | <0.001 | 1.000 | 0.001 | <0.001 | <0.001 | 1.000 | 1.000 | 1.000 | <0.001 | <0.001 |
| A vs G | 1.000 | 0.642 | 0.041 | 0.058 | 0.002 | 0.006 | 1.000 | 1.000 | 1.000 | 0.095 | 1.000 |
| B vs C | <0.001 | <0.001 | <0.001 | 0.009 | 0.321 | 0.132 | 1.000 | 1.000 | 1.000 | 0.001 | 1.000 |
| B vs D | <0.001 | <0.001 | <0.001 | <0.001 | 0.174 | 0.084 | 1.000 | 1.000 | 1.000 | <0.001 | 1.000 |
| B vs E | <0.001 | <0.001 | <0.001 | <0.001 | 0.180 | 0.052 | 1.000 | 1.000 | 1.000 | <0.001 | 0.975 |
| B vs F | 1.000 | <0.001 | 1.000 | 1.000 | <0.001 | <0.001 | 0.008 | 0.002 | 0.001 | <0.001 | 0.001 |
| B vs G | 0.043 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.016 | <0.001 | 0.001 | 0.001 | 0.036 |
| C vs D | 0.001 | 0.003 | 0.007 | <0.001 | 1.000 | 0.943 | 1.000 | 1.000 | 1.000 | 0.663 | 1.000 |
| C vs E | 0.034 | 0.012 | 0.456 | 0.163 | 1.000 | 0.099 | 1.000 | 0.109 | 0.001 | <0.001 | 1.000 |
| C vs F | 0.083 | <0.001 | 0.488 | 1.000 | <0.001 | <0.001 | 0.017 | 0.002 | <0.001 | 0.002 | 0.004 |
| C vs G | <0.001 | 0.116 | 1.000 | 0.025 | 1.000 | 1.000 | <0.001 | <0.001 | <0.001 | <0.001 | 0.051 |
| D vs E | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.408 | 1.000 | 0.014 | 1.000 |
| D vs F | 0.008 | <0.001 | 0.153 | 1.000 | <0.001 | <0.001 | 1.000 | 0.003 | <0.001 | 0.011 | 0.025 |
| D vs G | <0.001 | 0.046 | 1.000 | 0.005 | 1.000 | 1.000 | 1.000 | <0.001 | 0.001 | <0.001 | 0.061 |
| E vs F | 0.007 | <0.001 | 0.159 | 1.000 | <0.001 | <0.001 | 1.000 | <0.001 | <0.001 | 0.041 | 0.106 |
| E vs G | <0.001 | 0.045 | 1.000 | 0.006 | 1.000 | 1.000 | 1.000 | <0.001 | <0.001 | <0.001 | 0.041 |
| F vs G | 1.000 | <0.001 | 1.000 | 1.000 | <0.001 | <0.001 | 1.000 | 1.000 | 0.407 | <0.001 | <0.001 |
A, CB; B, CDCB0; C, CDCB10; D, CDCB15; E, CDCB20; F, OBDB; G, 5F-IMRT.
The current study predicted the optimal radiation technique strategy for left-sided advanced breast cancer by summing the specific weights of each parameter in each technique and then comparing the sums with the highest one and checking the comparisons by t-test. The specific weight for the comparison of each parameter in each technique was defined as “0”, “1” and “2” when the p values were > 0.05, ≤ 0.05 and ≤ 0.001, respectively. The sums of the expectations provided by the parameters of the OARs in CB, CDCB0, CDCB10, CDCB15, CDCB20, OBDB and 5F-IMRT were 22, 33, 52, 58, 61, 17 and 19, respectively. CDCB20 was better than CDCB0, OBDB and 5F-IMRT. In addition, CDCB20 was slightly better than CB. However, there were no differences among CDCB10, CDCB15 and CDCB20. A range of 10–20 degrees for the CDCB technique was considered the optimal restricted angle. (Table 6)
Table 6.
The sum of the specific weights of each parameter in each technique for left-sided advanced breast cancer, comparison with the highest sum and a check by t-test. The specific weight for the comparison of each parameter in each technique is defined as “0”, “1” and “2” when p values were > 0.05, ≤ 0.05 and ≤ 0.001, respectively
| CB | CDCB0 | CDCB10 | CDCB15 | CDCB20 | OBDB | 5F-IMRT | ||
|---|---|---|---|---|---|---|---|---|
| Ipsilateral lung | Mean | 0 | 3 | 6 | 8 | 8 | 0 | 0 |
| V5 | 2 | 2 | 6 | 8 | 9 | 0 | 2 | |
| V10 | 0 | 1 | 4 | 5 | 4 | 0 | 1 | |
| V20 | 0 | 2 | 4 | 7 | 5 | 1 | 0 | |
| V40 | 0 | 4 | 4 | 5 | 5 | 7 | 0 | |
| Contra. lung | Mean | 8 | 5 | 6 | 4 | 2 | 0 | 11 |
| V20 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | |
| Contra. breast | Mean | 2 | 2 | 3 | 3 | 3 | 0 | 2 |
| Heart | Mean | 10 | 2 | 2 | 2 | 2 | 0 | 2 |
| V30 | 0 | 4 | 5 | 5 | 6 | 0 | 0 | |
| V40 | 0 | 4 | 6 | 4 | 7 | 0 | 0 | |
| LAD | Mean | 0 | 3 | 5 | 6 | 9 | 9 | 0 |
| SUM | 22 | 33 | 52 | 58 | 61 | 17 | 19 | |
| p value | 0.06 | 0.015 | 0.211 | 0.60 | 0.003 | 0.03 |
Discussion
Breast cancer patients treated with RNI by IMRT have demonstrated dosimetric benefits compared with conventional delivery.14–16 HT has also been confirmed to have smaller hotspots than three-dimensional (3D) plans9 and with much greater dose homogeneity than the IMRT plan.8 However, these reports also claimed that HT plans designed with the directional block technique delivered a significantly greater volume of low-dose radiation to the lungs, contralateral breast and other normal tissues when compared with 3D or IMRT plans. Recently, the HT plan using the CB technique for early-stage left-sided breast irradiation was shown to decrease the volume of low-dose radiation delivered to the OARs.8 Nevertheless, the problem with using CB in RNI, especially IMNI, is the inadequate CI for the coverage of IMN. In the current study, the OBDB technique had the best CI value as a result of to the degrees of freedom of the beamlet entrance.
The average mean heart dose (MHD) is approximately 4 Gy for standard left-tangential irradiation without involving the IMN.17 However, when using different techniques to irradiate the IMN, the increased to 7.0–16.7 Gy,9,18–23 as shown in Table 7. Through tight heart dose constraints in the whole-breast IMRT plan, the MHDs could be decreased to 2 Gy.24 Breast cancer treated by rotational techniques without a special design may expose the heart to a relatively high MHD (7.0 to 14.4 Gy).9,18,19,22 However, when specially designed, rotational techniques such as VMAT are able to easily reduce the MHD on average from 10 Gy to ≤6.5 Gy when compared with 3D conformal planning.23 The OBDB technique has a better conformity for PTV coverage than others but with worse MHDs (10.4 Gy) and V5 of the heart (88.8%) compared with other techniques. With the CB or angular restriction techniques, the V5 of the heart could decrease by 55 to 69% when compared with OBDB. The angular restriction techniques have lower V30 and V40 of the heart than CB, OBDB and 5F-IMRT. Additionally, a recent review of the literature recommends that the V25 of the heart should be less than 10% for a probability of cardiac mortality below 1%.25 In the current study, the V25 of the heart in all techniques ranged from 3.41 to 5.45%, which made all plan designs safer but still exposed patients to a significant risk of major coronary events. Darby et al7 found that the incidence of ischemic heart disease increases linearly with the mean dose to the heart with no apparent threshold below which there was no risk. Jagsi R et al26 reported that IMRT with a deep inspiration breath hold technique reduced the percentage of the left ventricle receiving ≥5 Gy by approximately 10% for patients with left-sided disease in whom IMN was targeted and suggested a potential benefit for preservation of the cardiac ejection fraction. For these reasons, the best approach seems to be keeping the dose to the heart as low as possible, avoiding direct irradiation altogether and using a special design and cardiac-sparing techniques whenever possible.23,26–29
Table 7.
Comparison of the planning parameters of the target and OARs for helical tomotherapy (HT) plans in this study and HT/volumetric modulated arc therapy (VMAT) plans for other studies in the literature for locoregional irradiation, including the internal mammary chain in left-sided breast cancer
| Author | Goddu9 | Caudrelier18 | Sakumi21 | Nichols19 | Tyran20 | Dumane23 | Current study (HT) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target volumes | Breast/chest-wall + IMC+SCV + AX | |||||||||||
| Patient number | 10a | 10a | 5a | 15a | 10a | 10b | 10a | |||||
| Dp | 50.4 | 50 | 50/45 | 50.4 | 50/46.95 | 50.4 | 50 | |||||
| (breast/LN) | (breast/LN) | |||||||||||
| Technique | HT | HT | VMAT | HT | VMAT | VMAT | VMAT | OBDB | CB | CDCB10 | CDCB15 | CDCB20 |
| PTV | ||||||||||||
| V95% (%) | 98 | 99 | 95.2/98.7/99.4 | 96.0/96.0 | 96.3 | 98.8 | 99.1 | 98.7 | 98.6 | 98.7 | ||
| (breast/clavicular/IMN) | (breast/LN) | |||||||||||
| V107% (%) | 31.1 | |||||||||||
| V109% (%) | 1.5 | 0.2 | 0.4 | 0.7 | 1.4 | 0.8 | ||||||
| D95 (Gy) | 49.8 | 49.3 | 48.89 | 50.2 | 50.2 | 50.2 | 50.3 | 50.2 | ||||
| CI | 0.56 | 0.719 | 0.778 | 0.8 (combine) | 0.73 | 0.66 | 0.69 | 0.69 | 0.69 | |||
| Ipsilateral lung | ||||||||||||
| Dmean (Gy) | 11.9 | 8.3 | 12.7 | 15.2 | 15.3 | 15.1 | 15.8 | 13.1 | 12.8 | 12.8 | ||
| V5 (%) | 73.7 | 35.4 | 74.6 | 99.3 | 96.9 | 86.7 | 84.3 | 84.1 | 56.2 | 54.8 | 54.1 | 54.0 |
| V10 (%) | 34.7 | 9.2 | 44.5 | 55.3 | 46.9 | 50.7 | 48.0 | 42.8 | 41.5 | 41.7 | ||
| V20 (%) | 17.6 | 18.9 | 29.9 | 32.3 | 25.4 | 24.7 | 26.6 | 34.3 | 25.8 | 24.8 | 24.9 | |
| Contralateral lung | ||||||||||||
| Dmean (Gy) | 4.2 | 6.2 | 4.0 | 4.0 | 3.6 | 6.0 | 1.1 | 1.2 | 1.4 | 1.6 | ||
| Heart | ||||||||||||
| Dmean (Gy) | 12.2 | 7 | 11.4 | 14.4 | 12.9 | 8.6 | 6.5 | 10.4 | 5.1 | 6.1 | 6.2 | 6.2 |
| V5 (%) | 38.3 | 90.3 | 39.0 | 88.8 | 20 | 33.2 | 33.7 | 33.9 | ||||
| V25 (%) | 0.7 | 5.5 | 5.1 | 3.6 | 5.3 | 5.4 | ||||||
| V30 (%) | 1.5 | 3.0 | 1.3 | 3.5 | 3.6 | 2.1 | 2.1 | 1.9 | ||||
| V40 (%) | 1.2 | 1.5 | 0.7 | 0.7 | 0.6 | |||||||
| Contralateral breast | ||||||||||||
| Dmean (Gy) | 4.3 | 4.8 | 3.1 | 1.8 | 1.5 | 3.2 | 4.2 | 4.6 | 2.8 | 2.5 | 2.5 | 2.5 |
HT, helical tomotherapy; VMAT, volumetric modulated arc therapy
a. left-sided breast cancer patients
b. five left-sided and five right-sided breast cancer patients
The coronary artery dose estimates for females who received breast cancer radiotherapy were between 6 and 43 Gy.30 Patients with unfavorable anatomy may receive radiation doses greater than 30 Gy to parts of the distal LAD artery.31The Danish Breast Cancer Cooperative Group proposed potential benefits from respiratory gating to decrease the LAD constraint to a maximum dose of 20 Gy.32 The higher radiation doses to the coronary artery or LAD were strongly associated with more frequent injury.33 One of the reasons for this is that mid- or distal LAD coronary artery segments remained in the radiation fields in some females.27 The maximum dose values of the LAD in the current study were 35–48 Gy. In a study by Taylor et al,33 the left-versus-right ratios for injury to these segments from tangential radiotherapy after breast-conserving surgery were approximately three for the LV apex and approximately six for the mid- or distal LAD segment, indicating that the sparing priority should be the LAD segment. CB has a better MHD and V5 of the heart than the other plans. However, the CB technique also resulted in the second highest mean and maximum doses in the LAD. In contrast, OBDB has a better mean and maximum dose in the LAD in all techniques. However, OBDB resulted in the highest MHD (10 Gy) and V5 of the heart (88.8%). Darby et al7 demonstrated a dose–effect relationship based on the MHD. Additionally, the volume of the left ventricle receiving 5 Gy (LV-V5) seems to be a better predictor for acute coronary events than the MHD.34 Therefore, CBCD20 is better than CB and OBDB for heart protection. Moreover, the parameter priority for reducing the risk of cardiac injury would be maximum dose of LAD,33 followed by V5 of the heart,34the MHD,7 and V25.25
The risks of early and late radiogenic lung sequelae for patients with breast cancer are strongly related to the volume of the irradiated lung and the dose. A mean lung dose (MLD) >15.0 Gy and a V20 >31.1% for breast cancer patients treated with RT can easily cause Grade one radiation pneumonitis; therefore, the MLD should remain between 12 and 15 Gy and the V20 should remain below 24% to avoid lung toxicity.35 The addition of SCF and IMN irradiation will increase the MLD and V20, which are associated with a 2.5-fold higher risk of radiation pneumonitis and a two-fold risk of radiogenic fibrosis.35 Furthermore, SCF irradiation showed a strong association with the incidence of radiation pneumonitis (OR = 5.07).36
Compared to conventional techniques or 3D-CRT, IMRT can reduce mean lung V20 by approximately 4–10%.16,22,37 However, the ipsilateral lung V5 will increase approximately 9–68%,16,22,37,38 and the lung V5 could be as high as 85.8%.38 Lancellotta et al reported a better lung V20 by using the 3D-CRT technique than by using HT.22 Similarly, HT with a special design can provide lower ipsilateral lung V20 more easily than 3D-CRT9,39 or IMRT.8,18,39 Nevertheless, the ipsilateral lung V5 in HT without a special design could be as high as 70–99.3%.19,22,40,41 In contrast, the low-dose volume of the lung could be reduced efficiently from 33.1 to 24.7% by HT with the CB technique when compared with IMRT.8 Additionally, the CB or CDCB techniques used here not only meet the MLD (12.7–15.8 Gy) and V20 (24.8–34.3%) criteria but also decreased the ipsilateral lung V5 (54.0–58.3%) and the contralateral lung V5 (2.1–6.3%).
An overview analysis suggests that patients receiving radiation may have an elevated incidence of contralateral breast cancer compared with those who did not receive radiation.42,43 The mean dose to the contralateral breast during RNI using the OBDB technique treatment by HT was 4.3 to 4.8 Gy.9,18,22 The OBDB technique has better conformity for PTV (CI = 0.73) than the others; however, it could expose the other organs to a higher mean contralateral breast dose (4.6 Gy), contralateral lung dose (6.0 Gy) and heart dose (10.4 Gy). In contrast, the other techniques have lower mean doses of 2.5–2.8 Gy, 1.1–1.6 Gy and 5.1–6.2 Gy for the contralateral breast, contralateral lung and heart, respectively. However, Nichols et al19 reported that the mean dose to the contralateral breast was 1.8 Gy by the same technique. Nevertheless, the MHD, ipsilateral V5 of the lung and contralateral V5 of the lung in their report were higher, at 14.4 Gy, 99 and 82%, respectively.19 Similarly, the breast treated with RNI by a different arc therapy, such as volumetric-modulated arc therapy (VMAT), appears to have a lower contralateral breast dose (1.5–3.2 Gy). Nonetheless, this technique also has a higher MHD (9–13 Gy) and ipsilateral lung dose (4 Gy).19–21 Therefore, awareness of the potential risks of scatter dose to the contralateral breast or other organs by HT with the OBDB technique or arc therapy is prudent.
To take into account the parameters of the OARs in CB, CDCB0, CDCB10, CDCB15, CDCB20, OBDB and 5F-IMRT, the sums of the expectations were calculated to be 22, 33, 52, 58, 61, 17 and 19, respectively (Table 6). CDCB10-20 was better than CB, CDCB0, OBDB and 5F-IMRT. However, there are some limitations in the current study. First, we did not use cardiac-sparing techniques (such as breath hold, treatment in a prone position, treatment during deep inspiration (including the use of breath-hold and gating techniques)) for planning during the current study. In the current study, the maximum dose of LAD ranged from 39.7 to 47.7 Gy for all techniques. One of the reasons for this is that mid- or distal LAD coronary artery segments remained in the radiation fields. Taylor et al33 reported that the left-versus-right ratio for injury to these segments from tangential radiotherapy was approximately six for the mid- or distal LAD segment, indicating that irradiating these segments causes injury. Additionally, the Danish Breast Cancer Cooperative Group proposed potential benefits from respiratory gating to decrease the LAD constraint to a maximum dose of 20 Gy.32 Moreover, using the breath hold technique for the left-side-breast with RNI, remarkably low doses to the OARs have been reported.44Therefore, where possible, these segments should be excluded from fields using cardiac-sparing techniques.23,26–29However, cardiac V40 ranged from 0.55 to 1.47%, and CDCB20 reduced cardiac V40 by 53–63% compared with CB, OBDB and IMRT in the current study. For some institutes that do not use the breath hold technique, the CDCB technique provides a chance to possibly decrease cardiac toxicity.
Second, lying prone causes the breast to fall away from the chest wall, which allows for cardiac avoidance.45 However, in general, Asian females have smaller breast volumes compared to Caucasian females.46 For females with large breast volumes, using the prone positioning can ensure that the breast is far away from the chest wall and is expected to result in minimal cardiac doses. However, in females with smaller breast volumes, it can be theorized that the change between breast tissue and the chest wall is limited. Moreover, lying prone is described as uncomfortable,47 and patients may not be able to maintain their position throughout treatment; therefore, reproducibility of treatment is challenging, and as a result the OAR dose may increase.48 The prone position was not used in the current study based on these published observations.
Conclusions
HT with the CDCB technique successfully reduced radiation doses to the heart, LAD artery and lung compared to IMRT, OBDB and CB. Considering the balance with the minimal dose to the critical organs and the best homogeneity and conformity of the PTV, the optimal angle for CDCB is 10–20 degrees. Clinical studies of left-sided breast irradiation with angle-restricted HT are warranted in the future.
Footnotes
Acknowledgment: We thank Ms. Yu-Lin Hsieh for analyzing the data. This study was supported by research grants from Far Eastern Memorial Hospital (FEMH-2016-C-061, FEMH 107–2314-B-418–007).
Funding: This study was supported in part by research grants from Far Eastern Memorial Hospital grants (FEMH-2016-C-061, FEMH 107–2314-B-418–007)
Ethics approval and consent to participate:All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of our hospital (FEMH-IRB-104105-E).
Consent for publication:Consent to publish has been obtained from the applicable persons and is available.
Competing interests:The authors declare that they have no competing interests. All authors have disclosed that there are no financial relationships relevant to this publication.
Contributor Information
Hsin-Pei Yeh, Email: baibai2000313@gmail.com.
Yu-Chuen Huang, Email: yuchuen@mail.cmu.edu.tw.
Li-Ying Wang, Email: liying@ntu.edu.tw.
Pei-Wei Shueng, Email: shuengsir@gmail.com.
Hui-Ju Tien, Email: catju.tw@gmail.com.
Chiu-Han Chang, Email: chang.chiuhan@gmail.com.
San-Fang Chou, Email: sfchou1971@gmail.com.
Chen-Hsi Hsieh, Email: chenciab@gmail.com.
REFERENCES
- 1. Thorsen LBJ, Offersen BV, Danø H, Berg M, Jensen I, Pedersen AN, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol 2016; 34: 314–20. doi: 10.1200/JCO.2015.63.6456 [DOI] [PubMed] [Google Scholar]
- 2. Budach W, Bölke E, Kammers K, Gerber PA, Nestle-Krämling C, Matuschek C. Adjuvant radiation therapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials- an update. Radiat Oncol 2015; 10: 258. doi: 10.1186/s13014-015-0568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015; 373: 317–27. doi: 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
- 4. Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med 2015; 373: 307–16. doi: 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys 2007; 69: 1484–95. doi: 10.1016/j.ijrobp.2007.05.034 [DOI] [PubMed] [Google Scholar]
- 6. Krueger EA, Schipper MJ, Koelling T, Marsh RB, Butler JB, Pierce LJ. Cardiac chamber and coronary artery doses associated with postmastectomy radiotherapy techniques to the chest wall and regional nodes. Int J Radiat Oncol Biol Phys 2004; 60: 1195–203. doi: 10.1016/j.ijrobp.2004.04.026 [DOI] [PubMed] [Google Scholar]
- 7. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 8. Shiau A-C, Hsieh C-H, Tien H-J, Yeh H-P, Lin C-T, Shueng P-W, et al. Left-Sided whole breast irradiation with hybrid-IMRT and helical tomotherapy dosimetric comparison. Biomed Res Int 2014; 2014: 1–7. doi: 10.1155/2014/741326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goddu SM, Chaudhari S, Mamalui-Hunter M, Pechenaya OL, Pratt D, Mutic S, et al. Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys 2009; 73: 1243–51. doi: 10.1016/j.ijrobp.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 10. Radiation Therapy Oncology Group RTOG breast cancer contouring atlas..
- 11. Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011; 79: 10–18. doi: 10.1016/j.ijrobp.2009.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Zhang X, Dong L, Liu H, Gillin M, Ahamad A, et al. Effectiveness of noncoplanar IMRT planning using a parallelized multiresolution beam angle optimization method for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys 2005; 63: 594–601. doi: 10.1016/j.ijrobp.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 13. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. technical note. J Neurosurg 2000; 93 Suppl 3(Suppl 3): 219–22. doi: 10.3171/jns.2000.93.supplement_3.0219 [DOI] [PubMed] [Google Scholar]
- 14. Fogliata A, Nicolini G, Alber M, Asell M, Dobler B, El-Haddad M, et al. Imrt for breast. A planning study. Radiother Oncol 2005; 76: 300–10. doi: 10.1016/j.radonc.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 15. Popescu CC, Olivotto I, Patenaude V, Wai E, Beckham WA. Inverse-planned, dynamic, multi-beam, intensity-modulated radiation therapy (IMRT): a promising technique when target volume is the left breast and internal mammary lymph nodes. Med Dosim 2006; 31: 283–91. doi: 10.1016/j.meddos.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 16. Beckham WA, Popescu CC, Patenaude VV, Wai ES, Olivotto IA. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer? Int J Radiat Oncol Biol Phys 2007; 69: 918–24. doi: 10.1016/j.ijrobp.2007.06.060 [DOI] [PubMed] [Google Scholar]
- 17. Taylor CW, Wang Z, Macaulay E, Jagsi R, Duane F, Darby SC. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys 2015; 93: 845–53. doi: 10.1016/j.ijrobp.2015.07.2292 [DOI] [PubMed] [Google Scholar]
- 18. Caudrelier J-M, Morgan SC, Montgomery L, Lacelle M, Nyiri B, Macpherson M. Helical tomotherapy for locoregional irradiation including the internal mammary chain in left-sided breast cancer: dosimetric evaluation. Radiother Oncol 2009; 90: 99–105. doi: 10.1016/j.radonc.2008.09.028 [DOI] [PubMed] [Google Scholar]
- 19. Nichols GP, Fontenot JD, Gibbons JP, Sanders M. Evaluation of volumetric modulated Arc therapy for postmastectomy treatment. Radiat Oncol 2014; 9: 66. doi: 10.1186/1748-717X-9-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tyran M, Mailleux H, Tallet A, Fau P, Gonzague L, Minsat M, et al. Volumetric-modulated Arc therapy for left-sided breast cancer and all regional nodes improves target volumes coverage and reduces treatment time and doses to the heart and left coronary artery, compared with a field-in-field technique. J Radiat Res 2015; 56: 927–37. doi: 10.1093/jrr/rrv052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakumi A, Shiraishi K, Onoe T, Yamamoto K, Haga A, Yoda K, et al. Single-arc volumetric modulated Arc therapy planning for left breast cancer and regional nodes. J Radiat Res 2012; 53: 151–3. doi: 10.1269/jrr.11159 [DOI] [PubMed] [Google Scholar]
- 22. Lancellotta V, Iacco M, Perrucci E, Falcinelli L, Zucchetti C, de Bari B, et al. Comparing four radiotherapy techniques for treating the chest wall plus levels III-IV draining nodes after breast reconstruction. Br J Radiol 2018; 91: 20160874. doi: 10.1259/bjr.20160874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dumane VA, Bakst R, Green S. Dose to organs in the supraclavicular region when covering the internal mammary nodes (IMNs) in breast cancer patients: a comparison of volumetric modulated Arc therapy (VMAT) versus 3D and VMAT. PLoS One 2018; 13: e0205770. doi: 10.1371/journal.pone.0205770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caudell JJ, De Los Santos JF, Keene KS, Fiveash JB, Wang W, Carlisle JD, et al. A dosimetric comparison of electronic compensation, conventional intensity modulated radiotherapy, and tomotherapy in patients with early-stage carcinoma of the left breast. Int J Radiat Oncol Biol Phys 2007; 68: 1505–11. doi: 10.1016/j.ijrobp.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 25. Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl): S77–85. doi: 10.1016/j.ijrobp.2009.04.093 [DOI] [PubMed] [Google Scholar]
- 26. Jagsi R, Griffith KA, Moran JM, Ficaro E, Marsh R, Dess RT, et al. A randomized comparison of radiation therapy techniques in the management of node-positive breast cancer: primary outcomes analysis. Int J Radiat Oncol Biol Phys 2018; 101: 1149–58. doi: 10.1016/j.ijrobp.2018.04.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor CW, Kirby AM. Cardiac side-effects from breast cancer radiotherapy. Clin Oncol 2015; 27: 621–9. doi: 10.1016/j.clon.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 28. Boda-Heggemann J, Knopf A-C, Simeonova-Chergou A, Wertz H, Stieler F, Jahnke A, et al. Deep inspiration breath Hold—Based radiation therapy: a clinical review. Int J Radiat Oncol Biol Phys 2016; 94: 478–92. doi: 10.1016/j.ijrobp.2015.11.049 [DOI] [PubMed] [Google Scholar]
- 29. Pierce LJ, Feng M, Griffith KA, Jagsi R, Boike T, Dryden D, et al. Recent time trends and predictors of heart dose from breast radiation therapy in a large quality consortium of radiation oncology practices. Int J Radiat Oncol Biol Phys 2017; 99: 1154–61. doi: 10.1016/j.ijrobp.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 30. Taylor CW, Brønnum D, Darby SC, Gagliardi G, Hall P, Jensen M-B, et al. Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977-2001. Radiother Oncol 2011; 100: 176–83. doi: 10.1016/j.radonc.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor CW, Povall JM, McGale P, Nisbet A, Dodwell D, Smith JT, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008; 72: 501–7. doi: 10.1016/j.ijrobp.2007.12.058 [DOI] [PubMed] [Google Scholar]
- 32. Berg M, Lorenzen EL, Jensen I, Thomsen MS, Lutz CM, Refsgaard L, et al. The potential benefits from respiratory gating for breast cancer patients regarding target coverage and dose to organs at risk when applying strict dose limits to the heart: results from the DBCG HYPO trial. Acta Oncol 2018; 57: 113–9. doi: 10.1080/0284186X.2017.1406139 [DOI] [PubMed] [Google Scholar]
- 33. Taylor C, McGale P, Brønnum D, Correa C, Cutter D, Duane FK, et al. Cardiac structure injury after radiotherapy for breast cancer: cross-sectional study with individual patient data. JCO 2018; 36: 2288–96. doi: 10.1200/JCO.2017.77.6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Bogaard VAB, Ta BDP, van der Schaaf A, Bouma AB, Middag AMH, Bantema-Joppe EJ, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol 2017; 35: 1171–8. doi: 10.1200/JCO.2016.69.8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kahán Z, Csenki M, Varga Z, Szil E, Cserháti A, Balogh A, et al. The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys 2007; 68: 673–81. doi: 10.1016/j.ijrobp.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 36. Gokula K, Earnest A, Wong LC. Meta-Analysis of incidence of early lung toxicity in 3-dimensional conformal irradiation of breast carcinomas. Radiat Oncol 2013; 8: 268. doi: 10.1186/1748-717X-8-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Laan HP, Korevaar EW, Dolsma WV, Maduro JH, Langendijk JA. Minimising contralateral breast dose in post-mastectomy intensity-modulated radiotherapy by incorporating conformal electron irradiation. Radiother Oncol 2010; 94: 235–40. doi: 10.1016/j.radonc.2009.12.015 [DOI] [PubMed] [Google Scholar]
- 38. Haciislamoglu E, Colak F, Canyilmaz E, Dirican B, Gurdalli S, Yilmaz AH, et al. Dosimetric comparison of left-sided whole-breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and volumetric Arc therapy. Phys Med 2015; 31: 360–7. doi: 10.1016/j.ejmp.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 39. Coon AB, Dickler A, Kirk MC, Liao Y, Shah AP, Strauss JB, et al. Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys 2010; 78: 104–10. doi: 10.1016/j.ijrobp.2009.07.1705 [DOI] [PubMed] [Google Scholar]
- 40. Lancellotta V, Chierchini S, Perrucci E, Saldi S, Falcinelli L, Iacco M, et al. Skin toxicity after chest wall/breast plus level III-IV lymph nodes treatment with helical tomotherapy. Cancer Invest 2018; 36(9-10): 504–11. doi: 10.1080/07357907.2018.1545854 [DOI] [PubMed] [Google Scholar]
- 41. Lancellotta V, Iacco M, Perrucci E, Zucchetti C, Dipilato AC, Falcinelli L, et al. Comparison of helical tomotherapy and direct tomotherapy in bilateral whole breast irradiation in a case of bilateral synchronous grade 1 and stage 1 breast cancer. Am J Case Rep 2017; 18: 1020–3. doi: 10.12659/AJCR.905245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–106. doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 43. Boice JD, Harvey EB, Blettner M, Stovall M, Flannery JT. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med 1992; 326: 781–5. doi: 10.1056/NEJM199203193261201 [DOI] [PubMed] [Google Scholar]
- 44. Korreman SS, Pedersen AN, Aarup LR, Nøttrup TJ, Specht L, Nyström H. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2006; 65: 1375–80. doi: 10.1016/j.ijrobp.2006.03.046 [DOI] [PubMed] [Google Scholar]
- 45. Kainz K, White J, Chen G-P, Hermand J, England M, Li XA. Simultaneous irradiation of the breast and regional lymph nodes in prone position using helical tomotherapy. Br J Radiol 2012; 85: e899–905. doi: 10.1259/bjr/18685881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maskarinec G, Lyu LC, Meng L, Theriault A, Ursin G. Determinants of mammographic densities among women of Asian, native Hawaiian, and Caucasian ancestry. Ethn Dis 2001; 11: 44–50. [PubMed] [Google Scholar]
- 47. Mahe M-A, Classe J-M, Dravet F, Cussac A, Cuilliere J-C. Preliminary results for prone-position breast irradiation. Int J Radiat Oncol Biol Phys 2002; 52: 156–60. doi: 10.1016/S0360-3016(01)01741-2 [DOI] [PubMed] [Google Scholar]
- 48. Huppert N, Jozsef G, Dewyngaert K, Formenti SC. The role of a prone setup in breast radiation therapy. Front Oncol 2011; 1: 31. doi: 10.3389/fonc.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]