Abstract
Objective:
To introduce capped biparametric (bp) MRI slots for follow-up imaging of prostate cancer patients enrolled in active surveillance (AS) and evaluate the effect on weekly variation in the number of AS cases and total MRI workload.
Methods:
Three 20 min bpMRI AS slots on two separate days were introduced at Addenbrooke’s Hospital, Cambridge. The weekly numbers of total prostate MRIs and AS cases recorded 15 months before and after the change (Groups 1 and 2, respectively). An intergroup variation in the weekly scan numbers was assessed using the coefficient of variance (CV) and mean absolute deviation; the Mann–Whitney U test was used for an intergroup comparison of the latter.
Results:
In AS patients, a shift from considerable to moderate variation in weekly scan numbers was observed between the two groups (CV, 51.7 and 26.8%, respectively); mean absolute deviation of AS scans also demonstrated a significant decrease in Group 2 (1.28 vs 2.58 in Group 1; p < 0.001). No significant changes in the variation in total prostate MRIs were observed, despite a 10% increased workload in Group 2.
Conclusion:
A significant reduction in weekly variation of AS cases was demonstrated following the introduction of capped bpMRI slots, which can be used for more accurate long-term planning of MRI workload.
Advances in knowledge:
The paper illustrates the potential of introducing capped AS MRI slots using a bp protocol to reduce weekly variation in demand and allow for optimising workflow, which will be increasingly important as the demands on radiology departments increase worldwide.
Introduction
Prostate cancer (PCa) is the commonest malignancy in males in the UK, accounting for 26% of new cancer diagnoses, and has the second highest mortality rate at 14%.1 Following the results of several recent prospective trials including PROMIS and PRECISION, the 2019 European Association of Urology guidelines now recommend pre-biopsy multiparametric MRI (mpMRI) as a preferred diagnostic test in biopsy naïve patients and patients with prior negative biopsy.2–4 These recommendations are mirrored by a joint panel of the American Urological Association and the Society of Abdominal Radiology and the 2019 UK NICE guidelines, which also recommend mpMRI as the first-line investigation for patients with suspected clinically localised PCa.5,6
The global move to pre-biopsy mpMRI is associated with a number of scheduling challenges that will be relevant to all healthcare systems. In the UK, the current pressure on radiology departments is likely to further increase with the introduction of the new Faster Diagnosis Standard in April 2020, wherein 95% of patients should receive a definite cancer diagnosis within 28 days of referral. According to a recent review of cancer diagnostic process in the UK, PCa has a considerably longer median diagnostic interval at 55.5 days, compared to all cancers at 40.0, which highlights the importance of optimising workflow management to meet the increasing demand whilst striving to maintain quality.7 Although the proposed target is unlikely to have a significant effect on PCa clinical outcomes, the new standard is aimed at reducing patient anxiety and minimising unwarranted regional variation in time to diagnosis.8,9
Upfront mpMRI prior to biopsy has been shown to reduce time to diagnosis by 23% when compared to transrectal ultrasound-guided biopsy pathway.10 In addition, introduction of reserved MRI slots can further reduce time to diagnosis by 18%.11 However, limited flexibility in MRI capacity may restrict the ability of some centres to accommodate weekly or even day-to-day variation in demand, particularly when the frequency of new referrals from clinic cannot be easily predicted and reports require a quick turnaround time to avoid breaching the 28 day standard.
Simultaneously, the rising demand for MRI risks further increasing the number of patients enrolled on active surveillance (AS) programmes, the success of which relies heavily on the use of MRI and targeted biopsies as evidenced by the recent 2 year follow-up on the ASIST trial.12–14 As routine follow-up imaging for AS is normally scheduled within 12–18 months post-enrollment with no pathway-dependent reporting times, it can be planned in advance and controlling appointment times can therefore help limit variance in the weekly imaging workload. Moreover, introducing shorter protocols, such as single-plane biparametric MRI (bpMRI) within capped imaging slots, can further reduce the pressure on MRI capacity, thereby allowing more time for cancer pathway referred pre-biopsy scans that require the full multiparametric protocol.
In this study, we introduced six capped 20 min AS bpMRI slots performed on two separate days. The aim of this study was, therefore, to evaluate the effect of introducing capped AS bpMRI slots on weekly variation in AS scans and the overall MRI workload, evaluating 15 month time periods before and after this change.
Methods
This study was performed as a service evaluation of the PCa diagnostic pathway, with the need for informed consent for data analysis waived by the Local Ethics Committee (reference number: anonymised) for this retrospective analysis of the numbers of prostate MRI scans performed weekly at our institution.
Active surveillance cohort
Enrollment criteria for AS included treatment-naïve males aged 50–80 years suitable for radical therapy with histologically proven prostate adenocarcinoma, clinical stage T1–T2, prostate-specific antigen (PSA) ≤20 ng ml−1, histological Grade Group ≤ 2 and <50% overall tumour core involvement. A baseline mpMRI was performed at AS entry with repeat MRI performed 12 months post-enrolment and at variable time points afterwards, depending on clinical risk of progression. Weekly numbers of total prostate MRIs and AS cases were recorded during a 30-month period (9/05/2016 to 29/10/2018), 15 months before and after the introduction of reserved AS slots. The introduction of three 20-min AS slots performed on two separate days was based on our analysis of the preceding 12 months’ MRI workload that revealed an estimated number of AS cases as 6 per week/300 per year; Supplementary Figure 1.
MRI parameters
Patients underwent prostate MRI on a 1.5 T MR450 scanner or a 3 T HDx Discovery MR750 HDx (GE Healthcare, Waukesha) with a 16–32 channel phased array coil. Unless contraindicated, intravenous injection of hyoscine butylbromide (Buscopan, 20 mg ml−1, Boehringer, Germany) was administered prior to imaging to reduce peristaltic movement. Multiparametric MRI protocol included Axial T1, multiplanar T2:field of view (FOV) 18 × 18 cm; slice thickness 3–3.5 mm; gap 0–0.5 mm. Diffusion-weighted imaging (DWI) was performed using a spin-echo echoplanar imaging pulse sequence (slice thickness 3–4 mm; gap 0 mm) with b-values: b-150, b-750, and b-1,000; with additional small FOV DWI using b-1,400s/mm2 at 1.5 T and b-2,000s/mm2 at 3 T); apparent diffusion coefficient (ADC) maps were automatically calculated. The protocol additionally included dynamic contrast enhancement (DCE) imaging; Table 1. Abbreviated active surveillance protocol included Axial T1 and T2 FSE pelvis and DWI with multiplanar T2, DCE and small FOV DWI omitted; Table 2. Ranges stated for slice thickness and gap in Table 1 reflect the differences in imaging protocols used on 3 and 1.5 T scanners, respectively, and are in line with PI-RADS v. 2.1 technical requirements. The overall scan time for mpMRI was 25 min, 38 s with a 40 min slot allotted, and for the bpMRI AS protocol 11 min, 32 s, with 20 min slots.
Table 1.
Summary table of sequence parameters as part of a multiparametric prostate MRI protocol
| Parameter | Localiser | Axial T1 FSE | Axial T2 FSE | Sagittal T2 FSE | Axial DWI | Axial DWI focus | DCE LAVA |
|---|---|---|---|---|---|---|---|
| TE/TR, ms | 20/200 | 30/789 | 102/3743 | 102/3743 | 85/3775 | 60/4000 | min full/4.3 |
| FOV, cm | 30 | 32 | 18 | 22 | 28 | 24 | 24 |
| Matrix | 256 | 512 | 384 | 288 | 128 | 356 | 192 |
| Slice thickness, mm | 3–4 | 6 | 3–3.5 | 2 | 3–4 | 3 | 3–4 |
| Gap, mm | 0 | 2 | 0–0.5 | 0 | 0 | 0 | 0 |
| Phase | 128 | 320 | 224 | 224 | 128 | 80 | 192 |
| b-values | - | - | - | - | 100, 750, 1000 1400 (at 3T) |
1.5T: 100, 1400 3T: 100, 2000 |
- |
| Synthetic b-values | - | - | - | - | 2000, 2500 | 2500 | - |
| Scan time, min | 00:35 | 02:32 | 05:22 | 03:13 | 02:42 | 04:52 | 06:22 |
DCE, dynamic contrast enhancement; DWI, diffusion-weighted imaging; FOV, field of view; FSE, fast spin echo; LAVA, liver acquisition with volume acceleration; TE, echo time; TR, repetition time.
Table 2.
Summary table of sequence parameters as part of a biparametric prostate MRI protocol
| Parameter | Localizser | Axial T1 FSE | Axial T2 FSE | Axial DWI |
|---|---|---|---|---|
| TE/TR, ms | 20/200 | 30/789 | 102/3743 | 85/3775 |
| FOV, cm | 30 | 32 | 18 | 28 |
| Matrix | 256 | 512 | 384 | 128 |
| Slice thickness, mm | 3–4 | 6 | 3 | 3 |
| Gap, mm | 0 | 2 | 0 | 0 |
| Phase | 128 | 320 | 224 | 128 |
| b-values | - | - | - | 100, 750, 1400 |
| Synthetic b-values | - | - | - | 2000, 2500 |
| Scan time, min | 00:56 | 02:32 | 05:22 | 02:42 |
DCE, dynamic contrast enhancement; DWI, diffusion-weighted imaging; FOV, field of view; FSE, fast spin echo; LAVA, liver acquisition with volume acceleration; TE, echo time; TR, repetition time.
Statistics
The Shapiro–Wilk test was performed to assess the distribution of AS cases and MRI numbers with their intergroup comparison performed using the Mann–Whitney U test. An intergroup variation between the weekly numbers of AS cases and MRIs was evaluated using the coefficient of variation (CV) and mean absolute deviation (MAD) with the Mann–Whitney U test performed for intergroup comparison of the latter. For CV, a value of <0.25 was classified as low variation; 0.25 to 0.75 moderate variation and >0.75 high variation.
Results
Weekly numbers of total MRIs and AS slots were divided into two groups of 15 months duration, Group 1, before the introduction of capped slots and Group 2, after the introduced change. A total of 1667 MRIs for all indications were performed during the study period in Group 1 and 1848 MRIs in Group 2, representing a 10.9% increase in overall prostate MRI demand over the period. Of note, there was a 9.6-fold (from 34 to 327 per year) increase in the number of annual AS scans between 2010 and 2018; Supplementary Figure 1.
Impact of capped slots on weekly AS variance
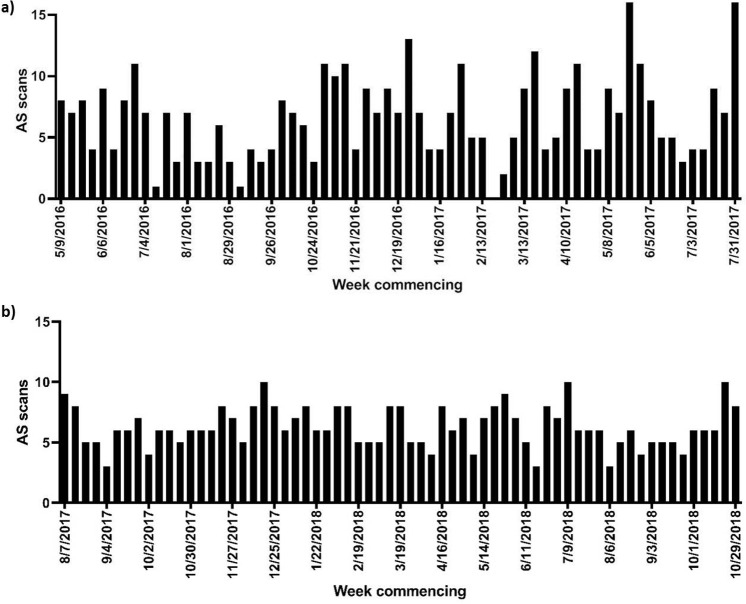
In AS patients, median age and PSA were 65 years [interquartile range (IQR) 60–65 years] and 6.0 ng ml−1 (IQR 4.2–8.4 ng ml−1). The number of scans performed in Groups 1 and 2 were 428 and 408, respectively. The median weekly numbers of AS showed no intergroup difference, Group 1: median 7 (IQR 4–9), Group 2: median 6 (IQR 5–8); p = 0.991. In addition to the marked intergroup difference in standard deviation (3.40 vs 1.68), a considerable decrease in variation was observed between the weekly AS case numbers in Group 1 (CV = 51.7%) vs Group 2 (CV = 26.8%); Figure 1, Supplementary Table 1. This was supported by an intergroup analysis of the mean absolute deviation of AS cases, which demonstrated a significant decrease in Group 2 (1.28) compared to Group 1 (2.58); p < 0.0001).
Figure 1.
Monthly variance in AS cases performed at our institution 15 months pre- (a) andpost-introduction (b) of capped AS slots. AS = active surveillance.
Impact of capped slots on overall MRI variance
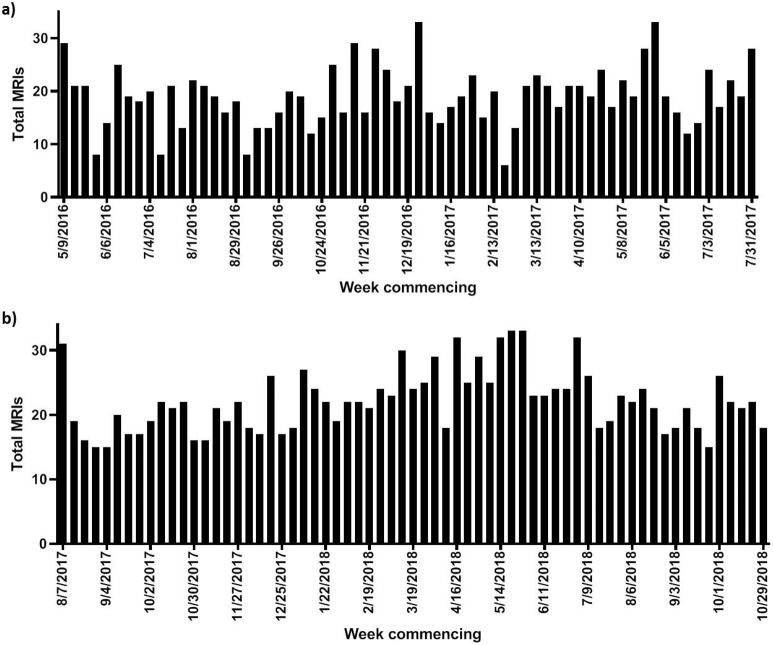
The total number of MRIs performed for non-AS indications was 1239 in Group 1 and 1440 in Group 2. The median number of weekly MRIs was significantly higher in Group 2: median 22 (IQR 18–25) compared to Group 1: median 19 (IQR 16–22), p = 0.001; however, the maximum number of scans per week remained constant at 33. Variation in the total numbers of MRIs decreased from moderate (CV = 29.6%) to low (CV = 21.7%) in Groups 1 and 2, respectively; Figure 2, Supplementary Table 1. However, no intergroup difference was noted between the mean absolute deviation of the total numbers of MRIs (Group 1, 3.15 vs Group 2, 3.06; p = 0.725).
Figure 2.
Monthly variance in overall MRI workload recorded at our institution 15 months pre- (a) and post-introduction (b) of capped AS slots. MRI = magnetic resonance imaging, AS = active surveillance.
Discussion
The present study investigates the effect of introducing capped 20 min active surveillance abbreviated bpMRI slots on variation in weekly AS scans and on overall MRI workload. The introduction of reserved AS slots significantly reduced variation in the weekly numbers of AS scans and kept the variation in total weekly number of prostate MRI scans unchanged, despite the marked increase in demand observed during the study period. Given the likely increased burden on imaging services following the introduction of pre-biopsy MRI and the upcoming Faster Diagnosis Standard in the UK, the introduction of capped AS imaging slots using shorter protocols may allow for more adequate planning of MRI workload. Times of increased and reduced demand could even be mapped based on historic local data to allow for seasonal variations in demand and allow for “dynamic” adjustments to the numbers of dedicated AS slots to further limit weekly fluctuations.
Active surveillance is now the standard of care for males with low/intermediate clinical risk, with studies showing that AS is a safe and effective management option for such patients.5,15,16 Disease progression rates for patients on AS are low (15.9% at median follow-up 39 months) due to the appropriate risk stratification of males at enrollment based on mpMRI and targeted biopsy.17 With males remaining on AS for longer periods, it is highly likely that AS numbers will increase over time, adding further pressure on imaging services. However, this may be partially mitigated by upfront mpMRI and biopsy avoidance in MRI negative cases, which has been shown to reduce the detection of clinically insignificant (Gleason score 3 + 3) disease.4 Frequency of follow-up MRI in AS can also be tailored depending on clinical risk, based on PSA, PSA density, MRI lesion presence/absence and Gleason grade, and MR imaging safely being performed at 3-yearly intervals in those categorised as lowest risk.18 Another promising approach towards reducing imaging workload from a radiologist’s perspective is the development of a dedicated software allowing automated lesion size comparison between sequential scans on AS according to the Prostate Cancer Radiological Estimation of Change in Sequential Evaluation recommendations.19
The use of an abbreviated bpMRI protocol allowed us to achieve a 55% reduction in scan time comparing to our standard multiplanar multiparametric protocol. This, in turn, made it possible for us to utilise two separate 1 h time slots (3 scans of 20 min each) on separate days. The value of bpMRI for the detection of PCa in biopsy-naïve males has been demonstrated in several prospective studies, with potential benefits including the avoidance of gadolinium retention, reduced costs and shortened scan times.20–22 Furthermore, a recent head-to-head comparison of mpMRI, bpMRI and “fast” bpMRI (images obtained in the axial plane only) demonstrated an identical diagnostic performance to mpMRI and bpMRI with only a slight increase in the number of indeterminate PI-RADS category three calls; this is supported by a recent meta-analysis comparing diagnostic efficacy of bpMRI and mpMRI..23,24 Finally, although the recently updated PI-RADS v. 2.1 guidelines still advocate DCE for the majority of MRI indications, the use of a biparametric approach is allowed in stable AS patients fully characterised at baseline and in whom no signs indicating possible clinical progression are present, such as raised PSA, PSA-density, PSA doubling time or symptoms suggestive of advanced disease.25 However, although this represents consensus expert opinion, the current lack of comparative trials of these protocols in the AS setting means caution should be applied when attempting to make them a standard of care.
One of the limitations to this study was the use of different slice thickness and gap parameters as part of multi- and biparametric MRI protocols, which was done to ensure that optimal imaging quality is achieved on both 3 and 1.5 T scanning systems.26 Incorporating T 1 weighted imaging in the abbreviated protocol was justified by its ability to detect post-biopsy change and spontaneous prostate haemorrhage that may occur in patients with benign prostatic hyperplasia, as well as its usefulness in staging the bony pelvis and assessing pelvic lymph nodes. Only axial T 2 weighted images were obtained as part of the biparametric protocol, which was in line with the supportive results in current literature.23,27,28
It is of note that following the introduction of dedicated slots there remained variation from 4 to 10 scans per week, reflecting missed appointments and urgent imaging requests due to suspicion of AS progression based on the aforementioned clinical grounds. Although the results showed significantly reduced variance in weekly AS scans, there was no difference in variance for the total MRI studies performed, due to the highly variable weekly requests for pre-biopsy scans. However, despite the 10% increase in total scans in the 15 months post-change, there was unchanged variation in the total number of weekly scans and the maximum number of scans per week remained at 33, which can be likely be explained by the introduction of capped AS slots.
In conclusion, a significant reduction in weekly AS variance was demonstrated following the introduction of capped bpMRI slots for routine follow-up imaging of AS patients, and helped keep variation in the total number of MRIs per week unchanged despite a 10% increase in overall imaging workload.
Footnotes
Funding: The authors acknowledge support from Cancer Research UK, National Institute of Health Research Cambridge Biomedical Research Centre, Cancer Research UK and the Engineering and Physical Sciences Research Council Imaging Centre in Cambridge and Manchester and the Cambridge Experimental Cancer Medicine Centre.
Contributor Information
Nikita Sushentsev, Email: ns784@medschl.cam.ac.uk.
Iztok Caglic, Email: iztokcaglic@gmail.com.
Evis Sala, Email: evis.sala@gmail.com.
Nadeem Shaida, Email: nadeemshaida@doctors.org.uk.
Rhys A Slough, Email: rhys.slough@gmail.com.
Bruno Carmo, Email: bruno.carmo@gmail.com.
Vasily Kozlov, Email: kvv.doc@gmail.com.
Vincent J. Gnanapragasam, Email: vjg29@cam.ac.uk.
Tristan Barrett, Email: tb507@medschl.cam.ac.uk.
REFERENCES
- 1. UK CR Prostate cancer statistics 2015. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer [2015-05-14].
- 2. Urology EAo EAU Guidelines: Prostate Cancer | Uroweb 2019.
- 3. Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet 2017; 389: 815–22. doi: 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 4. Kasivisvanathan V, Emberton M, Moore CM. MRI-Targeted biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 379: 1767–77. doi: 10.1056/NEJMoa1801993 [DOI] [PubMed] [Google Scholar]
- 5. NICE Recommendations | Prostate cancer: diagnosis and management | Guidance | NICE: NICE. 2019. Available from: https://www.nice.org.uk/guidance/ng131/chapter/Recommendations#assessment-and-diagnosis.
- 6. American Urological Association SoAR Standard Operating Procedure for Multiparametric Magnetic Resonance Imaging in the Diagnosis, Staging and Management of Prostate Cancer - American Urological Association. 2019. Available from: https://www.auanet.org/guidelines/mri-of-the-prostate-sop.
- 7. Swann R, McPhail S, Witt J, Shand B, Abel GA, Hiom S, et al. Diagnosing cancer in primary care: results from the National cancer diagnosis audit. Br J Gen Pract 2018; 68: e63–72. doi: 10.3399/bjgp17X694169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. England N NHS England » Diagnosing cancer earlier and faster. 2019. Available from: https://www.england.nhs.uk/cancer/early-diagnosis/#faster.
- 9. Redaniel MT, Martin RM, Gillatt D, Wade J, Jeffreys M. Time from diagnosis to surgery and prostate cancer survival: a retrospective cohort study. BMC Cancer 2013; 13: 559. doi: 10.1186/1471-2407-13-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panebianco V, Valerio MC, Giuliani A, Pecoraro M, Ceravolo I, Barchetti G, et al. Clinical utility of multiparametric magnetic resonance imaging as the first-line tool for men with high clinical suspicion of prostate cancer. Eur Urol Oncol 2018; 1: 208–14. doi: 10.1016/j.euo.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 11. Barrett T, Slough R, Sushentsev N, Shaida N, Koo BC, Caglic I, et al. Three-Year experience of a dedicated prostate mpMRI pre-biopsy programme and effect on timed cancer diagnostic pathways. Clin Radiol 2019; 74: 894.e1–894.e9. doi: 10.1016/j.crad.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 12. Oberlin DT, Casalino DD, Miller FH, Meeks JJ. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom Radiol 2017; 42: 1255–8. doi: 10.1007/s00261-016-0975-5 [DOI] [PubMed] [Google Scholar]
- 13. Klotz L, Loblaw A, Sugar L, Moussa M, Berman DM, Van der Kwast T, et al. Active surveillance magnetic resonance imaging study (ASIST): results of a randomized multicenter prospective trial. Eur Urol 2019; 75: 300–9. doi: 10.1016/j.eururo.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 14. Klotz L, Pond G, Loblaw A, Sugar L, Moussa M, Berman D, et al. Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2-year Postbiopsy follow-up. Eur Urol 2019. doi: 10.1016/j.eururo.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 15. Large MC, Eggener SE. Active surveillance for low-risk localized prostate cancer. Oncology 2009; 23: 974–9. [PubMed] [Google Scholar]
- 16. Dall'Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012; 62: 976–83. doi: 10.1016/j.eururo.2012.05.072 [DOI] [PubMed] [Google Scholar]
- 17. Thurtle D, Barrett T, Thankappan-Nair V, Koo B, Warren A, Kastner C, et al. Progression and treatment rates using an active surveillance protocol incorporating image-guided baseline biopsies and multiparametric magnetic resonance imaging monitoring for men with favourable-risk prostate cancer. BJU Int 2018; 122: 59–65. doi: 10.1111/bju.14166 [DOI] [PubMed] [Google Scholar]
- 18. Gnanapragasam VJ, Barrett T, Massie C, Pacey S, Warren A. Using prognosis to guide early detection and treatment selection in non-metastatic prostate cancer. BJU Int 2019; 123: 562–3. doi: 10.1111/bju.14637 [DOI] [PubMed] [Google Scholar]
- 19. Giganti F, Allen C, Piper JW, Mirando D, Stabile A, Punwani S, et al. Sequential prostate MRI reporting in men on active surveillance: initial experience of a dedicated precise software program. Magn Reson Imaging 2019; 57: 34–9. doi: 10.1016/j.mri.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 20. Boesen L, Nørgaard N, Løgager V, Balslev I, Bisbjerg R, Thestrup K-C, et al. Assessment of the diagnostic accuracy of Biparametric magnetic resonance imaging for prostate cancer in Biopsy-Naive men: the Biparametric MRI for detection of prostate cancer (BIDOC) study. JAMA Netw Open 2018; 1: e180219. doi: 10.1001/jamanetworkopen.2018.0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jambor I, Boström PJ, Taimen P, Syvänen K, Kähkönen E, Kallajoki M, et al. Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD trial). J Magn Reson Imaging 2017; 46: 1089–95. doi: 10.1002/jmri.25641 [DOI] [PubMed] [Google Scholar]
- 22. Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019; 76: 340–51. doi: 10.1016/j.eururo.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 23. van der Leest M, Israël B, Cornel EB, Zámecnik P, Schoots IG, van der Lelij H, et al. High diagnostic performance of short magnetic resonance imaging protocols for prostate cancer detection in Biopsy-naïve men: the next step in magnetic resonance imaging accessibility. Eur Urol 2019; 76: 574–81. doi: 10.1016/j.eururo.2019.05.029 [DOI] [PubMed] [Google Scholar]
- 24. Kang Z, Min X, Weinreb J, Li Q, Feng Z, Wang L. Abbreviated Biparametric versus standard multiparametric MRI for diagnosis of prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol 2019; 212: 357–65. doi: 10.2214/AJR.18.20103 [DOI] [PubMed] [Google Scholar]
- 25. Barrett T, Rajesh A, Rosenkrantz AB, Choyke PL, Turkbey B. PI-RADS version 2.1: one small step for prostate MRI. Clin Radiol 2019; 74: 841–52. doi: 10.1016/j.crad.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 26. Burn PR, Freeman SJ, Andreou A, Burns-Cox N, Persad R, Barrett T. A multicentre assessment of prostate MRI quality and compliance with UK and international standards. Clin Radiol 2019; 74: 894.e19–894.e25. doi: 10.1016/j.crad.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 27. Lee DH, Nam JK, Lee SS, Han JY, Lee JW, Chung MK, et al. Comparison of multiparametric and Biparametric MRI in first round cognitive targeted prostate biopsy in patients with PSA levels under 10 ng/mL. Yonsei Med J 2017; 58: 994–9. doi: 10.3349/ymj.2017.58.5.994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Junker D, Steinkohl F, Fritz V, Bektic J, Tokas T, Aigner F, et al. Comparison of multiparametric and biparametric MRI of the prostate: are gadolinium-based contrast agents needed for routine examinations? World J Urol 2019; 37: 691–9. doi: 10.1007/s00345-018-2428-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.