Abstract
Objective:
To determine the utility of low-dose gelatin sponge particles and 5% ethanolamine oleate iopamidol (EOI) mixture in retrograde transvenous obliteration (GERTO) for gastric varices (GV).
Methods:
57 consecutive patients who underwent balloon-occluded retrograde transvenous obliteration (B-RTO) for GV were divided into three groups with Hirota’s grade by balloon-occluded retrograde transvenous venography. Hirota’s Grade 1 patients were assigned to G1 group and underwent treatment with 5% EOI. Grade ≥ 2 patients prior to August 2015 were G ≥ 2 group treated with 5% EOI, and those treated thereafter were GERTO group. The amount of EOI used per unit GV volume (EOI/GV ratio), the times to embolization and recurrence rate of GV were evaluated.
Results:
The EOI/GV ratio was 0.66 ± 0.19 in G1, 1.5 ± 0.8 in G ≥ 2, and 0.58 ± 0.23 in GERTO (G ≥ 2 vs GERTO, p < 0.0001). The times to embolization were 26.5 ± 10.5 min for G1, 39.2 ± 26.8 for G ≥ 2, and 21.4 ± 9.4 for GERTO (G ≥ 2 vs GERTO, p = 0.005). The recurrence rate was not significantly different in any of the groups.
Conclusion:
GERTO was performed in lower amount of sclerosants and in less time compared to conventional B-RTO in Hirota’s grade ≥2.
Advances in knowledge:
Feasibility of low-dose gelatin sponge particles and 5% EOI mixture as sclerosants for GV.
Introduction
Embolic agents used for gastric varix sclerotherapy in balloon-occluded retrograde transvenous obliteration (B-RTO) include ethanolamine oleate (EO), polidocanol foam, sodium tetradecyl sulfate (STS), ethanol, N-butyl-2-cyanoacrylate (NBCA), and, more recently, contrast agents containing gelatin sponge (GS) particles to achieve plug-assisted retrograde transvenous obliteration (PARTO).1–4
EO was first applied as a sclerosant against esophageal varices by Trolle and Patterson in 1946.5–7 B-RTO with EO has been in use since the 1996 by Kanagawa.1 EO induces thrombus formation by directly damaging the venous endothelium. The vessel is eventually obstructed due to the accumulation of platelets and fibrin on damaged endothelium and mural thrombi.7 EO functions well as sclerosants but has the maximum tolerable amount. It is therefore unsuited for large-volume varices with substantial collateral draining veins (i.e. Hirota’s classification of high-grade), since the agent could escape before embolization is achieved in an amount exceeding the tolerable level.8 Draining veins are therefore often embolized with a coil before treatment to downgrade the lesion. Selective catheter insertion into the vessels to be embolized is difficult in some patients, and larger amounts of sclerosant must be used when many narrow draining veins are present. Both these situations increase the time required to complete the procedure.
First introduced in 2013, PARTO is a new modality that uses GS particles as a embolic agent.9 It is technically easier to perform and can decrease procedure time because it does not require a balloon catheter and does not perform selective embolization of collateral efferent veins.10 Recurrence of varices, however, is controversial. The author of PARTO is 100% obliteration of gastric varices (GV) at 6 months; however, Gwon et al mentions that recurrence is higher compared with EO-based treatment, standing at 32.8% after 1 year.2,9 The purpose of this study is to examine the utility of 5% ethanolamine oleate iopamidol (EOI) with low-dose gelatin sponge particles mixture as sclerosants in B-RTO.
Methods and materials
Patients
The ethics committee at osaka city university hospital deemed this retrospective study to be appropriate for publication. The study included 57 consecutive patients who underwent B-RTO for GV between November 2013 and March 2017. Informed consent was obtained from all patients before the procedure. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Treatment indications and procedure
Treatment criteria for GV were as follows: isolated varices which are moderately enlarged, beady form, or markedly enlarged, nodular, tumor-shaped form, and/or red spot as defined by the Japan Gastroenterological Endoscopy Society.11,12 Bleeding GV which was not achieved primary hemostasis cases were excluded. The indications for procedure were a major portocaval shunt that, on the basis of pre-operative CT, could anatomically be reached transvenously using a catheter, e.g. by gastrorenal (GR) shunt, gastrocaval (GC) shunt continuing the inferior phrenic vein (IPV), or pericardiacophrenicl vein (PCV).
Balloon occluded retrograde transvenous venography was used to classify the patients according to Hirota’s classification (Grade 1: GV are well opacified without collateral veins, Grade 2: GV remain opacified despite small and few collateral veins, Grade 3: GV are opacified partially with medium to large collaterals, Grade 4: GV are not opacified due to many large collaterals, and Grade 5: left adrenal vein cannot be occluded with a balloon catheter).13 Patients assessed as Grade ≥ 2 underwent coil embolization of the collateral draining veins where possible to downgrade the grade followed by balloon occluded retrograde transvenous venography again.14 Patients successfully downgraded to Grade 1 were assigned to a G1 group and underwent treatment with 5% EOI mixed with 10% ethanolamine oleate (Oldamin; Takeda Pharmaceutical, Osaka, Japan) and the same volume of nonionic contrast medium (iopamidol 300 mg I/mL, Iopamiron 300; Bayer Schering Pharma, Osaka, Japan). Grade ≥ 2 patients treated from November 2013 to July 2015 were assigned to a G ≥ 2 group and treated with 5% EOI, and those treated from August 2015 to March 2017 were assigned to a GERTO group and underwent embolization with 5% EOI containing GS particles. If stagnation of sclerosants is insufficient, stepwise injection was performed at intervals of 5 min.15
Particles measuring 1–5 mm in diameter were used. After embolic agent injection in varices under fluoroscopy, intravariceal sclerosants were confirmed by abdominal plain CT. To prevent renal dysfunction, an intravenous drip infusion of 4000 U of haptoglobin was given before injection of 5% EOI.16
Procedure was performed by one expert radiologist and one senior resident. Expert radiologists were two independent interventional radiologists who had experienced approximately 30 and 20 cases per year of treatment with B-RTO for recent 5 years, respectively. They were randomly assigned by case. Senior resident who did not acquire qualification of specialist had experienced less than 10 cases per year for recent 2 years.
CT scan protocol and measurement of GV volume
Pre-operative abdominal CT scans were obtained using a 64-row multidetector CT scanner (Light Speed VCT; GE Healthcare, Milwaukee, WI). The iopamidol (Iopamiron 370 mgI ml−1; Bayer, Osaka, Japan) were administered via the cubital vein at a flow rate of 5 ml s−1 with a mechanical injector. Using the bolus-tracking method, arterial phase scanning was initiated 6 s after enhancement of the celiac artery reached 100 Hounsfield units (HUs). Portal phase scanning was initiated 23 s after the arterial phase. The agent was injected at 600 mgI/kg as iodine per kilogram for 20 s. GV volume was measured on portal phase axial images of 0.625 mm thickness and 0.625 mm interval with an add-vessel function of a volume analyzer (SYNAPSE VINCENT; Fujifilm, Tokyo, Japan). This function is a method for selectively extracting only the blood vessels in CT images, as the boundaries between blood vessels and surrounding tissues can be delineated based on differences in CT values.17 First, the inside of imaged GV as enhanced area was marked arbitrarily. Second, adjacent blood vessels with similar densities were automatically traces by setting the marked site as the origin, and only the blood vessels were extracted (Figure 1). Blood vessel extraction and measurement of GV volume was performed by two independent radiologists who were unaware of the actual injected amount of 5% EOI (Figure 2).
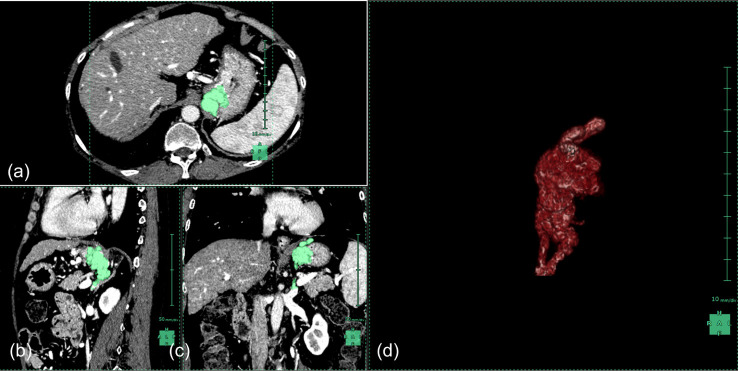
Figure 1.
Volumetry of gastric varices. Synapse Vincent was used for volumetric analysis. Areas in green represent traces of the gastric varices. MDCT scans are (a) axial, (b) sagittal, (c) coronal, and (d) a three-dimensional representation of the traces. The variceal volume of this patient is 26.9 ml. MDCT, multidetector row computed tomography.
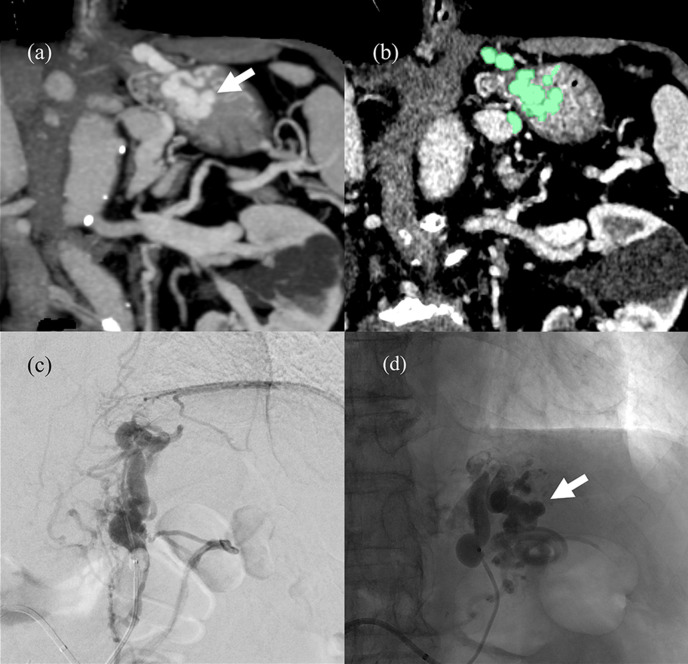
Figure 2.
CECT, DSA and fluoroscopic images of Hirota’s Grade 4 case (a) Pre-procedural CECT coronal MIP image. The arrow indicates the areas identified as varices. (b) Green traces of varices analyzed with Synapse Vincent volumetry. The variceal volume of this patient is 12.9 ml. (c) This patient was classified as Hirota’s Grade 4 because variceal extraction was not possible despite the presence of many narrow draining veins in B-RTV from the GR shunt. (d) This unenhanced X-ray taken after the injection of 9 ml of 5% EOI with gelatin sponge particles shows satisfactory retention of the sclerosant in the varices (arrow head). B-RTV, balloon occluded retrograde transvenous venography; CECT, contrast-enhanced CT; DSA, digital subtraction angiography; EOI, ethanolamine oleateiopamidol; GR, gastrorenal; MIP, maximum intensity projection.
Evaluations
Technical success was defined as stagnation of sclerosants in GV on unenhanced CT imaging immediately after procedure. All patients underwent contrast-enhanced multidetector CT of the chest–abdomen–pelvis within 7 days of the procedure. Clinical success was defined as absence of residual enhancement within the gastric varices on this CT. This scan was also used to check the complications such as pulmonary embolism (PE), venous embolism, portal embolism, pleural effusion and/or ascites on the basis of Clavien–Dindo classification.18 The sclerosants-related adverse effects such as symptomatic cerebral infarction, disseminated intravascular coagulation (DIC), acute respiratory distress syndrome (ARDS) and EOI-induced renal dysfunction were also clinically evaluated. The amount of 5% EOI used, volume of varices, amount used per unit volume (EOI/GV ratio), time to embolization, and change in estimate glomerular filtration rate (eGFR) from before to after the procedure were evaluated. The time to embolization was defined as the time from the start of sclerosant injection to the time of unenhanced CT imaging to confirm sclerosant hardening within the varices. The time to GV recurrence or rupture were additionally evaluated. Recurrence of GV was defined as enlargement of GV was revealed on endoscopy, enhancing that gastric varices were revealed on CT or rebleeding was occurred. CT and/or endoscopy was performed every 3–6 months after B-RTO basically. The cumulative recurrence rates of GV after B-RTO in each group were analyzed.
Statistical analyses
The statistical analysis was conducted using the paired t-test to compare renal function of pre- and post-treatment and the Steel-Dwass multiple test to compare EOI/volume ratio and embolization time between three procedure groups (SAS for Windows v. 9.3, SAS Institute, Cary, NC). The cumulative recurrence rates of gastric varices after BRTO in each group were analyzed using the Kaplan–Meier method and compared using the log-rank test using GraphPad Prism v. 7 software (GraphPad Software, San Diego, CA). A p-value less than 0.05 was considered to indicate a significant difference.
Results
We obtained follow-up data in 57 patients (20 patients in G1, 17 in G ≥ 2, and 20 in GERTO). There was no statistical difference in each group in demographic factors, such as age (GERTO vs G ≥ 2 p = 0.96, G ≥ 2 vs G1 p = 0.66, GERTO vs G = 1 p = 0.55) and Child-Pugh score (G ≥ 2 vs G = 1 p = 0.97, GERTO vs G = 1 p = 0.96, GERTO vs G ≥ 2 p = 0.92) (Table 1). Of the 37 patients who were Hirota’s Grade 2 or higher, 17 underwent conventional B-RTO (7 were Grade 2, 6 were Grade 3, 4 were Grade 4, and 0 were Grade 5), and 20 underwent GERTO (2 were Grade 2, 6 were Grade 3, and 12 were Grade 4). Hirota’s grade in GERTO group was significantly higher than G ≥ 2 (p = 0.03) (Table 2). The main draining vein approached was the GR shunt (n = 49), GC shunt (n = 7), and PCV (n = 1).
Table 1.
Patient demographics
| G1 group (n = 20) |
G ≥ 2 group (n = 17) |
GERTO group (n = 20) |
||
|---|---|---|---|---|
| Male:Female | 14:6 | 8:9 | 13:7 | |
| Age (years) | 68.4 ± 11.2 | 65.4 ± 11.6 | 65.2 ± 10.8 | an.s. |
| Underlying cause (virus:alcohol:other) |
13:5:2 | 12:4:1 | 6:9:5 | |
| Child-Pugh score | 6.4 ± 1.0 | 6.6 ± 1.42 | 6.5 ± 1.6 | an.s. |
Data are shown as mean ± standard deviation, or number.
Variables analyzed using Steel–Dwass test.
Table 2.
Main drainage vein, Hirota’s grade, and change in eGFR from before to after the procedure in each group
| G1 | G ≥ 2 | GERTO | Overall | |
|---|---|---|---|---|
| group | group | group | rate | |
| (n = 20) | (n = 17) | (n = 20) | (n=57) | |
| Main drainage vein | ||||
| GR shunt | 18 | 15 | 16 | 86% |
| GC shunt | 2 | 2 | 3 | 12% |
| PCV | 0 | 0 | 1 | 1.8% |
| Hirota’s Grade | a p = 0.03 | |||
| 1 | 20 | - | - | |
| 2 | - | 7 | 2 | |
| 3 | - | 6 | 6 | |
| 4 | - | 4 | 12 | |
| 5 | - | 0 | 0 | |
| Pre-eGFR (mL/min/1.73 m2) | 70.6 ± 19.0 | 77.1 ± 25.0 | 78.1 ± 17.4 | |
| Post-eGFR (mL/min/1.73 m2) | 66.3 ± 21.1 | 77.1 ± 26.4 | 81.6 ± 19.2 | |
| bp value | 0.13 | 0.91 | 0.33 | |
EOI, ethanolamine oleate iopamidol; GC, gastrocaval; GERTO, retrograde transvenous obliteration; GR, gastrorenal; PCV, pericardial vein;eGFR, estimate glomerular filtration rat.
Data are shown as mean ± standard deviation, or number.
Variables analyzed using Fisher exact probability test about 5% EOI group and 5% EOI containing GS Particles Group in Grade 2–5.
Variables analyzed using fisher exact propability test.
The technical and clinical success rate of B-RTO and GERTO was 100%. As complications transient left-sided pleural effusion is most observed in 15.8% (Table 3). Splenic thrombosis or portal thrombosis was also found in 1.8% respectively, however, it was transient in any cases. Ascites was found in 12.3%. Refractory ascites in Clavien Dindo Grade Ⅲa was found in 1.8%. Symptomatic cerebral infarction or pulmonary embolism was not noted as post-procedural complications in the all patients. DIC or ARDS which is reported previously was not also noted.19,20
Table 3.
Complications
| Clavien Dindo classification (Grade) | Overall rate (n = 57) | G1 Group (n = 20) | G ≥ 2 Group (n = 17) | GERTO Group (n = 20) | |
|---|---|---|---|---|---|
| Left-sided pleural effusion | 15.8% | ||||
| Ⅰ | 14.0% | 20% (4) | 5.9% (1) | 15% (3) | |
| Ⅱ | 1.8% | 5.9% (1) | |||
| Ascites | 12.3% | ||||
| Ⅰ | 8.8% | 15% (3) | 10% (2) | ||
| Ⅱ | 1.8% | 5.9% (1) | |||
| Ⅲa | 1.8% | 5% (1) | |||
| Portal vein thrombosis | 1.8% | ||||
| Ⅱ | 1.8% | 5% (1) | |||
| Splenic vein thrombosis | 1.8% | ||||
| Ⅰ | 1.8% | 5% (1) | |||
| Pulmonary embolism | 0% | ||||
| Symptomatic cerebral infarction | 0% | ||||
| DIC | 0% | ||||
| ARDS | 0% |
ARDS, Acute respiratory distress syndrome;DIC, Disseminated intravascular coagulation;GERTO, retrograde transvenous obliteration.
Data are shown as %, or number.
The EOI/GV ratio was 0.66 ± 0.19 in the G1 patients, 1.5 ± 0.8 in the G ≥ 2 patients, and 0.58 ± 0.23 in the GERTO patients (Table 4). A significantly lower EOI/GV ratio was used in the patients with a Hirota’s Grade 2–5 who underwent GERTO compared with those who underwent conventional B-RTO (p < 0.0001). It was 61.3% less on average. On the other hand, Hirota’s Grade 1 patients were significantly lower amounts of sclerosants than Grade 2–5 in conventional B-RTO groups (p = 0.0001).
Table 4.
Comparison among G1, G ≥ 2 and GERTO groups
| G1 group (n = 20) | G ≥ 2 group (n = 17) | GERTO group (n = 20) | |
|---|---|---|---|
| Amount of 5% EOI (mL) | 18.5 ± 11.0 | 27.2 ± 17.8 | 13.9 ± 7.0 |
| Volume of varices (mL) | 30.1 ± 23.0 | 21.6 ± 15.8 | 26.7 ± 16.0 |
| EOI/GV ratio | 0.66 ± 0.19 | 1.5 ± 0.8 | 0.58 ± 0.23 |
| a p=0.0001 | a p<0.0001 | ||
| Embolization time (min) | 26.5 ± 10.5 | 39.2 ± 26.8 | 21.4 ± 9.4 |
| a p=0.005 | |||
EOI, ethanolamine oleate iopamidol;GERTO, retrograde transvenous obliteration; GV, gastric varices.
Data are shown as mean ± standard deviation, or number.
Variables analyzed using Steel–Dwass test.
The times to embolization were 26.5 ± 10.5 min for G1, 39.2 ± 26.8 min for G ≥ 2, and 21.4 ± 9.4 min for GERTO. The time required for GERTO was significantly shorter compared with that required for conventional B-RTO in the Grade ≥ 2 patients (p = 0.005). It was 45.5% shorter on average.
The post-procedural eGFR was 66.3 ± 21.1 for Grade 1 patients who underwent conventional B-RTO, 77.1 ± 26.4 for Grade ≥ 2 patients who underwent conventional B-RTO, and 81.6 ± 19.2 for Grade ≥ 2 patients who underwent GERTO. Renal function therefore did not significantly deteriorate in any of the patient groups (Grade 1 p = 0.13, Grade ≥ 2 with conventional B-RTO p = 0.91, GERTO p = 0.33) (Table 2).
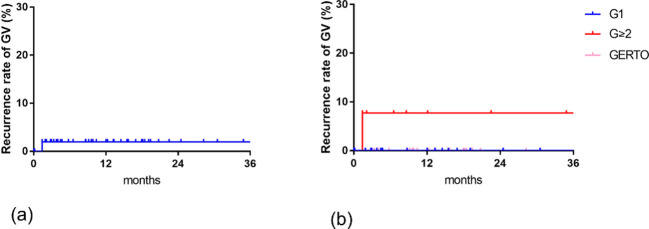
The mean follow-up duration was 15.1 ± 13.0 in Grade 1, 12.7 ± 12.8 in Grade 2–5, 16.2 ± 4.6 months in GERTO group. Expected 6 month, 1-, and 2 year recurrence rates of gastric varices were 2.0, 2.0, and 2.0% in all patients, 0, 0, and 0% in Grade 1 group, 7.7, 7.7, and 7.7% in Grade 2–5 group with EO, 0, 0, and 0% in GERTO group (Figure 3). Recurrence rate was not significantly different in any of the patient groups (G1 vs G ≥ 2 p = 0.91, G ≥ 2 vs GERTO p = 0.33, G1 vs GERTO p = 0.35).
Figure 3.
Expected recurrence rate of GV after B-RTO by Kaplan–Meier method (a) 6 month, 1-, and 2 year overall recurrence rates of gastric varices were 2.0, 2.0, and 2.0%. (b) 6 month, 1-, and 2 year recurrence rates of gastric varices were 0, 0, and 0% in G1 group, 7.7, 7.7, and 7.7% in G ≥ 2 group with EO, 0, 0, and 0% in GERTO group. Significant difference was not found in any of the patient groups. B-RTO, balloon-occluded retrograde transvenous obliteration; GERTO, retrograde transvenous obliteration; GV, gastric varices.
Discussion
This is a first report about utility of low dose of GS particles and 5% EOI mixture as sclerosants in B-RTO. B-RTO with EO features a low recurrence rate,21 while embolization with GS particles can be performed quickly.2 The present hybrid procedure we investigated combines both these advantages.
In the treatment of GV with B-RTO in Hirota’s Grade ≥ 2 patients, who are difficult to treat, the patients treated with GS particles required 61.3% less 5% EOI per milliliter of variceal volume than the conventional B-RTO patients even though Hirota’s grade in GERTO group was higher than G ≥ 2. Moreover, the time to embolization was 45.5% shorter in the patients treated with GS particles compared with those undergoing conventional B-RTO, that is to say, stepwise injection was hardly necessary for B-RTO with 5% EOI containing GS Particles. Even downgrading method may be unneeded.14
On the topic of variceal volume and the amount of sclerosant used, Kageyama et al state GV volume measured on CT showed a significant correlation with the amount of 5% EOI injected.17 The Hirota’s Grade ≥ 2 patients we treated with conventional B-RTO required 5% EOI in an amount approximately 1.5-fold the variceal volume. Sclerosant loss from collateral draining veins may partially explain why extra EOI is required. The Hirota’s Grade ≥ 2 patients we treated with added GS particles, however, achieved embolization with an amount of 5% EOI 0.58-fold the variceal volume. We attribute this smaller required volume to the plugging of the narrow draining veins in these patients by GS particles. In other words, the particles, like coil embolization, produce downgrading (Figure 4). We performed conventional B-RTO with EO in hirota's Grade 1 case because sclerosants were not washed out into collateral draining veins.
Figure 4.
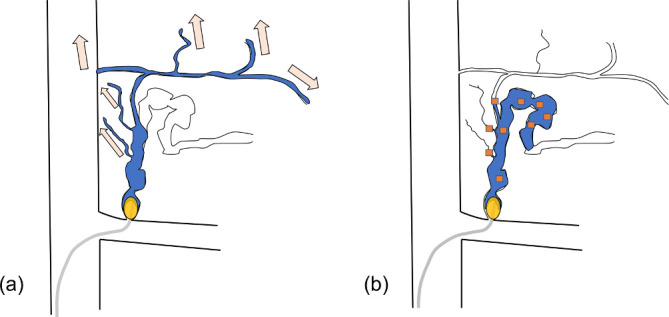
Schema about injection of sclerosant from gastrorenal shunt under balloon occlusion in Hirota’s high grade case (a) 5% EOI is washed out from fine collateral veins in conventional B-RTO and sclerosant loss arises. As a result varices is difficult to be visualized. Further stepwise injection of EO to embolize narrow draining veins takes time. (b) In 5% EOI containing GS particles (orange square grains), GS particles produce downgrading to embolize collateral veins. So much less amount of sclerosant is required. Good stagnation of EO is obtained and makes robust damage of vascular endothelium. Stepwise injection is unneeded, so embolization takes less time. B-RTO, balloon-occluded retrograde transvenous obliteration; EOI, ethanolamine oleateiopamidol; GS, gelatin sponge.
EO is a liquid embolic agent that damages the vascular endothelium. Since intravascular sclerosis is not instantaneous, some EO can leak into the systemic circulation via the draining veins, when present. The maximum tolerable amount of 5% EOI of 0.4 mL/kg may be exceeded in patients with large-volume varices or a high Hirota’s grade, making the procedure difficult.8 Although a large amount of sclerosant was needed in the Grade ≥ 2 patients who underwent conventional B-RTO, much less was required when GS particles were used.
NBCA is also a liquid embolic agent. It has been used in endoscopic sclerotherapy for esophageal varices and has come to be used in the treatment of bleeding disorders, endoleaks, portal vein embolization, and arterial embolization.22 It polymerizes into solid form after contact with ions and is mixed with lipiodol to adjust its polymerization time. Handling for injection is somewhat difficult compared to GS particle or EO because of the instant viscosity. Therefore, it must be precisely administered by an experienced operator to prevent non-target embolization such as PE or unintentional sticking to the catheter.
In PARTO, contrast agent containing GS particles is injected into varices under the placement of vascular plug in the drainage vein.9 PARTO can be performed in countries where EO use is not approved. GS is widely recognized as a temporary occlusive agent in arterial embolization.23 It can be absorbed within 2–6 weeks, and thereafter embolized vessels are fully recanalized. However, the attenuation of embolized vessels occurs due to vasculitis and intimal proliferation. Permanent vascular occlusion can also develop, depending on the amount and compactness of GS particles and intensity of inflammatory reactions.24,25 Discussing the mechanism of GS particles embolization of GV, Kim et al also state, “Gelfoam works as a matrix for the formation of thrombus. This thrombosis causes vascular spasm, platelet agglutination, and rapid clot formation”.10 In this study, we noted intravariceal flow stagnation as the GS particles embolized the collateral draining veins, which also appears to contribute to this mechanism. Recurrence rate of GV after PARTO is controversial (0–16% after 6 months, 32.8% after 1 year, and 55.2% after 2 years for PARTO vs 2.7% recurrence and 1.5% re-bleeding after 5 years and 14% after 8 years for conventional B-RTO).2,10,21,26 In our study, GV recurrence rate at 2 year after GERTO was 0%. We believe that endothelial destruction caused by EO is also an important factor in permanent embolization.
The critical complications were not found in all patients; however, there are a few reports about DIC or ARDS after intravariceal injection of EO.19,20 In prospective study, the moderate or serious adverse event related to EO such as severe ascites or sepsis was observed in 2.3% respectively.27 There is a report about respiratory effects after B-RTO using EO.28 Therefore, we tried to decrease the volume of sclerosant. Patients with portopulmonay venous anastomosis or patent foramen ovale are at risk of infarction of the brain or other organs from leaking sclerosant. The prevalence of portopulmonay venous anastomosis ranges from 8 to 33%.29,30 The prevalence of patent foramen ovale is 34% to age 30 and 20% at age 80.31 Although cerebral infarction has yet to be reported in association with B-RTO, which uses EO, caution and further investigation are needed because GS particles are a solid embolic agent but not liquid agent. Cerebral infarction has been reported following percutaneous transhepatic variceal obliteration with glue and lipiodol for esophageal varices.32 Paradoxical embolism and cerebral infarction from lipiodol have been reported following transcatheter arterial chemoembolization for hepatocellular carcinoma, the authors concluding that a right-to-left shunt in the heart or lung may have been involved.33
The limitations of the study include a relatively small sample size in each group and relatively short follow-up for recurrence and retrospective study. Recurrence, however, is unlikely to be more frequent than in conventional B-RTO because there is less sclerosant loss and EO remains in contact with the target vessel for a longer time.
Conclusion
GERTO was performed in lower amount of sclerosants and in less time compared to conventional B-RTO in Hirota’s grade ≥2.
Contributor Information
Atsushi Jogo, Email: atsushijz@gmail.com.
Akira Yamamoto, Email: loveakirayamamoto@gmail.com.
Toshio Kaminoh, Email: kaminout@osaka.zaq.jp.
Mariko Nakano, Email: mana_zoe@yahoo.co.jp.
Ken Kageyama, Email: kageyama@med.osaka-cu.ac.jp.
Etsuji Sohgawa, Email: es35@hotmail.co.jp.
Shinichi Hamamoto, Email: hamashin_tigers1975@yahoo.co.jp.
Yukimasa Sakai, Email: sakaiy@trust.ocn.ne.jp.
Masao Hamuro, Email: hamuro@med.osaka-cu.ac.jp.
Norifumi Nishida, Email: irinvestz@gmail.com.
Yukio Miki, Email: yukio.miki@med.osaka-cu.ac.jp.
REFERENCES
- 1. Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 1996; 11: 51–8. doi: 10.1111/j.1440-1746.1996.tb00010.x [DOI] [PubMed] [Google Scholar]
- 2. Gwon DI, Kim YH, Ko G-Y, Kim JW, Ko HK, Kim JH, et al. Vascular Plug-Assisted retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy: a prospective multicenter study. J Vasc Interv Radiol 2015; 26: 1589–95. doi: 10.1016/j.jvir.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 3. Itou C, Koizumi J, Hashimoto T, Myojin K, Kagawa T, Mine T, et al. Balloon-Occluded retrograde transvenous obliteration for the treatment of gastric varices: Polidocanol foam versus liquid ethanolamine oleate. AJR Am J Roentgenol 2015; 205: 659–66. doi: 10.2214/AJR.14.13389 [DOI] [PubMed] [Google Scholar]
- 4. Mosli MH, Aljudaibi B, Almadi M, Marotta P. The safety and efficacy of gastric fundal variceal obliteration using n-butyl-2-cyanoacrylate; the experience of a single Canadian tertiary care centre. Saudi J Gastroenterol 2013; 19: 152–9. doi: 10.4103/1319-3767.114508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trolle E, Trolle D. Treatment of oesophageal varices by injections of sclerosing agents through oesophagoscope in splenectomized patient suffering from splenic phlebostenosis (splenic anemia); a case with autopsy. Acta Chir Scand 1946; 94: 385–96. [PubMed] [Google Scholar]
- 6. Patterson CO, Rouse MO. The sclerosing therapy of esophageal varices. Gastroenterology 1947; 9: 391–5. [PubMed] [Google Scholar]
- 7. Masaki M, Obara K, Suzuki S, Orikasa K, Mitsuhashi H, Iwasaki K, et al. The destructive effects of sclerosant ethanolamine oleate on mammalian vessel endothelium. Gastroenterol Jpn 1990; 25: 230–5. doi: 10.1007/BF02776821 [DOI] [PubMed] [Google Scholar]
- 8. Iso Y, Kitano S, Iwanaga T, Hashizume M, Sugimachi K. Morphological and Hemodynamic Changes of the Lung after Injection of 5% Ethanolamine Oleate into Dogs. Eur Surg Res 1989; 21(3-4): 175–83. doi: 10.1159/000129021 [DOI] [PubMed] [Google Scholar]
- 9. Gwon DI, Ko G-Y, Yoon H-K, Sung K-B, Kim JH, Shin JH, et al. Gastric varices and hepatic encephalopathy: treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration--a primary report. Radiology 2013; 268: 281–7. doi: 10.1148/radiol.13122102 [DOI] [PubMed] [Google Scholar]
- 10. Kim YH, Kim YH, Kim CS, Kang UR, Kim SH, Kim JH. Comparison of Balloon-Occluded retrograde transvenous obliteration (BRTO) using ethanolamine oleate (Eo), BRTO using sodium tetradecyl sulfate (STS) foam and vascular Plug-Assisted retrograde transvenous obliteration (PARTO. Cardiovasc Intervent Radiol 2016; 39: 840–6. doi: 10.1007/s00270-015-1288-8 [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell A. Management of gastric varices. Clin Gastroenterol Hepatol 2014; 12: 919–28 e1; quiz e51-2. doi: 10.1016/j.cgh.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 12. Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 2010; 22: 1–9. doi: 10.1111/j.1443-1661.2009.00929.x [DOI] [PubMed] [Google Scholar]
- 13. Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology 1999; 211: 349–56. doi: 10.1148/radiology.211.2.r99ma25349 [DOI] [PubMed] [Google Scholar]
- 14. Fukuda T, Hirota S, Sugimoto K, Matsumoto S, Zamora CA, Sugimura K. "Downgrading" of gastric varices with multiple collateral veins in balloon-occluded retrograde transvenous obliteration. J Vasc Interv Radiol 2005; 16: 1379–83. doi: 10.1097/01.RVI.0000175336.05823.EB [DOI] [PubMed] [Google Scholar]
- 15. Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices: Part 2. strategy and techniques based on hemodynamic features. Radiographics 2003; 23: 921–37 discussion 37. doi: 10.1148/rg.234025135 [DOI] [PubMed] [Google Scholar]
- 16. Miyoshi H, Ohshiba S, Matsumoto A, Takada K, Umegaki E, Hirata I. Haptoglobin prevents renal dysfunction associated with intravariceal infusion of ethanolamine oleate. Am J Gastroenterol 1991; 86: 1638–41. [PubMed] [Google Scholar]
- 17. Kageyama K, Nishida N, Yamamoto A, Jogo A, Tsukamoto T, Miki Y. Usefulness of CT volumetry for gastric varix before balloon-occluded retrograde transvenous obliteration. Hepatogastroenterology 2014; 61: 1806–11. [PubMed] [Google Scholar]
- 18. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250: 187–96. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 19. Vallgren S, Sigurdsson GH, Moberger G, Christenson JT. Influence of intravenous injection of sclerosing agents on the respiratory function. Acta Chir Scand 1988; 154: 271–6. [PubMed] [Google Scholar]
- 20. Bellary SV, Isaacs P. coagulation Dintravascular Dic) after endoscopic injection sclerotherapy with ethanolamine oleate. Endoscopy 1990; 22: 151. [DOI] [PubMed] [Google Scholar]
- 21. Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol 2005; 184: 1340–6. doi: 10.2214/ajr.184.4.01841340 [DOI] [PubMed] [Google Scholar]
- 22. Takeuchi Y, Morishita H, Sato Y, Hamaguchi S, Sakamoto N, Tokue H, et al. Guidelines for the use of NBCA in vascular embolization devised by the Committee of practice guidelines of the Japanese Society of interventional radiology (CGJSIR), 2012 edition. Jpn J Radiol 2014; 32: 500–17. doi: 10.1007/s11604-014-0328-7 [DOI] [PubMed] [Google Scholar]
- 23. Miyayama S, Yamakado K, Anai H, Abo D, Minami T, Takaki H, et al. Guidelines on the use of gelatin sponge particles in embolotherapy. Jpn J Radiol 2014; 32: 242–50. doi: 10.1007/s11604-014-0292-2 [DOI] [PubMed] [Google Scholar]
- 24. Satoh M, Yamada R. Experimental and clinical studies on hepatic artery embolization for the treatment of hepatoma. Nihon Igaku Hoshasen Gakkai Zasshi 1983; 43: 977–1005. [PubMed] [Google Scholar]
- 25. Abada HT, Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol 2007; 10: 257–60. doi: 10.1053/j.tvir.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 26. Akahoshi T, Hashizume M, Tomikawa M, Kawanaka H, Yamaguchi S, Konishi K, et al. Long-Term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol 2008; 23: 1702–9. doi: 10.1111/j.1440-1746.2008.05549.x [DOI] [PubMed] [Google Scholar]
- 27. Kobayakawa M, Kokubu S, Hirota S, Koizumi J, Nishida N, Yasumoto T, et al. Short-Term safety and efficacy of Balloon-Occluded retrograde transvenous obliteration using ethanolamine oleate: results of a prospective, multicenter, single-arm trial. J Vasc Interv Radiol 2017; 28: 1108–15. doi: 10.1016/j.jvir.2017.03.041 [DOI] [PubMed] [Google Scholar]
- 28. Arai H, Abe T, Takayama H, Toyoda M, Mori K, Ueno T, et al. Respiratory effects of balloon occluded retrograde transvenous obliteration of gastric varices - A prospective controlled study. J Gastroenterol Hepatol 2011; 26: no–94. doi: 10.1111/j.1440-1746.2011.06727.x [DOI] [PubMed] [Google Scholar]
- 29. Sano A, Nishizawa S, Sasai K, Imanaka K, Tanaka K, Hashimura T, et al. Contrast echocardiography in detection of portopulmonary venous anastomosis. AJR Am J Roentgenol 1984; 142: 137–40. doi: 10.2214/ajr.142.1.137 [DOI] [PubMed] [Google Scholar]
- 30. Kariya S, Komemushi A, Nakatani M, Yoshida R, Kono Y, Shiraishi T, et al. Portopulmonary venous anastomosis in balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices. J Gastroenterol Hepatol 2014; 29: 1522–7. doi: 10.1111/jgh.12583 [DOI] [PubMed] [Google Scholar]
- 31. Kedia G, Tobis J, Lee MS. Patent foramen ovale: clinical manifestations and treatment. Rev Cardiovasc Med 2008; 9: 168–73. [PubMed] [Google Scholar]
- 32. Lee YM, Lee Y, Choe YH. Stroke after percutaneous transhepatic variceal obliteration of esophageal varix in caroli syndrome. Korean J Pediatr 2013; 56: 500–4. doi: 10.3345/kjp.2013.56.11.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim J-T, Heo S-H, Choi S-M, Lee S-H, Park M-S, Kim B-C, et al. Cerebral embolism of iodized oil (lipiodol) after transcatheter arterial chemoembolization for hepatocellular carcinoma. J Neuroimaging 2009; 19: 394–7. doi: 10.1111/j.1552-6569.2009.00380.x [DOI] [PubMed] [Google Scholar]