Abstract
Objective:
Chemical shift artefact (CSA) is often encountered during MRI evaluation of superficial soft tissue masses. The study aim was to determine the incidence and diagnostic relevance of CSA in a consecutive series of superficial soft tissue masses referred to a specialist musculoskeletal sarcoma service.
Methods:
All patients referred over a 6 month period with a non-lipomatous superficial soft tissue mass were prospectively analysed. Patients characteristics (age, gender), lesion features (anatomical location, size, relationship to the skin and deep fascia), presence of CSA and final histopathological diagnosis were collected. The presence of CSA was statistically analysed against these clinical, imaging and histopathological variables.
Results:
128 patients fulfilled the inclusion criteria [63 males, 65 females; mean age = 50.6 years (7–96 years)]. CSA was present in 50 cases (39.1%) overall, but in 39 (41.5%) of 94 cases with histological diagnosis. There was no statistically significant relationship to any assessed variable apart from relationship to the deep fascia, CSA being more frequent in lesions contacting the fascia compared to lesions contacting both skin and fascia (p-value 0.02). In particular, the presence of CSA did not allow differentiation between non-malignant and malignant lesions.
Conclusion:
The presence of CSA is a not infrequent finding in the MRI assessment of superficial soft tissue masses but does not appear to be of any significance in differentiating between non-malignant and malignant lesions.
Advances in knowledge:
CSA is a relatively common finding in association with superficial soft tissue masses, but does not indicate a particular histological diagnosis or help in the differentiation of non-malignant from malignant lesions.
Introduction
MRI has been established for many years as the technique of choice for the identification, characterisation and local staging of soft tissue tumours.1–3 There are also many non-neoplastic and benign lesions which have typical MR imaging features that allow a confident diagnosis to be made without the requirement for needle biopsy,4–6 but the characterisation of most soft tissue sarcomas is less reliable. However, MRI plays a major role in the pre-operative planning of soft tissue sarcomas.7
Further characterisation of soft tissue tumours is possible with diffusion-weighted imaging (DWI), which is an MRI technique that assesses the movement of free water molecules within tissues as measured by the apparent diffusion coefficient (ADC).8 Variation in ADC values can differentiate benign from malignant lesions,9–11 can differentiate desmoid tumours from malignant soft tissue tumours,12 and can also differentiate histological grade in musculoskeletal soft tissue sarcomas.13
Chemical shift artefact (CSA) occurs due to the slight difference in resonance frequency of protons in fat and water molecules, being identified at any fat–fluid interface on short TE sequences along the frequency encoding axis.14,15 It appears as a line of low signal intensity (SI) on one side of a lesion/organ with corresponding high SI at the opposite margin, and is commonly identified around various organs such as the orbit, kidneys and urinary bladder. Therefore, it might be expected that soft tissue masses which have a relatively high fluid content and are surrounded by fat may demonstrate CSA. CSA has been utilised in a variety of clinical settings. The presence of CSA can differentiate between benign and malignant lymphadenopathy with a diagnostic accuracy approaching 90%.16,17 However, the potential of CSA as a marker of increased tissue fluid to further characterise musculoskeletal soft tissue masses has not been formally investigated, although the artefact has been noted in cases of tenosynovial giant cell tumour and epidermoid cyst.18
During the reporting of MRI studies for superficial soft tissue lesions, CSA was frequently encountered by the senior author at the margin of the tumour with subcutaneous fat, suggesting that the lesion had a relatively high fluid content, therefore potentially being cystic, vascular or highly cellular. The current study aims to determine the incidence and diagnostic relevance of CSA in a consecutive series of superficial non-lipomatous soft tissue masses referred to a specialist musculoskeletal sarcoma service.
Methods
The study was approved by the Research and Development Committee, with no requirement for informed patient consent.
All patients referred to a specialist musculoskeletal sarcoma service for assessment of a superficial soft tissue mass were entered prospectively onto a database over a 6-month period between June and December 2018. The inclusion criteria were as follows: (1) the mass was located within the subcutaneous compartment (i.e. between the skin and the deep fascia); (2) all MRI studies were performed on a 3T MRI scanner; (3) simple lipomata (based on the typical MRI features of lesions with pure fat signal intensity on all pulse sequences) were excluded; (4) the lesion had not undergone previous biopsy or surgery.
Data collected included patient age and gender, anatomical location of the lesion, maximal dimension, relationship to the skin and deep fascia (defined as contact with skin only, contact with fascia only, contact with both or contact with neither), presence of CSA, and final histological diagnosis for those cases that underwent image-guided core needle biopsy (IGCNB) or primary surgical excision.
The routine MRI sequences included sagittal or coronal T 1 weighted turbo spin echo (T 1W TSE), sagittal or coronal short tau inversion recovery (STIR), coronal or sagittal T 2 weighted fast spin echo (T 2W FSE), axial proton density weighted fast spin echo (PDW FSE) and axial spectral adiabatic inversion recovery (SPAIR), with dedicated repetition time, echo time and coil selection depending upon the body region being imaged. CSA was considered to be present if the lesion was associated with linear hyperintensity on one side and corresponding linear hypointensity at its opposite margin on any sequence (Figures 1 and 2). The absence/presence of CSA was assessed independently by two Consultant Musculoskeletal Radiologists with 9 (Reader 1) and 24 years’ (Reader 2) experience of musculoskeletal tumour imaging. Where there was discrepancy, the final decision was made independently by a third Consultant Musculoskeletal Radiologist with 5 years’ experience of musculoskeletal tumour imaging. All radiologists were blinded to the final histological diagnosis.
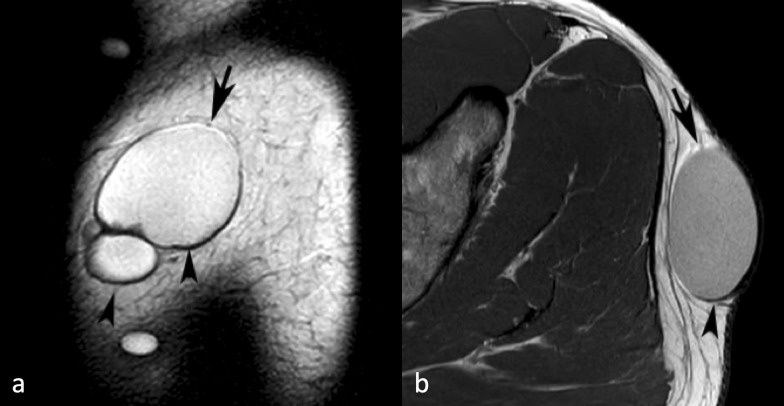
Figure 1.
A 44-yr-old male with an epidermoid cyst over the lateral aspect of the left buttock. (a) Sagittal T 2W FSE MR image showing a well-defined multilocular fluid signal intensity lesion with linear hyperintensity around its upper margin (arrow) and hypointensity around its lower margin (arrowheads) due to chemical shift artefact. (b) Similarly, axial PDW FSE MR image showing linear hyperintensity around its anterior margin (arrow) and hypointensity around its posterior margin (arrowhead). PDW FSE, proton densityweighted fast spin echo; T 2W FSE, T 2 weightedfast spin echo.
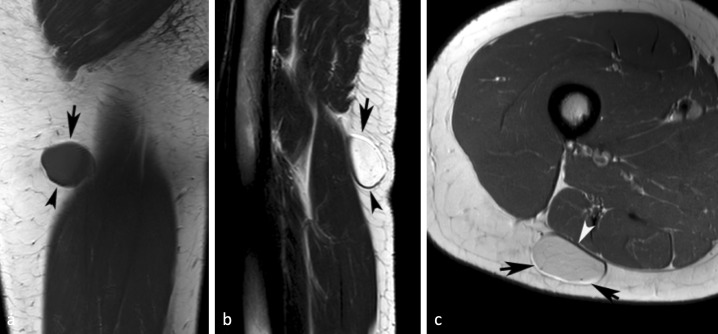
Figure 2.
A 27-yr-old female with a myxoid liposarcoma of the posterior right thigh. (a) Coronal T 1W SE and (b) sagittal T 2W FSE MR images showing a well-defined lesion with linear hyperintensity around its upper margin (arrows) and hypointensity around its lower margin (arrowheads) due to chemical shift artefact. (c) Similarly, axial PDW FSE MR image showing linear hyperintensity around its superficial margin (arrows) and hypointensity around its deep margin (arrowhead). PDW FSE, proton densityweighted fast spin echo; T 1W SE, T 1 weighted spin echo; T 2W FSE,T 2 weightedfast spin echo.
All IGCNB/resection specimens were reported by Consultant Histopathologists working within a specialist musculoskeletal sarcoma service. The histological diagnosis was obtained by review of histopathology reports for those cases that underwent IGCNB and/or surgical excision. For lesions excised following IGCNB, the diagnosis was based on the final resection specimen histology. Tumours were graded as non-neoplastic, benign, intermediate or malignant based on the 2013 World Health Organisation Classification of soft tissue tumours. However, for statistical analysis intermediate grade lesions were combined with benign lesions due to their small number.19
Statistical analysis
Interobserver correlation was assessed using the Cohen κ statistic. The presence of CSA was correlated with patient age and gender, anatomical location in the body, relationship to the skin and fascia, lesion dimension and histological grade. Continuous variables were compared between groups using the unpaired t-test for normally distributed variables, whilst the Mann–Whitney test was preferred for non-normally distributed variables. Categorical variables were compared between groups using the χ2 test, or Fisher’s exact test for variables where the number of patients in some categories was small.
Results
A total of 128 patients fulfilled the inclusion criteria, 63 males and 65 females with mean age of 50.6 years (range 7–96 years). The lesion location is listed in Table 1. The upper limb was involved in 51 cases (39.8%), the lower limb in 70 cases (54.7%), and the trunk in 7 (5.5%). 37 lesions (28.3%) made contact with the skin, 17 (13.4%) with the deep fascia, 58 (45.7%) with both and 16 (12.6%) with neither.
Table 1.
Anatomical distribution of 128 lesions
| Body region | Subdivision | Number of lesions | |
|---|---|---|---|
| Location | Upper limb | Shoulder girdle | 6 |
| Upper arm | 14 | ||
| Elbow | 6 | ||
| Forearm | 6 | ||
| Wrist & hand | 19 | ||
| Lower limb | Pelvis, groin & buttock | 12 | |
| Thigh | 16 | ||
| Knee | 12 | ||
| Calf | 13 | ||
| Ankle & foot | 17 | ||
| Trunk | Chest | 2 | |
| Abdomen | 5 |
Discrepancy between the first two readers occurred in 27 cases (21.1%). Although this was not assessed formally, consensus review suggested that the commonest cause was cases where CSA was subtle and usually called positive by Reader 2 and negative by Reader 1. Interobserver correlation for assessment of the presence of CSA between Readers 1 and 2 was calculated at 0.581, indicating a moderate degree of agreement. Following review of discrepant cases by Reader 3, a final diagnosis of CSA was made in 50 cases (39.1%).
A histological diagnosis was available in 3 cases (2.3%) based on IGCNB alone, and in 91 cases (71.9%) based on surgical resection. Of these 94 cases, 29 (30.9%) were non-neoplastic in nature, 51 (54.2%) were benign neoplasms and 14 (14.9%) were malignant. The histological grading and final diagnoses are presented in Table 2. The remaining lesions were not resected based on the decision of the treating surgeon following clinical and imaging review. Of these, 11 showed CSA, the imaging diagnoses being as follows: four epidermoid cysts, three slow-flow vascular malformations, one ganglion and three lesions which were classed as indeterminate.
Table 2.
Histological diagnoses for 94 cases categorised into non-neoplastic, benign and malignant (alphabetical order and number in parentheses)
| Non-neoplastic (n = 29) | Benign (n = 51) | Malignant (n = 14) |
|---|---|---|
| Abscess1 | Angioleiomyoma11 | Dermatofibrosarcoma1 |
| Calcinosis cutis1 | Angiolipoma1 | Epithelioid sarcoma (high-grade)1 |
| Distorted erector pili muscle1 | Bland fibroblastic tumour1 | Leiomyosarcoma4 |
| Endometriosis2 | Deep fibrous histiocytoma1 | Merkel cell carcinoma1 |
| Epidermoid cyst9 | Fibroma of tendon sheath1 | Metastatic endometrial adenocarcinoma1 |
| Foreign body inflammatory reaction1 | Giant cell (tenosynovial) tumour2 | Metastatic squamous cell sarcoma1 |
| Ganglion cyst4 | Glomus tumour2 | Myxofibrosarcoma (Grade 3)1 |
| Lymph node1 | Granular cell tumour1 | Myxoid liposarcoma3 |
| Non-specific cyst1 | Haemangioma/vascular malformation10 | Soft tissue osteosarcoma1 |
| Pilar cyst1 | Myopericytoma3 | |
| Pilonidal abscess1 | Myopericytoma/glomangiopericytoma1 | |
| Post-traumatic lesion1 | Myxoma2 | |
| Reactive lymphoid tissue1 | Neurofibroma5 | |
| Nodular fasciitis1 | ||
| Tophaceous gout1 | Pilomatrixoma2 | |
| Traumatic neuroma1 | Schwannoma2 | |
| Tumoral calcinosis1 | Solitary fibrous tumour1 | |
| Xanthomatous inflammation1 | Spindle cell lipoma2 | |
| Superficial plantar fibromatosis1 | ||
| Superficial myositis ossificans1 |
Of the 94 cases with histological diagnosis, 39 (41.5%) showed CSA. Of these 39 cases, 7 (17.9%) were non-neoplastic, 25 (64.1%) benign neoplasms and 7 (18%) were malignant. The details of histological diagnoses are presented in Table 3.
Table 3.
Histological diagnoses for 39 resected cases that showed CSA (alphabetical order and number in parentheses)
| Non-neoplastic (n = 7) | Benign (n = 25) | Malignant (n = 7) |
|---|---|---|
| Epidermoid cyst2 | Angioleiomyoma3 | Dermatofibrosarcoma1 |
| Ganglion cyst2 | Glomus tumour2 | Leiomyosarcoma1 |
| Lymph node1 | Haemangioma/vascular malformation7 | Merkel cell carcinoma1 |
| Non-specific cyst1 | Myopericytoma3 | Metastatic squamous cell carcinoma1 |
| Pilar cyst1 | Neurofibroma4 | Myxoid liposarcoma3 |
| Pilomatrixoma1 | ||
| Schwannoma2 | ||
| Solitary fibrous tumour1 | ||
| Spindle cell lipoma1 | ||
| Superficial myositis ossificans1 |
Table 4 presents details of the relationship between the presence of CSA and age, gender, lesion size, relationship to skin and deep fascia, anatomical distribution and histological grade. The results indicated no significant differences between the two groups for most patient characteristics. However, lesion location in relation to the skin and fascia was found to be significantly different between groups. The CSA present group had a larger percentage of lesions located in contact with the fascia (22% vs 8%), and a smaller percentage of lesions contacting both skin and fascia (30% vs 56%) when compared to the CSA absent group. This difference was statistically significant with a p-value of 0.02. CSA was more common in benign neoplastic lesions and less common in non-neoplastic lesions, but this difference was not significant. Also, when comparing all non-malignant lesions with malignant lesions, there was no statistical difference in the presence or absence of CSA.
Table 4.
Relationship between clinical and imaging variables and CSA for all 128 cases
| Variable | Category | CSA absent (n = 78) |
CSA present (n = 50) |
p-value |
|---|---|---|---|---|
| Age | - | 50.9 ± 17.3 | 49.9 ± 16.7 | 0.73 |
| Gender | Male | 39 (50%) | 24 (48%) | 0.83 |
| Female | 39 (50%) | 26 (52%) | ||
| Size (mm) | - | 22 [14-37] | 21 [14-32] | 0.80 |
| Lesion location related to fascia | Skin | 20 (26%) | 17 (34%) | 0.02 |
| Fascia | 6 (8%) | 11 (22%) | ||
| Both | 43 (56%) | 15 (30%) | ||
| Neither | 9 (12%) | 7 (14%) | ||
| Anatomical distribution | Upper Limb | 29 (37%) | 22 (44%) | 0.42 |
| Lower Limb | 46 (59%) | 24 (48%) | ||
| Trunk | 3 (4%) | 4 (8%) | ||
| Histologya | Non-neoplastic | 22 (40%) | 7 (18%) | 0.14 |
| Benign | 26 (47%) | 25 (64%) | ||
| Malignant Non-malignant Malignant |
7 (13%) 48 (87%) 7 (13%) |
7 (18%) 32 (82%) 7 (18%) |
0.48 |
Statistics based on 94 cases with histological diagnosis.
The commonest non-neoplastic lesions were epidermoid cysts (n = 9), for which CSA was demonstrated in two cases (22.2%) (Figure 1), and ganglion cysts (n = 4) for which CSA was seen in two cases (50%). The commonest benign neoplastic lesions were angioleiomyomas (n = 11) for which CSA was demonstrated in three cases (27.3%) (Figure 3), and vascular malformations (n = 10) for which CSA was seen in seven cases (70%) (Figure 4). The commonest malignant lesions were leiomyosarcoma (n = 4) for which CSA was demonstrated in one case (25%) (Figure 5), and myxoid liposarcoma (n = 3) for which CSA was seen in three cases (100%) (Figure 2). Conversely, lesions for which CSA was seen in all cases included glomus tumours (2 of 2), schwannomas (2 of 2), myopericytomas (3 of 3), myxoid liposarcomas (3 of 3), dermatofibrosarcoma (1 of 1) and Merckel cell carcinoma (1 of 1).
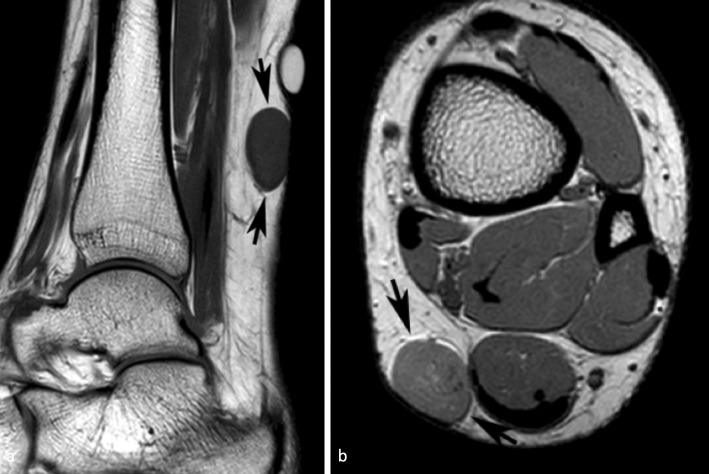
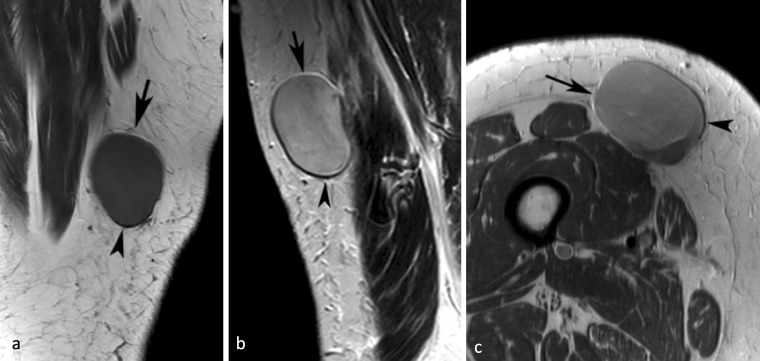
Figure 3.
A 26-yr-old female with an angioleiomyoma of the left distal calf. (a) Sagittal T 1W SE MR image showing an oval intermediate signal intensity lesion (arrows) with linear hyperintensity around its upper margin and hypointensity around its lower margin due to chemical shift artefact. (b) Similarly, axial PDW FSE MR image showing linear hyperintensity around its anterior margin and hypointensity around its posterior margin (arrows). PDW FSE, proton densityweighted fast spin echo; T 1W SE, T 1 weighted spin echo.
Figure 4.
A 45-yr-old male with a slow-flow vascular malformation of the left upper arm. (a) Sagittal T 2W FSE MR image showing a lobular lesion containing small phleboliths with linear hyperintensity around its upper margin (arrow) and hypointensity around its lower margin (arrowhead) due to chemical shift artefact. (b) Similarly, axial PDW FSE MR image showing linear hyperintensity around its medial margin (arrows) and hypointensity around its lateral margin (arrowhead). PDW FSE, proton densityweighted fast spin echo; T 2W FSE, T 2 weightedfast spin echo.
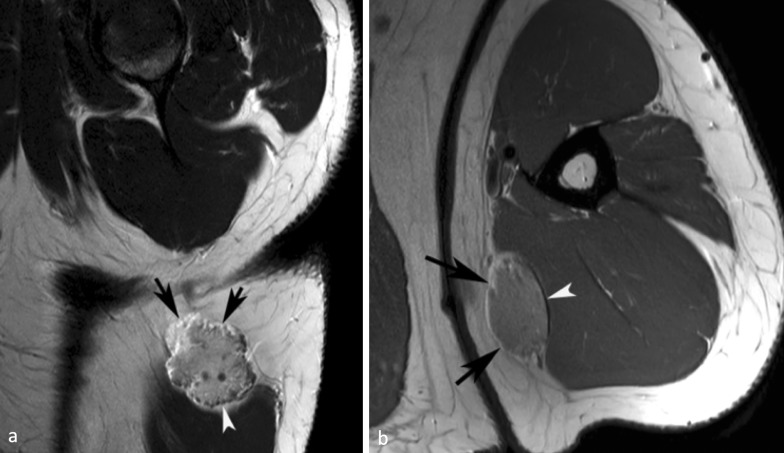
Figure 5.
An 80-yr-old female with a high-grade leiomyosarcoma of the medial right thigh. (a) Coronal T 1W SE and (b) sagittal T 2W FSE MR images showing a well-defined lesion with linear hyperintensity around its upper margin (arrows) and hypointensity around its lower margin (arrowheads) due to chemical shift artefact. (c) Similarly, axial PDW FSE MR image showing linear hyperintensity around its medial margin (arrow) and hypointensity around its lateral margin.
Discussion
The wide differential diagnosis of superficial soft tissue masses has been reviewed in several recent articles. Blacksin et al20 discussed differential diagnosis based on lesion origin from the epidermis, dermis and subcutaneous fat layer. In a further review article, Beaman et al21 classified superficial masses as being of mesenchymal origin, skin appendage tumours, metastatic lesions, other tumours and tumour-like lesions and inflammatory lesions. Further differential diagnosis was based on patient age and location within the subcutaneous compartment, lesions being related to the dermis/epidermis, to the fascia or purely within the subcutaneous fat. Most recently, Zhang et al22 described the CT and MRI features of superficial soft tissue masses. All three articles were based on literature review and gave no indication as to the relative frequency of the different lesions described.
Hung et al23 reviewed the ultrasound features of 714 superficial soft tissue tumours, 247 of which had a confirmed histological diagnosis. 27 different lesions were encountered, of which lipomas (n = 105), vascular malformations (n = 30) and epidermoid cysts (n = 30) were the commonest, and there were only 11 malignant tumours comprising 4.5% of all cases, but 7.7% of non-lipomatous tumours. Khoo et al24 reported on the safety of primary surgical excision in 58 patients with small (<3 cm) superficial soft tissue masses that were indeterminate based on their MRI appearances. 48 were neoplastic and 4 (6.9%) were found to be malignant. All lesions were completely excised, supporting the safety of this practise in a specialist sarcoma service as recommended by recent European guidelines.3 Most recently, Pham et al25 reviewed the histological diagnoses of small (<2 cm) indeterminate soft tissue masses referred to a specialist sarcoma service, finding 7 of 39 cases (17.9%) to be malignant with no differentiating MRI features.
It is known that inadvertent excision of soft tissue sarcomas in non-specialist centres results in a significant proportion with residual tumour ranging from 31 to 72%, as recently reviewed by Grimer et al.26 This requires re-excision of the surgical bed resulting in a more extensive scar, and often post-operative radiotherapy,27 although such management may not compromise overall patient prognosis.28 In a study looking at the patient characteristics of unplanned surgical excision of soft tissue sarcomas, most tumours arose in the thoracic region. Leiomyosarcoma was the commonest tumour, with a diagnosis of lipoma and fibroma/dermatofibroma most commonly being initially made. Half of tumours were small and superficial in location, and just over 60% had received no pre-operative imaging. Approximately, two-thirds of the lesions did not fulfil criteria for referral to a sarcoma service.29
Considering the above findings, any imaging features which can help to distinguish between non-malignant (non-neoplastic lesions and benign neoplasms) and malignant superficial solid soft tissue tumours would be of added value. Galant et al30 described the relationship of subcutaneous soft tissue tumours to the superficial fascia on MRI, finding that if the lesion formed an obtuse angle with the fascia, it had a 6.3 times greater likelihood of being malignant while if the lesion penetrated the fascia it was 6.88 times more likely to be malignant. Calleja et al31 reviewed the clinical and MRI features that helped distinguish superficial sarcomas from benign lesions, with increased patient age, lobular contour, intratumoral haemorrhage/necrosis, fascial oedema and skin thickening all being features suggestive of sarcoma. The addition of DWI to standard MRI criteria has also been advocated as useful in determining the malignancy of a superficial non-fatty mass. Unfortunately, in this study no details were given of the standard MRI criteria used to differentiate benign from malignant tumours.32
The current study including 94 superficial soft issue masses with histological diagnosis arising in the trunk and extremities once again illustrated the wide differential diagnosis of non-fatty lesions, with 18 different non-neoplastic lesions, 18 different benign tumours and 9 different malignant tumours, including 2 metastases. The 14.9% incidence of malignant tumours is likely biased by the fact that 34 lesions were not removed based on a combination of clinical and imaging features. Therefore, if it is assumed that all these 34 lesions were either non-neoplastic or benign, then the incidence of malignant superficial tumours is likely closer to 10.9%. This is still higher than the 7.7% incidence of malignant tumours reported by Hung et al once lipomas had been excluded,23 and the 6.9% reported by Khoo et al,24 but lower than that reported by Pham et al.16
The current study describes a new feature in the MRI assessment of superficial soft tissue tumours, namely CSA at the tumour margin with subcutaneous fat. Although this phenomenon has been previously illustrated in the literature,21,24,32–35 we are unaware of any formal assessment of its incidence or diagnostic relevance. The presence of CSA would suggest that the mass in question has relatively high water content, resulting in CSA similar to that commonly seen at other fluid–lipid interfaces such as the orbits, kidneys and urinary bladder.14 In a series of 128 consecutive non-lipomatous superficial soft tissue masses presenting to a specialist musculoskeletal sarcoma service, CSA was identified in 50 (39.1%) cases with a wide variety of histological diagnoses including non-neoplastic, benign neoplastic and malignant lesions. 20 histologically different lesions demonstrated CSA, of which 7 were non-neoplastic, 25 were benign neoplasms and 7 were malignant tumours. No relationship was found between patient age or sex, lesion size or location within the body and the presence of CSA. However, lesion location adjacent to the fascia was more commonly associated with the presence of CSA, which is difficult to explain. Non-neoplastic lesions were less commonly associated with CSA while benign neoplastic lesions were more commonly associated with CSA, but this difference did not reach statistical significance. Similarly, the presence of CSA could not distinguish non-malignant from malignant lesions. Therefore, it appears that CSA at the tumour–fat interface is not a useful MRI sign for guiding management as to whether a lesion requires excision, or with what degree of margin.
It might have been expected that CSA was a marker of ‘cystic’ lesions such as ganglia and epidermoid cysts, but only 6 of 15 (40%) of ‘cysts’ were associated with CSA. This could be related to the presence of a true cyst wall. As expected, CSA was present in a large proportion of vascular tumours (7 of 10; 70%). Benign neoplasms for which CSA was seen in all cases included glomus tumours (2 of 2), schwannomas (2 of 2) and myopericytomas (3 of 3), while all cases of myxoid liposarcoma (3 of 3), dermatofibrosarcoma (1 of 1) and Merckel cell carcinoma (1 of 1) showed CSA. These findings are of unclear relevance considering the small number of cases. However, the association between CSA and myxoid liposarcoma is not surprising, considering the high fluid content in the myxoid matrix of such lesions.36
The study has several limitations. Interobserver correlation for the presence of CSA was only moderate, which may be due to several reasons. In some cases CSA was obvious (Figure 1), whereas in others it was subtle and may have been considered differently by the two readers. Also, the differentiation between a hypointense cyst wall and possible CSA was occasionally problematic, although it would have been expected that a cyst wall would result in a circumferential line of hypointensity. CSA was the only factor assessed, and it is unclear if combining this with other MRI features that have previously been assessed to differentiate benign from malignant superficial soft tissue masses would have had an impact on the findings. This would likely require a further study with larger patient numbers. The number of malignant lesions was relatively small, although greater than that described in other studies of superficial soft tissue tumours.23,24 Also, the study was performed at 3 T, and it is not known if the findings would be relevant at 1.5 T since CSA is known to be more prominent at higher field strengths.
In conclusion, the current study has documented the presence of CSA at the margin of superficial non-lipomatous soft tissue masses referred to specialist musculoskeletal sarcoma service in 39.1% of cases. However, this sign does not appear to be of added value in differentiating between non-neoplastic, benign neoplastic and malignant superficial soft tissue lesions.
REFERENCES
- 1. Mendenhall WM, Indelicato DJ, Scarborough MT, Zlotecki RA, Gibbs CP, Mendenhall NP, et al. The management of adult soft tissue sarcomas. Am J Clin Oncol 2009; 32: 436–42. doi: 10.1097/COC.0b013e318173a54f [DOI] [PubMed] [Google Scholar]
- 2. Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010; 2010: 1–15. doi: 10.1155/2010/506182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noebauer-Huhmann IM, Weber M-A, Lalam RK, Trattnig S, Bohndorf K, Vanhoenacker F, et al. Soft tissue tumors in adults: ESSR-Approved guidelines for diagnostic imaging. Semin Musculoskelet Radiol 2015; 19: 475–82. doi: 10.1055/s-0035-1569251 [DOI] [PubMed] [Google Scholar]
- 4. Crundwell N, O'Donnell P, Saifuddin A. Non-Neoplastic conditions presenting as soft-tissue tumours. Clin Radiol 2007; 62: 18–27. doi: 10.1016/j.crad.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 5. Goodwin RW, O'Donnell P, Saifuddin A. Mri appearances of common benign soft-tissue tumours. Clin Radiol 2007; 62: 843–53. doi: 10.1016/j.crad.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 6. Walker EA, Fenton ME, Salesky JS, Murphey MD. Magnetic resonance imaging of benign soft tissue neoplasms in adults. Radiol Clin North Am 2011; 49: 1197–217. doi: 10.1016/j.rcl.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 7. De La Hoz Polo M, Dick E, Bhumbra R, Pollock R, Sandhu R, Saifuddin A. Surgical considerations when reporting MRI studies of soft tissue sarcoma of the limbs. Skeletal Radiol 2017; 46: 1667–78. doi: 10.1007/s00256-017-2745-z [DOI] [PubMed] [Google Scholar]
- 8. Subhawong TK, Jacobs MA, Fayad LM. Insights into quantitative diffusion-weighted MRI for musculoskeletal tumor imaging. AJR Am J Roentgenol 2014; 203: 560–72. doi: 10.2214/AJR.13.12165 [DOI] [PubMed] [Google Scholar]
- 9. Teixeira PAG, Gay F, Chen B, Zins M, Sirveaux F, Felblinger J, et al. Diffusion-Weighted magnetic resonance imaging for the initial characterization of non-fatty soft tissue tumors: correlation between T2 signal intensity and ADC values. Skeletal Radiol 2016; 45: 263–71. doi: 10.1007/s00256-015-2302-6 [DOI] [PubMed] [Google Scholar]
- 10. Song Y, Yoon YC, Chong Y, Seo SW, Choi Y-L, Sohn I, et al. Diagnostic performance of conventional MRI parameters and apparent diffusion coefficient values in differentiating between benign and malignant soft-tissue tumours. Clin Radiol 2017; 72: 691.e1–691.e10. doi: 10.1016/j.crad.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 11. Lim HK, Jee W-H, Jung J-Y, Paek MY, Kim I, Jung C-K, et al. Intravoxel incoherent motion diffusion-weighted MR imaging for differentiation of benign and malignant musculoskeletal tumours at 3 T. Br J Radiol 2018; 91: 20170636. doi: 10.1259/bjr.20170636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oka K, Yakushiji T, Sato H, Fujimoto T, Hirai T, Yamashita Y, et al. Usefulness of diffusion-weighted imaging for differentiating between desmoid tumors and malignant soft tissue tumors. J Magn Reson Imaging 2011; 33: 189–93. doi: 10.1002/jmri.22406 [DOI] [PubMed] [Google Scholar]
- 13. Chhabra A, Ashikyan O, Slepicka C, Dettori N, Hwang H, Callan A, et al. Conventional Mr and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur Radiol 2019; 29: 4485–94. doi: 10.1007/s00330-018-5845-9 [DOI] [PubMed] [Google Scholar]
- 14. Hood MN, Ho VB, Smirniotopoulos JG, Szumowski J. Chemical shift: the artifact and clinical tool revisited. Radiographics 1999; 19: 357–71. doi: 10.1148/radiographics.19.2.g99mr07357 [DOI] [PubMed] [Google Scholar]
- 15. Morelli JN, Runge VM, Ai F, Attenberger U, Vu L, Schmeets SH, et al. An image-based approach to understanding the physics of Mr artifacts. Radiographics 2011; 31: 849–66. doi: 10.1148/rg.313105115 [DOI] [PubMed] [Google Scholar]
- 16. Farshchian N, Tamari S, Farshchian N, Madani H, Rezaie M, Mohammadi-Motlagh H-R. Diagnostic value of chemical shift artifact in distinguishing benign lymphadenopathy. Eur J Radiol 2011; 80: 594–7. doi: 10.1016/j.ejrad.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 17. Zhang H, Zhang C, Zheng Z, Ye F, Liu Y, Zou S, et al. Chemical shift effect predicting lymph node status in rectal cancer using high-resolution MR imaging with node-for-node matched histopathological validation. Eur Radiol 2017; 27: 3845–55. doi: 10.1007/s00330-017-4738-7 [DOI] [PubMed] [Google Scholar]
- 18. Weis J, Aström G, Vinnars B, Wanders A, Ahlström H. Chemical-shift micro-imaging of subcutaneous lesions. MAGMA 2005; 18: 59–62. doi: 10.1007/s10334-004-0099-8 [DOI] [PubMed] [Google Scholar]
- 19. Bridge J. A, Hogendoorn P. C, Fletcher C. eds. WHO Classifiation of Tumours of Soft Tisse and Bone 2013, 4th ed ; 2013. . [Google Scholar]
- 20. Blacksin MF, Ha D-H, Hameed M, Aisner S. Superficial soft-tissue masses of the extremities. Radiographics 2006; 26: 1289–304. doi: 10.1148/rg.265055729 [DOI] [PubMed] [Google Scholar]
- 21. Beaman FD, Kransdorf MJ, Andrews TR, Murphey MD, Arcara LK, Keeling JH. Superficial soft-tissue masses: analysis, diagnosis, and differential considerations. RadioGraphics 2007; 27: 509–23. doi: 10.1148/rg.272065082 [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Li Y, Zhao Y, Qiao J. Ct and MRI of superficial solid tumors. Quant Imaging Med Surg 2018; 8: 232–51. doi: 10.21037/qims.2018.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hung EHY, Griffith JF, Ng AWH, Lee RKL, Lau DTY, Leung JCS. Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. AJR Am J Roentgenol 2014; 202: W532–40. doi: 10.2214/AJR.13.11457 [DOI] [PubMed] [Google Scholar]
- 24. Khoo M, Pressney I, Hargunani R, Saifuddin A. Small, superficial, indeterminate soft-tissue lesions as suspected sarcomas: is primary excision biopsy suitable? Skeletal Radiol 2017; 46: 919–24. doi: 10.1007/s00256-017-2635-4 [DOI] [PubMed] [Google Scholar]
- 25. Pham K, Ezuddin NS, Pretell-Mazzini J, Subhawong TK. Small soft tissue masses indeterminate at imaging: histological diagnoses at a tertiary orthopedic oncology clinic. Skeletal Radiol 2019; 48: 1555–63. doi: 10.1007/s00256-019-03205-0 [DOI] [PubMed] [Google Scholar]
- 26. Grimer R, Parry M, James S. Inadvertent excision of malignant soft tissue tumours. EFORT Open Rev 2019; 4: 321–9. doi: 10.1302/2058-5241.4.180060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pretell-Mazzini J, Barton MD, Conway SA, Temple HT. Unplanned excision of soft-tissue sarcomas: current concepts for management and prognosis. J Bone Joint Surg Am 2015; 97: 597–603. doi: 10.2106/JBJS.N.00649 [DOI] [PubMed] [Google Scholar]
- 28. Bianchi G, Sambri A, Cammelli S, Galuppi A, Cortesi A, Righi A, et al. Impact of residual disease after "unplanned excision" of primary localized adult soft tissue sarcoma of the extremities: evaluation of 452 cases at a single Institution. Musculoskelet Surg 2017; 101: 243–8. doi: 10.1007/s12306-017-0475-y [DOI] [PubMed] [Google Scholar]
- 29. Dyrop HB, Safwat A, Vedsted P, Maretty-Kongstad K, Hansen BH, Jørgensen PH, et al. Characteristics of 64 sarcoma patients referred to a sarcoma center after unplanned excision. J Surg Oncol 2016; 113: 235–9. doi: 10.1002/jso.24137 [DOI] [PubMed] [Google Scholar]
- 30. Galant J, Martí-Bonmatí L, Soler R, Saez F, Lafuente J, Bonmatí C, et al. Grading of subcutaneous soft tissue tumors by means of their relationship with the superficial fascia on MR imaging. Skeletal Radiol 1998; 27: 657–63. doi: 10.1007/s002560050455 [DOI] [PubMed] [Google Scholar]
- 31. Calleja M, Dimigen M, Saifuddin A. Mri of superficial soft tissue masses: analysis of features useful in distinguishing between benign and malignant lesions. Skeletal Radiol 2012; 41: 1517–24. doi: 10.1007/s00256-012-1385-6 [DOI] [PubMed] [Google Scholar]
- 32. Jeon JY, Chung HW, Lee MH, Lee SH, Shin MJ. Usefulness of diffusion-weighted MR imaging for differentiating between benign and malignant superficial soft tissue tumours and tumour-like lesions. Br J Radiol 2016; 89: 20150929. doi: 10.1259/bjr.20150929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hong SH, Chung HW, Choi J-Y, Koh YH, Choi J-A, Kang HS. Mri findings of subcutaneous epidermal cysts: emphasis on the presence of rupture. AJR Am J Roentgenol 2006; 186: 961–6. doi: 10.2214/AJR.05.0044 [DOI] [PubMed] [Google Scholar]
- 34. Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. Mr imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics 2011; 31: 1321–40. doi: 10.1148/rg.315105213 [DOI] [PubMed] [Google Scholar]
- 35. Wadhwa V, Lee PP, Strome GM, Suh KJ, Carrino JA, Chhabra A. Spectrum of superficial nerve-related tumor and tumor-like lesions: MRI features. Acta Radiol 2014; 55: 345–58. doi: 10.1177/0284185113494983 [DOI] [PubMed] [Google Scholar]
- 36. Sung MS, Kang HS, Suh JS, Lee JH, Park JM, Kim JY, et al. Myxoid liposarcoma: appearance at MR imaging with histologic correlation. Radiographics 2000; 20: 1007–19. doi: 10.1148/radiographics.20.4.g00jl021007 [DOI] [PubMed] [Google Scholar]