Abstract
Remote and minimally-invasive modulation of biological systems with light has transformed modern biology and neuroscience. However, light absorption and scattering significantly prevents penetration to deep brain regions. Here we describe the use of gold-coated mechanoresponsive nanovesicles, which consist of liposomes made from the artificial phospholipid Rad-PC-Rad as a tool for the delivery of bioactive molecules into brain tissue. Near-infrared picosecond laser pulses activated the gold-coating on the surface of nanovesicles, creating nanomechanical stress and leading to near-complete vesicle cargo release in sub-seconds. Compared to natural phospholipid liposomes, the photo-release was possible at 40 times lower laser energy. This high photosensitivity enables photorelease of molecules down to a depth of 4 mm in mouse brain. This promising tool provides a versatile platform to optically release functional molecules to modulate brain circuits.
Keywords: mechanoresponsive vesicles, near-infrared light, gold shell, uncaging, brain
Graphical Abstract

Ultra-photosensitive nanovesicles are developed by coating gold shell on the surface of mechanoresponsive nanovesicles. Gold shell transduces near-infrared laser pulses to nanomechanical force, leading to efficent cargo release in sub-seconds. This high photosensitivity enables photorelease of molecules down to a depth of 4 mm in mouse brain.
Introduction
Remote modulation of biological activity and behavior has important implications in medicine and biology.[1] Various triggers such as light,[2] ultrasound[3] and magnetic fields[4] have been used to remotely control biological processes, including in the brain. Light is an important tool to modulate biological systems, such as for photo-induced drug release or uncaging in the treatment of cancer and neurological disorders,[5] modifying cell signaling,[6] and optogenetics[7] due to the high spatial resolution and wide availability of low-cost lasers. However, light absorption and scattering in biological tissue significantly limits the penetration,[8] with 1/e penetration depth of only 1–2 mm for visible light.[9] Alternatively, optical fibers can deliver light deep into the tissue, but permanent implants are fraught with problems such as inflammation, tissue damage and scar formation.[10] Using near-infrared (NIR) light (650 to 1350 nm) has promise to reach deeper tissue regions.[11] Recently, Chen et al. demonstrated deep brain stimulation by combination of NIR and upconversion nanoparticle-mediated optogenetics.[2a] Several studies reported NIR-stimulated drug release, however, this approach is still limited to superficial tissue regions[12] or transparent organisms.[13] The light energy available in deep tissue regions is a limiting factor to trigger the release. Thus, remote photorelease of biomolecules into deep tissue regions represents a significant challenge since it requires high dose of light energy at the surface that can be possibly toxic.
Here, we present a new approach to engineer highly photosensitive nanovesicles which can be used to remotely release biomolecules into deep brain regions with NIR light. We build upon recently formulated mechanoresponsive gel phase nanovesicles with a non-spherical shape and defect edges (Figure 1).[14] These nanovesicles release their entrapped cargo upon a physical trigger, such as an increase in shear stress as found in atherosclerotic stenosis.[15] Gold-coating on the nanovesicles makes them plasmonically active and enables efficient absorption of NIR light.[16] Activating the gold-coated mechanoresponsive vesicles with ultrashort laser pulses, generates nanoscale photomechanical effects (such as pressure wave and nanoscale cavitation) and leads to rapid and more efficient release of encapsulated molecules when compared with nanovesicles made from natural lipids. The high photosensitivity further effectively doubles the penetration depth for biomolecule release in the brain compared with the standard nanovesicles, or to around 4 mm, a depth that covers most of the brain regions in a mouse model. We anticipate that the ability to release biomolecules at deeper tissue will find broad biomedical applications from cancer treatment to deep brain modulation.
Figure 1. Schematic illustration of near-infrared (NIR) laser pulse-triggered release in deep brain regions using gold-coated mechanoresponsive nanovesicles (Au-m-nV).

Au-m-nV were injected locally to the mouse deep brain regions. After activating Au-m-nV with NIR ultrashort laser pulses, nanoscale photomechanical effects (such as nanoscale cavitation) are generated and break the defect line of mechanoresponsive nanovesicles, leading to rapid and efficient release of encapsulated molecules. Illustration of mouse was adapted with permission from Ref 2a.
Results and Discussion
First, we prepared and characterized plasmonic mechanoresponsive nanovesicles. 1,3-Diamidophospholipid Rad-PC-Rad was synthesized as previously reported.[14b] The carboxylic acid ester moiety of the natural phospholipid was exchanged by an amide bond and the configuration at the glycerol backbone was changed from the natural sn-1,2 to a sn-1,3 arrangement (Figure 2a). The amide moieties allow additional tight hydrogen-bonding interactions between the phospholipids. Furthermore, the increased spacing of non-natural 1,3-arrangement between the lipid tails allows for an interdigitation of the two bilayer leaflets.[17] This leads to very stiff bilayers in the gel phase and therefore significant changes in the vesicle morphology that is now heavily faceted (Supporting information, Figure S1). We then coated Rad-PC-Rad nanovesicles with gold (Au-m-nV) to obtain near-infrared plasmon resonances from 700 to 900 nm (Figure 2b) and observed hydrodynamic diameter increase due to gold-coating by dynamic light scattering (Figure S1). Au-m-nV show highly faceted geometries and non-spherical morphology in transmission electron microscopy (TEM) images (Figure 2c). Compact and continuous gold shell instead of individual nanoparticle was observed on the surface of Au-m-nV by high-resolution scanning electron microscopy (SEM) and TEM (Figure 2c, Supporting information, Figure S2). The formation of gold lattice and presence of Au in the energy-dispersive X-ray spectrum (EDS) further demonstrates gold shells formation on the surface. The hybridization interaction of plasmons at the inner and outer surfaces of the shell leads to the near-infrared absorption.[18] Gold-coated nanovesicles (Au-nV) were also prepared as a control from natural DPPC lipid with a phase transition temperature of 41.3 °C[19] (Supporting information, Figure S3, Table S1). Individual small gold nanoparticles deposit on the surface of Au-nV. The difference of the gold-coating between Au-m-nV and Au-nV might be attributed to the difference of shape or stiffness between these two nanovesicles. Au-m-nV showed high stability without changes of size and polydispersity index in 0.01 M phosphate-buffered saline (PBS) at 4 °C over two weeks (Supporting information, Figure S4), consistent with the previous report.[16b]
Figure 2. Characterization and NIR laser pulse-triggered release of Au-m-nV.
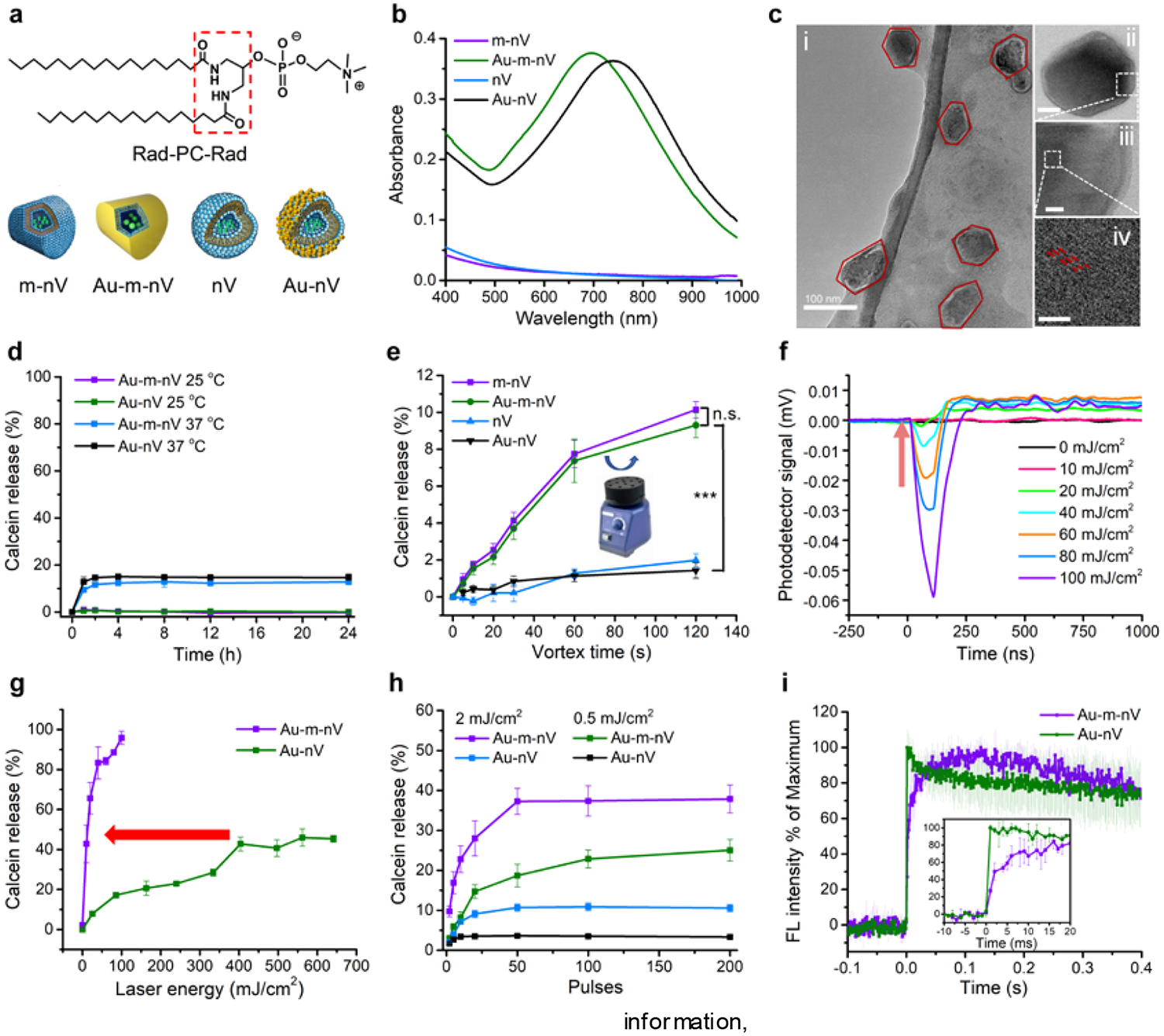
(a) Molecular structure of Rad-PC-Rad and nomenclature of different nanovesicles used in this work. m-nV were made from Rad-PC-Rad lipid, and nV were made from natural DPPC lipid. Gold-coating was prepared on the surface of these nanovesicles (Au-m-nV, Au-nV). (b) UV-Vis spectra of different nanovesicles. (c) Transmission electron microscopy (TEM) images of Au-m-nV. Scar bar: i, 100 nm; ii, 50 nm; iii, 10nm; iv, 2 nm. (d) Spontaneous release after keeping nanovesicles in 10% fetal bovine serum (FBS) at 25 °C and 37 °C. (e) Mechanical stimulation (i.e. vortex shaking) leads to molecule release from mechanoresponsive nanovesicles (m-nV, Au-m-nV,), but not standard nanovesicles (nV, Au-nV) (n=6). n.s., not significant, *** p < 0.001. (f) Measurement of cavitation nanobubbles generated by high-energy near-infrared laser pulse stimulation of Au-m-nV. Arrow indicates that laser pulse was given. (g) Comparison of photorelease efficiency of Au-m-nV and Au-nV under single pulse irradiation (740 nm). Arrow shows the reduction in laser pulse energy that leads to approximately 40% release of encapsulated content (404 mJ/cm2 for Au-nV and 10 mJ/cm2 for Au-m-nV). (h) Comparison of photorelease efficiency of Au-m-nV and Au-nV under multiple pulses irradiation (740 nm) at low laser energies. (i) Kinetics of photoreleasing calcein dye from Au-m-nV and Au-nV (740 nm, 40 mJ/cm2, 1 pulse). Insert figure shows the initial ultra-fast release from Au-m-nV within 2 ms. Data were expressed as Mean ± SD (n = 3).
To test their mechanoresponsivity, nanovesicles containing calcein at a self-quenching concentration during encapsulation were prepared. There was no calcein release at 25 °C when nanovesicles were kept in 0.01 M PBS or 10% fetal bovine serum (FBS) and left untouched on the laboratory bench for 24 hours (Figure 2d and Supporting information, Figure S5). Less than 13% release was measured at 37 °C for Au-m-nV. The high stability of Au-m-nV is attributed to the high main phase transition temperature of Rad-PC-Rad at 44.7 °C and the tight interdigitation of lipid chains.[17] The nanovesicles were then vortexed using a laboratory vortex mixer (Figure 2e). Both the m-nV and Au-m-nV released the encapsulated dye molecules, while no release was observed for DPPC vesicles in the presence or absence of gold-coating (Au-nV and nV). There were no significantly different responses on the mechanical stress for Au-m-nV and m-nV. This indicates that the vortexing disrupts the m-nV and Au-m-nV due to its mechanoresponsivity and gold-coating does not affect the mechanoresponsivity. With their unique mechanoresponsive feature, Au-m-nV are ideally suited for photorelease based on nanomechanical effects. Ultrashort laser pulse excitation of plasmonic nanoparticles leads to nanomechanical effects such as photoacoustic pressure wave and, at high laser intensities, vapour nanobubble formation,[20] where in both cases the ultrashort picosecond laser stimulation can lead to nanomechanical forces to induce membrane stress and rupture.[21] We detected the presence of water vapour nanobubbles above 20 mJ/cm2 fluence when irradiating Au-m-nV with ultrashort picosecond laser pulses (Figure 2f). By measuring the calcein dye release from the nanovesicles on a capillary system (Supporting information, Figure S6), single ultrashort laser pulse irradiation (28 ps, 740 nm) leads to 40% calcein release from Au-m-nV at 10 mJ/cm2, compared with Au-nV at 404 mJ/cm2. Thus, Au-m-nV allow 40-fold less laser pulse energy to reach the same release as Au-nV does and show the potential for complete release (Figure 2g). We further compared the release efficiency by using lower pulse energy and multiple pulses (Figure 2h), and found that the maximum release efficiency for Au-m-nV was 4–5 folds higher than that of Au-nV., There is no temperature increase of the solution under the laser irradiation (Supporting information, Figure S7), suggesting that nanoscale heating exists and, when dissipated, it is insufficient to cause bulk heating.[22] Real-time fluorescent imaging shows that Au-m-nV exhibits an interesting two-step release kinetics, with an initial fast release (approximately 50% of fluorescence increase within 2 ms) and a slower and continuous release in the next 0.1 s (Figure 2i, Supporting information, Figure S8). In comparison, Au-nV shows a one-step fast fluorescence increase within 1 ms. Comparison of the release efficiency and kinetics shows an interesting difference between Au-m-nV and Au-nV. Au-nV shows an ultrafast (<1 ms) but inefficient release, while Au-m-nV shows a two-step and highly efficient release at much lower laser energy. Considering the higher phase transition temperature of Rad-PC-Rad (44.7 °C) than that of DPPC (41.3 °C), the highly efficient photorelease from Au-m-nV may be due to the fact that the photomechanical stress breaks the packing defects and thus the integrity of the Rad-PC-Rad vesicles that are already under internal stress. This also excludes the effect of nanoscale heating as the dominant mechanism since the DPPC has a lower phase transition temperature than Rad-PC-Rad.
Next, we investigated the photorelease of plasmonic mechanoresponsive liposomes in live cells (murine macrophage Raw 264.7 cells). We tested NIR laser pulse-induced photorelease of fluorescent dye (calcein) inside the cells using a real-time imaging system (Figure 3a). Calcein-loaded Au-m-nV were allowed to be taken up by Raw 264.7 cells through endocytosis (Supporting information, Figure S9). Once in the endosomes, laser stimulation of nanovesicles triggered the release of calcein into the cell cytosol (Figure 3b). The quantitative analysis shows 3-fold higher fluorescence intensity for Au-m-nV compared with Au-nV under the same conditions (Figure 3c). Next, we investigated the cellular response of photoreleasing a secondary messenger molecule (inositol trisphosphate, IP3) from Au-m-nV (Figure 3d). IP3 can bind to the receptor located on the endoplasmic reticulum membrane, which causes calcium ion (Ca2+) release from endoplasmic reticulum into cytosol.[23] Fluo-4 was used as Ca2+ indicator. We monitored the Ca2+ responses following photorelease of IP3 from Au-m-nV and observed a prolonged and higher Ca2+ response in Au-m-nV as compared to Au-nV (Figure 3e,f and Supporting information, Figure S10). There was no Ca2+ response by laser irradiation alone on the cells. These results indicate a higher release efficiency of Au-m-nV causes larger amounts of intracellular calcium release, which takes the cells longer to sequester the calcium back into intracellular compartments.[24] We further tested the cytotoxicity of Au-m-nV and laser irradiation using WST-1 and live/dead viability assays. The cell viability is over 95% even at 1 mM of lipid and laser pulses irradiation did not affect the cell viability at 30 mJ/cm2, an energy level for calcein and IP3 uncaging (Supporting information, Figure S11 and Figure S12). These results indicate that the intracellular uncaging technique shows no significant toxicity to living cells.
Figure 3. NIR laser pulse-triggered release inside living cells.
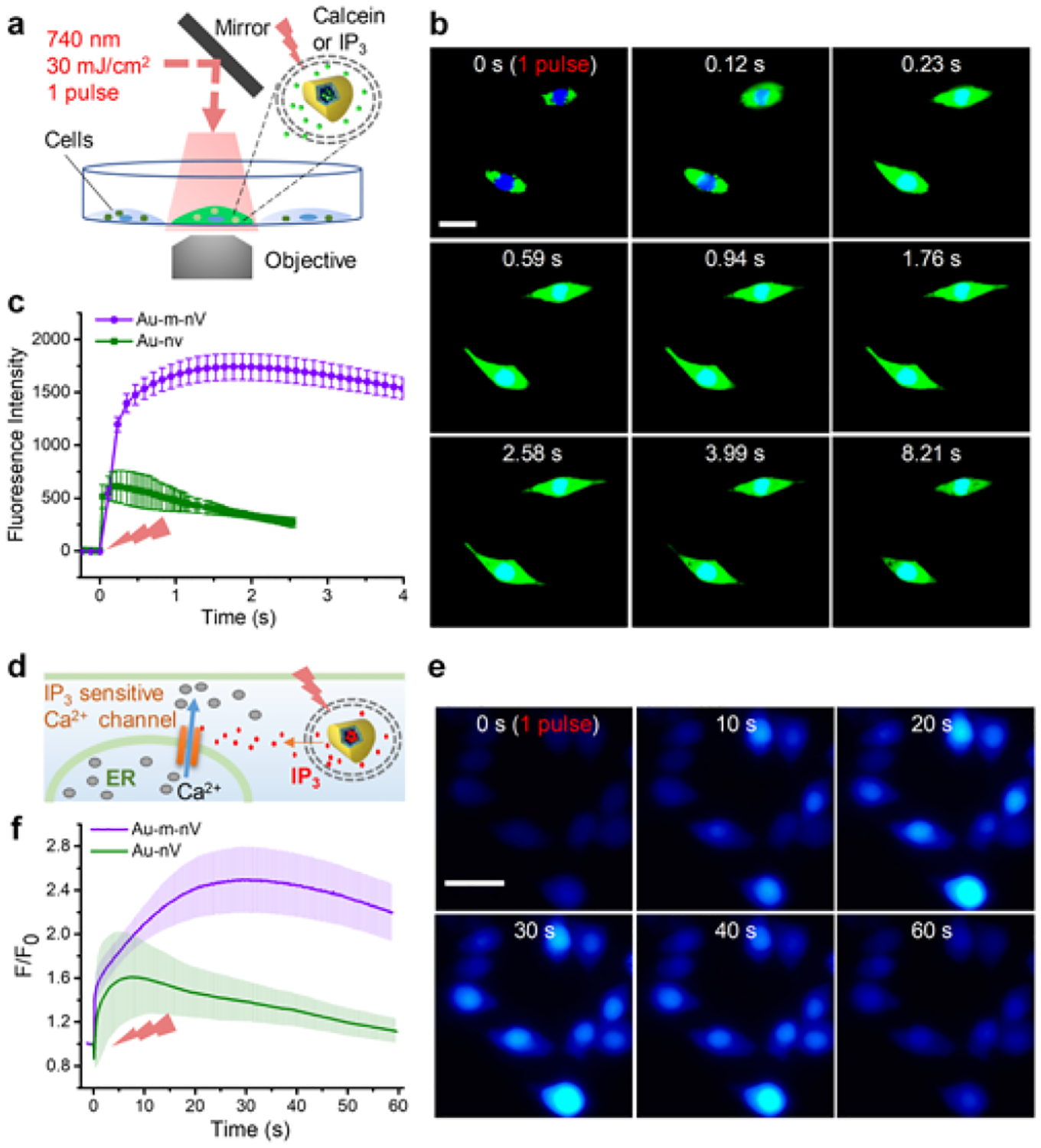
(a) Schematic of experimental setup for NIR laser pulse-triggered intracellular release. (b) Snapshots intracellular release of calcein in live cells (Raw 264.7). (c) Real-time changes of fluorescence intensity for the photo-stimulated cell. (d) Schematic and (e) snapshots of calcium imaging after photoreleasing secondary messenger molecule IP3 from nanovesicles in live cells (Raw 264.7). (f) Quantification of the calcium signal after photorelease from Au-m-nV and Au-nV. Single pulse, NIR (740 nm) laser was applied at 30 mJ/cm2 in all the experiments. Scale bar: 20 μm. Data were expressed as Mean ± SD (n = 3).
Finally, we explored the capability of remotely releasing biomolecules in deep brain regions of C57BL/6 mice using NIR light. To study this, we stereotaxically injected1 μL of calcein-loaded Au-m-nVs, or Au-nVs, at 2 mm and 4 mm depths into the striatum (0.14 mm anterior, 2 mm lateral relative to bregma) (Figure 4a). After a NIR laser beam (740 nm, 170 mJ/cm2) was applied to the cortical surface, the brain was immediately dissected, frozen and cut into 40 μm coronal slices for fluorescent imaging. Calcein released from nanovesicle is expected to increase the green fluorescence, compared to the low fluorescence signal from calcein-loaded nanovesicles due to self-quenching at high concentrations (75 mM). Imaging of brain slices revealed fluorescent spots with higher intensity for the Au-m-nV laser-treated group at both 2 and 4 mm depth (Figure 4b and Supporting information, Figure S13). We measured and confirmed the actual injection and photorelease depth to be 2 mm and 4mm in brain (Supporting information, Figure S14). We collected brain slices around the injection site and determined the total calcein fluorescence intensity for each section as a function of the anterior-posterior axis. At the 2 mm depth, the fluorescence distribution shows an increase in intensity with laser stimulation that is higher for Au-m-nV as compared to Au-nV groups (Figure 4c). We integrated the fluorescence intensity for each mouse (i.e. area under the curve for Figure 4c) and found the calcein fluorescence is 2.3-fold higher in Au-m-nV group than that in Au-nV group under laser irradiation (Figure 4d). This indicates a higher photorelease efficiency in vivo for Au-m-nV than Au-nV under the same laser irradiation. There was no significant release from Au-m-nV in the absence of NIR laser pulses, suggesting that the mechanoenvironment in the brain is insufficient to cause molecule release from Au-m-nV.[15,25] At 4 mm depth, laser stimulation does not lead to an observable photorelease for Au-nV, while the Au-m-nV group continues to show a clear fluorescent signal (Figure 4c,d). Calcein fluorescence is 3.9-fold higher in the Au-m-nV group than that in the Au-nV group at 4 mm depth. These results suggest Au-m-nV can be photoreleased at 4 mm in the brain, while there is insufficient laser energy to photorelease Au-nV. To confirm the results, we mixed nanovesicles with a fluorescent dye (dextran-Texas red) which serves as a reference and does not change with laser stimulation (Supporting information, Figure S15). This allows us to analyze the fluorescent intensity ratios between calcein and dextran-Texas red. The fluorescent ratio shows similar results, thus further confirming calcein release at 4 mm depth using Au-m-nV while Au-nV can only be released at 2 mm depth (Figure 4e). These results demonstrate that the high photosensitivity of plasmonic mechanoresponsive nanovesicles (Au-m-nV) enable remote release of biomolecules in deep brain regions down to 4 mm, covering most brain regions in mouse. This technique provides significant potential to modulate deep brain activity.
Figure 4. NIR laser pulse-triggered release in mouse brain.
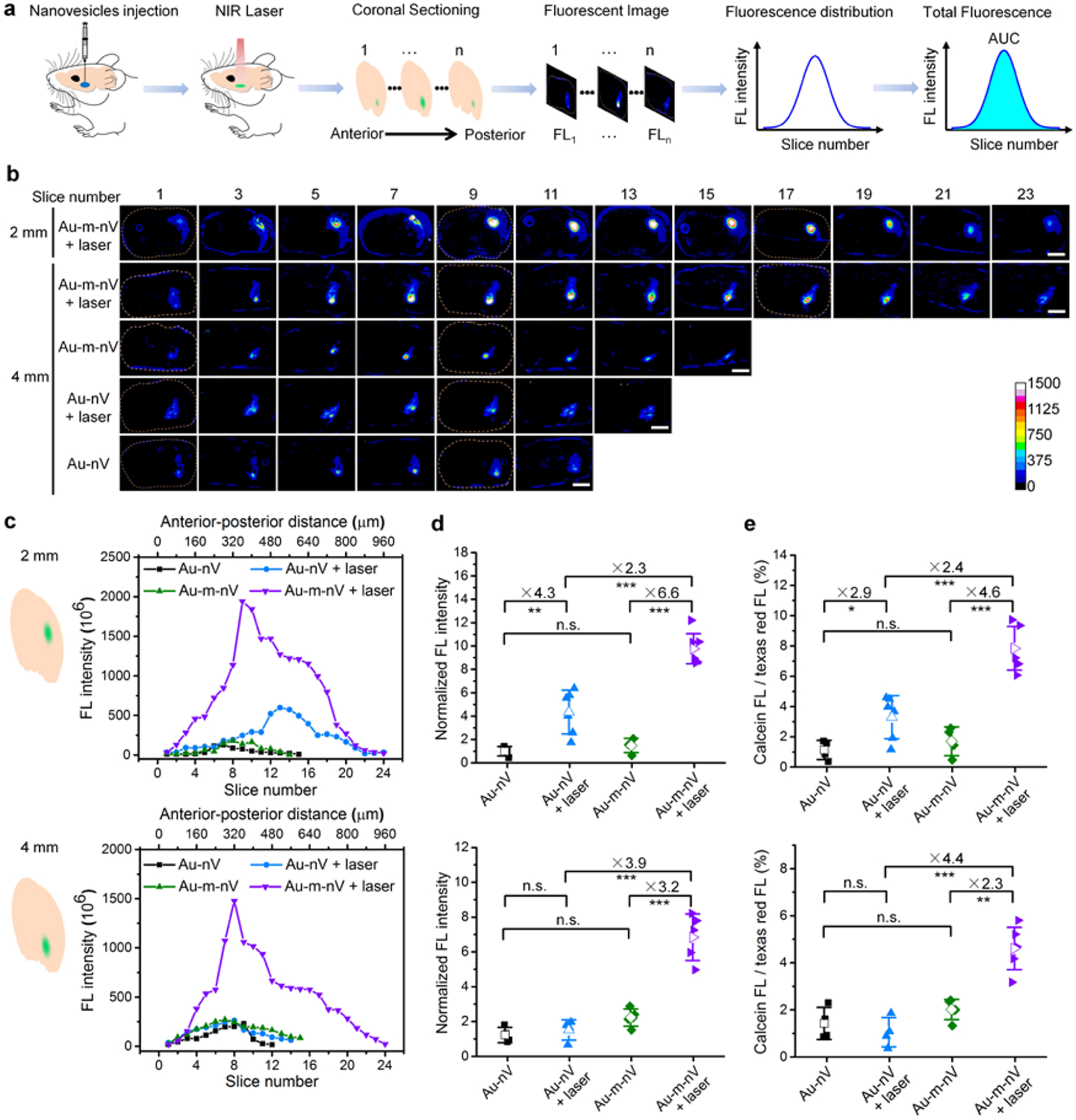
(a) Schematic of experimental procedures. Nanovesicle solution (1 μL) was stereotaxically injected to different depths in the brain and then NIR laser pulses (740 nm, 170 mJ/cm2, 400 pulses) were applied on the brain surface. Afterwards, brain was immediately extracted and frozen to obtain coronal slices (40 μm) for imaging. Calcein fluorescence intensity was analyzed for each slice and integrated as total fluorescence intensity for each mouse. (b) Representative fluorescent images of brain sections. Scale bar: 2 mm. (c) Fluorescence distribution in each slice at different depths in the brain (top: 2 mm, bottom: 4 mm). The difference in the peak location is due to the different amount of brain slices that give fluorescent signal in each case. (d) Normalized total fluorescence intensity for each mouse at different depths (top: 2 mm, bottom: 4 mm, n = 4–6 for each group). (e) Ratio of calcein and dextran-Texas red fluorescence for each mouse at different depths (top: 2 mm, bottom: 4 mm, n = 4–6 for each group). Data were expressed as Mean ± SD; n.s., not significant, * p < 0.05, ** p < 0.01, *** p < 0.001.
We further examined any phototoxocity and the laser attenuation by pathology analysis and Monte Carlo simulations, respectively. First, we carried out immunostaining of brain sections 24 h after remote laser irradiation with injected nanovesicles. Images and quantification both show there is no difference in immunoreactivity as quantified by fluorescent intensity for neuron-specific nuclear protein (NeuN) and glial fibrillary acidic protein (GFAP) between laser-stimulated and the contralateral control hemispheres (Figure 5a,b). This suggests that there is no photodamage in the cortex where the laser intensity is the highest since the laser is applied on the cortical surface. We also examined the striatum where the nanovesicles were injected. The dark contrast in the bright field images shows the presence of gold-coated nanovesicles (Figure 5c). No differences in cell nuclei number or size were observed in the area around the injection site (318 μm × 318 μm) or the enlarged view (100 μm × 100 μm) (Figure 5c–e). This suggests that laser stimulation under these conditions does not evoke phototoxicity. To examine the light attenuation in the brain, we performed Monte Carlo simulation using MCML program.[26] 2D map of laser fluence distribution shows that the laser fluence drops dramatically within the cortex (first layer of grey matter) after entering the brain (Figure 5f). Furthermore, the white matter (corpus callosum) behaves like a “light barrier” and significantly attenuates the light intensity (Supporting information, Figure S16).[9] The light intensity quickly decays as one moves deeper in the brain (Figure 5g). The estimated laser pulse energy at 4 mm depth is around 0.1 mJ/cm2 and significantly lower than the pulse energy at cortical surface (170 mJ/cm2). Nanovesicles were not considered in the Monte Carlo since they only reside in the injected site (<100 μm2, Figure 5c). We then tested the release efficiency in vitro under similar conditions. The result indicates that Au-m-nV show 3-fold higher release than Au-nV (Figure 5h), which is consistent with the in vivo results.
Figure 5. Assessment of laser phototoxicity and attenuation in the brain.

(a) Hoechst, NeuN and GFAP labeled sections of a mouse brain 24 h after laser irradiation on the right hemisphere. The area inside the dotted line was used for quantitative fluorescence intensity analysis. Scale bar: 1 mm. (b) The ratio of fluorescence intensity for Hoechst, NeuN, and GFAP on two hemispheres of the corresponding sections (n=5). (c) Confocal images of particles injected area in the brain sections from laser irradiated and non-irradiated mice. Black signal in bright field shows the existence of gold. Area (100 μm × 100 μm) in the center was enlarged. Scale bar: 50 μm. (d) Nuclei number in particles injected area from laser irradiated and non-irradiated mice (n=3). (e) Box plot of nuclei size (n=3). (f) 2D map of laser fluence distribution in brain (740 nm, laser fluence at surface is 170 mJ/cm2). (g) Laser fluence at 2 mm and 4 mm depth. (h) Comparison of in vitro release efficiency for Au-m-nV and Au-nV at 0.1 mJ/cm2 at the same laser pulse conditions as in vivo (Figure 4). Data were expressed as Mean ± SD; *** p < 0.001; n.s., not significant.
Numerous molecules contribute to modulating brain activity and controlling the release of these molecules in deep brain regions remain a significant challenge. To date, caged compounds, such as caged glutamate or GABA, are widely employed to study cell signaling in glia and neurons, and have contributed significantly to our understanding of the nervous system.[27] However, uncaging with UV or blue light is limited by the photo-toxicity and tissue penetration. The gold-coated mechanoresponsive nanovesicles are responsive to NIR light and promising to address this challenge by packaging and releasing various neuroactive molecules. Packaged molecules are separated from the physiological environment and prevent possible degradation or off-target activity. The high NIR photosensitivity enables the release with ideal temporal and spatial resolution even in deep brain regions. Furthermore, the ultra-photosensitive nanovesicles are taking advantage of nanoscale mechanical force without creating a global heating,[16] which can reduce the toxicity. Therefore, results from this work will have significant impact to allow remote photorelease of neuroactive molecules in the brain in live and behaving animals.
Conclusion
We have developed a novel photorelease system that can be stimulated by near-infrared light in deep brain regions. Gold shell on the surface of mechanoresponsive nanovesicles efficiently transduced NIR laser pulse to nanomechanical force for fast (< 0.1s) and efficient release. Photorelease from plasmonic mechanoresponsive nanovesicles could be realized at much lower laser exposure (40 times less) compared with standard liposomes. We demonstrated the mechanoresponsive nanovesicles can be used for fast intracellular IP3 uncaging, which triggers intracellular Ca2+-dependent signaling pathways in living cells. Importantly, the highly photosensitive nanovesicles enabled biomolecular release down to 4 mm in the rodent brain, making the technology accessible to most brain regions of interest. We anticipate that this novel ultra-photosensitive nanovesicle system will offer a versatile platform for ultra-rapid uncaging of a wide range of neuroactive molecules and find broad applications in remotely stimulating brain activity in deep tissue regions.
Supplementary Material
Acknowledgements
We thank Dr. Jinguo Wang and Dr. Qingxiao Wang (University of Texas at Dallas) for the technical advice and assistance with HAADF-STEM. This work was partially supported by the University of Texas System Neuroscience and Neurotechnology Research Institute (UTSNNRI) under award number 362492 (Z.Q.), National Science Foundation under award number 1631910 (Z.Q.), National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under award number RF1NS110499 (Z.Q., P.A.S.), and National Institute of Mental Health of NIH under award number R01MH111499 (P.A.S.), National Institute of Biomedical Imaging and Bioengineering under award number K99EB023993 (H.W.), and National Heart, Lung, and Blood Institute under award number R01HL51177 (J.A.Z.). A.Z. was supported by a stipend professorship of the Swiss National Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Supporting information for this article is given via a link at the end of the document.
Contributor Information
Hejian Xiong, Department of Mechanical Engineering, The University of Texas at Dallas, Richardson, Texas 75080 (United States).
Xiuying Li, Department of Mechanical Engineering, The University of Texas at Dallas, Richardson, Texas 75080 (United States).
Peiyuan Kang, Department of Mechanical Engineering, The University of Texas at Dallas, Richardson, Texas 75080 (United States).
John Perish, School of Behavioral and Brain Sciences, The University of Texas at Dallas, Richardson, Texas 75080 (United States).
Frederik Neuhaus, National Centre of Competence in Research in Chemical Biology, 30 quai Ernest Ansermet, CH-1211 Geneva 4 (Switzerland).
Jonathan E. Ploski, School of Behavioral and Brain Sciences, The University of Texas at Dallas, Richardson, Texas 75080 (United States)
Sven Kroener, School of Behavioral and Brain Sciences, The University of Texas at Dallas, Richardson, Texas 75080 (United States).
Maria O. Ogunyankin, Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, Minnesota 55455 (United States)
Jeong Eun Shin, Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, Minnesota 55455 (United States).
Joseph A. Zasadzinski, Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, Minnesota 55455 (United States)
Hui Wang, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital/Harvard Medical School, Charlestown, MA 02129 (United States).
Paul A. Slesinger, Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, New York 10029-5674 (United States)
Andreas Zumbuehl, Acthera Therapeutics Ltd., Peter Merian-Str. 45, 4052 Basel (Switzerland).
Zhenpeng Qin, Department of Mechanical Engineering, The University of Texas at Dallas, Richardson, Texas 75080 (United States); Department of Bioengineering, The University of Texas at Dallas, Richardson, Texas 75080 (United States); Center for Advanced Pain Studies, The University of Texas at Dallas, Richardson, Texas 75080 (United States); Department of Surgery, The University of Texas at Southwestern Medical Center, Dallas, Texas 75390 (United States).
References
- [1].a) Chen R, Canales A, Anikeeva P, Nat. Rev. Mater 2017, 2, 16093; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang Y, Kohane DS, Nat. Rev. Mater 2017, 2, 17020: [Google Scholar]; c) Zhu D, Roy S, Liu Z, Weller H, Parak WJ, Feliu N, Adv. Drug Deliv. Rev 2019, 138, 117–132. [DOI] [PubMed] [Google Scholar]
- [2].a) Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y, Huang AJY, Hashimotodani Y, Kano M, Iwasaki H, Parajuli LK, Okabe S, Teh DBL, All AH, Tsutsui-Kimura I, Tanaka KF, Liu X, McHugh TJ, Science 2018, 359, 679–684; [DOI] [PubMed] [Google Scholar]; b) Yoo S, Park J-H, Nam Y, ACS Nano 2019, 13, 544–551; [DOI] [PubMed] [Google Scholar]; c) Parameswaran R, Carvalho-de-Souza JL, Jiang Y, Burke MJ, Zimmerman JF, Koehler K, Phillips AW, Yi J, Adams EJ, Bezanilla F, Tian B, Nat. Nanotechnol 2018, 13, 260–266; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li J, Pu K, Chem. Soc. Rev 2019, 48, 38–71. [DOI] [PubMed] [Google Scholar]
- [3].a) Cotero V, Fan Y, Tsaava T, Kressel AM, Hancu I, Fitzgerald P, Wallace K, Kaanumalle S, Graf J, Rigby W, Kao T-J, Roberts J, Bhushan C, Joel S, Coleman TR, Zanos S, Tracey KJ, Ashe J, Chavan SS, Puleo C, Nat. Commun 2019, 10, 952; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sato T, Shapiro MG, Tsao DY, Neuron 2018, 98, 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P, Science 2015, 347, 1477; [DOI] [PubMed] [Google Scholar]; b) Christiansen MG, Senko AW, Anikeeva P, Annu. Rev. Neurosci 2019, 42, 271–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Li W, Lin J, Wang T, Huang P, Curr. Med. Chem 2019, 26, 1406–1422; [DOI] [PubMed] [Google Scholar]; b) Liu L, Chen Q, Wen L, Li C, Qin H, Xing D, Adv. Funct. Mater 2019, 29, 180860; [Google Scholar]; c) Silva JM, Silva E, Reis RL, J. Control. Release 2019, 298, 154–176; [DOI] [PubMed] [Google Scholar]; d) Lyu Y, He S, Li J, Jiang Y, Sun H, Miao Y, Pu K, Angew. Chem. Int. Ed 2019, 58, 18197–18201. [DOI] [PubMed] [Google Scholar]
- [6].a) Kang H, Zhang K, Pan Q, Lin S, Wong DSH, Li J, Lee WY-W, Yang B, Han F, Li G, Li B, Bian L, Adv. Funct. Mater 2018, 28, 1802642; [Google Scholar]; b) Mao C, Xiang Y, Liu X, Cui Z, Yang X, Li Z, Zhu S, Zheng Y, Yeung KWK, Wu S, ACS Nano 2018, 12, 1747–1759; [DOI] [PubMed] [Google Scholar]; c) Nakagawa H, Chem. Rec 2018, 18, 1708–1716; [DOI] [PubMed] [Google Scholar]; d) Lyu Y, Tian J, Li J, Chen P, Pu K, Angew. Chem. Int. Ed 2018, 57, 13484–13488; [DOI] [PubMed] [Google Scholar]; e) Lyu Y, Xie C, Chechetka SA, Miyako E, Pu K, J. Am. Chem. Soc 2016, 138, 9049–9052. [DOI] [PubMed] [Google Scholar]
- [7].a) Chen X, Venkatachalapathy M, Dehmelt L, Wu Y-W, Angew. Chem. Int. Ed 2018, 130, 12169–12173; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rost BR, Schneider-Warme F, Schmitz D, Hegemann P, Neuron 2017, 96, 572–603; [DOI] [PubMed] [Google Scholar]; c) Yu N, Huang L, Zhou Y, Xue T, Chen Z, Han G, Adv. Healthc. Mater 2019, 8, 1801132. [DOI] [PubMed] [Google Scholar]
- [8].a) Ash C, Dubec M, Donne K, Bashford T, Lasers Med. Sci 2017, 32, 1909–1918; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Richardson DS, Lichtman JW, Cell 2015, 162, 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jacques SL, Phys. Med. Biol 2013, 58, R37–R61. [DOI] [PubMed] [Google Scholar]
- [10].a) Feiner R, Dvir T, Nat. Rev. Mater 2017, 3, 17076; [Google Scholar]; b) Spearman BS, Desai VH, Mobini S, McDermott MD, Graham JB, Otto KJ, Judy JW, Schmidt CE, Adv. Funct. Mater 2018, 28, 1701713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Abdo A, Ersen A, Sahin M, J. Biomed. Opt 2013, 18, 075001; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Smith AM, Mancini MC, Nie S, Nat. Nanotechnol 2009, 4, 710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Carter KA, Shao S, Hoopes MI, Luo D, Ahsan B, Grigoryants VM, Song W, Huang H, Zhang G, Pandey RK, Geng J, Pfeifer BA, Scholes CP, Ortega J, Karttunen M, Lovell JF, Nat. Commun 2014, 5, 3546; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rwei AY, Lee J-J, Zhan C, Liu Q, Ok MT, Shankarappa SA, Langer R, Kohane DS, Proc. Natl. Acad. Sci. U.S.A 2015, 112, 15719–15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ambrosone A, Marchesano V, Carregal-Romero S, Intartaglia D, Parak WJ, Tortiglione C, ACS Nano 2016, 10, 4828–4834. [DOI] [PubMed] [Google Scholar]
- [14].a) Frederik N, Dennis M, Radu T, Sandor B, Takashi I, Gerald B, Andreas Z, Angew. Chem. Int. Ed 2017, 56, 6515–6518; [Google Scholar]; b) Neuhaus F, Mueller D, Tanasescu R, Balog S, Ishikawa T, Brezesinski G, Zumbuehl A, Langmuir 2018, 34, 3215–3220. [DOI] [PubMed] [Google Scholar]
- [15].Holme MN, Fedotenko IA, Abegg D, Althaus J, Babel L, Favarger F, Reiter R, Tanasescu R, Zaffalon PL, Ziegler A, Muller B, Saxer T, Zumbuehl A, Nat. Nanotechnol 2012, 7, 536–543. [DOI] [PubMed] [Google Scholar]
- [16].a) Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA, J. Am. Chem. Soc 2008, 130, 8175–8177; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li X, Che Z, Mazhar K, Price TJ, Qin Z, Adv. Funct. Mater 2017, 27, 1605778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Buscema M, Deyhle H, Pfohl T, Zumbuehl A, Müller B, Mater. Today Bio 2019, 1, 100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Brinson BE, Lassiter JB, Levin CS, Bardhan R, Mirin N, Halas NJ, Langmuir 2008, 24, 14166–14171; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ, Chem. Phys. Lett 1998, 288, 243–247; [Google Scholar]; c) Randrianalisoa J, Li X, Serre M, Qin Z, Adv. Opt. Mater 2017, 5, 1700403. [Google Scholar]
- [19].Biltonen RL, Lichtenberg D, Chem. Phys. Lipids 1993, 64, 129–142. [Google Scholar]
- [20].a) Anderson LJE, Hansen E, Lukianova-Hleb EY, Hafner JH, Lapotko DO, J. Control. Release 2010, 144, 151–158; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lukianova-Hleb E, Hu Y, Latterini L, Tarpani L, Lee S, Drezek RA, Hafner JH, Lapotko DO, ACS Nano 2010, 4, 2109–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Romanov OG, Romanov GS, Bull. Russ. Acad. Sci.: Phys 2014, 78, 1299–1302; [Google Scholar]; b) Lukianova-Hleb EY, Ren X, Sawant RR, Wu X, Torchilin VP, Lapotko DO, Nat. Med 2014, 20, 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].a) Kang P, Chen Z, Nielsen SO, Hoyt K, D’Arcy S, Gassensmith JJ, Qin Z, Small 2017, 13, 1700841; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Qin Z, Bischof JC, Chem. Soc. Rev 2012, 41, 1191–1217. [DOI] [PubMed] [Google Scholar]
- [23].Berridge MJ, Bootman MD, Lipp P, Nature 1998, 395, 645–648. [DOI] [PubMed] [Google Scholar]
- [24].Caride AJ, Filoteo AG, Penheiter AR, Pászty K, Enyedi Á, Penniston JT, Cell Calcium 2001, 30, 49–57. [DOI] [PubMed] [Google Scholar]
- [25].a) Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, Spassky N, Nat. Cell Biol 2010, 12, 341–350; [DOI] [PubMed] [Google Scholar]; b) Park MG, Jang H, Lee S-H, Lee CJ, Exp. Neurobiol 2017, 26, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang L, Jacques SL, Zheng L, Comput. Methods Programs Biomed 1995, 47, 131–146. [DOI] [PubMed] [Google Scholar]
- [27].a) Callaway EM, Katz LC, Proc. Natl. Acad. Sci. U.S.A 1993, 90, 7661–7665; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nerbonne JM, Curr. Opin. Neurobiol 1996, 6, 379–386; [DOI] [PubMed] [Google Scholar]; c) Ellis-Davies GCR, Nat. Methods 2007, 4, 619–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


