Abstract
BACKGROUND
Bacterial vaginosis affects 15 to 50% of women of reproductive age, and recurrence is common after treatment with an antibiotic agent. The high incidence of recurrence suggests the need for new treatments to prevent recurrent bacterial vaginosis.
METHODS
We conducted a randomized, double-blind, placebo-controlled, phase 2b trial to evaluate the ability of Lactobacillus crispatus CTV-05 (Lactin-V) to prevent the recurrence of bacterial vaginosis. Women 18 to 45 years of age who had received a diagnosis of bacterial vaginosis and who had completed a course of vaginal metronidazole gel as part of the eligibility requirements were randomly assigned, in a 2:1 ratio, to receive vaginally administered Lactin-V or placebo for 11 weeks; follow-up occurred through week 24. The primary outcome was the percentage of women who had a recurrence of bacterial vaginosis by week 12.
RESULTS
A total of 228 women underwent randomization: 152 to the Lactin-V group and 76 to the placebo group; of these participants, 88% in the Lactin-V group and 84% in the placebo group could be evaluated for the primary outcome. In the intention-to-treat population, recurrence of bacterial vaginosis by week 12 occurred in 46 participants (30%) in the Lactin-V group and in 34 participants (45%) in the placebo group (risk ratio after multiple imputation for missing responses, 0.66; 95% confidence interval [CI], 0.44 to 0.87; P = 0.01). The risk ratio for recurrence by week 24 (also calculated with multiple imputation for missing responses) was 0.73 (95% CI, 0.54 to 0.92). At the 12-week visit, L. crispatus CTV-05 was detected in 79% of participants in the Lactin-V group. The percentage of participants who had at least one adverse event related to Lactin-V or placebo by week 24 did not differ significantly between the groups. The percentage of participants with local or systemic adverse events was similar in the two groups.
CONCLUSIONS
The use of Lactin-V after treatment with vaginal metronidazole resulted in a significantly lower incidence of recurrence of bacterial vaginosis than placebo at 12 weeks. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT02766023.)
BACTERIAL VAGINOSIS AFFECTS 15 TO 50% of women of reproductive age worldwide.1 After treatment with an antibiotic agent, 20 to 75% of women have recurrent bacterial vaginosis within 3 months.2 Bacterial vaginosis has been associated with an increased risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV) infection,3–6 as well as premature birth7,8 and other reproductive health sequelae.9,10 Furthermore, recurrent bacterial vaginosis leads to negative emotional, sexual, and social effects, as well as a substantial economic burden on the health care system.11,12 The high incidence of recurrence suggests the need for new agents to prevent bacterial vaginosis.
Lactobacillus crispatus CTV-05 (Lactin-V, Osel) is a live biotherapeutic product that contains a naturally occurring vaginal strain of L. crispatus. It is composed of a powder containing 2×109 colony-forming units (CFU) of L. crispatus CTV-05 preserved with inactive ingredients and is administered with the use of a prefilled vaginal applicator. Lactin-V is designed to promote a community state type (i.e., microbial composition) with a predominance of L. crispatus11 after treatment with vaginal 0.75% metronidazole gel. In previous clinical trials, no serious or grade 3 adverse events that were considered to be related to the use of Lactin-V were reported.13–18 On the basis of the results from a phase 2a clinical trial of Lactin-V,16 the current phase 2b trial was designed to assess whether treatment with Lactin-V after a 5-day course of metronidazole to treat bacterial vaginosis would result in a significantly lower incidence of recurrence of bacterial vaginosis than placebo.
METHODS
TRIAL DESIGN AND OVERSIGHT
From April 2016 through February 2019, we conducted a multicenter, randomized, double-blind, placebo-controlled, phase 2b trial to assess the efficacy of repeated doses of Lactin-V over the course of 11 weeks in preventing the recurrence of bacterial vaginosis in women who had received a diagnosis of bacterial vaginosis at the screening visit. The trial protocol and statistical analysis plan are available with the full text of this article at NEJM.org. The protocol, which was approved by the institutional review board at each of four participating clinical sites, was written by the trial investigators and personnel at Emmes (a contract research organization). Representatives of the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) reviewed the protocol. Staff members at Osel reviewed the portions of the protocol that described Lactin-V and placebo, including storage requirements, and one of the staff members (the penultimate author) was involved in writing the manuscript. Osel also provided both Lactin-V and placebo. The first, second, and last authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
Premenopausal women 18 to 45 years of age were screened for eligibility after providing written informed consent to be screened. At the screening visit, the participant’s medical history was obtained, and physical and pelvic examinations were performed. If a potentially eligible woman met at least three of four Amsel criteria (i.e., thin, white or yellow, homogeneous discharge; >20% clue cells on microscopic examination; vaginal fluid with a pH of >4.5; and release of a fishy odor when 10% potassium hydroxide is added to a vaginal specimen), a vaginal swab was obtained and sent to a central laboratory at Magee–Womens Research Institute. Gram’s staining of the vaginal smear was used to determine the Nugent score (0 to 3 [normal], 4 to 6 [intermediate], or 7 to 10 [indicative of bacterial vaginosis]).19 A blood sample was obtained to test for HIV infection and syphilis; a vaginal swab was obtained for molecular testing for gonorrhea, chlamydia, and trichomonas infections; and a urine specimen was obtained to test for beta human chorionic gonadotropin and urinary tract infection. Potentially eligible women completed a standard 5-day course of vaginal 0.75% metronidazole within 30 days before the screening visit.
Eligible women who met at least three Amsel criteria and who had a Nugent score of 4 to 10 at the screening visit and negative results on tests for STIs then returned to the trial clinic within 48 hours after completion of the metronidazole regimen for reevaluation of their eligibility. After providing written informed consent to participate in the trial, eligible women were randomly assigned, in a 2:1 ratio, to receive Lactin-V at 2×109 CFU per dose or matching placebo and subsequently received a carton containing 25 vaginal applicators. The placebo formulation contained the same inactive ingredients as Lactin-V, without L. crispatus CTV-05.
Participants were instructed on proper administration of the assigned treatment and were observed during administration of the first dose in the clinic. Subsequently, participants were to administer four consecutive daily doses of Lactin-V or placebo during week 1, followed by twice-weekly doses for 10 weeks. Participants used a paper-based memory aid to log administration of Lactin-V or placebo and menstruation and sexual activity and to record symptoms and adverse events. The memory aid was to be completed daily during the first 12 weeks of the trial and weekly thereafter.
Clinic follow-up visits were scheduled 4, 8, 12, and 24 weeks after enrollment. At each time point, vaginal swabs were obtained for assessment of Amsel criteria, determination of the Nugent score, and detection of L. crispatus (all strains and the CTV-05 strain specifically) by means of quantitative polymerase-chain-reaction (PCR) assays; the swabs were also used for repeat STI testing if clinically indicated. A positive L. crispatus result was defined as a level above the lower limit of detection for both the assay for all strains and the assay for the CTV-05 strain (see the Supplementary Appendix, available at NEJM.org, for details of the PCR methods). Vaginal applicators that were returned at the follow-up visits were stained with 0.5% trypan blue as a proxy for adherence to the assigned treatment.20 Further details of the trial, including trial procedures and the complete list of eligibility criteria, are provided in the Supplementary Appendix.
TREATMENT ASSIGNMENT
Women underwent randomization after completion of the course of metronidazole. Randomization was performed in permuted blocks with the use of the online enrollment module of AdvantageEDC software (Emmes). The randomization sequence was prepared by statisticians at Emmes.
At the time of randomization, a kit number was generated electronically, and the site pharmacist selected the corresponding carton containing either Lactin-V or matching placebo. Trial personnel and participants were unaware of the treatment-group assignment. Before un-blinding of the treatment-group assignments occurred, a case-review committee identified participants who had had protocol deviations, such as the use of prohibited medications, trial visits outside the protocol-specified time window, or poor adherence to the assigned treatment; these participants were excluded from the per-protocol analyses (see the Methods section in the Supplementary Appendix).
EFFICACY OUTCOMES
The primary efficacy outcome was the percentage of participants who had recurrent bacterial vaginosis (defined by the presence of at least three Amsel criteria and a Nugent score of 4 to 10) at any follow-up visit up to and including the week 12 visit. Prespecified secondary efficacy outcomes included the percentage of participants who had recurrent bacterial vaginosis at any follow-up visit up to and including the week 24 visit, after completion of the 12-week post-treatment phase of the trial; the percentage of participants in the Lactin-V group who had detectable L. crispatus CTV-05 at 12 weeks and at 24 weeks, overall and stratified according to the occurrence of menses and of sexual intercourse; and the acceptability of Lactin-V, as assessed by the percentage of participants in each treatment group who were adherent to the assigned treatment and by responses on questionnaires. (The data from the questionnaire are not reported here.)
SAFETY OUTCOMES
Participants recorded the presence and intensity of solicited (expected and prespecified) local (involving the genitourinary tract) and systemic adverse events in a memory aid, starting on the first day of dosing, daily through week 12, and then weekly through week 24. In addition, at each trial visit, participants were asked about any other (unsolicited) adverse events. The lists of solicited local and systemic adverse events can be found in the protocol. The severity of all adverse events was graded according to grading tables provided by the Division of AIDS of the NIH.21,22 The percentage of participants who discontinued Lactin-V or placebo early owing to adverse events was also evaluated.
The primary safety outcome was the percentage of participants who had at least one adverse event by week 24 that was considered to be related to Lactin-V or placebo. All solicited adverse events were considered to be related to Lactin-V or placebo; the relatedness of unsolicited adverse events was determined by trial staff.
STATISTICAL ANALYSIS
The efficacy analyses were performed in several analysis populations. The intention-to-treat analyses included all participants who underwent randomization. The modified intention-to-treat, complete-case-analysis, and per-protocol populations are described in the Supplementary Appendix.
We estimated that with a sample of 228 participants, the trial would have 70% power to detect a cumulative percentage of participants with recurrence of bacterial vaginosis by week 12 that was 50% lower in the Lactin-V group than in the placebo group, using Pearson’s chi-square test at a two-sided alpha level. This calculation was based on an anticipated dropout rate of 10% in the complete-case-analysis population and an assumed cumulative percentage of participants in the placebo group with recurrence of bacterial vaginosis by week 12 of 30%.
We calculated the cumulative percentage of participants who had a recurrence of bacterial vaginosis by the 12-week and 24-week visits in each treatment group, as well as the risk ratios and 95% confidence intervals. Pearson’s chi-square test was used to test the null hypothesis that the cumulative percentages of participants who had recurrence of bacterial vaginosis by weeks 12 and 24 in the Lactin-V group would be equal to those in the placebo group, with a two-sided alternative hypothesis. For the intention-to-treat and modified intention-to-treat analyses, when diagnoses of bacterial vaginosis were missing, the data were imputed with the use of two single-imputation methods, one of which was the last observation carried forward. Post hoc sensitivity analyses for missing data were performed to calculate risk ratios and corresponding confidence intervals; the hypothesis tests were performed with the use of logistic-regression multiple imputation, under the assumption of a monotone pattern of missing data.23,24 The results of the prespecified intention-to-treat analyses in which missing data were imputed with the use of the last-observation-carried-forward method were included in the final clinical trial report and were reported to ClinicalTrials.gov. Here, we present the results of the post hoc multiple-imputation analyses, which corroborated the last-observation-carried-forward analyses; the results of the latter analyses are included in Tables S1 and S2.
The percentage of participants in each group who had detectable L. crispatus species (any strain) and L. crispatus CTV-05 at any time after baseline through week 24 were calculated, along with risk ratios for these outcomes. For the intention-to-treat and modified intention-to-treat analyses, missing data regarding the concentrations of L. crispatus species and L. crispatus CTV-05 were not imputed. In addition, the associations of adherence to the assigned treatment (≥75% of doses administered vs. <75% of doses administered), reported occurrence of menses since the last visit (menses vs. no menses), and reported occurrence of sexual activity (sex without the use of a condom vs. abstinence or sex with the use of a condom) with the presence of L. crispatus species and L. crispatus CTV-05 were assessed as exploratory analyses, as specified in the statistical analysis plan.
Because the statistical analysis plan did not include a provision for correcting for multiplicity for the secondary or other outcomes, results are reported as point estimates and 95% confidence intervals. The widths of the confidence intervals were not adjusted for multiplicity, so the intervals should not be used to infer definitive treatment effects for the secondary or other outcomes.
RESULTS
PARTICIPANTS
A total of 522 women were screened, of whom 228 across four trial sites underwent randomization: 152 were assigned to the Lactin-V group and 76 to the placebo group (Fig. 1). The demographic and clinical characteristics of the two groups were generally well balanced at baseline. More than half the participants reported a history of five or more episodes of bacterial vaginosis (Table 1).
Figure 1.
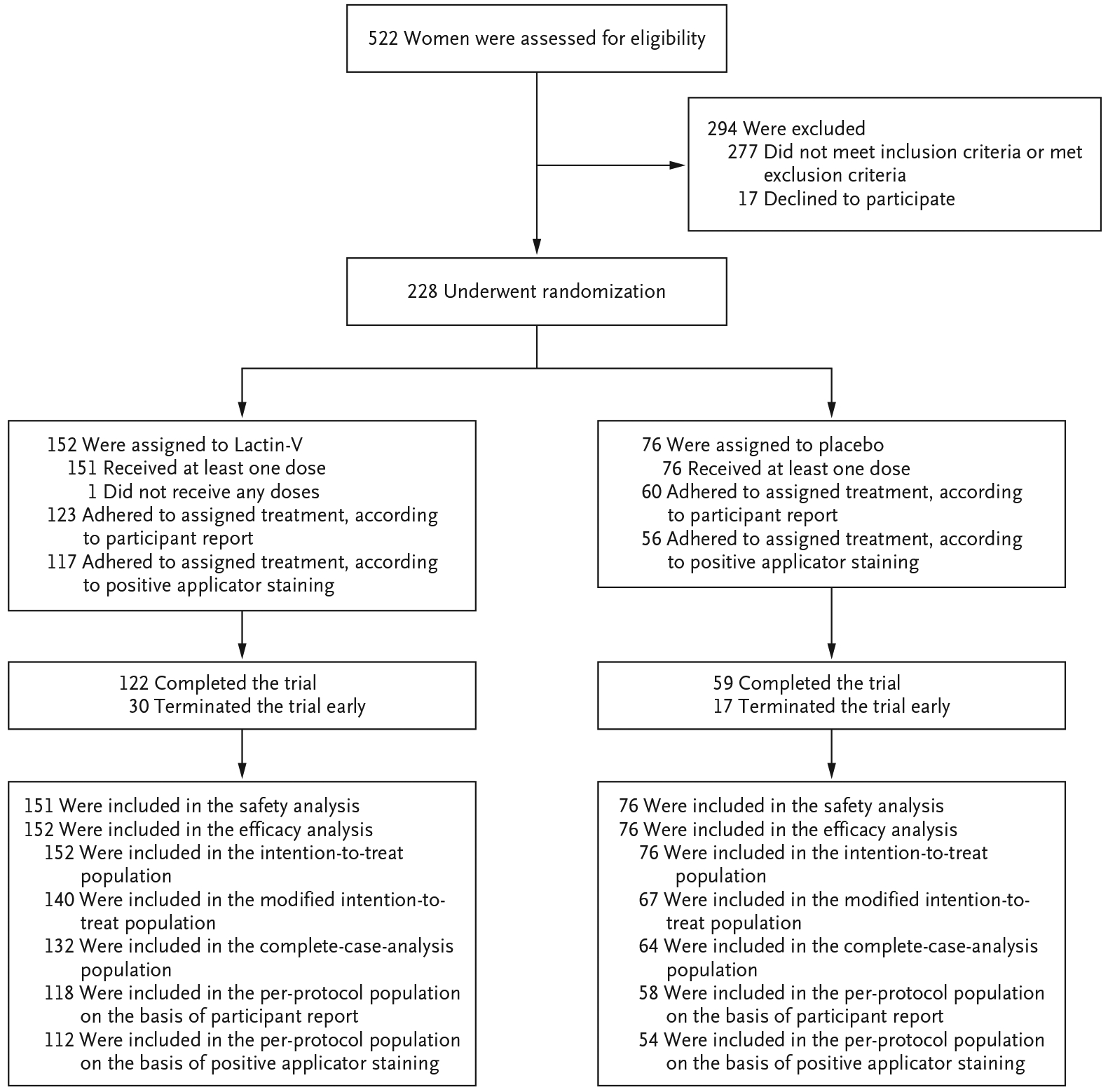
Enrollment, Randomization, and Follow-up.
Table 1.
Demographic and Clinical Characteristics at Baseline in the Intention-to-Treat Population.*
| Variable | Lactin-V (N = 152) | Placebo (N = 76) |
|---|---|---|
| Age — yr | 30.7±6.8 | 31.4±7.1 |
| Race or ethnic group — no. (%)† | ||
| American Indian or Alaskan Native | 2 (1) | 1 (1) |
| Asian | 6 (4) | 3 (4) |
| Native Hawaiian or other Pacific Islander | 1 (1) | 0 |
| Black | 64 (42) | 31 (41) |
| White | 55 (36) | 26 (34) |
| Multiracial | 10 (7) | 7 (9) |
| Unknown | 14 (9) | 8 (11) |
| Hispanic or Latino ethnic group — no. (%)† | 30 (20) | 15 (20) |
| Current relationship status — no. (%) | ||
| Married | 7 (5) | 10 (13) |
| Divorced or separated | 7 (5) | 2 (3) |
| Single, never married | 49 (32) | 20 (26) |
| Widowed | 2 (1) | 0 |
| Steady partner, cohabitating | 37 (24) | 15 (20) |
| Steady partner, not cohabitating | 37 (24) | 21 (28) |
| Casual partner | 13 (9) | 8 (11) |
| Sexual intercourse in the previous 30 days — no. (%) | 123 (81) | 69 (91) |
| No. of pregnancies | 1.7±2.0 | 1.5±1.7 |
| No. of male sexual partners in the previous 6 mo | 1.6±1.8 | 1.7±1.5 |
| Previous episodes of bacterial vaginosis — no. (%) | ||
| None | 7 (5) | 4 (5) |
| 1 or 2 | 33 (22) | 14 (18) |
| 3 or 4 | 23 (15) | 13 (17) |
| ≥5 | 78 (51) | 38 (50) |
| Unknown | 11 (7) | 7 (9) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the participant.
One participant in the Lactin-V group did not receive any doses, and Lactin-V was discontinued in 2 participants in the Lactin-V group who had spontaneous abortions (both of which were categorized as unsolicited adverse events). In addition, the assigned treatment was discontinued in 2 participants in the Lactin-V group and in 2 participants in the placebo group owing to genital itching or burning. Adherence to the assigned treatment was 81% according to participant report and 77% according to the number of applicators that had positive staining in the Lactin-V group, as compared with 79% and 74%, respectively, in the placebo group.
EFFICACY
Primary Outcome
Among the 228 participants who underwent randomization, the status of recurrence of bacterial vaginosis was known for 197 participants (86%). In the intention-to-treat population, recurrence of bacterial vaginosis by week 12 occurred in 46 participants (30%) in the Lactin-V group and in 34 participants (45%) in the placebo group (risk ratio, 0.66; 95% confidence interval [CI], 0.44 to 0.87; P = 0.01) (Table 2). The results of the intention-to-treat (last-observation-carried-forward), modified intention-to-treat, complete-case, and per-protocol analyses were similar to those of the intention-to-treat analysis (Table S1).
Table 2.
Bacterial Vaginosis Recurrence Status in the Intention-to-Treat Population.
| Variable | Lactin-V (N = 152) |
Placebo (N = 76) | Risk Ratio (95% CI)* | P Value |
|---|---|---|---|---|
| no. (%) | ||||
| Status of recurrence by wk 12 | ||||
| Recurrence† | 46 (30) | 34 (45) | 0.66 (0.44–0.87) | 0.01 |
| No recurrence | 87 (57) | 30 (39) | ||
| Unknown | 19 (12) | 12 (16) | ||
| Status of recurrence by wk 24 | ||||
| Recurrence | 59 (39) | 41 (54) | 0.73 (0.54–0.92) | |
| No recurrence | 63 (41) | 21 (28) | ||
| Unknown | 30 (20) | 14 (18) | ||
Risk ratios were calculated with the use of logistic-regression multiple imputation, under the assumption of a monotone pattern of missing data. 95% confidence intervals (CIs) were not adjusted for multiplicity and should not be used to make definitive conclusions regarding treatment effects.
This variable represents the primary outcome.
Secondary Outcomes
Among the participants without known recurrence of bacterial vaginosis by week 12, a total of 13 of 106 participants (12%) in the Lactin-V group and 7 of 42 participants (17%) in the placebo group had a recurrence by week 24. The risk ratio for recurrence by week 24 was 0.73 (95% CI, 0.54 to 0.92) (Table 2). The recurrence status at week 24 was unknown for 30 participants (20%) in the Lactin-V group and for 14 participants (18%) in the placebo group. Results across the analysis populations were consistent (Table S2).
In the Lactin-V group, L. crispatus CTV-05 was detected in 79% (at week 12) to 84% of participants during weeks 4, 8, and 12 and in 48% of participants at week 24 (Fig. 2). In contrast, in the placebo group, L. crispatus CTV-05 was detected in 2 to 6% of participants during weeks 4, 8, and 12 and in 2% of participants at week 24. The results were materially unchanged across the analysis populations. Among the participants with detectable L. crispatus CTV-05, the median concentration in the Lactin-V group ranged from 1.7×106 to 6.2×106 CFU per milliliter during the treatment phase through week 12 and was 5.6×106 CFU per milliliter at week 24 (Fig. 2).
Figure 2. Median Concentration and Incidence of Detectable Lactobacillus crispatus CTV-05 in the Lactin-V Group, According to Trial Visit.
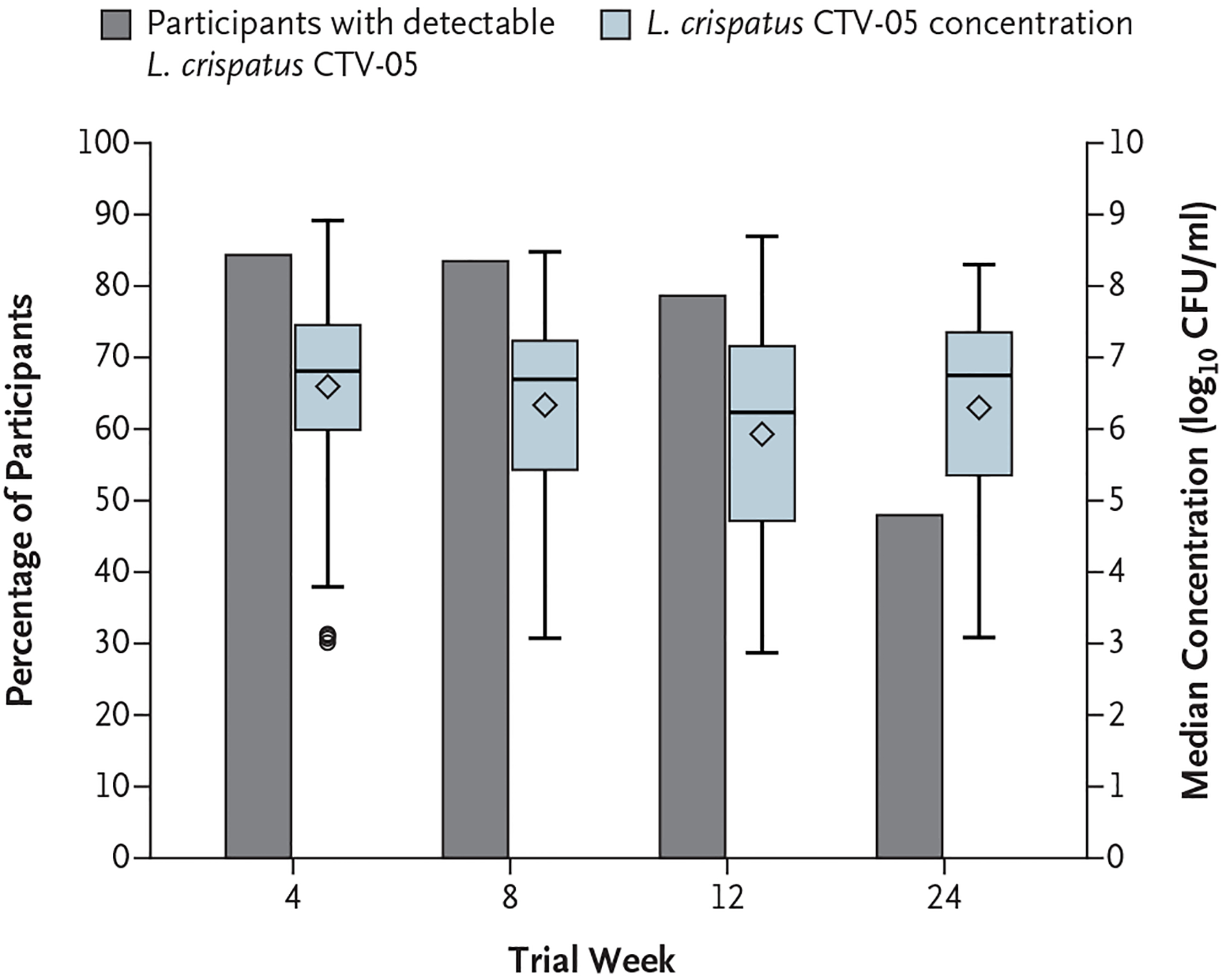
I bars indicate maximum and minimum values, the horizontal lines inside the blue bars indicate median values, and the diamonds indicate mean values. (The circles below the blue bar at week 4 represent outliers.) The size of each blue bar represents the interquartile range. CFU denotes colony-forming units.
There were no apparent associations between the detection of L. crispatus CTV-05 at week 12 or week 24 and adherence to the assigned treatment, occurrence of menses since the last visit, or occurrence of sex without the use of a condom. The incidence and risk ratio for each variable are provided in Table S3.
SAFETY
After the exclusion of 1 participant in the Lactin-V group who did not receive any doses, 151 participants in the Lactin-V group and 76 participants in the placebo group were evaluated for safety; data on solicited adverse events were missing for 11 and 10 participants, respectively, owing to early loss to follow-up. The percentage of participants who had at least one treatment-related adverse event (i.e., any solicited adverse event or an unsolicited adverse event that was assessed by the trial staff as being related to Lactin-V or placebo) by week 24 did not differ significantly between the groups (132 participants [87%] in the Lactin-V group and 60 participants [79%] in the placebo group; risk ratio, 1.11; 95% CI, 0.98 to 1.33; P = 0.12).
Across the two treatment groups, the most common solicited local adverse events were abnormal vaginal discharge, abnormal vaginal odor, external genital irritation, and genital itching (Fig. 3), and the most common solicited systemic adverse events were abdominal pain or cramps, headache, and frequent urination. In general, the incidence and severity of solicited local and systemic adverse events were similar in the two groups (Tables S4 through S6).
Figure 3. Solicited Local (Genitourinary) Adverse Events, According to Treatment Group.
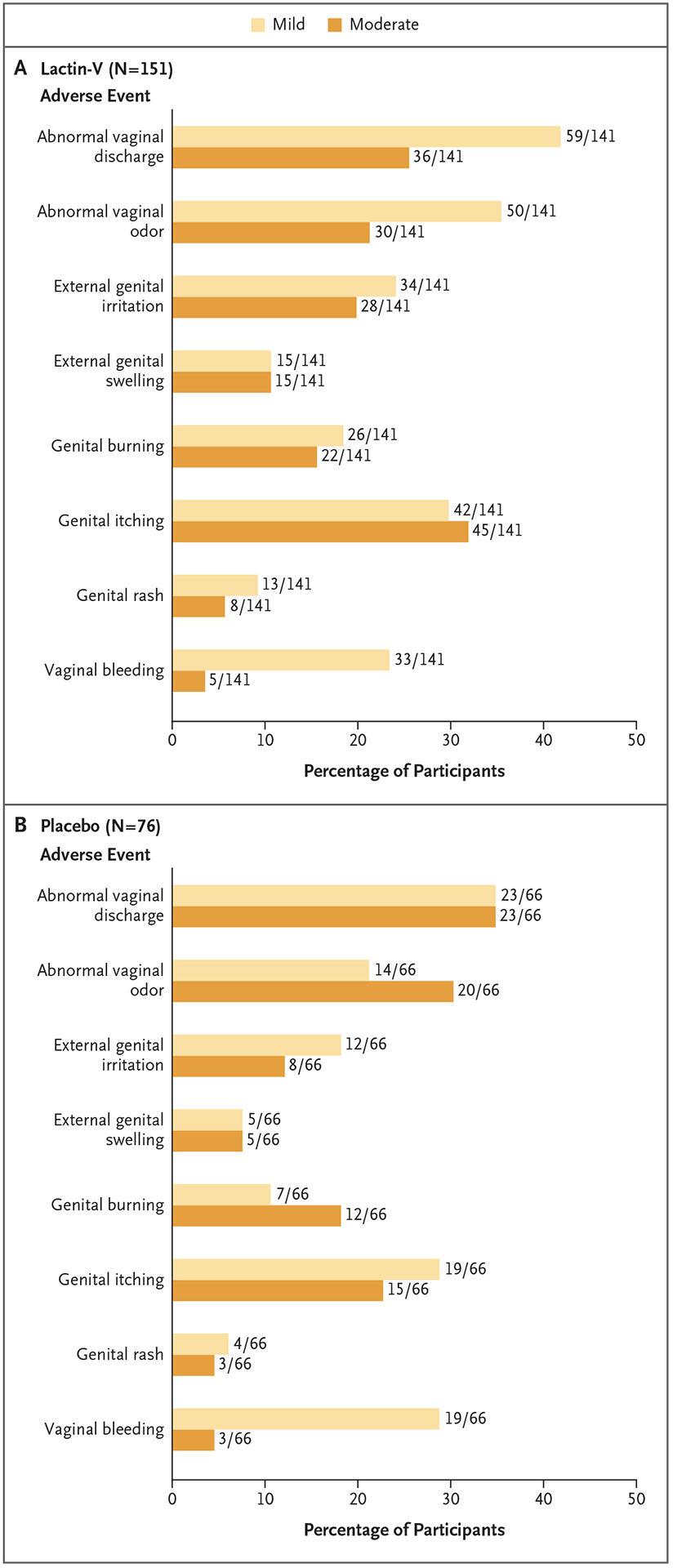
No severe solicited local adverse events were reported in either group.
Unsolicited adverse events were reported in 63 participants (42%) in the Lactin-V group and in 22 participants (29%) in the placebo group (risk ratio, 1.44; 95% CI, 0.97 to 2.30) (Tables S7 and S8). A total of 7 participants (4 in the Lactin-V group and 3 in the placebo group) had a severe grade 3 adverse event, one of which was reported as a serious adverse event (Lactin-V group) (Table S9). None of the severe adverse events were classified as being related to Lactin-V or placebo.
DISCUSSION
The use of Lactin-V after treatment with vaginal metronidazole for bacterial vaginosis resulted in a significantly lower incidence of recurrence of bacterial vaginosis at 12 weeks than placebo, and the benefit appeared to persist through week 24. Overall, adherence to the assigned treatment was high. In the Lactin-V group, L. crispatus CTV-05 was detected in almost 80% of the participants at the week 12 visit and in almost 50% of the participants at the week 24 visit after completion of the 12-week post-treatment phase of the trial.
In addition to live biotherapeutic products such as Lactin-V, other products and strategies have been tested to determine their effectiveness in reducing the risk of recurrence of bacterial vaginosis; these include intravaginal metronidazole at a dose of 750 mg plus miconazole at a dose of 200 mg,25 boric acid,26 and, most recently, vaginal microbiome transplantation.27 Although combination metronidazole–probiotic regimens have been tested previously and some have been shown to reduce the risk of recurrence of bacterial vaginosis, the trials have generally been small and have lacked the use of standardized methods, including objective outcome measures for bacterial vaginosis recurrence and colonization by the active trial medication.28–30
We found no material differences between the treatment groups in the percentage of participants who had a treatment-related adverse event or in the number, type, or severity of solicited local adverse events during the course of the 24 weeks of follow-up. These results extend earlier findings seen with Lactin-V and support the general safety of the current formulation and dosing strategy.16
The results of our phase 2a trial raised the possibility that exposure to semen during sex without the use of a condom, the presence of menstrual blood, and the reestablishment of bacteria associated with bacterial vaginosis soon after completion of antibiotic treatment may reduce the colonization of L. crispatus after administration of Lactin-V.16,31 In the current trial, the initial dose of Lactin-V or placebo was administered within 48 hours after the last dose of vaginal metronidazole gel, and the frequency of dosing after the first week was increased from once weekly to twice weekly through week 11. We did not find that the occurrence of menses since the last visit or the occurrence of sex without the use of a condom affected the detection of L. crispatus CTV-05.
Among the enrolled women, 14% were ineligible for the analysis of the primary outcome, a percentage that was higher than the assumed percentage of 10% that was used to estimate the sample size. However, the results of multiple-imputation analyses and several sensitivity analyses yielded findings that were consistent with those of the complete-case analysis. The current trial evaluated 11 weeks of treatment and 24 weeks of follow-up; further study should be considered to assess longer-term sustainability of colonization and prevention of bacterial vaginosis.
Among women with a history of bacterial vaginosis, the use of Lactin-V after treatment with vaginal metronidazole resulted in a lower incidence of recurrence of bacterial vaginosis at 12 weeks than placebo.
Supplementary Material
Acknowledgments
Supported by the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases of the NIH (contracts HHSN2722013000141 and HHSN27200007).
Footnotes
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFRENCES
- 1.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109: 114–20. [DOI] [PubMed] [Google Scholar]
- 2.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193: 1478–86. [DOI] [PubMed] [Google Scholar]
- 3.Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervico-vaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017; 46: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 2012; 9(6): e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdool Karim SS, Baxter C, Passmore JS, McKinnon LR, Williams BL. The genital tract and rectal microbiomes: their role in HIV susceptibility and prevention in women. J Int AIDS Soc 2019; 22(5): e25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180: 1863–8. [DOI] [PubMed] [Google Scholar]
- 7.Peelen MJ, Luef BM, Lamont RF, et al. The influence of the vaginal microbiota on preterm birth: a systematic review and recommendations for a minimum data-set for future research. Placenta 2019; 79: 30–9. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case– control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988; 319: 972–8. [DOI] [PubMed] [Google Scholar]
- 9.Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988; 158: 819–28. [DOI] [PubMed] [Google Scholar]
- 10.Hillier SL, Kiviat NB, Hawes SE, et al. Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecol 1996; 175: 435–41. [DOI] [PubMed] [Google Scholar]
- 11.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46: 304–11. [DOI] [PubMed] [Google Scholar]
- 12.Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One 2013; 8(9): e74378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 2011; 52: 1212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czaja CA, Stapleton AE, Yarova-Yaro-vaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol 2007; 2007: 35387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmerling A, Harrison W, Schroeder A, et al. Phase 1 dose-ranging safety trial of Lactobacillus crispatus CTV-05 for the prevention of bacterial vaginosis. Sex Transm Dis 2009; 36: 564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmerling A, Harrison W, Schroeder A, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex Transm Dis 2010; 37: 745–50. [DOI] [PubMed] [Google Scholar]
- 17.Antonio MA, Hillier SL. DNA finger-printing of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J Clin Microbiol 2003; 41: 1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonio MA, Meyn LA, Murray PJ, Busse B, Hillier SL. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J Infect Dis 2009; 199: 1506–13. [DOI] [PubMed] [Google Scholar]
- 19.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991; 29: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmerling A, Harrison WG, Brown JM, et al. Trypan blue staining to determine vaginal exposure in two types of plastic vaginal applicators containing two different microbicide formulations. Sex Transm Dis 2012; 39: 710–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Allergy and Infectious Diseases. Division of AIDS table for grading the severity of adult and pediatric adverse events, December 2004 — Addendum 1: female genital grading table for use in microbicide studies. November 2007. (https://rsc.niaid.nih.gov/sites/default/files/addendum-1-female-genital-grading-table-v1-nov-2007.pdf).
- 22.National Institute of Allergy and Infectious Diseases. Division of AIDS table for grading the severity of adult and pediatric adverse events version 1.0, December 2004 (clarification dated August 2009) (https://rsc.niaid.nih.gov/sites/default/files/table-for-grading-severity-of-adult-pediatric-adverse-events.pdf).
- 23.Rubin DB. Inference and missing data. Biometrika 1976; 63: 581–92. [Google Scholar]
- 24.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley, 1987. [Google Scholar]
- 25.McClelland RS, Balkus JE, Lee J, et al. Randomized trial of periodic presumptive treatment with high-dose intravaginal metronidazole and miconazole to prevent vaginal infections in HIV-negative women. J Infect Dis 2015; 211: 1875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeron Mullins M, Trouton KM. BASIC study: is intravaginal boric acid non-inferior to metronidazole in symptomatic bacterial vaginosis? Study protocol for a randomized controlled trial. Trials 2015; 16: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma D, Chen Y, Chen T. Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiol Lett 2019; 366: fnz025. [DOI] [PubMed] [Google Scholar]
- 28.Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev 2009; 4: CD006289. [DOI] [PubMed] [Google Scholar]
- 29.van de Wijgert J, Verwijs MC. Lactoba-cilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG 2020; 127: 287–99. [DOI] [PubMed] [Google Scholar]
- 30.van de Wijgert J, Verwijs MC, Agaba SK, et al. Intermittent lactobacilli-containing vaginal probiotic or metronidazole use to prevent bacterial vaginosis recurrence: safety and preliminary efficacy by microscopy and sequencing. medRxiv. July 8, 2019. (https://www.medrxiv.org/content/10.1101/19001156v1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngugi BM, Hemmerling A, Bukusi EA, et al. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex Transm Dis 2011; 38: 1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


