Abstract
Context:
The definitive surgical treatment of severe endometriosis remains to be hysterectomy whether done by laparoscopy or laparotomy.
Aim:
The aim of this study was to assess the feasibility and outcome of laparoscopic hysterectomy in severe pelvic endometriosis.
Settings and Design:
This retrospective study was carried out in a tertiary center over a period of 5 years (January 2013–December 2017).
Subjects and Methods:
A total of 70 patients who underwent laparoscopic hysterectomy for severe pelvic endometriosis with a score of more than 40, which was defined by the revised American Fertility Society classification, were included in the study. Feasibility of laparoscopic hysterectomy and other clinical parameters such as operative time, blood loss, recurrence of the disease, and need for postoperative medical treatment was analyzed.
Results:
The mean age of the patients was 43.2 ± 4.56. Majority of the women (62.8%) had dysmenorrhea as the primary complaint, followed by menorrhagia (21.4%). Intraoperatively rectovaginal septum was involved in 95% of the cases with complete obliteration of the pouch of Douglas in 80% of the cases. The ureter was involved in 34% of the cases. The bladder was densely adherent in 71.4% of the patients. There was no conversion to laparotomy in any of these patients and no visceral injuries. The mean duration of surgery was 3 h. The estimated blood loss ranged from 100 to 500 ml. The duration of hospital stay was 2–5 days. There was no recurrence during follow-up in any of these patients.
Conclusions:
Laparoscopy in experienced hands is feasible and safe even in difficult cases of Stage IV pelvic endometriosis apart from offering superior results.
KEYWORDS: Feasibility, hysterectomy, laparoscopy, severe endometriosis
INTRODUCTION
Pelvic endometriosis has always been an enigmatic disease. The overall prevalence of pelvic endometriosis has been reported to be variably around 5%–15%.[1,2,3] Laparoscopy is emerging to be the mode of treatment, and the definitive surgical management of severe endometriosis remains to be hysterectomy.[4] Tiny lesions and dense adhesions of the rectum and bladder can be precisely dissected due to laparoscopic magnification. The use of advanced energy sources and minimal tissue handling prevents the formation of adhesions to a greater extent. Although conventional laparoscopy has gained wide acceptance in the diagnosis of pelvic endometriosis, the feasibility of laparoscopy in the definitive management of severe pelvic endometriosis has not been well studied. This is probably due to the fact that laparoscopic hysterectomy in severe pelvic endometriosis still remains to be a complex procedure which requires good skill and expertise. The aim of our study is to evaluate the feasibility of laparoscopic hysterectomy in patients with severe pelvic endometriosis.
SUBJECTS AND METHODS
This retrospective study was carried out in a tertiary laparoscopic center over a period of 5 years (2013 January–2017 December). A total of seventy patients who underwent laparoscopic hysterectomy for severe pelvic endometriosis were analyzed in our study. All these patients had the revised American Fertility score of more than 40. All the surgeries were done by a single senior surgeon.
The following patients who were not willing for medical treatment/conservative surgery underwent total laparoscopic hysterectomy with unilateral or bilateral salpingo-oophorectomy:
Patients with Stage IV pelvic endometriosis with failed medical management
Nullipara with Stage IV pelvic endometriosis with multiple failed in vitro fertilization treatments
Patients with ovarian endometriomas and had completed family with Stage IV pelvic endometriosis
Patients aged >40 years with Stage IV pelvic endometriosis willing for definitive treatment
Patients with extensive involvement of bowel, bladder, and ureter.
For all these women, hysterectomy was planned in the same sitting or after 3 doses of injection gonadotropin-releasing hormone (GnRH).
The following patients were excluded from our study:
Patients with transmural involvement of rectum and sigmoid/bowel endometriosis which requires resection and anastomosis/repair
Patients with ureteric endometriosis which requires resection and anastomosis or reimplantation
Patients desirous of children
Patients not willing for definitive surgery.
The primary outcome measure was the feasibility of laparoscopic hysterectomy in severe pelvic endometriosis. The secondary outcome includes operative time, blood loss, intraoperative/postoperative complications, postoperative recovery, duration of stay, recurrence of the disease, and need for postoperative medical management. In addition, patients’ clinical profile and correlation with intraoperative findings were analyzed.
Preoperative preparation
Three doses of preoperative GnRH injections were given for selected cases
All patients were on liquid diet for 2 days before procedure
Bowel preparation was done with Colo-Prep (sodium phosphate anhydrous) enema on the previous day of surgery
All patients received preoperative thromboprophylaxis.
Surgical steps
Veress needle was routinely put in the supraumbilical region
Primary camera port was created in the supraumbilical region except for large-size uteri in which case epigastric port was used
Careful adhesiolysis was done using Harmonic scalpel
Ureter was traced on both sides before starting the procedure in all cases and ureterolysis was done
Following this, adnexectomy (unilateral or bilateral) was done in the beginning of the procedure for adequate visualization
Bladder was dissected by sharp dissection/lateral window technique with Harmonic scalpel as it is adherent in most of the cases
Coagulation of uterine pedicles
In cases where rectum and sigmoid were adherent to the uterus, it was carefully shaved off from the surface of the uterus
Following Total Laparoscopic Hysterectomy, Endometriotic nodules over the rectum, uterosacral ligaments, and peritoneum were excised
Routine ureteric stenting was not done in any of our cases
Specimen retrieval was done vaginally, and in cases of large uterus, morcellation in endobag was done
Vault was closed transversely with 2-0 vicryl or 2-0 PDS.
Postoperatively, our patients were started on clear liquids after 6 h and soft diet after 24 h and were discharged on the Post operative day-2. All our patients were given injection Clexane till the day of discharge.
RESULTS
In our present study, a total of 70 women who underwent total laparoscopic hysterectomy for Stage IV endometriosis were included. Table 1 shows patient characteristics among these women. The mean age of the patients was 43.2 ± 4.56. The youngest patient operated was 34 years and the oldest was 53 years. Majority of the patients were in the age group of 40–49 years. 68.5% had a history of previous abdominal surgeries. Twenty-four patients were nulliparous, whereas majority of the patients had completed their families. Women with Stage IV endometriosis presented with varied symptoms, as shown in Table 2. Majority of the women(62.8%) had dysmenorrhea alone as the primary complaint, followed by menorrhagia (21.4%). All patients who presented with dysmenorrhea and dyspareunia had the involvement of Pouch of Douglas and the uterosacral ligaments. Table 2 shows the distribution of clinical features among women with severe pelvic endometriosis.
Table 1.
Patient characteristics among women with severe pelvic endometriosis (n=70)
| Patient characteristics | Number of patients (%) |
|---|---|
| Age (years) | |
| 30-39 | 16 (22.8) |
| 40-49 | 49 (70) |
| 50-59 | 5 (7.2) |
| Parity | |
| 0 | 24 (34.2) |
| Secondary infertility | 2 (2.8) |
| 1 | 19 (27.2) |
| 2 | 23 (32.8) |
| 3 | 2 (2.8) |
| Previous surgery | 48/70 (68.57) |
| LSCS | 13 |
| Laparotomy | 5 |
| Sterilization | 5 |
| Diagnostic laparoscopy | 9 |
| TLH deferred | 16 |
LSCS: Lower segment cesarean section, TLH: Total laparoscopic hysterectomy
Table 2.
Distribution of clinical features among women with severe pelvic endometriosis (n=70)
| Signs and symptoms | Number of patients (%) |
|---|---|
| Dysmenorrhea | 44 (62.8) |
| With dyschezia | 2 |
| With dysuria | 1 |
| With dyspareunia | 16 |
| Menorrhagia | 15 (21.4) |
| Both (menorrhagia with dysmenorrhea) | 8 (11.4) |
| Incidental USG finding of ovarian endometrioma | 1 (1.4) |
| Mass per abdomen | 1 (1.4) |
| Premenstrual spotting | 1 (1.4) |
| Size of the uterus | |
| ≤10 weeks uterus | 40 (57) |
| >10 weeks uterus | 24 (34) |
| ≥20 weeks uterus | 6 (9) |
| Mobility of uterus | |
| Mobile | 20 (29) |
| Restricted | 50 (71) |
| Position of uterus | |
| Anteverted | 27 (39) |
| Retroverted | 43 (61) |
| POD | |
| Deep POD | 22 (31) |
| POD nodules | 7 (10) |
| POD tenderness | 20 (28) |
| Uterosacral nodularity | 15 (21) |
| Palpable adnexa | 24 (34) |
| Adnexal tenderness | 17 (24) |
POD: Pouch of Douglas
Clinical diagnosis of pelvic endometriosis was made preoperatively in 46 patients (65%) by bimanual pelvic examination. In pelvic examination, 9% of patients had associated adenomyosis with uterus size varying between 20 and 24 weeks. Majority of the patients had restricted uterine mobility (71%) and retroverted uterus (61%).
Table 3 depicts investigations. CA125 was done in a total of 47 patients. It was raised in 24 patients (51%) and was within the normal range in 23 patients. The lowest value was 7 U/ml and the highest was 600 U/ml. Ultrasonography (USG) was done for all patients, of which 43 patients (61%) were either diagnosed with adenomyosis or endometriotic cyst in USG. However, intraoperatively, ovaries were involved in more than half of the patients (65%) [Figure 1].
Table 3.
Distribution of investigations
| Investigation | Number of patients (%) |
|---|---|
| CA125 (n=47) | |
| ≤35 | 23 (49) |
| >35 | 15 (32) |
| >100 | 9 (19) |
| CA 19.9 (n=15) | |
| ≤37 | 10 (66) |
| >37 | 5 (34) |
| USG | |
| Adenomyosis | 16 (23) |
| Endometriotic cyst | 17 (24) |
| Both | 10 (14) |
| Normal | 27 (38) |
| MRI | 16 (22) |
| Cystoscopy | 1 (1.4) |
| Sigmoidoscopy | 7 (10) |
MRI: Magnetic resonance imaging, USG: Ultrasound
Figure 1.
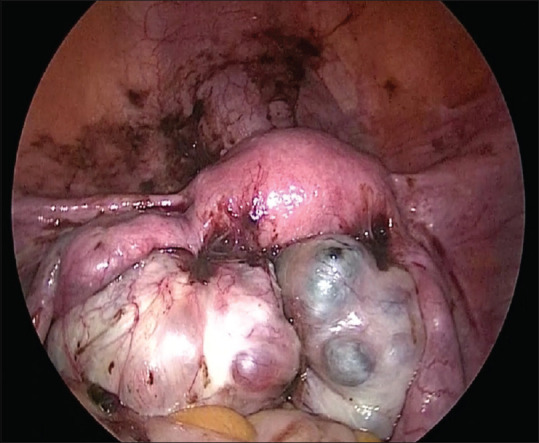
Ovarian endometrioma
The intraoperative findings are shown in Table 4. Intraoperatively, the bladder was densely adherent and drawn up in 71.4% due to previous surgeries and disease per se [Figure 2]. The rectum was involved in 95% of the cases with complete obliteration of POD in 56 cases and partial obliteration in 11 cases [Figures 1 and 3]. POD obliteration and ovarian endometrioma were found intraoperatively in majority of the patients, which were missed in both clinical and radiological examinations in majority of the patients.
Table 4.
Intraoperative findings among women with severe pelvic endometriosis
| Intraoperative findings | Number of patients (%) |
|---|---|
| Obliteration of POD (n=67) | |
| Complete | 56 (80) |
| Partial | 11 (15) |
| No involvement | 3 (4) |
| Contracted pelvic wall | 24 (34) |
| Adherent bladder | 50 (71.4) |
| Ovarian endometrioma (n=46) | |
| 1-3 cm | 9 (12) |
| >3 cm | 37 (52) |
| Ovarian adhesions (n=40) | |
| <2/3rd enclosure | 7 (10) |
| >2/3rd enclosure | 33 (47) |
| Fallopian tube adhesions | 1 (1) |
| Peritoneal endometriosis | 2 (2) |
| Organs involved with endometriosis | |
| Unilateral/bilateral ovary | 46 (65) |
| Ureter | 24 (34) |
| Rectovaginal septum | 67 (95) |
| Uterosacral ligaments | 32 (45) |
| Bladder | 1 (1) |
| Bowel serosa | 36 (51) |
| Fallopian tube | 1 (1) |
| Appendix | 5 (7) |
POD: Pouch of Douglas
Figure 2.
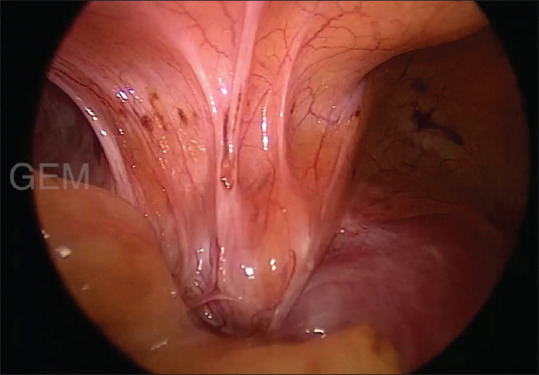
Bladder involvement in severe pelvic endometriosis
Figure 3.
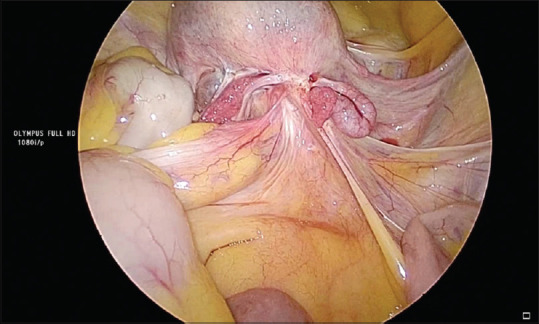
Obliteration of the pouch of Douglas
The estimated blood loss during surgery varied from 100 to 500 ml with the precise use of advanced energy sources such as Harmonic and LigaSure. The mean duration of surgery which included adhesiolysis, ureterolysis, and shaving of the rectum was 3 h [Figure 4]. In cases with bladder involvement, which required excision of bladder wall endometriosis, the mean duration was 4 h and 30 min. The duration of hospital stay was ranging from 2 to 5 days.
Figure 4.
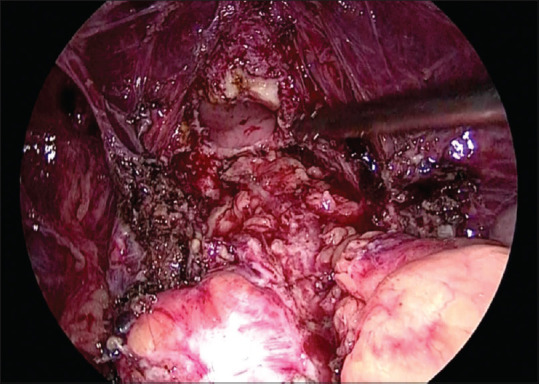
After shaving off the rectum
Postoperative suppression therapy was given in 27 cases (38%), of which GnRH agonist was given in 18 cases (66%), danazol alone was given for 2 cases (7%), and dienogest alone was given for 7 cases (25%). For 43 cases, no postoperative suppression therapy was given.
DISCUSSION
Endometriosis is reported to be more common in women aged between 40 and 44 years,[3] and similarly, in our study, the prevalence was noted to be high in women aged between 40 and 49 years (70%).
Infertile women are 6–8 times more likely to have endometriosis than fertile women, and apparently, 34% of the patients in our study were nulliparous with primary infertility, which is in correlation with Mao and Anastasi study,[5] and the causative factors include distorted pelvic anatomy, altered peritoneal function, ovulatory abnormalities, and altered hormonal function in the endometrium.
The most frequent reason for consultation in patients with severe pelvic endometriosis remains to be dysmenorrhea in our study. Although the relation between severity of dysmenorrhea and type and site of endometriotic lesion has been debatable, Koninckx et al.[6] have correlated the degree of pain with volume and depth of the lesion. In our study, most of the cases with dysmenorrhea and dyspareunia had endometriosis of the rectovaginal septum and uterosacral ligaments. Dyspareunia was noted in 16 of our cases (22%) and is probably due to the location of the lesions in the retrocervical region with uterocervical involvement.
The incidence of bladder endometriosis has been variably reported to be around 1%–11% in women diagnosed with pelvic endometriosis.[7,8] In our study, one patient had associated bladder endometriosis with extensive peritoneal endometriosis. Total laparoscopic hysterectomy with bilateral salpingo-oophorectomy with laparoscopic excision of bladder wall endometriosis was done. Postoperatively, injection GnRH was given for 3 months, and there was no recurrence in her follow-up period and she was asymptomatic. Laparoscopic excision of bladder endometriosis ensures complete removal of the disease by enhanced visualization of the pelvic cavity and gives long-term results without any recurrence.
Two patients in our study with dyschezia with normal preoperative sigmoidoscopy had rectosigmoid adhesions to the posterior surface of the uterus intraoperatively. Seracchioli et al.[8] have reported that the severity of dyschezia was directly associated with the diameter of rectovaginal endometriosis and not with anterior rectal wall involvement. This explains why both our patients with dyschezia were not associated with transmural involvement of the rectum.
One patient in our study who presented with complaints of mass per abdomen had a 30-week size uterus clinically which reduced to 28 weeks following 3 doses of GnRH. Intraoperatively, she had enlarged uterus with huge myoma and severe pelvic endometriosis. GnRH injections were helpful in reducing the size of uterus as evident in this case, and this is supported by many studies.[9]
Clinical diagnosis has always been proven to be helpful in the diagnosis of pelvic endometriosis. Eskenazi et al.[10] state that pelvic examination has 76% sensitivity and 74% specificity. In a tertiary center like ours, as we have good exposure to pelvic endometriosis cases, we were able to diagnose 65% of our patients by clinical examination alone. Majority of our patients had bulky, retroverted uterus with restricted mobility (57%, 61%, and 71%, respectively). POD nodules were palpated only in 10% of the patients, which is comparatively low when compared to Chapron et al.’s study[11] who reported palpable POD nodules in 43% of their patients. However, in our study, majority of the patients were nulliparous, and pelvic examination is technically difficult because of narrow introitus and poor co-operation by the patient.
Serum CA125 and its role in endometriosis are well known with sensitivity and specificity for the diagnosis of endometriosis being 61.1% and 87.5%, respectively.[12] In our study group, CA125 was raised in 24 cases (51%), which is consistent with Barbieri et al.’s[13] study which showed that 54% of the patients with Stage IV endometriosis had raised CA125 levels. CA125 has been reported to be proportionate with the severity of pelvic adhesions.[14] Similarly, all our cases with raised CA125 levels had severe adhesions intraoperatively. However, CA 19.9 has a low sensitivity when compared with CA125.[15] The highest CA 19.9 level in our study was 1204 U/ml, and this patient had a 4 cm × 4 cm left ovarian endometrioma with extensive superficial bowel endometriosis. This extremely high level of CA 19.9 would have been probably due to the extensive bowel involvement in that patient.
USG is useful in diagnosing ovarian endometrioma which has a classical ground glass appearance as a result of the hemorrhagic debris. USG has a limited advantage as it lacks in identifying pelvic adhesions and superficial peritoneal implants. Bazot et al.[16] reported the sensitivity of USG in detecting pelvic endometriosis to be around 78% and a specificity to be 95%. Magnetic resonance imaging (MRI) is more sensitive than USG as it can help in precise mapping of deep infiltrating endometriotic implants and adhesions.[17] However, MRI has limitations in cases of the retroflexed uterus, wherein endometriotic involvement of uterosacral ligaments can be masked.
In our study, we had deferred hysterectomy in 16 cases in the first sitting in view of dense bowel adhesions engulfing the uterus and were given 3 doses of GnRH and then taken up for hysterectomy. On the contrary, 12 cases in whom pelvic endometriosis was not anticipated preoperatively were successfully operated in the first sitting.
The role of GnRH injections in pelvic endometriosis is debatable. Although it remains controversial with studies from Muzii et al.[18] which report that there were no better surgical outcomes or a decrease in recurrence rates with preoperative GnRH injections, in our experience, we found a optimal surgical outcome in patients taking GnRH doses in terms of reduced uterine size, decreased operative time, decreased vascularity, and reduced adhesions.
A special mention to a 50-year-old woman who was planned for hysterectomy in view of symptomatic adenomyosis uterus, intraoperatively, she had a 24-week adenomyosis uterus with dense sigmoid and rectal adhesions over the uterus with involvement of bilateral ovaries. As pelvic endometriosis was not anticipated preoperatively, hysterectomy was deferred in view of severe bowel adhesions and proceeded with bilateral salpingo-oophorectomy in the same sitting which was excluded in our study. During her regular follow-up, she was completely asymptomatic and uterus shrunk to normal size at the end of 1 year and hence was advised observation. While the benefits of oophorectomy are still debatable, Namnoum et al.[19] reported that 62% of the women had persistent pain when the ovaries were conserved, compared with 10% when the ovaries were removed. Hence, in very difficult situations where hysterectomy is not feasible, oophorectomy carries a very good prognosis in terms of overall symptomatic relief.
Total laparoscopic hysterectomy with bilateral salpingectomy, thereby retaining one or both ovaries, was performed in three of our cases as the ovaries were healthy. No postoperative medical treatment was given for these three cases, and on follow-up, there was no recurrence of symptoms.
In our study, 95% of the patients had involvement of the rectovaginal septum and the surgical technique employed in these cases involved shaving off the rectum from the uterus, followed by excision of endometriotic nodules over the rectum, uterosacral ligaments, and peritoneum. In all these cases, there was no bowel injury during adhesiolysis, and none of them required resection anastomosis. Many authors suggest radical extirpation of the diseased bowel for the complete resolution of symptoms and to reduce the risk of recurrence.[20,21] However, in our study, in the process of shaving off the rectum, around 2–3 mm of the adherent area might have been left out, and all these patients were given oral hormones postoperatively for a period of 6–12 months. Surprisingly, all are symptom free and no recurrence was noted.
The ureter was involved in 24 cases (34%), and all these cases had contracted the lateral pelvic wall. Abrao et al.[22] state that the involvement of ureter is most likely to be associated with rectosigmoid adhesions, which is similar to our study. Ureteric stent was not used in any of the cases, and there were no Ureteric injuries. Ureteral involvement can lead to serious complications such as stenosis with hydronephrosis and finally loss of renal function if the diagnosis is delayed. Hence, early diagnosis is crucial for the prognosis. In the case of ureteral endometriosis, the aim of the treatment is to liberate the ureter from all endometriotic tissue to allow normal function and to avoid morbidity, and so in our study, all our patients with ureter involvement underwent ureterolysis.
Forty-six (65%) of our patients had endometriotic cysts involving one or both ovaries with the largest endometriotic cyst being 15 cm × 12 cm. Few authors have stated that the left ovary is commonly involved than the right.[23,24] This could be due to the presence of sigmoid colon in the left hemipelvis which avoids regurgitation of endometrial cells through the left tube that prevents the clearance of endometrial cells by the clockwise peritoneal macrophage disposal system. However, in our study, the right ovary was commonly involved (65%) than the left ovary.
Seven percent of our patients had the involvement of the appendix though the incidence of appendiceal endometriosis is rare. Gustofson et al.[25] report an incidence of 2.8% in their study. The high incidence of appendiceal involvement in our study could be attributed to the fact that the right ovary was most commonly involved in our study. Endometriosis of the appendix can mimic appendicitis, and it should always be considered in the differential diagnosis of young women complaining of nonspecific recurrent lower abdominal pain, especially with a history of infertility. Incidental appendicectomy during surgical treatment of pelvic endometriosis is controversial.[26]
The mean operative time in our study for total laparoscopic hysterectomy was 3 h except in certain cases like those involving resection of bladder endometriosis, wherein the operating time increased to 4 h and 30 min, similar to Chalermchockchareonkit et al.[27] In our study, we had a 0% conversion to laparotomy, and there were no major complications including visceral injuries in our study. Three of our patients had paralytic ileus due to dense bowel adhesions, which was managed conservatively. Two patients received a blood transfusion in the postoperative period. The duration of hospital stay on an average was 3 days.
Forty-five percent of our cases had more than a 3-year follow-up. Few studies[28,29] have shown no difference in benefit of postsurgical medical management in whom disease has been completely extirpated. Thirty-eight percent of our patients received postoperative medical management as a prophylaxis for microscopic residual disease. There was no recurrence in this group of patients, and surprisingly, there was no recurrence in the remaining group of patients who had not received any postoperative medical management.
CONCLUSIONS
Laparoscopic hysterectomy in severe pelvic endometriosis is feasible, and it should be the treatment of choice with the availability of expertise. It offers major patient benefits by avoiding major complications with quick recovery and reducing the recurrence with precise excision.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank our chairman and our mentor Dr. C. Palanivelu, GEM Hospital, for his contribution and support.
REFERENCES
- 1.Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: Epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: An update for clinicians. Hum Reprod Update. 2006;12:179–89. doi: 10.1093/humupd/dmi049. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy S. Who gets endometriosis? Women’s Health Med. 2005;2:18–9. [Google Scholar]
- 4.Shah PR, Adlakha A. Laparoscopic management of moderate: Severe endometriosis. J Minim Access Surg. 2014;10:27–33. doi: 10.4103/0972-9941.124463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao AJ, Anastasi JK. Diagnosis and management of endometriosis: The role of the advanced practice nurse in primary care. J Am Acad Nurse Pract. 2010;22:109–16. doi: 10.1111/j.1745-7599.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 6.Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55:759–65. doi: 10.1016/s0015-0282(16)54244-7. [DOI] [PubMed] [Google Scholar]
- 7.Mettler L. New Delhi: Jaypee Brothers Medical Publishers; 2006. Manual for Laparoscopic and Hysteroscopicgynecological Surgery. [Google Scholar]
- 8.Seracchioli R, Mabrouk M, Guerrini M, Manuzzi L, Savelli L, Frascà C, et al. Dyschezia and posterior deep infiltrating endometriosis: Analysis of 360 cases. J Minim Invasive Gynecol. 2008;15:695–9. doi: 10.1016/j.jmig.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2000;2:CD000547. doi: 10.1002/14651858.CD000547. [DOI] [PubMed] [Google Scholar]
- 10.Eskenazi B, Warner M, Bonsignore L, Olive D, Samuels S, Vercellini P. Validation study of nonsurgical diagnosis of endometriosis. Fertil Steril. 2001;76:929–35. doi: 10.1016/s0015-0282(01)02736-4. [DOI] [PubMed] [Google Scholar]
- 11.Chapron C, Dubuisson JB, Pansini V, Vieira M, Fauconnier A, Barakat H, et al. Routine clinical examination is not sufficient for diagnosing and locating deeply infiltrating endometriosis. J Am Assoc Gynecol Laparosc. 2002;9:115–9. doi: 10.1016/s1074-3804(05)60117-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen FP, Soong YK, Lee N, Lo SK. The use of serum CA-125 as a marker for endometriosis in patients with dysmenorrhea for monitoring therapy and for recurrence of endometriosis. Acta Obstet Gynecol Scand. 1998;77:665–70. doi: 10.1034/j.1600-0412.1998.770615.x. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri RL, Niloff JM, Bast RC, Jr, Scaetzl E, Kistner RW, Knapp RC. Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril. 1986;45:630–4. doi: 10.1016/s0015-0282(16)49333-7. [DOI] [PubMed] [Google Scholar]
- 14.Cheng YM, Wang ST, Chou CY. Serum CA-125 in preoperative patients at high risk for endometriosis. Obstet Gynecol. 2002;99:375–80. doi: 10.1016/s0029-7844(01)01731-8. [DOI] [PubMed] [Google Scholar]
- 15.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: A systematic review. Hum Reprod Update. 2010;16:651–74. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazot M, Thomassin I, Hourani R, Cortez A, Darai E. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obstet Gynecol. 2004;24:180–5. doi: 10.1002/uog.1108. [DOI] [PubMed] [Google Scholar]
- 17.Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, Johnson N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;7:CD012281. doi: 10.1002/14651858.CD012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muzii L, Marana R, Caruana P, Mancuso S. The impact of preoperative gonadotropin-releasing hormone agonist treatment on laparoscopic excision of ovarian endometriotic cysts. Fertil Steril. 1996;65:1235–7. doi: 10.1016/s0015-0282(16)58346-0. [DOI] [PubMed] [Google Scholar]
- 19.Namnoum AB, Hickman TN, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898–902. doi: 10.1016/s0015-0282(16)57899-6. [DOI] [PubMed] [Google Scholar]
- 20.Thomassin I, Bazot M, Detchev R, Barranger E, Cortez A, Darai E. Symptoms before and after surgical removal of colorectal endometriosis that are assessed by magnetic resonance imaging and rectal endoscopic sonography. Am J Obstet Gynecol. 2004;190:1264–71. doi: 10.1016/j.ajog.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Fedele L, Bianchi S, Zanconato G, Bettoni G, Gotsch F. Long-term follow-up after conservative surgery for rectovaginal endometriosis. Am J Obstet Gynecol. 2004;190:1020–4. doi: 10.1016/j.ajog.2003.10.698. [DOI] [PubMed] [Google Scholar]
- 22.Abrao MS, Dias JA, Jr, Bellelis P, Podgaec S, Bautzer CR, Gromatsky C. Endometriosis of the ureter and bladder are not associated diseases. Fertil Steril. 2009;91:1662–7. doi: 10.1016/j.fertnstert.2008.02.143. [DOI] [PubMed] [Google Scholar]
- 23.Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164–6. doi: 10.1016/s0029-7844(02)02446-8. [DOI] [PubMed] [Google Scholar]
- 24.Chapro C, Fauconnier A, Dubuisson JB, Vieira M, Bonte H, Vacher-Lavenu MC. Does deep endometriosis infiltrating the uterosacral ligaments present an asymmetric lateral distribution? BJOG. 2001;108:1021–4. doi: 10.1111/j.1471-0528.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 25.Gustofson RL, Kim N, Liu S, Stratton P. Endometriosis and the appendix: A case series and comprehensive review of the literature. Fertil Steril. 2006;86:298–303. doi: 10.1016/j.fertnstert.2005.12.076. [DOI] [PubMed] [Google Scholar]
- 26.Wie HJ, Lee JH, Kyung MS, Jung US, Choi JS. Is incidental appendectomy necessary in women with ovarian endometrioma? Aust N Z J Obstet Gynaecol. 2008;48:107–11. doi: 10.1111/j.1479-828X.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 27.Chalermchockchareonkit A, Tekasakul P, Chaisilwattana P, Sirimai K, Wahab N. Laparoscopic hysterectomy versus abdominal hysterectomy for severe pelvic endometriosis. Int J Gynaecol Obstet. 2012;116:109–11. doi: 10.1016/j.ijgo.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Busacca M, Vignali M. Ovarian endometriosis: From pathogenesis to surgical treatment. Curr Opin Obstet Gynecol. 2003;15:321–6. doi: 10.1097/01.gco.0000084247.09900.4f. [DOI] [PubMed] [Google Scholar]
- 29.Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev. 2004;3:CD003678. doi: 10.1002/14651858.CD003678.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


