Abstract
Background:
Perimenopause refers to the period around menopause (40-55 years). This includes the period before menopause and the first year after menopause. Perimenopausal age is an important stage in a women’s life. Many women are diagnosed with hypothyroidism at midlife. Hypothyroidism - both overt and subclinical are associated with increased risk of CVS diseases. Subclinical hypothyroidism is more important as this stage is usually ignored from treatment point of view and if early intervention is done in SCH worsening of metabolic derangement may be avoided.
Objectives:
The present study was aimed to know the prevalence of subclinical hypothyroidism and associated dyslipidemia in perimenopausal females.
Material and Methods:
In our retrospective study we took 100 perimenopausal females (40-55years) who were investigated for thyroid and lipid profile. Atherogenic indices like TC/HDL-c, LDL-c/HDL-c, TG/HDL-c ratios were calculated from the individual lipid profile parameters. The reference guidelines for lipid profile was according to NCEP ATP III.
Result:
Subclinical hypothyroidism was found to be present in 18% of perimenopausal females The mean TSH levels were found to be higher in SCH as compared to euthyroid females with a mean value of 7.56±3.54(μIU/ ml). Dyslipidemia was seen in patients with SCH. TSH levels were found to be positively correlated with total cholesterol.
Conclusion:
We conclude that subclinical hypothyroidism is present in 18% females of perimenopausal age group. Increased TSH levels are associated with hypertension, hypertriglyceridemia, and elevated TC/HDL-C ratio and non cholesterol HDL. In perimenopausal women the condition is usually underdiagnosed and ignored but subclinical hypothyroidism in these females should be screened and treated timely to decrease the risk of accelerated atherosclerosis and premature coronary artery disease in them.
KEYWORDS: Atherogenic indices, perimenopausal females, subclinical hypothyroidism
INTRODUCTION
Perimenopause refers to the period around menopause (40–55 years).[1] This includes the period before menopause (when the endocrinological, biological, and clinical features of approaching menopause commence) and the 1st year after menopause. This is the natural transition period which encompasses changes from normal ovulatory cycles to cessation of menses.[2] Perimenopausal age is an important stage in a woman’s life. She is exposed to several social and psychological stress factors at this period. External stress factors as well as fluctuating hormonal levels make women vulnerable to various disorders. Many women are diagnosed with hypothyroidism at midlife. Statistics show that 1 in 8 women between the ages of 35 and 65 years has thyroid disease, and 1 in 5 women over 65 years is affected. Approximately 26% of women in or near perimenopause are diagnosed with hypothyroidism.
While many women with these problems are completely asymptomatic, others may have a wide variety of symptoms; most commonly are mood disturbances (usually in the form of depression and irritability), low energy levels, weight gain, mental confusion, and sleep disturbances.
Many of the symptoms of hypothyroidism are the same as during perimenopause. However, it is still possible to have hypothyroidism, yet have completely normal thyroid function. According to Dr. John Lee, when excess estrogen exists, it can block the action of the thyroid hormone, so that even when the thyroid is producing normal levels of the hormone, the hormone is rendered ineffective and the symptoms of hypothyroidism appear. In this case, laboratory tests may show normal thyroid hormone levels in a woman’s system because the thyroid gland itself is not malfunctioning.[3]
More than 10 million Americans have been diagnosed with thyroid disease, but interestingly, women are at greatest risk, developing thyroid problems seven times more often than men. Thyroid hormone regulates metabolic rate, so low levels tend to cause unwanted weight gain, depression, low energy, and cold intolerance. On the other hand excessive thyroid hormones lead to increased BMR and weight loss. But it’s hypothyroidism, or low thyroid, that is most common in women during the perimenopausal and postmenopausal years.[4]
Moreover, thyroid disorder, especially hypothyroidism, is associated with dyslipidemia, weight gain, and adverse metabolic profile for cardiovascular risk. Both overt and subclinical hypothyroidism are associated with increased risk of CVS diseases.
Hypothyroidism is common, especially in older females. There is increasing evidence that SCH may have serious consequences in peri- and postmenopausal females. It has also been found that SCH is an independent risk factor for atherosclerosis and myocardial infarction in postmenopausal females.
The impact of thyroid hormone on lipid levels may be mediated through genes or via increased catabolism of low-density lipoprotein (LDL). Increased thyroid-stimulating hormone (TSH) levels are also associated with hypercholesterolemia independently.
Subclinical hypothyroidism (SCH) is relatively common among hypercholesterolemic patients. Thus, the measurement of serum TSH levels should be included in the screening of patients with dyslipidemia.[5,6] Hypercholesterolemic patients with SH may be treated with thyroxine substitution therapy because the restoration of euthyroidism can effectively lower the lipid levels, relieve certain symptoms, and may prevent progression to overt hypothyroidism.[7]
The present study was aimed to know the prevalence of SCH and associated dyslipidemia in perimenopausal females.
MATERIALS AND METHODS
In our retrospective study, we included perimenopausal females who were investigated for thyroid and lipid profile. The fasting sample was taken for both the parameters. Lipid profile measurement was based on enzymatic method, on fully automated chemistry analyzer (AU480, Beckman Coulter, USA). Further, LDL-cholesterol (LDL-C) was calculated using the Friedewald formula.[8] Plasma lipid abnormality was based on the expert panel of the National Cholesterol Education Program cutoff values.[9] Further, atherogenic indices such as total cholesterol (TC)/high-density lipoprotein cholesterol (HDL-C), LDL-C/HDL-C, and triglyceride (TG)/HDL-C ratios were calculated from the individual parameters of lipid profile. Non-HDL-C has become a commonly used marker for a blood lipid pattern associated with increased risk of heart disease. It was calculated as non-HDL-C = TC – HDL – C.
Thyroid profile included estimation of free T3, free T4, and TSH. The measurement was done on fully automated chemiluminescence (Access 2 Beckman Coulter, USA).
Statistical analysis
The collected data were analyzed using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp 2011. The differences in groups were determined by performing the Student’s t-test and the Pearson’s correlation and linear regression tests. The data were expressed as mean ± standard deviation. The statistical significance was set at P < 0.05.
RESULTS
Of 100 perimenopausal females, 18% of females were found to be subclinical hypothyroid. Lipid profile was found to be deranged in subclinical hypothyroid patients as compared to euthyroid females. The mean and standard deviation for various biochemical parameters are given in Tables 1-3 and Figures 1-3.
Table 1.
Thyroid profile and general characteristics in perimenopausal female
| General features | Euthyroid | Subclinical hypothyroid |
|---|---|---|
| n | 88 | 18 |
| Age (years) | 44.1±2.38 | 43.3±3.2 |
| BMI (kg/m2) | 20.11±0.56 | 25.0±3.82 |
| Menstrual irregularities (%) | 5 | 37 |
| TSH (mIU/ml) | 3.11±0.62 | 7.56±3.54 |
| FT3 (pg/ml) | 3.2±0.59 | 3.0±0.61 |
| FT4 (ng/dl) | 0.81±0.21 | 0.72±0.19 |
TSH: Thyroid-stimulating hormone, BMI: Body mass index
Table 3.
Atherogenic profile in euthyroid and subclinical hypothyroid perimenopausal females
| Atherogenic profile | Euthyroid | Subclinical hypothyroid females |
|---|---|---|
| TC/HDL | 3.44±0.6 | 4.30±1.1 |
| LDL/HDL | 2.16±0.98 | 2.80±1.2 |
| TG/HDL | 1.94±1.0 | 2.95±1.32 |
| Non HDL Cholesterol (mg%) | 121.9±19.8 | 155.2±17.9 |
P < 0.05. LDL: Low-density lipoprotein, HDL: High-density lipoprotein, TG: Triglyceride, TC: Total cholesterol
Figure 1.
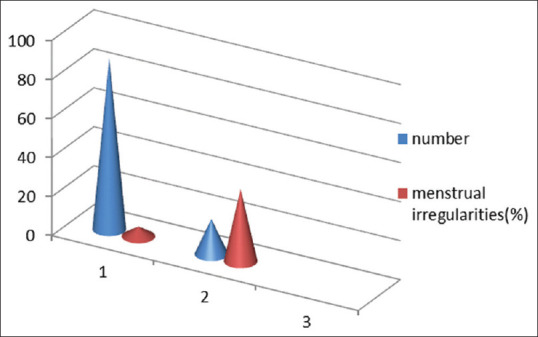
Menstrual irregularities in euthyroid and subclinical hypothyroid perimenopausal females
Figure 3.
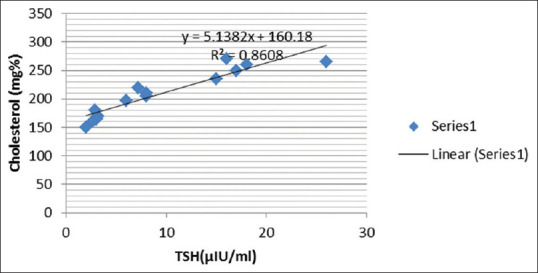
Correlation between thyroid-stimulating hormone and serum cholesterol in subclinical hypothyroid patients
Figure 2.
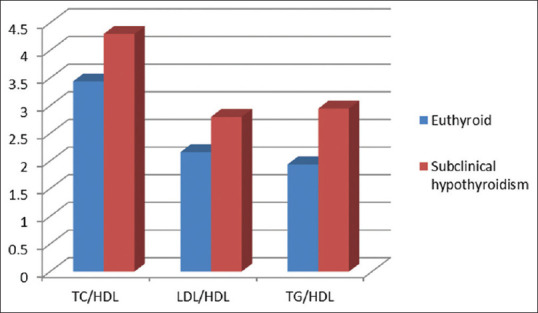
Atherogenic profile in euthyroid and subclinical hypothyroid females
DISCUSSION
Diseases of the thyroid gland are among the most abundant disorders worldwide second only to diabetes.[10] Onset increases with age, and it is estimated that 26% of perimenopausal and menopausal women are diagnosed with thyroid disease. The prevalence and incidence of thyroid disorders is influenced primarily by sex and age. Thyroid disorders are more common in women than men and in older adults compared with younger age groups.[11]
SCH is defined as a syndrome with normal free thyroxine and total thyroxine, but elevated basal thyrotropin levels and/or an exaggerated TSH response to oral thyrotropin-releasing hormone.
The prevalence of SCH in our study was 18% in perimenopausal females [Table 1].
Menstrual irregularities were more in patients with SCH as compared to euthyroid females [Figure 1].
In a study, it was found that 68% of hypothyroid women had menstrual abnormalities in 22 women with hypothyroidism, compared to only 12% in 49 controls. The most common abnormalities observed by hypothyroid women are changes in characteristic of the uterine bleeding and length of the intermenstrual interval; prolonged and heavy flow is commonly noted. It was reported that 23.4% of hypothyroid female patients had irregular cycles.[12] In adult women, the condition results in changes in cycle length and amount of bleeding and has been reported in association with the ovarian hyperstimulation syndrome. In an Indian study, 68.2% of hypothyroid women had menstrual abnormalities, compared to 12.2% of healthy controls.[13]
In our study, the menstrual irregularities were present in 5% of euthyroid females only, whereas 37% of subclinical hypothyroid females had menstrual irregularities.
In SCH, there is an elevation in TSH with normal levels of thyroxine (T4) and triiodothyronine (T3).[14] This condition which is more common in women and older populations may progress to overt hypothyroidism.[15,16] There is growing evidence that SCH is a risk factor for cardiovascular diseases, particularly in elderly women.[17,18,19]
SCH is associated with increased body mass index (BMI) and dyslipidemia as shown in Table 2.
Table 2.
Lipid profile in euthyroid and subclinical hypothyroid perimenopausal females
| Lipid profile (mg %) | Euthyroid | SCH |
|---|---|---|
| Cholesterol | 172±19 | 202±18 |
| TG | 97±18 | 136±37 |
| HDL-C | 50±8.8 | 46±9 |
| LDL-C | 100±8 | 129±4 |
| VLDL | 19.4±4 | 27.2±7.6 |
P<0.05. SCH: Subclinical hypothyroidism, HDL-C: High-density lipoprotein cholesterol, LDL-C: Low-density lipoprotein cholesterol, VLDL: Very low-density lipoproteins, TG: Triglyceride
In females with increased TSH, gain in body weight was observed having BMI value 24.32 ± 4.21 kg/m2 (P < 0.01), which was higher than hyperthyroid and control groups. This weight gain in hypothyroidism may be due to the reduction in removal rate of TGs and cholesterol, which is due to the decrease in the plasma postheparin lipolytic activity. From the data obtained, general reduction in body weight of hyperthyroid patients was observed. This reduction in weight may be due to the accelerated rate of degradation of most lipids out of proportion to synthesis, so body lipid depots consequently become depleted and levels of various plasma lipid components fall.[20]
The role of thyroid hormone in lipid metabolism may be due to its effect in causing dyslipidemia.
Enhanced catabolism of the LDL particles, lowering the bad cholesterol from body, stimulation of the cholesteryl ester transfer protein, an enzyme which transports cholesteryl esters from HDL2 to the very LDLs and the intermediate density lipoproteins, and TGs to the opposite direction, activation of the lipoprotein lipase, and stimulation of hepatic lipase, which catabolizes interconversion of HDL and IDL to LDL, and inhibition of LDL oxidation and thus preventing its accumulation in macrophages[21]
The impact of thyroid hormone on lipid levels is primarily mediated through triiodothyronine (T3)-bound thyroid protein binding and activation of the promoter regions of the LDL receptor and 3-hydroxy-3-methylglutaryl coenzyme A-reductase genes, leading to a reduction in serum cholesterol levels. Thus, the decreased T3 seen in hypothyroidism may result in increased serum cholesterol.[22]
However, in SCH, as the levels of thyroid hormones are normal with elevated TSH levels, this elevated TSH has been independently correlated with hypercholesterolemia.
TSH has extrathyroid targets; also, under healthy circumstances, a negative feedback regulatory system ensures normal thyroid hormone levels. With mild thyroid gland failure (SCH), elevated levels of TSH are required to maintain thyroid function, and these higher TSH levels may accelerate adipocyte lipolysis. TSH can increase cholesterol synthesis by upregulating HMG CoA reductase independent of the thyroid hormones.[23]
In premenopausal women, SCH has a negative effect on the lipoprotein profile and may translate into a sizable cardiovascular risk if left untreated.[24]
Studies enrolling hypothyroid participants receiving suboptimal T4 doses reported significantly larger decreases in serum TC after TSH normalization than studies enrolling previously untreated individuals with mild thyroid failure (P = 0.05). The change in serum LDL-C concentration was with a 95% confidence interval (CI) of −0.12–−0.41. These results suggest that T4 therapy in individuals with mild thyroid failure lowers mean serum total and LDL-C concentrations. The reduction in serum TC may be larger in individuals with higher pretreatment cholesterol levels and in hypothyroid individuals taking suboptimal T4 doses.[25]
Thyroid abnormalities affect a considerable portion of the population, and overt hypothyroidism is associated with an elevated risk of cardiovascular disease and adverse changes in blood lipids. SCH is also associated with an increased risk of cardiovascular disease.[26]
Adverse atherogenic ratios as seen in our study [Table 3] are in concordance with another study which states that SCH in middle-aged women is associated with hypertension, hypertriglyceridemia, and elevated TC/HDL-C ratio. This may increase the risk of accelerated atherosclerosis and premature coronary artery disease in some patients.[27]
In conclusion, it was found; that serum TSH levels are positively and linearly associated with serum TC levels after adjustments for the thyroid hormones in coronary heart disease [CHD] patients. In addition, it was found that TSH alone can increase the TC level in CHD patients independent of the thyroid hormones. Furthermore, the prevalence of hypercholesterolemia increased with an increasing serum TSH level. Each 1 mIU/L increase in the TSH level was estimated to elevate the TC level by 0.015580712 mmol/L. Although this is a very small effect, it is biologically and clinically significant. In accordance with their report that, the present finding that TSH elevations increased the TC level showed a direct clinical effect of TSH on the TC level. Because there is a high prevalence of thyroid dysfunction in CHD patients, it is necessary to routinely test the thyroid function of CHD patients. Maintaining the serum TSH levels in an appropriate range will achieve a homeostasis of the lipid levels and slow the progression of atherosclerosis in CHD patients.[28]
The positive correlation between TSH and cholesterol [Figure 3] indicates the role of increased TSH in hypercholesterolemia and increased TC/HDL ratio in SCH in perimenopausal females.
Non-HDL-C and lipid ratios, including TC/HDL-C and LDL-C/HDL-C, are better predictors for atherosclerosis than conventional lipid profiles.[29] Non-HDL-C has been recommended as a primary target of therapy of coronary artery disease. Non-HDL-C has also been shown to correlate more stronglywith atherogenic lipoprotein subtractions compared to LDL-C.[30],31] In our study, non-HDL-C is significantly increased in patients with SCH [Table 3] as compared to euthyroid patients (P < 0.05, 95% CI: 23.29–43.30).
CONCLUSION
We conclude that SCH is present in 18% of females of perimenopausal age group. Increased TSH levels are associated with hypertension, hypertriglyceridemia, and elevated TC/HDL-C ratio and increased non-HDL-C. In perimenopausal women, the condition is usually underdiagnosed and ignored, but SCH in these females should be screened and treated timely to decrease the risk of accelerated atherosclerosis and premature coronary artery disease in them.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Topper YJ. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- 2.Poppe K, Glinoer D. Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum Reprod Update. 2003;9:149–61. doi: 10.1093/humupd/dmg012. [DOI] [PubMed] [Google Scholar]
- 3.Massoudi MS, Meilahn EN, Orchard TJ, Foley TP, Jr, Kuller LH, Costantino JP, et al. Prevalence of thyroid antibodies among healthy middle-aged women Findings from the thyroid study in healthy women. Ann Epidemiol. 1995;5:229–33. doi: 10.1016/1047-2797(94)00110-f. [DOI] [PubMed] [Google Scholar]
- 4.Sulabha AJ, Bhaloero A, Somalwar S, Jain S, Vaidya M, Sherawat N. Screening of peri and postmenopausal females for hypothyroidism. J South Asian Fed Obstet Gynaecol. 2011;3:14–6. [Google Scholar]
- 5.Cooper DS. Subclinical thyroid disease: A clinician’s perspective. Ann Intern Med. 1998;129:135–8. doi: 10.7326/0003-4819-129-2-199807150-00016. [DOI] [PubMed] [Google Scholar]
- 6.Helfand M, Redfern CC. Clinical guideline, part 2 Screening for thyroid disease: An update American College of Physicians. Ann Intern Med. 1998;129:144–58. doi: 10.7326/0003-4819-129-2-199807150-00020. [DOI] [PubMed] [Google Scholar]
- 7.Ayala AR, Danese MD, Ladenson PW. When to treat mild hypothyroidism. Endocrinol Metab Clin North Am. 2000;29:399–415. doi: 10.1016/s0889-8529(05)70139-0. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 10.Mohanty S, Amruthlal W, Reddy GC, Kusumanjali G, Kanagasabapathy AS, Rao P. Diagnostic strategies for subclinical hypothyroidism. Indian J Clin Biochem. 2008;23:279–82. doi: 10.1007/s12291-008-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metabol. 2003;88:2438–44. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 12.Joshi JV, Bhandarkar SD, Chadha M, Balaiah D, Shah R. Menstrual irregularities and lactation failure may precede thyroid dysfunction or goitre. J Postgrad Med. 1993;39:137–41. [PubMed] [Google Scholar]
- 13.Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Paunkovic J, Paunkovic N, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf) 1999;50:655–9. doi: 10.1046/j.1365-2265.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 14.Higham JM, Shaw RW. The effect of thyroxine replacement on menstrual blood loss in a hypothyroid patient. Br J Obstet Gynaecol. 1992;99:695–6. doi: 10.1111/j.1471-0528.1992.tb13859.x. [DOI] [PubMed] [Google Scholar]
- 15.Efstathiadou Z, Bitsis S, Milionis HJ, Kukuvitis A, Bairaktari ET, Elisaf MS, et al. Lipid profile in subclinical hypothyroidism: Is L-thyroxine substitution beneficial? Eur J Endocrinol. 2001;145:705–10. doi: 10.1530/eje.0.1450705. [DOI] [PubMed] [Google Scholar]
- 16.Bell RJ, Rivera-Woll L, Davison SL, Topliss DJ, Donath S, Davis SR. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease – A community-based study. Clin Endocrinol (Oxf) 2007;66:548–56. doi: 10.1111/j.1365-2265.2007.02771.x. [DOI] [PubMed] [Google Scholar]
- 17.Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia. Ann Fam Med. 2004;2:351–5. doi: 10.1370/afm.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: The Rotterdam study. Ann Intern Med. 2000;132:270–8. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Neves C, Alves M, Medina JL, Delgado JL. Thyroid diseases, dyslipidemia and cardiovascular pathology. Rev Port Cardiol. 2008;27:1211–36. [PubMed] [Google Scholar]
- 20.Farasat T, Liaqat A, Mughal T. Assessment of thyroid hormones level in premenopausal and postmenopausal females. J Appl Pharm. 2010;1:165–78. [Google Scholar]
- 21.Liberopoulos EN, Elisaf MS. Dyslipidemia in patients with thyroid disorders. Hormones (Athens) 2002;1:218–23. doi: 10.14310/horm.2002.1170. [DOI] [PubMed] [Google Scholar]
- 22.Feld S, Dickey RA. An association between varying degrees of hypothyroidism and hypercholesterolemia in women: The thyroid-cholesterol connection. Arch Intern Med. 2000;160:526–34. [Google Scholar]
- 23.Sorisky A, Gagnon A. Freedom of expression beyond the thyroid: the thyroid-stimulating hormone receptor in the adipocyte. OA Biochem. 2014;2:2. [Google Scholar]
- 24.Mikhail GS, Alshammari SM, Alenezi MY, Mansour M, Khalil NA. Increased atherogenic low-density lipoprotein cholesterol in untreated subclinical hypothyroidism. J Clin Endocrinol Metab. 2000;85:2993–3001. doi: 10.4158/EP.14.5.570. [DOI] [PubMed] [Google Scholar]
- 25.Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: Effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: A quantitative review of the literature. J Biol Chem. 2003;278:34114–8. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- 26.Luboshitzky R, Aviv A, Herer P, Lavie L. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid. 2002;12:421–5. doi: 10.1089/105072502760043512. [DOI] [PubMed] [Google Scholar]
- 27.Luboshitzky R, Aviv A, Herer P, Lavie L. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Vojnosanit Pregl. 2007;64:749–52. doi: 10.1089/105072502760043512. [DOI] [PubMed] [Google Scholar]
- 28.Xu C, Yang X, Liu W, Yuan H, Yu C, Gao L, et al. Thyroid stimulating hormone, independent of thyroid hormone, can elevate the serum total cholesterol level in patients with coronary heart disease: A cross-sectional design. Nutr Metab (Lond) 2012;9:44. doi: 10.1186/1743-7075-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou Q, Li S, Gao Y, Tian H. Relations of lipid parameters, other variables with carotid intima-media thickness and plaque in the general Chinese adults: An observational study. Lipids Health Dis. 2018;17:107. doi: 10.1186/s12944-018-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 1 – Full report. J Clin Lipidol. 2015;9:129–69. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhu CG, Zhang Y, Xu RX, Li S, Wu NQ, Guo YL, et al. Circulating non-HDL-C levels were more relevant to atherogenic lipoprotein subfractions compared with LDL-C in patients with stable coronary artery disease. J Clin Lipidol. 2015;9:794–800. doi: 10.1016/j.jacl.2015.08.010. [DOI] [PubMed] [Google Scholar]


