Abstract
CNS trauma is a prominent cause of mortality and morbidity, and although much effort has focused on developing treatments for CNS trauma-related pathologies, little progress has been made. Pre-clinical models of TBI and SCI suffer from significant drawbacks, which result in substantial failures during clinical translation of promising pre-clinical therapies. Here, we review recent advances made in the development of in vitro models of CNS trauma, the promises and drawbacks of current in vitro CNS injury models, and the attributes necessary for future models to accurately mimic the trauma microenvironment and facilitate CNS trauma drug discovery. The goal is to provide insight for the development of future CNS injury models and to aid researchers in selecting effective models for pre-clinical research of trauma therapeutics.
Keywords: CNS trauma, traumatic brain injury, spinal cord injury, ex vivo injury model, in vitro injury model
1. Introduction
Traumatic brain injury (TBI) and spinal cord injury (SCI) are major causes of death and disability in the world. Thus far, little substantial progress has been made in the development of effective therapies to treat TBI- and SCI-associated neuropathologies. Current models of TBI and SCI do not accurately recapitulate many of the neurophysiological and heterogenous aspects of CNS trauma, leading to a bottleneck in the research and development of novel therapeutics. Animal models, often utilizing mouse or rat, are currently the gold standard for pre-clinical CNS trauma drug development. However, the heterogeneity of types of injury in humans, lack of standardization across different animal models, and fundamental differences between murine and human physiology complicate clinical translation of discovered pharmaceuticals, many of which fail in Phase II and Phase III TBI clinical trials [1]. The use of higher order mammals in CNS trauma models mitigates some of these issues for translation, but these models are costly and significantly limit throughput of candidate drug studies. Nonetheless, animal models are critical for pre-clinical assessment of behavioral outcomes and systemic effects of drug administration and will remain a necessary component of pre-clinical drug development. In contrast, in vitro models provide researchers with several advantages over animal models, which include significantly decreased costs, higher throughput capabilities, and greater control over experimental conditions. Due to these advantages, in vitro models are ideal for drug discovery and mechanism of action studies. However, significant improvements can be made to facilitate drug development for CNS trauma through the development of in vitro CNS injury models, which accurately mimic the physiological structure and function of in vivo tissue, are reproducible, allow for precise control over injury and treatment, and are suited for high throughput candidate assessment. Here, we review recent advances made in in vitro TBI and SCI models, promises and drawbacks of current in vitro models, and necessary features of future models to facilitate drug discovery.
2. Applications for in vitro models of CNS trauma
The primary goal of in vitro trauma models is to recapitulate injury pathology in a controlled system and decrease the influence of confounding systemic effects, allowing for discernment of specific mechanistic injury cascades and effects of therapeutic interventions on injury parameters. Well-developed models promote basic science research into mechanistic sequalae of CNS injury, facilitate discovery and validation of new therapy targets, and promote discovery of novel therapeutics. Brain and spinal cord trauma are highly heterogenous, and although both types of trauma share similarities in pathogenesis, there are distinct differences between TBI and SCI in the mechanisms of secondary injury progression. Furthermore, barriers to therapeutic success and types of therapeutic strategies applied in research of potential treatments vary between TBI and SCI. Many models in use today either recreate aspects of mechanical injury directly or induce facets of secondary injury cascades by means of chemical intervention or co-culture of affected cell types. Due to fundamental differences in pathobiology between TBI and SCI, distinct in vitro models of injury are used to mimic the human condition to discern and understand specific elements of the injury cascade and to test the efficacy of potential therapeutic interventions in overcoming the barriers to successful therapy.
3. Aspects of TBI and SCI pathogenesis modeled in vitro
CNS trauma results from mechanical loading onto CNS tissue, propagating a series of molecular events that over time, lead to neuronal cell death in regions proximal and distal to the injury site. Injuries can be subdivided into focal and diffuse, depending on the mechanics of the forces acting on the tissue (Fig. 1a,b). Focal injury occurs as a result of direct impact to tissue, and depending on injury severity, can mediate formation of a primary lesion. Diffuse injury results from rapid linear and/or rotational acceleration/deceleration of respective tissue that induces shear, compression, and tension stress in multifocal regions of affected brain or spinal cord [2]. While focal injury is common in both SCI and TBI, diffuse injury and resulting axonal stretch damage are more prevalent in TBI [2]. Thus, while penetrating brain injury and SCI share common pathology, there are distinct differences between mild TBI or concussion and SCI [2,3]. Additionally, direct damage to axonal tracts projecting through the spinal cord is common in SCI, while acceleration-induced axonal injury in TBI is diffuse and rarely involves axonal transection [3]. This is reflected in the clinical treatment strategies currently applied to SCI and TBI, as many SCI treatments aim to promote axonal regeneration while therapies for TBI commonly aim to decrease neuronal cell death resulting from glutamate excitotoxicity and excessive generation of reactive oxidative species [2–4].
Fig. 1. Aspects of TBI and SCI pathogenesis modeled in vitro.
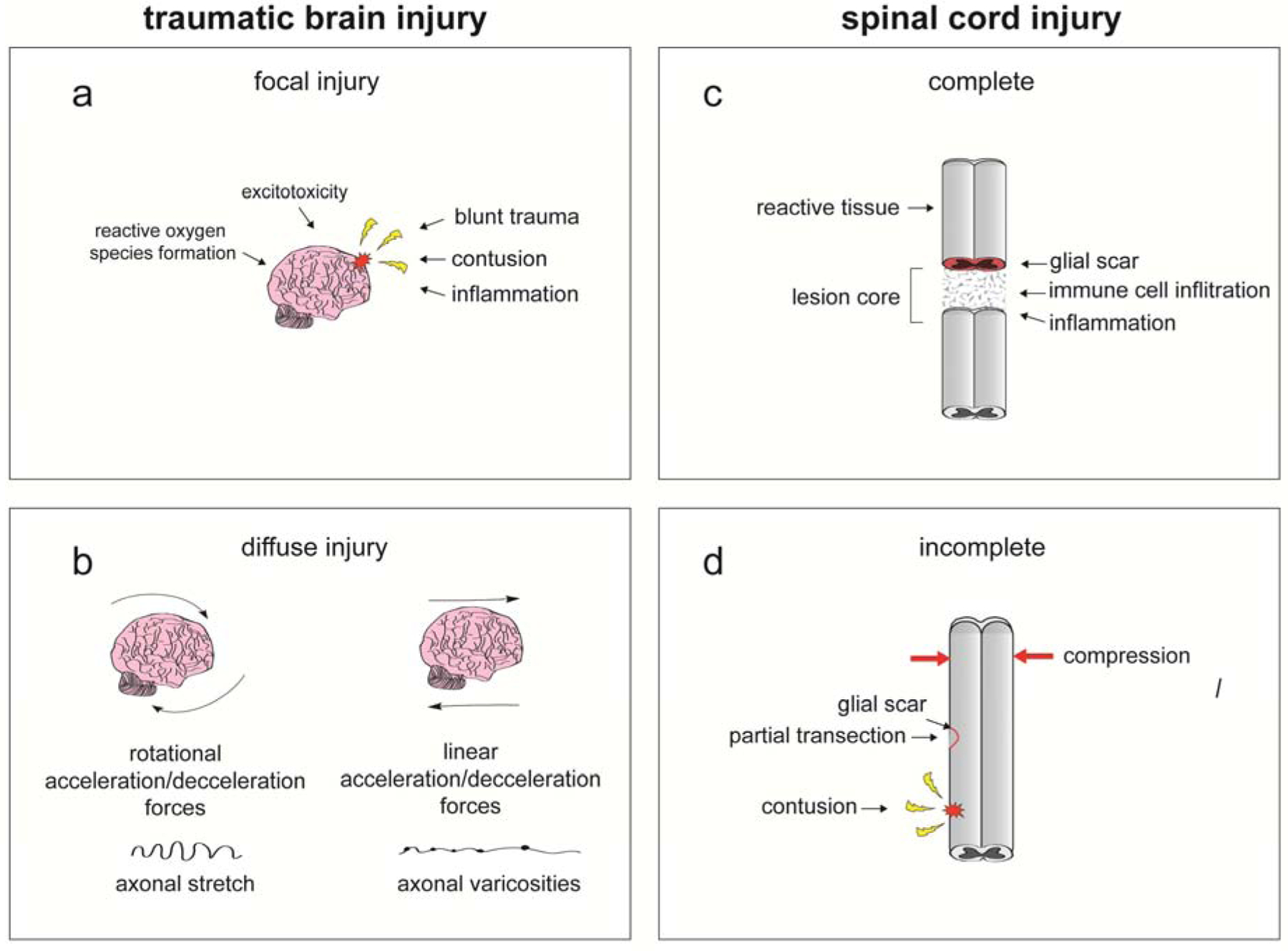
CNS injury is highly heterogenous and is categorized the biomechanics of the forces acting on the brain or spinal cord during trauma, injury severity, and other clinical complications. a. Focal injury results from direct impact to tissue and can lead to formation of a lesion. b. Diffuse injury results from rapid rotational and/or linear acceleration of brain or spinal cord that propagates shear, compression, and tension stress in multiple regions of the respective tissue. c-d Spinal cord injury can be further classified based on whether the spinal cord is completely severed (c) or is partially damaged (d). Investigators should utilize appropriate in vitro models of CNS trauma based on the severity and biomechanics of injury that is the focus of study.
During the mechanical phase of TBI and SCI, direct impact to or acceleration of the skull or the spinal column, respectively, can result in contusion, compression, hemorrhage, or direct damage to brain or spinal cord tissue. In the case of SCI, deformation of the spinal cord results in the formation of cystic cavitations, which are filled with extracellular fluid, bands of connective tissue, and macrophages, which ultimately develop into a lesion [5]. Following TBI or SCI, there is physical damage to neurons and glial cells, disruption of the blood-brain barrier (BBB), and damage to cerebral and spinal cord vasculature. Disruptions in microvasculature and breakdown of the BBB can induce ischemic conditions and inflammation. Furthermore, following injury, glial cells release proinflammatory cytokines, and macrophages and neutrophils may infiltrate the spinal cord or brain. Thus, neural cell viability (particularly for neurons), ischemic conditions, and the inflammatory microenvironment represent important components of SCI and TBI for researchers to study [3,5].
After SCI, heightened astrocyte activation and proliferation proximal to the lesion site during the subacute phase also result in the formation of a perilesional cellular barrier. Extracellular matrix proteins secreted from microglia, macrophage, and astrocyte-mediated signaling during the acute phase associate with this cellular barrier to form the glial scar and prevent axonal regeneration during earlier stages after mechanical injury. Although glial scar formation is generally associated with adverse effects following injury, recent research indicates that glial scars may also play a beneficial role during recovery, thus complicating current treatment strategies [3,4]. The glial scar plays an important role in restricting inflammatory cells to the injury site, thereby preserving healthy surrounding neural tissue, and recent data support the idea that glial scar borders promote axonal regeneration during later stages post-injury [3,4]. Thus, modeling astrogliosis and glial scar formation is important for more comprehensive characterization of SCI pathophysiology [5].
During the intermediate and chronic phases of SCI and TBI, some level of endogenous recovery, in the form of neuron remyelination, axonal outgrowth, and neural progenitor cell proliferation and differentiation, may occur. However, the extent of recovery is largely dependent upon injury severity. In the case of a complete injury, the spinal cord is severed, and little to no recovery of tissue integrity and function below the injury site occurs (Fig. 1c). Patients with injuries that involve only a partial transection or less severe compression or contusion may have a greater chance of recovery [5] (Fig. 1d). It is important for investigators to choose appropriate in vitro models of injury respective to the severity of injury that is being studied. Thus, developing accurate models of injury that can reliably recapitulate the effects of varying severities of SCI and TBI is essential for advancements in the field and the development of new therapies to more effectively address the unmet needs of these patient populations.
4. Mechanical in vitro CNS injury models
To mimic impact trauma in vitro, models of TBI and SCI apply many of the same mechanical types of injuries as those used in the past [6], including mechanical stretch, transection or scratch, blunt impact, and compression (Fig. 2). Mechanical injury models aim to mimic the biophysical phenomena that occur in clinical TBI or SCI to recapitulate the pathobiological mechanisms and microenvironment of secondary injury. These models are used to elucidate and characterize the biomechanics and pathology of trauma, to test the efficacy of potential therapeutic interventions, and to facilitate drug discovery. In contrast with animal trauma models, mechanical in vitro models allow for precise control of applied injury biomechanics, observation of live cell injury response, and acquisition of high-content data from large sets of experimental conditions. Thus, these types of models are used to further characterize the cellular and molecular response to injury and the biomechanics of different injuries at the tissue and cellular levels to better understand trauma pathology and uncover new therapeutic targets. Due to the heterogeneous nature of clinical injuries, characterization of biophysical and molecular responses to distinct injury subtypes is important as a personalized therapeutic approach may become more relevant to treatment of TBI and SCI in lieu of a ‘one-fits-all’ standardized treatment.
Fig. 2. In vitro models of CNS trauma.
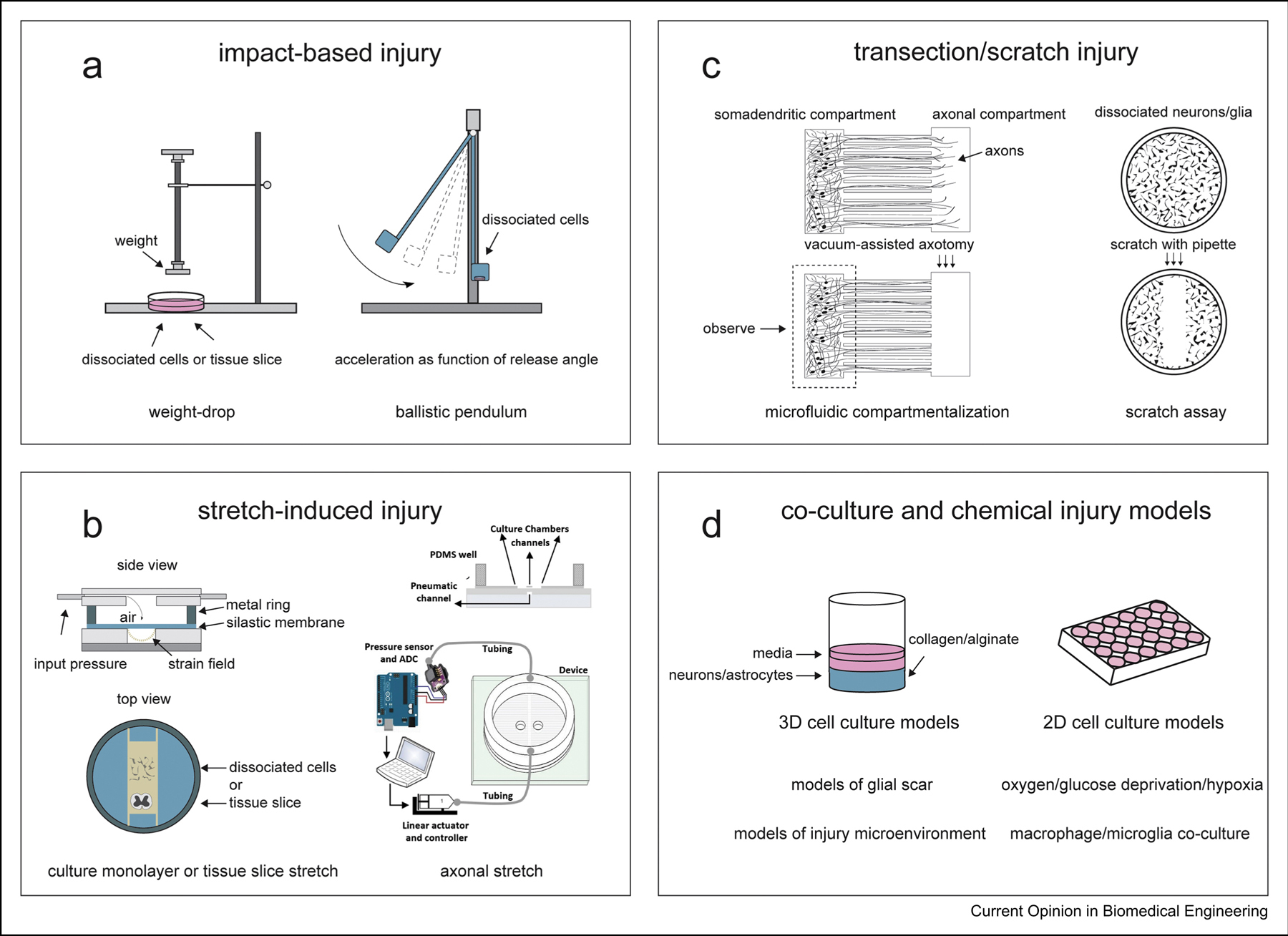
Mechanical models of CNS injury aim to recreate the biomechanical parameters that underlie specific injury subtypes to recapitulate secondary injury mechanisms and the injury microenvironment. a. Impact-based models utilize a weight-drop or ballistic pendulum mechanism to induce blunt tissue damage or to recreate collisional impact, respectively. b. Stretch-induced injury models deform surrogate tissue or cells cultured on a flexible substrate, such as polydimethylsiloxane (PDMS), using compressed gas or by lowering the substrate onto an indenter platform. Biaxial and uniaxial strain can be applied based on the type of apparatus and setup employed. Microfabricated devices are used to compartmentalize and isolate axonal projections in microgrooves for investigation of axonal stretch injury. c. Transection models induce axotomy using laser or vacuum in investigations of axonal degeneration and regeneration. Microfabricated platforms with microgrooves are used in transection models to separate somatodendritic and axonal compartments to isolate axotomy and observe injury-induced alterations in synapse remodeling and synaptic function. d. Aspects of secondary injury pathogenesis are modeled in isolation to control for confounding pathology, especially in investigations of mechanism of action. 3D co-culture models have been developed using collagen or alginate to encapsulate primary neural cells in studies of perilesional tissue architecture alterations following injury. 2D monolayer cultures are used to investigate post-injury cell-cell interactions and injury-induced biochemical changes in neurons and glial cells. Modified culture conditions, such as oxygen/glucose deprivation, and treatments with chemicals are used to mimic the injury microenvironment and to control for confounding factors. Part of Figure 2b is adapted from [26] and is licensed under a Creative Commons Attribution 4.0 International License, which allows use, sharing, adaptation, distribution and reproduction. Copy of license: http://creativecommons.org/licenses/by/4.0/.
4.1. Impact-based injury
Blunt injury to the brain or spinal cord is modeled using weight-drop-based impactors that use a controlled weight-drop mechanism to drive a stylus [7] or flat impactor head [8,9] directly onto ex vivo cultures or acute preparations of brain or spinal cord tissue (Fig. 2a). These models mimic focal contusion and compression injuries and are used to study severe TBI and SCI, typically using rodent tissue explants or slice preparations. Alternatively, engineered 3D neural tissue models can be used as surrogates for trauma studies [10,11]. 3D brain models, in which cell bodies and axonal tracts are compartmentalized, can provide crucial information about effects of injury on white matter processes. To investigate the effects of impact at the single cell level, a microfluidic device has been developed in which individual cells can be impacted and compressed with up to 90% strain by a nickel-iron armature and subsequently cultured or analyzed [12].
Recently, an innovative in vitro model was developed to mimic acceleration conditions during impact, without concomitant blunt force trauma-induced tissue damage or deformation. The ballistic pendulum injury system allows for study of neural networks cultured on microelectrode arrays (MEAs) after high accelerations (30 to 300 g) and subsequent monitoring of cellular morphology and neural network electrophysiological function post-injury [13]. Investigation of alterations in network activity after rapid acceleration is of high significance for research and development of therapeutics for protection from concussion-induced deficits in neural network function.
4.2. Stretch-induced injury
Stretch-induced injury models aim to recreate traumatic brain and spinal cord deformation and are the most widely used in vitro TBI and SCI models. Typically, surrogate tissues or cultured cells are placed or grown on a flexible substrate, such as polydimethylsiloxane (PDMS), which is deformed by a hollow indenter [14–18] or pulses of compressed air [19] or nitrogen gas [20] (Fig.2b). Mechanical strain models have been extensively characterized and are commercially available as flexible silicone-based multi-well plates and accompanying validated injury controller systems [21–24]. Commercially available stretch culture systems have been developed over two decades and offer flexible membrane 6 and 24 multi-well plates, significantly increasing throughput capabilities of such systems. Specialized multi-well plates and apparatus modifications allow for induction of both equiaxial and uniaxial strain at a maximum of 20% strain. Moreover, a high-throughput 96 well stretch injury device driven by electromagnetic voice coils, which induces injury by stretching a silicone membrane with cultured cells onto hollow posts, was recently developed [25]. Applications of similar high-throughput systems will greatly improve future drug discovery and combination therapy screens. Importantly, as stretch models provide a high level of control and reproducibility, these models are becoming more widely used to study effects of repetitive TBI on brain function and recovery [16,17].
While the aforementioned models use stretch applied to whole organotypic slices or cultured cell monolayers, microfabricated microfluidic devices are used to compartmentalize and separate neuronal soma and axons for localized axonal stretch injury [26,27] (also reviewed in [28]). Diffuse axonal injury is a notable component of mild TBI neuropathology, and thus, these models induce stretch injury to axons alone to monitor post-injury axonal pathology at the subcellular level. Alternatively, a silicone barrier can be employed to compartmentalize neuronal soma and axons in commercially available elastic chambers [29]. After cell attachment, the barrier is removed, allowing axons to traverse through the previously blocked off area towards soma on the other side.
4.3. Transection and scratch injury
Transection and scratch injury models are commonly used to characterize trauma-induced axotomy and assess efficacy of therapeutics to promote axonal regeneration post-injury. While primary axotomy is relatively rare in TBI, damage to axonal tracts is more prevalent in SCI, especially in cases of complete SCI. It is important to develop models for axonal regeneration that accurately mimic the injury microenvironment and increase throughput relative to animal SCI models. Transection is typically performed by using a scalpel blade [30–32], laser [33], plastic stylet [34] or needle [35] to cut ex vivo tissue [30,31] or isolated axonal tracts [36] (Fig. 2c). A novel transection method was recently developed in which cultured neurons are transected with a custom-built tool while mounted on a light sheet microscope, allowing observation of post-injury actin reorganization, which is difficult to do using conventional microscopy [35]. While induction of transection injury aims to mimic primary physical injury, it also leads to the activation of the secondary injury responses observed in vivo. The injury microenvironment is recapitulated through promotion of glutamate-induced excitotoxicity [36], release of proinflammatory cytokines [37], expression of growth factors and axonal growth inhibiting molecules, such as chondroitin sulfate proteoglycan [32,36,37], and alterations in cell metabolism and generation of reactive oxygen species [32].
Tissue or cultured cells can be transected in a dish or on a cell culture insert for studies of macroscopic injury in which damage is not localized or cell-specific. Microfabricated microfluidic devices can be utilized to compartmentalize or isolate neuronal dendrites and axons into separate chambers for localized transection. This type of microfluidics model was used to compartmentalize dissociated hippocampal neurons into somatodendritic and axonal compartments to investigate the effects of distal axotomy on synaptic remodeling [36]. Axons traverse from the somatodendritic compartment to the axonal compartment through 8μm wide microgrooves, which separate the two compartments [36]. To perform axotomy, fluid was aspirated from the axonal compartment, allowing for examination of the effects of axotomy on retrograde spine loss, synaptic vesicle release, and miniature excitatory postsynaptic currents of axotomized neurons [36].
An additional transection in vitro model is the scratch assay, in which primary neurons or astrocyte cultures or immortalized cell lines are scraped using a pipette tip [38] or plastic needle [39] to induce secondary damage and initiate secondary injury molecular cascades. This model is simple and accessible and is commonly used to induce astrocyte reactivity in studies of inflammation [37] and assess astrocytic response and wound closure following injury [38,40]. Moreover, because of its simplicity, the model is also used to induce generalized injury to neurons [34,39,41] or cell lines [42] as a means to investigate secondary injury.
5. Secondary injury models
In addition to modeling the primary impact that occurs as the first phase of CNS trauma, several groups have more directly assessed specific molecular pathologies associated with secondary injury. Many of the models used aim to characterize specific features of the injury microenvironment. Most commonly assessed secondary injury pathologies include perilesional tissue architecture, oxidative stress, and cell-cell interactions. In the case of tissue and lesion architecture models, 3D culturing methods have played an instrumental role in their development. Models of the glial scar, a hallmark of secondary SCI, are constructed by implanting mixed neural cell or astrocyte cultures in collagen or alginate gels [43–45] (Fig. 2d). These 3D models successfully recapitulate several important characteristics of glial scar pathophysiology and architecture, including hyperplasia [43,44], astrocyte scar clusters formation [44], changes in gene expression [45], and increased extracellular matrix production [45]. Thus, such models more accurately recapitulate the structure of injured tissue after CNS injury.
Structural alterations to the injury microenvironment are further augmented by biochemical changes. Oxidative stress, which occurs under ischemic conditions as a result of heightened ROS generation and release, is another molecular component of both secondary TBI and SCI. Ischemia is modeled through oxygen-glucose deprivation of dissociated neural cell [46,47] and organotypic slice cultures [48]. Subjecting microglial cultures to hypoxia/hypoglycemia (1% oxygen, 250μM glucose) followed by normoxia/normoglycemia (21% oxygen, 25mM glucose) was shown to induce targeted expression of interferon-stimulated genes [46], important signaling mediators of neuroinflammation and neuroimmune response.
Additional studies on CNS injury implement co-culturing models to examine interactions between specific cell types within or proximal to the injured tissue. Macrophage-microglia studied with this type of model demonstrated that macrophages suppress pro-inflammatory and phagocytic functions of microglia [49]. Additionally, investigation of pericyte-oligodendrocyte dynamics demonstrated that pericytes promote the differentiation of oligodendrocyte precursor cells, which ultimately contribute to CNS remyelination [50]. Cumulatively, in vitro studies on secondary CNS injury have expanded our understanding of injury-associated signaling, gene and protein expression, and cell-type specific interactions. However, additional advancements in the field and the continued development of new systems are needed to provide more diverse and versatile models of secondary injury pathophysiology.
6. Limitations and future improvements
Although in vitro CNS trauma models are useful tools for investigation of injury neuropathology and discovery of new treatments, there are several limitations which hinder their utility. The most prominent is disparity between cultured primary cells and their counterparts in vivo due to differences in microenvironment. Preparation of ex vivo brain or spinal cord slices, especially using a vibratome, results in tissue and cell damage, which may affect cellular and molecular responses following experimental injury procedures and therapy treatments. Culture conditions, while mimicking the in vivo microenvironment, are still generally distinct from conditions in vivo, making it difficult to predict how patient cells will behave during the process of clinical translation. Isolation of primary cells and tissue may affect gene regulation, and thus, affect downstream gene expression during experimental procedures. Furthermore, it is difficult to deduce drug dosages for in vivo applications from drug concentrations applied in vitro, further complicating studies of toxicity and mechanism of action.
In addition to general constraints of in vitro studies, there are several drawbacks to in vitro trauma models, limiting their potential. While animal trauma models will remain necessary during pre-clinical drug development, in vitro models could be significantly improved to bolster existing strengths and further utility for different drug discovery applications. Few models, beside the commercially sold stretch microwell plates, offer medium to high throughput assessment capabilities. As in vitro models can serve as important tools in discovery of new drug and therapy candidates and in studies of drug toxicity, the ability to test a multitude of compounds and concentrations in one experiment becomes of significant importance. Medium- to high-throughput in vitro injury models should also be highly controlled and reproducible, to ensure a similar level of injury across conditions and to decrease technical and biological variability. In addition, there is also a need for additional in vitro models that utilize co-culture of different cell types whereby groups of cells and their interactions can be controlled to further dissect the roles that different glial cell types play during the manifestation of post-injury sequalae. Future developments of in vitro CNS injury models should aim to improve cell culture conditions to better mimic the in vivo microenvironment, allowing for greater dynamic control over cellular signaling, behavior, and response, and the composition of and alterations in the extracellular environment in response to injury. Improvements to current ex vivo culture systems include incorporating technologies used for 3D culture to improve the congruence between current in vitro models and corresponding in vivo phenomena, promoting retention of tissue or explant 3D structure and cellular composition.
Acknowledgements
This work was funded by National Science Foundation grant #CBET 1512170 and New Jersey Commission on Brain Injury Research grant #CBIR14IRG019 and #CBIR17IRG006 to B.L.F. A.O. and N.S. were supported by National Institutes of Health Biotechnology Training Grant T32 GM008339-20. A.O. was supported by Predoctoral Fellowship from the New Jersey Commission on Brain Injury Research #CBIR19FEL018. N.S. was supported by Predoctoral Fellowship from the New Jersey Commission on Spinal Cord Injury Research #CSCR20FEL004.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Stein DG: Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Injury 2015, 29:1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill CS, Coleman MP, Menon DK: Traumatic Axonal Injury: Mechanisms and Translational Opportunities. Trends Neurosci. 2016, 39:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courtine G, Sofroniew MV: Spinal cord repair: advances in biology and technology. Nature Medicine 2019, 25:898–908. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV: Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG: Traumatic spinal cord injury. Nature Reviews Disease Primers 2017, 3:17018. [DOI] [PubMed] [Google Scholar]
- 6.Morrison III B, Elkin BS, Dolle JP, Yarmush ML: In vitro models of traumatic brain injury. Annu. Rev. Biomed. Eng 2011, 13:91–126. [DOI] [PubMed] [Google Scholar]
- 7.Llufriu-Dabén G, Meffre D, Massaad C, Jafarian-Tehrani M: A novel model of trauma-induced cerebellar injury and myelin loss in mouse organotypic cerebellar slice cultures using live imaging. Journal of Neuroscience Methods 2019, 311:385–393. [DOI] [PubMed] [Google Scholar]
- 8.Pandamooz S, Salehi MS, Zibaii MI, Safari A, Nabiuni M, Ahmadiani A, Dargahi L: Modeling traumatic injury in organotypic spinal cord slice culture obtained from adult rat. Tissue and Cell 2019, 56:90–97. [DOI] [PubMed] [Google Scholar]
- 9.Pandamooz S, Salehi MS, Zibaii MI, Ahmadiani A, Nabiuni M, Dargahi L: Epidermal neural crest stem cell-derived glia enhance neurotrophic elements in an ex vivo model of spinal cord injury. J Cell Biochem 2018, 119:3486–3496. [DOI] [PubMed] [Google Scholar]
- 10.Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL: Bioengineered functional brain-like cortical tissue. Proceedings of the National Academy of Sciences 2014, 111:13811–13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chwalek K, Tang-Schomer MD, Omenetto FG, Kaplan DL: In vitro bioengineered model of cortical brain tissue. Nature Protocols 2015, 10:1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson LHC, Walker JL, Rodriguez-Mesa E, Shields K, Foster JS, Valentine MT, Doyle AM, Foster KL: Investigating Cellular Response to Impact With a Microfluidic MEMS Device. Journal of Microelectromechanical Systems 2019:1–11. [Google Scholar]
- 13.Rogers EA, Gross GW: Simultaneous electrophysiological and morphological assessment of functional damage to neural networks in vitro after 30–300 g impacts. Scientific Reports 2019, 9:14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Pietro V, Amorini AM, Lazzarino G, Yakoub KM, D’Urso S, Lazzarino G, Belli A: S100B and Glial Fibrillary Acidic Protein as Indexes to Monitor Damage Severity in an In Vitro Model of Traumatic Brain Injury. Neurochemical Research 2015, 40:991–999. [DOI] [PubMed] [Google Scholar]
- 15.Lamprecht MR, Elkin BS, Kesavabhotla K, Crary JF, Hammers JL, Huh JW, Raghupathi R, Morrison B: Strong Correlation of Genome-Wide Expression after Traumatic Brain InjuryIn VitroandIn VivoImplicates a Role for SORLA. Journal of Neurotrauma 2017, 34:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effgen GB, Morrison B: Electrophysiological and Pathological Characterization of the Period of Heightened Vulnerability to Repetitive Injury in an in Vitro Stretch Model. Journal of Neurotrauma 2016, 34:914–924. [DOI] [PubMed] [Google Scholar]
- 17.Effgen GB, Morrison B: Memantine Reduced Cell Death, Astrogliosis, and Functional Deficits in an in vitro Model of Repetitive Mild Traumatic Brain Injury. Journal of Neurotrauma 2016, 34:934–942. [DOI] [PubMed] [Google Scholar]
- 18.Hu F, Lamprecht MR, Wei L, Morrison B, Min W: Bioorthogonal chemical imaging of metabolic activities in live mammalian hippocampal tissues with stimulated Raman scattering. Sci Rep 2016, 6:39660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel MV, Sewell E, Dickson S, Kim H, Meaney DF, Firestein BL: A Role for Postsynaptic Density 95 and Its Binding Partners in Models of Traumatic Brain Injury. Journal of Neurotrauma 2019, 36:2129–2138. [DOI] [PubMed] [Google Scholar]
- 20.Xu Z, Liu Y, Yang D, Yuan F, Ding J, Chen H, Tian H: Sesamin protects SH-SY5Y cells against mechanical stretch injury and promoting cell survival. BMC Neuroscience 2017, 18:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-García I, Gerő D, Szczesny B, Szoleczky P, Olah G, Módis K, Zhang K, Gao J, Wu P, Sowers LC, et al. : Development of a stretch-induced neurotrauma model for medium-throughput screening in vitro: identification of rifampicin as a neuroprotectant. British Journal of Pharmacology 2018, 175:284–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chierto E, Simon A, Castoldi F, Meffre D, Cristinziano G, Sapone F, Carrete A, Borderie D, Etienne F, Rannou F, et al. : Mechanical Stretch of High Magnitude Provokes Axonal Injury, Elongation of Paranodal Junctions, and Signaling Alterations in Oligodendrocytes. Molecular Neurobiology 2019, 56:4231–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rachmany L, Tweedie D, Rubovitch V, Li Y, Holloway HW, Kim DS, Ratliff WA, Saykally JN, Citron BA, Hoffer BJ, et al. : Exendin-4 attenuates blast traumatic brain injury induced cognitive impairments, losses of synaptophysin and in vitro TBI-induced hippocampal cellular degeneration. Sci Rep 2017, 7:3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Chen Y, Meng J, Wu M, Bi F, Chang C, Li H, Zhang L: Ablation of caspase-1 protects against TBI-induced pyroptosis in vitro and in vivo. Journal of Neuroinflammation 2018, 15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman SA, Phillips JK, Costa JT, Cho FS, Oungoulian SR, Finan JD: Stretch Injury of Human Induced Pluripotent Stem Cell Derived Neurons in a 96 Well Format. Scientific Reports 2016, 6:34097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dollé JP, Morrison III B, Schloss RS, Yarmush ML: Brain-on-a-chip microsystem for investigating traumatic brain injury: Axon diameter and mitochondrial membrane changes play a significant role in axonal response to strain injuries. Technology 2014, 2:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omelchenko A, Shrirao AB, Bhattiprolu AK, Zahn JD, Schloss RS, Dickson S, Meaney DF, Boustany NN, Yarmush ML, Firestein BL: Dynamin and reverse-mode sodium calcium exchanger blockade confers neuroprotection from diffuse axonal injury. Cell Death & Disease 2019, 10:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrirao AB, Kung FH, Omelchenko A, Schloss RS, Boustany NN, Zahn JD, Yarmush ML, Firestein BL: Microfluidic platforms for the study of neuronal injury in vitro. Biotechnol Bioeng 2018, 115:815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Li C, Gan C, Zhao K, Chen J, Song J, Lei T: A Precise, Controllable in vitro Model for Diffuse Axonal Injury Through Uniaxial Stretch Injury. Frontiers in Neuroscience 2019, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patar A, Dockery P, Howard L, McMahon SS: Cell viability in three ex vivo rat models of spinal cord injury. J Anat 2019, 234:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashworth BE, Stephens E, Bartlett CA, Serghiou S, Giacci MK, Williams A, Hart NS, Fitzgerald M: Comparative assessment of phototherapy protocols for reduction of oxidative stress in partially transected spinal cord slices undergoing secondary degeneration. BMC neuroscience 2016, 17:21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guijarro-Belmar A, Viskontas M, Wei Y, Bo X, Shewan D, Huang W: Epac2 Elevation Reverses Inhibition by Chondroitin Sulfate Proteoglycans In Vitro and Transforms Postlesion Inhibitory Environment to Promote Axonal Outgrowth in an Ex Vivo Model of Spinal Cord Injury. The Journal of Neuroscience 2019, 39:8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stivers NS, Pelisch N, Orem BC, Williams J, Nally JM, Stirling DP: The toll-like receptor 2 agonist Pam3CSK4 is neuroprotective after spinal cord injury. Exp Neurol 2017, 294:1–11. [DOI] [PubMed] [Google Scholar]
- 34.Luo P, Li X, Wu X, Dai S, Yang Y, Xu H, Jing D, Rao W, Xu H, Gao X, et al. : Preso regulates NMDA receptor-mediated excitotoxicity via modulating nitric oxide and calcium responses after traumatic brain injury. Cell Death & Disease 2019, 10:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips JK, Sherman SA, Cotton KY, Heddleston JM, Taylor AB, Finan JD: Characterization of neurite dystrophy after trauma by high speed structured illumination microscopy and lattice light sheet microscopy. Journal of Neuroscience Methods 2019, 312:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagendran T, Larsen RS, Bigler RL, Frost SB, Philpot BD, Nudo RJ, Taylor AM: Distal axotomy enhances retrograde presynaptic excitability onto injured pyramidal neurons via trans-synaptic signaling. Nature Communications 2017, 8:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong HN, Shim JH, Won YJ, Yoo JY, Hwang CH: Therapeutic time window for the effects of erythropoietin on astrogliosis and neurite outgrowth in an in vitro model of spinal cord injury. Medicine 2018, 97:e9913–e9913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cragnolini AB, Montenegro G, Friedman WJ, Mascó DH: Brain-region specific responses of astrocytes to an in vitro injury and neurotrophins. Molecular and Cellular Neuroscience 2018, 88:240–248. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Wang H, Fan Y, Gao Y, Li X, Hu Z, Ding K, Wang Y, Wang X: Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Scientific Reports 2017, 7:46763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baez-Jurado E, Hidalgo-Lanussa O, Guio-Vega G, Ashraf GM, Echeverria V, Aliev G, Barreto GE: Conditioned Medium of Human Adipose Mesenchymal Stem Cells Increases Wound Closure and Protects Human Astrocytes Following Scratch Assay In Vitro. Molecular Neurobiology 2018, 55:5377–5392. [DOI] [PubMed] [Google Scholar]
- 41.Bae Y-H, Joo H, Bae J, Hyeon SJ, Her S, Ko E, Choi HG, Ryu H, Hur E-M, Bu Y, et al. : Brain injury induces HIF-1α-dependent transcriptional activation of LRRK2 that exacerbates brain damage. Cell Death & Disease 2018, 9:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kappy NS, Chang S, Harris WM, Plastini M, Ortiz T, Zhang P, Hazelton JP, Carpenter JP, Brown SA: Human adipose-derived stem cell treatment modulates cellular protection in both in vitro and in vivo traumatic brain injury models. Journal of Trauma and Acute Care Surgery 2018, 84:745–751. [DOI] [PubMed] [Google Scholar]
- 43.Spencer KC, Sy JC, Falcón-Banchs R, Cima MJ: A three dimensional in vitro glial scar model to investigate the local strain effects from micromotion around neural implants. Lab on a chip 2017, 17:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang A, Hao Z, Wang L, He J, Gao L, Mao X, Paz R: In vitro model of the glial scar. International Journal of Bioprinting 2019, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocha DN, Ferraz-Nogueira JP, Barrias CC, Relvas JB, Pego AP: Extracellular environment contribution to astrogliosis-lessons learned from a tissue engineered 3D model of the glial scar. Front Cell Neurosci 2015, 9:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonough A, Lee RV, Noor S, Lee C, Le T, Iorga M, Phillips JLH, Murphy S, Möller T, Weinstein JR: Ischemia/Reperfusion Induces Interferon-Stimulated Gene Expression in Microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience 2017, 37:8292–8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salvador E, Burek M, Foerster C: Stretch and/or oxygen glucose deprivation (OGD) in an in vitro traumatic brain injury (TBI) model induces calcium alteration and inflammatory cascade. Frontiers in Cellular Neuroscience 2015, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones EV, Bernardinelli Y, Zarruk JG, Chierzi S, Murai KK: SPARC and GluA1-Containing AMPA Receptors Promote Neuronal Health Following CNS Injury. Frontiers in Cellular Neuroscience 2018, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenhalgh AD, Zarruk JG, Healy LM, Baskar Jesudasan SJ, Jhelum P, Salmon CK, Formanek A, Russo MV, Antel JP, McGavern DB, et al. : Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol 2018, 16:e2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De La Fuente AG, Lange S, Silva ME, Gonzalez GA, Tempfer H, van Wijngaarden P, Zhao C, Di Canio L, Trost A, Bieler L, et al. : Pericytes Stimulate Oligodendrocyte Progenitor Cell Differentiation during CNS Remyelination. Cell reports 2017, 20:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]


