Abstract
Nitric oxide (NO) is a broad-spectrum antibacterial agent, making it an attractive alternative to traditional antibiotics for treating infections. To date, a direct comparison of the antibacterial activity of gaseous NO (gNO) versus water-soluble NO-releasing biopolymers has not been reported. In this study, the bactericidal action of NO-releasing chitosan oligosaccharides was compared to gNO treatment against cystic fibrosis-relevant Gram-positive and Gram-negative bacteria. A NO exposure chamber was constructed to enable the dosing of bacteria with gNO at concentrations up to 800 ppm under both aerobic and anaerobic conditions. Bacteria viability, solution properties (i.e., pH, NO concentration), and toxicity to mammalian cells were monitored to ensure a thorough understanding of bactericidal action and reproducibility for each delivery method. The NO-releasing chitosan oligosaccharides required significantly lower NO doses relative to gNO therapy to elicit antibacterial action against Pseudomonas aeruginosa and Staphylococcus aureus under both aerobic and anaerobic conditions. Reduced NO doses required for bacteria eradication using water-soluble NO-releasing chitosan were attributed to the release of NO in solution, removing the need to transfer from gas to liquid phase and the associated long diffusion distances of gNO treatment.
Keywords: Cystic fibrosis, nitric oxide, antibacterial, Pseudomonas aeruginosa, Staphylococcus aureus, biofilm
Graphical Abstract
Improper use and over prescription of antibiotics in the medical field has led to the widespread development of antibiotic-resistant bacteria. Antimicrobial resistant (AMR) infections are now a leading cause of healthcare-related mortality, resulting in over 23,000 deaths annually in the United States and billions of dollars in associated medical costs.1 If current trends continue, annual global AMR mortality rates are predicted to exceed 10 million deaths by the year 2050.2 While a portion of this epidemic may be addressed through improved medical practices (e.g., limiting unnecessary prescriptions), certain chronic diseases, including cystic fibrosis (CF), require constant antibiotic administration to prolong patient life expectancy. Cystic fibrosis is an incurable, genetic disease that results in the dehydration of the airway epithelium, thickening airway mucus until it becomes difficult to clear via traditional mucociliary mechanisms.3,4 Bacteria thrive in this static environment, promoting the formation of complex communities of pathogenic bacteria known as biofilms.4 The exopolysaccharide matrix produced by these biofilms inhibits oxygen diffusion, altering bacterial metabolism and increasing antibiotic resistance.5 This combination of thickened mucus and biofilm formation severely decreases the efficacy of most antibiotics, requiring large, consistent doses to mitigate infection.4
Traditional CF therapies treat either the infection or the stagnant mucus using antibiotics (e.g., aztreonam lysine6–8 and colistin9–11) or hypertonic saline, respectively.12–14 Inhalable tobramycin, either in nebulized or powder form, is currently considered the gold standard CF antibiotic treatment.15–18 Unfortunately, the routine use of antibiotics, such as tobramycin, has led to the emergence of antibiotic resistance in both the CF and general populations.19–21 Bacterial regrowth following deficient treatment often produces stronger, more resilient bacterial strains.3,22,23 The rise of CF-relevant AMR infections highlights the need for new therapeutics capable of effectively eradicating bacteria within the airway mucus while minimizing the chance of bacteria acquiring resistance.22,24
Nitric oxide (NO), an endogenously produced free radical, has been shown to eradicate bacteria through both oxidative and nitrosative stressors, including lipid peroxidation, nitrosation of membrane proteins, and DNA damage via reactive nitrogen species (e.g., peroxynitrite, dinitrogen trioxide).25–28 Nitric oxide’s multiple antibacterial mechanisms of action significantly diminish the risk of bacterial resistance.29,30 Indeed, serial exposure (20 passages) of NO to five bacteria species at sub-biocidal doses did not result in any acquired resistance.28 Furthermore, Reighard et al. reported NO’s ability to actively disrupt both the biofilm matrix and mucus structure, thus allowing for improved bactericidal action and mucociliary clearance.31,32
Miller and coworkers previously studied the therapeutic potential of gaseous NO (gNO) against CF-related pathogens using a custom-built NO exposure chamber.33–35 They reported potent antibacterial activity (1–3 log reduction in bacterial viability) without extensive cytotoxicity to epithelial lung cells with both continuous and intermittent exposure to 160 ppm gNO. In vivo studies further highlighted the ability of gNO to reduce the number of colonized bacteria in rat and human lungs.36,37 Unfortunately, the delivery of gNO is challenging as adverse side effects associated with systemic exposure to NO, primarily methemoglobinemia, must be avoided. Additionally, untargeted interactions may arise with gNO due to NO’s role in many physiological processes. As an alternative, water-soluble NO-releasing biopolymers are capable of controlled and more targeted release.38–42 Chemical modification of macromolecular scaffolds including silica nanoparticles, liposomes, and biopolymers with NO donor moieties has facilitated therapeutically useful NO storage and release.38–42 For example, water-soluble chitosan has become an appealing biopolymer for NO delivery due to its favorable toxicity, mucoadhesive properties, and ability to release NO directly in solution, thereby avoiding the transfer of NO from gas to liquid phases.5,31,32,43,44 Additionally, the NO-releasing chitosan is amenable to pulmonary delivery via solution nebulization, analogous to traditional CF antibiotics (i.e., tobramycin,17 aztreonam,8 and colistin.9)
To date, a direct comparison of the antibacterial action of gNO with that of macromolecular NO-release has not been performed, perhaps because a suitable conversion of NO concentration from the gas to solution phase is not apparent in the literature. Furthermore, reported exposure conditions are typically quite different between gNO- and NO-releasing macromolecule-treated bacteria. We thus sought to measure and directly compare the antibacterial activity of gNO and NO-releasing chitosan oligosaccharides. Treatment conditions for gNO (i.e., buffer capacity and solution depth) were examined to determine an appropriate configuration for comparison. The concentration of NO in solution required to elicit antibacterial action was measured in real-time using a NO-selective electrochemical sensor and end-point quantification via the Griess assay. The bacteria eradication ability of gNO and NO-releasing chitosan was evaluated against Gram-positive and Gram-negative CF-relevant bacteria strains. Further, aerobic and anaerobic conditions were investigated to determine how environmental conditions impact antibacterial activity, as both are relevant in thick CF lung mucus. Antibiofilm activity was probed against Pseudomonas aeruginosa biofilms. Finally, human lung cells were exposed to NO via both delivery methods at their respective bactericidal concentrations to assess cytotoxicity.
Experimental
Materials.
Medium molecular weight chitosan (43 kDa) was purchased from Primex (Siglufjörður, Iceland). Ethanolamine, p-anisaldehyde, p-toluenesulfonyl chloride, N-(1-naphthyl)ethylenediamine dihydrochloride, sulfanilamide, trimethoxymethylsilane (MTMOS), 5-amino-1-naphthol (5A1N), hydrochloric acid (HCl), fetal bovine serum (FBS), and sodium nitrite standards were obtained from Sigma-Aldrich (St. Louis, MO). Phosphoric acid, anhydrous ethanol, saline (0.9%, w/v), phenazine methosulfate (PMS), Roswell Park Memorial Institute (RPMI) 1640 cell culture medium, and common laboratory salts were purchased from Fisher Scientific (Fair Lawn, NJ). Heptadecafluoro-1,1,2,2-tetrahydrodecyl)trimethoxysilane (17FTMS) was obtained from Gelest (Morrisville, PA). The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfo-phen-yl)-2H-tetrazolium inner salt (MTS) was purchased from Promega (Madison, WI). Tryptic soy broth (TSB) and tryptic soy agar (TSA) were purchased from Becton, Dickinson, and Company (Franklin Lakes, NJ). Nitric oxide (NO; 99.5% and 800 ppm balance N2), nitrogen (N2; 99.998%), argon (Ar; 99.995%), and NO calibration (25.87 ppm, balance N2) gases were obtained from Airgas National Welders (Durham, NC). A Millipore Milli-Q UV Gradient A10 System (Bedford, MA) was used to purify distilled water to a resistivity of 18.2 MΩ·cm and a total organic content ≤6 ppb. Pseudomonas aeruginosa (#19143) and Staphylococcus aureus (#29213) were obtained from American Type Culture Collection (ATCC; Manassas, VA). All other chemicals were reagent grade and used as received.
Gaseous nitric oxide exposure chamber.
A modified version of the chamber reported by Miller et al. was fabricated according to literature.33 The exposure chamber consisted of medical grade air and 800 ppm NO (balance N2) gas cylinders equipped with regulators connected to a gas-tight polypropylene chamber (Figure 1). A Sievers Chemiluminescence Nitric Oxide Analyzer (NOA; Boulder, CO) was attached to the output of the chamber to allow for real-time analysis of NO in the gas phase. The output gas was scrubbed of water vapor and bacteria using a 0.2 μm polytetrafluoroethylene (PTFE) and a high-efficiency particulate air (HEPA)-CAP filter, respectively (Sigma-Aldrich; St. Louis, MO). The flow rate of each individual gas was controlled via separate digital mass flow controllers (Alicat Scientific; Tucson, AZ). The combined flow rate was ~250 mL/min. The interior dimensions of the chamber were 24.8×18.4×13.3 cm, thus giving a total volume of ~6.07 L. The entire chamber was kept at 37 °C by placing it within a VWR 1545 incubator (VWR International; Radnor, PA). A humidified environment (>90% relative humidity) was maintained using paper towels soaked in ~40 mL of sterile water in a water reservoir. Sensors were attached to the interior ceiling of the chamber to monitor temperature, humidity, and oxygen levels in real-time. A water-resistant coating was applied to the sensors using an acrylic conformal spray (Techspray; Amarillo, TX) to prevent corrosion due to the high humidity levels. The sensors were controlled by a single ATMEGA 328P-PU microcontroller (Mouser; Mansfield, TX) with data collected via a computer.
Figure 1.
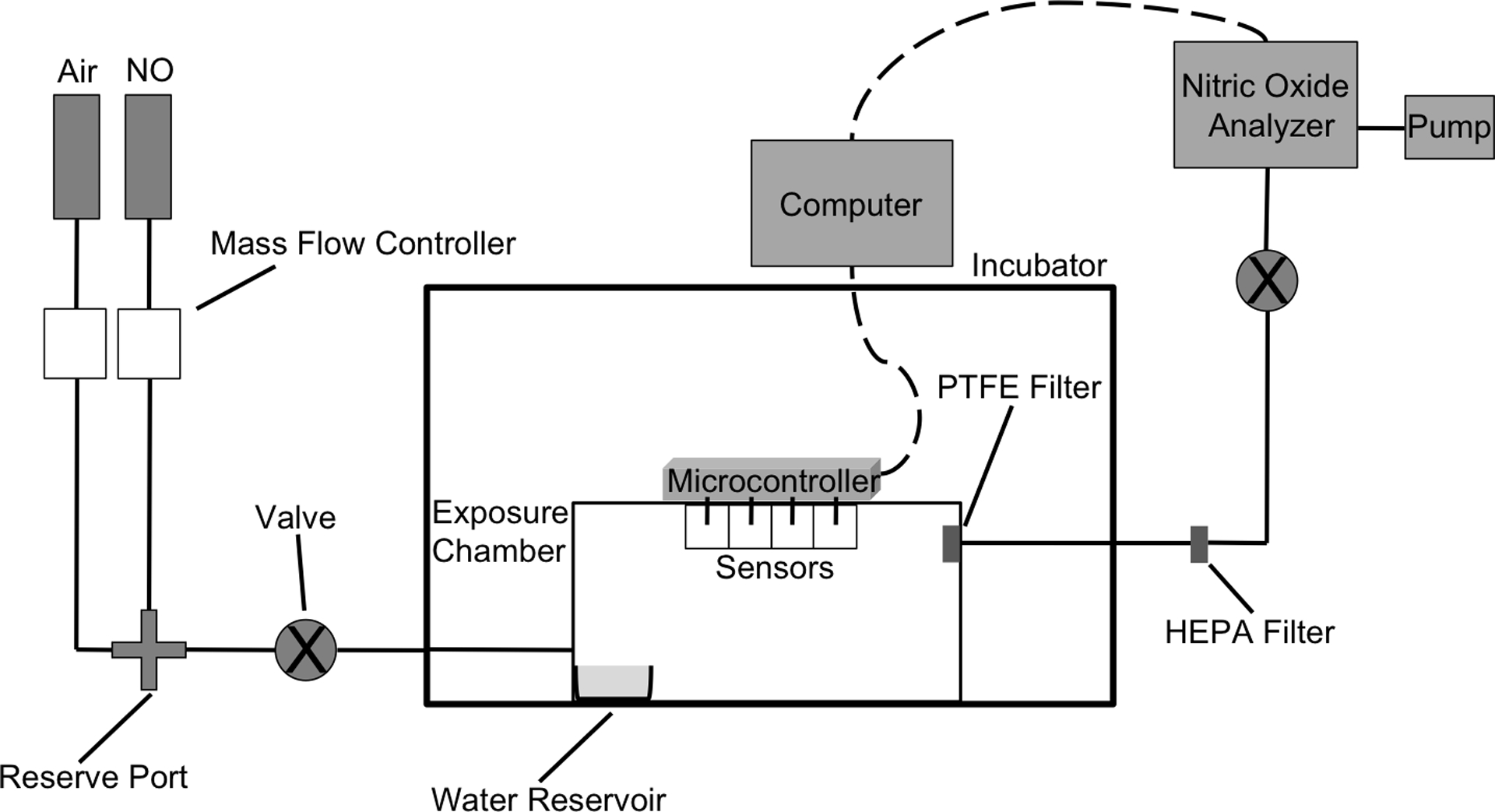
Schematic of gaseous NO exposure chamber. The flow rates of the gases were separately controlled to obtain precise concentrations of gNO. The combined mixture enters the humidified chamber through a single port while exiting through PTFE and HEPA filters to remove water vapor and aerosolized bacteria, respectively. The NO concentration of the output gas was analyzed using a chemiluminescence nitric oxide analyzer (NOA). The valves, connecting the gas cylinders to the exposure chamber and between the HEPA filter and NOA, allow for the creation of a closed, gas-tight system.
Synthesis of NO-releasing chitosan oligosaccharides (COS-EA/NO).
Nitric oxide-releasing ethanolamine-modified chitosan oligosaccharides were prepared as previously described.31,43 Detailed procedure is included in the Supporting Information.
Quantification of nitric oxide via NOA.
Nitric oxide-release kinetics and levels within the exposure chamber were measured using a NOA.45,46 Prior to analysis, the instrument was calibrated with air passed through a NO zero filter (0 ppm NO) and 25.87 ppm of NO standard gas (balance N2). Solid COS-EA/NO (~1 mg) was added to 30 mL of deoxygenated PBS (37 °C, pH 6.5). Nitric oxide released from COS-EA/NO was carried to the analyzer using nitrogen gas flowing through the solution at a rate of 80 mL/min. Additional nitrogen was supplied to the reaction flask to achieve the required instrument collection rate of 200 mL/min. The measurement period was terminated when NO levels decreased below 10 ppb/mg chitosan oligosaccharide. Total NO content inside the exposure chamber was performed during the final 5 min of the 30-min fill period. The output valve between the HEPA filter and NOA (Figure 1) was opened allowing the NOA to sample from the exposure gas inside the chamber. The total NO content was recorded when the value measured stabilized. At this time, the valve was closed to prevent the NOA from removing additional NO from the chamber.
Bactericidal assays.
Frozen ATCC bacteria stocks were reconstituted in tryptic soy broth (TSB) and cultured overnight at 37 °C. An aliquot of culture (1 mL) was grown in TSB (30 mL) to a concentration of 108 colony forming units per mL (CFU/mL), collected by centrifugation, resuspended in 10% glycerol (v/v in PBS), and stored at −80 °C in 1 mL aliquots. For daily experiments, a frozen 1 mL aliquot was reconstituted in 3 mL TSB overnight at 37 °C and recultured in fresh TSB (30 mL) the next day. Daily cultures were grown to 108 CFU/mL and diluted to 106 CFU/mL with PBS (10 mM, pH 6.5) or saline (0.9%, w/v). The suspended bacteria were added to 6-, 12-, 24-, or 96-well plates. For NO-releasing chitosan exposure, a fresh solution of COS-EA/NO (100 mg/mL) was diluted to the target concentrations (50.0–0.0031 mg/mL) and added to the wells. The plates were then incubated at 37 °C for 5 h. For gNO treatment, duplicate plates were placed into the gas exposure chamber. The atmosphere of the chamber was removed by vacuum and then filled with a mixture of medical grade air and NO for 30 min. The valves on the input and output gas lines were sealed to create a closed environment leaving the bacteria exposed for 5 h at 37 °C. After this period, the treated and control samples were spiral plated at 1-, 10-, and 100-fold dilutions on tryptic soy agar (TSA) plates using an Eddy Jet 2 spiral plater (IUL; Farmingdale, NY). Bacterial viability was assessed via colony counting using a Flash & Go colony counter (IUL; Farmingdale, NY). The minimum bactericidal concentration after 5 h (MBC5h) was defined as the lowest concentration that resulted in at least a 3-log (99.9%) reduction in viability. Of note, this counting method has a limit of detection of 2.5 × 103 CFU/mL.47 Anaerobic gNO treatments were carried out by substituting the medical grade air for nitrogen gas. For anaerobic COS-EA/NO treatments, exposures were performed inside an anaerobic chamber (Coy Laboratory Products, Ann Arbor, MI).
Biofilm growth and exposure.
Pseudomonas aeruginosa cultures were grown to 108 CFU/mL, subsequently diluted to 106 CFU/mL with TSB in a 24-well plate, and incubated at 37 °C for 3 d. The viscous biofilm (250 μL) was removed with a pipette, added to PBS (1 mL) to remove adhered planktonic bacteria, and treated with either COS-EA/NO (750 μL) or gaseous NO (500 ppm) in PBS (750 μL, pH 6.5) for 18 h. After the exposure period, the control (untreated) and treated biofilms were disrupted by repeated pipetting and vortexing, serially diluted (10- through 10,000-fold dilutions), plated, and enumerated. The minimum biofilm eradication concentration after 18 h (MBEC18h) was defined as the concentration resulting in a 5-log (99.999%) reduction in viability.
Quantification of NO via Griess assay.
Following exposure to gNO, the end-point, solution concentration of NO was determined indirectly via the Griess assay, a spectrophotometric measurement of nitrite, NO’s oxidation byproduct. Aliquots (50 μL) were taken from each well, combined with 50 μL of aqueous sulfanilamide (1% (w/v) in 5% (v/v) phosphoric acid), and incubated in the dark at room temperature for 10 min. After this period, 50 μL of N-(1-naphthyl)ethylenediamine dihydrochloride (0.1% (w/v) in water) was added and allowed to react for 10 min in the dark. The absorbance of the colorimetric product was measured at 540 nm using a SpectraMax M2e microplate spectrophotometer (Molecular Devices; Sunnyvale, CA). Nitrite concentrations were calculated using calibration curves constructed with a sodium nitrite standard.
Electrochemical detection of nitric oxide and nitrite.
Electrochemical experiments were carried out using a CH Instruments 730B Electrochemical Analyzer (Austin, TX). The four-electrode arrangement consisted of two inlaid 2 mm dia. polycrystalline platinum (Pt) disc working electrodes sealed in Kel-F (6 mm total dia.; CH Instruments), a silver-silver chloride (Ag|AgCl) reference electrode (3.0 M KCl; CH Instruments), and a coiled Pt wire counter electrode. Nitric oxide-selective sensors were prepared by modifying the Pt electrode surface with electropolymerized poly(5A1N) and a highly fluorinated xerogel as previously described.48,49 Briefly, electrodes were cycled from 0 to +1.0 V (10 mVs−1, 20 cycles) in a 10 mM monomer solution of 5A1N in 0.1 M HCl and 10 mM NaCl. A fluoroalkoxysilane sol solution was prepared via acid-catalyzed hydrolysis/co-condensation of MTMOS and 17FTMS precursors (30% 17FTMS, v/v balance MTMOS). After ordered additions of 3.6 mL ethanol, 630 μL MTMOS, 270 μL 17FTMS, 960 μL H2O, and 60 μL 0.5 M HCl, the sol was stirred rapidly for 1 h and then spray-coated onto the poly(5A1N)-modified electrode with an airbrush gun (Iwata HP-BC1 Plus; Yokohama, Japan).
Bare and modified electrodes enabling total NOx and NO-selective measurements, respectively, were polarized at +0.8 V vs. Ag|AgCl in 1 vol% TSB-supplemented PBS (10 mM; pH 7.4) for 2 h prior to calibration to enable background stabilization. Saturated NO solutions were prepared by purging 20 mL of PBS with argon for 20 min followed by purified NO gas (99.5%) for 20 min over ice. Amperometric calibration curves were collected with successive injections of either saturated NO or NaNO2 solution (1.9 mM and 1.0 M, respectively, in PBS). The selectivity of the NO sensor response for NO over nitrite was 10,000-fold. Sensors were then positioned midway in 4 mL of 1% (v/v) TSB-supplemented PBS in a 6-well culture plate (~2 mm above the well bottom). Amperograms were collected at +0.8 V vs. Ag|AgCl over a 5-h period with 160 and 500 ppm gaseous NO levels in the chamber. Because the NOx sensor (electrode 2) was sensitive to both NO and NOx species, with nitrite being the predominant oxidation byproduct, the current response was corrected according to Eq. 1, where is the current response specific to nitrite, I2 is the total current response of electrode 2, I1 is the current response of the NO sensor (electrode 1), S2,NO is the NO sensitivity of electrode 2, and S1,NO is the NO sensitivity of electrode 1.
| (Eq. 1) |
In vitro toxicity against A549 cells.
Human lung epithelial cells (A549) grown in RPMI 1640 media supplemented with FBS (10% v/v) and penicillin streptomycin (1% wt/wt) were incubated in CO2 (5% v/v) under humidified conditions at 37 °C. For cell viability assessment, the MTS assay was used as previously described.50 Briefly, cells (2 × 104 cells/mL) were seeded onto 96-well polystyrene plates in duplicate (100 μL) and exposed to either COS-EA/NO in the incubator or gNO in the treatment chamber. After 5 h incubation at 37 °C, the supernatant was removed and the cells were washed with PBS (100 μL) to remove remaining NO or COS-EA/NO. Cells were then incubated with 100 μL of RPMI/MTS/PMS (105/20/1, v/v/v) for 1 h at 37 °C. The absorbance of the colored solutions was quantified at 490 nm using a microplate spectrophotometer. Cell viability was calculated as follows:
| (Eq. 2) |
A mixture of RPMI 1640/MTS/PMS was used as the blank (Absblank) and the media above untreated cells as the control (Abscontrol).
Data analysis.
Values for pH, bacterial viability, and NO and NOx concentrations are expressed as the mean ± the standard error of the mean. Comparisons of data sets were made using a 2-tailed student’s t test.
Results and Discussion
In order to reproducibly dose bacteria with gNO, a simple, yet versatile exposure chamber was engineered following the schematic provided by Ghaffari et al.33 The chamber consisted of a gas- and water-tight polypropylene box able to withstand the applied pressures within an incubator to maintain an exposure temperature of 37 °C. A humidified environment (~90% rh) was created using a water reservoir. Real-time sensors were used to measure humidity, oxygen, and NO levels in the chamber (Figure 1). Input gas (e.g., air, NO) flow rate was tuned using mass flow controllers to adjust the ratio of gases entering the chamber.
Impact of gNO on solution properties.
Traditional bactericidal assays administer the antibiotic to planktonic bacteria suspended in solution. The properties of the chosen solution are critical, as changing the solution composition (e.g., pH, ionic strength) may alter the antibacterial activity. The selection of an appropriate solution is especially important for reactive molecules like NO, that will readily form secondary compounds in solution. For example, nitrous acid will form upon diffusion of gNO into an oxygenated solution as follows:51
| (Eq. 3) |
Depending on the solution characteristics, the formation of nitrous acid may lower the pH. Acidified solutions are problematic when evaluating antibacterial activity, as bacteria growth and viability are altered through pH modification alone.52 Therefore, the pH change of aqueous media (200 μL in a 96-well plate) was measured before and after exposure to gNO as a function of solution composition (Table 1). The initial gNO concentration selected for this study was 160 ppm as others have identified this concentration as sufficient to eradicate CF-related pathogens.33–37 Unbuffered saline (0.9%, w/v), PBS (10 mM), and HEPES (50 mM) were chosen as solutions due to their widespread use in biological testing and diverse buffering capacities. A pH drop was observed in each solution, suggesting the formation of acidic species (e.g., HNO2) (Table 1). Only the unbuffered saline solution exhibited a significant decrease in pH (35%) relative to the other solutions because of its poor buffering capacity. At a pH of 4.5, it was hypothesized that bacteria eradication would occur irrespective of NO exposure. Indeed, a ~1.5-log reduction in viability was observed after exposing bacteria to saline adjusted to pH 4.5 for 5 h, possibly a result of denatured proteins or membrane/DNA damage from extended exposure to acidic conditions (Figure S2).53 At the more representative pH of the airway surface liquid in CF lungs (i.e., pH 6.5),54–56 no significant change in bacterial viability was observed, indicating that more acidic solutions (pH <6) are required to bias the antibacterial activity (Figure S2).
Table 1.
pH change of oxygenated aqueous solutions after exposure to 160 ppm NO for 5 h.a
| Media | Initial pH | Post exposure pH | Percent change (%) |
|---|---|---|---|
| PBS | 6.48 | 6.41 ± 0.07 | 1.1 ± 1.0 |
| Salineb | 6.90 | 4.51 ± 0.05 | 34.6 ± 0.7 |
| HEPES | 7.13 | 7.02 ± 0.06 | 1.5 ± 0.9 |
| PBS | 7.34 | 7.19 ± 0.06 | 2.0 ± 0.8 |
All solutions contained 1% (v/v) TSB to represent an experiment containing bacteria. Concentration of buffers were 10 mM.
Concentration was 0.9% (w/v).
Enhanced bacterial killing against Pseudomonas aeruginosa was observed for unbuffered saline relative to PBS following exposure to 160 ppm NO for 5 h (Figure 2). Corroborated by Miller et al.,34 a 3-log reduction in bacterial viability was obtained in saline after exposure to 160 ppm NO for 5 h. However, the change in P. aeruginosa viability in PBS (pH 6.5) was only a 1.5-log reduction. Such disparity highlights the importance of selecting an appropriate medium for experiments when investigating the biological utility of gNO. It is imperative that solutions with suitable buffering capacities be employed in order to mitigate the impact of pH change, which may convolute the true antibacterial nature of NO.
Figure 2.
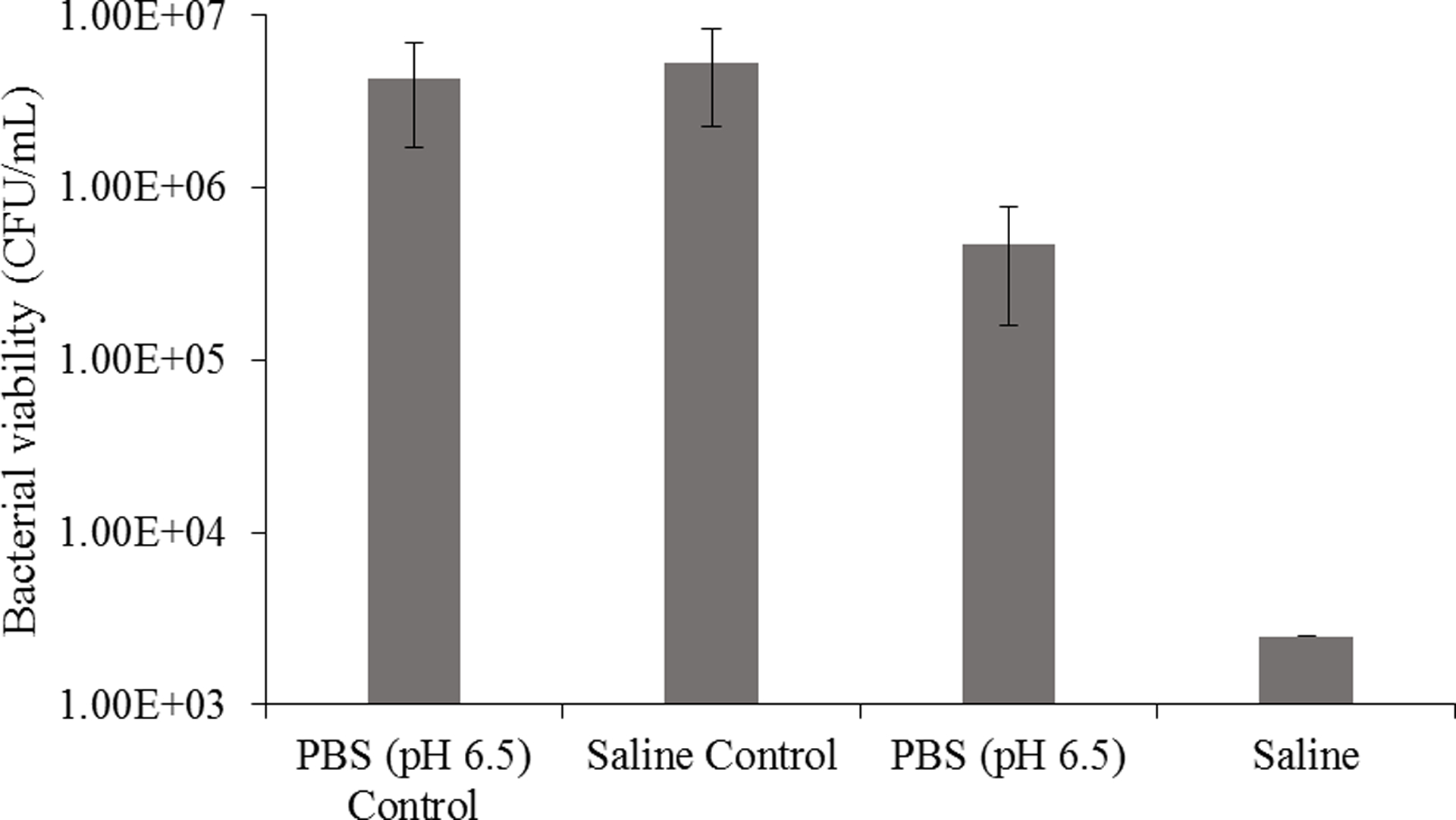
Bactericidal action of 160 ppm gNO against Pseudomonas aeruginosa in saline (0.9% w/v) and PBS (pH 6.5, 10 mM) following a 5-h exposure period. Performed in 96-well plate with 200 μL per well. All solutions contained 1% (v/v) TSB. Controls were not exposed to gNO.
In addition to pH, solution depth is a critical parameter to control for when studying the effects of gNO. Transfer of gNO to solution phase occurs at the gas/liquid interface. Under a constant molar flux of NO (JNO), the concentration of NO in solution (CNO,aq) will depend on the interfacial surface area (Sint), the volume of the aqueous phase (Vaq), and the total time of exposure (t) according to Eq. 4:
| (Eq. 4) |
For cylindrical containers (or any container whose cross-sectional area remains the same along the axis of depth), the dependence on Sint and Vaq simplifies to an inverse dependence on aqueous phase depth (daq):
| (Eq. 5) |
Verification of this relationship was performed by quantifying the amount of NO, indirectly via nitrite (NO2−), in solution using the Griess assay in 96-, 24-, 12-, and 6-well plates as a function of solution depth (Table 2). Importantly, cells and bacteria produce many reactive molecules, such as superoxide, that convert NO to byproducts, while NO in buffer alone will only follow the oxidative path to form nitrite.57 The quantity of nitrite in solution can therefore be directly linked to NO that diffused into solution. The concentration of nitrite that accumulated in solution was approximately equivalent across 24-, 12-, and 6-well plates at each depth, even though both the surface area and volume of the wells changed. However, the 96-well plate exhibited consistently larger nitrite concentrations, attributable perhaps to the smaller solution volumes partially evaporating over the course of the experiment. Use of the 96-well plate was thus abandoned to mitigate undesirable deviation. Increasing the solution depth by using greater volumes led to lower measured nitrite levels as expected since the volume increased while the rate of NO diffusion into solution remained constant. Clearly, it is critical to report solution depth for any gNO experiments in order to ensure reproducibility by others. Subsequent experiments herein were performed in 24-well plates with a 1.5 mm solution depth (297 μL) to maximize dissolved NO concentrations.
Table 2.
Nitric oxide accumulation in solution as a function of solution depth following a 5-h exposure to 160 ppm NO.a
| Plate size (wells) | Nitric oxide concentration (mM) | ||
|---|---|---|---|
| 1.5 mm depth | 3.0 mm depth | 6.0 mm depth | |
| 96 | 2.09 ± 0.16 | 1.16 ± 0.07 | 0.67 ± 0.06 |
| 24 | 1.67 ± 0.16 | 0.91 ± 0.08 | 0.50 ± 0.06 |
| 12 | 1.50 ± 0.05 | 0.81 ± 0.05 | 0.45 ± 0.04 |
| 6 | 1.80 ± 0.04 | 0.91 ± 0.08 | 0.48 ± 0.05 |
All solutions were 10 mM PBS (pH 6.5, 37 °C) containing 1% (v/v) TSB to represent an experiment containing bacteria. NO concentrations were measured using the Griess assay.
Antibacterial action of gNO versus COS-EA/NO against a Gram-negative bacterium.
Chronic P. aeruginosa infections are common in CF patients, resulting in prolonged inflammation, lung degradation, and eventually respiratory failure.4,58 Planktonic P. aeruginosa was treated with either gNO or COS-EA/NO under oxygenated conditions to compare the antibacterial activity of both NO delivery modes (Table 3). Unexpectedly, gNO was unable to eradicate (i.e., achieve a 3-log reduction) the bacteria at 160 ppm exposures for 5 h. In fact, a 3-log reduction in P. aeruginosa viability was not observed, even after 24-h exposure at 160 ppm gNO. In PBS, the minimum bactericidal concentration (MBC5h; i.e., the concentration required to elicit a 3-log reduction after 5-h gNO exposure) was only achieved at a much greater concentration (500 ppm) than previously reported.34 These discrepancies stem from the prior studies employing saline as the test medium, thereby eliciting enhanced bactericidal action due to changes in pH (Figure 2). Buffered systems prevent the significant portion of antibacterial activity caused by the acidic environment, which bias the MBC5h (Figure S2).
Table 3.
Bactericidal action of gNO and COS-EA/NO against Pseudomonas aeruginosa and Staphylococcus aureus exposure under aerobic or anaerobic conditions for 5 h.a
| Bacteria species | NO exposure method | Aerobic exposure | Anaerobic exposure | ||
|---|---|---|---|---|---|
| MBC5h | NO dose (μmol/mL) | MBC5h | NO dose (μmol/mL) | ||
| P. aeruginosa | COS-EA/NO | 0.20 mg/mL | 0.09b | 0.39 mg/mL | 0.17b |
| gNO | 500 ppm | 1.8c | >800 ppm | n.q.d | |
| S. aureus | COS-EA/NO | 3.12 mg/mL | 1.37b | 0.78 mg/mL | 0.34b |
| gNO | >600 ppm | n.q.d | >800 ppm | n.q.d | |
All exposures performed in 10 mM PBS (pH 6.5, 37 °C).
Quantified using NOA.
Determined using amperometric sensor.
Not quantified; unable to quantify NO dose due to MBC requiring higher gNO concentration than our system could supply.
In order to accurately compare the MBC values between the modes of delivery, the concentration of NO in the solution phase during the gNO treatment was ascertained, as the amount of NO in the gas phase is not equivalent to the antibacterial concentration. An electrochemical sensor was used to quantify the NO that diffused from the gas phase to the liquid phase due to its high temporal resolution, dual-detection capabilities (for NO and NOx), and the ability to facilitate real-time measurements. A dual electrode system, where one working electrode was coated with a permselective membrane, was designed to quantify NO through NOx background subtraction by exploiting the sensor’s 10,000-fold increased sensitivity to NO over NOx.48,49 Real-time accumulation of both nitrite and NO in 10 mM PBS (pH 6.5, 37 °C) over 5 h is shown in Figure 3. Upon diffusion into solution, NO is oxidized to nitrite with nitrite levels steadily increasing over the 5-h period to 1.8 mM. This concentration represents the total possible NO concentration in solution that elicited antibacterial action over the 5-h exposure. The MBC5h of 500 ppm gNO was thus determined to be 1.8 μmol NO/mL. The temporal resolution of electrochemical detection allows for the quantification of unoxidized NO throughout the exposure. Of note, the NO concentration peaked at 12 μM for the 500 ppm gNO treatment. At 2.5 h, the NO concentration began to decline due to most of the gNO in the headspace volume being scavenged by oxygen to form nitrogen dioxide gas. At this point, the rate of NO diffusion is no longer faster than the rate of NO reacting with dissolved oxygen to produce nitrite, producing a linear increase in nitrite concentration. Logically, the peak NO concentration was altered by simply changing the gNO concentration in the headspace (Figure S3). We hypothesize that the height of this peak concentration correlates to the bactericidal activity of gNO, as a one-time 160 ppm gNO treatment failed to achieve full eradication (peak concentration of 3 μM), while 500 ppm gNO elicited potent bactericidal activity (peak concentration of 12 μM).
Figure 3.
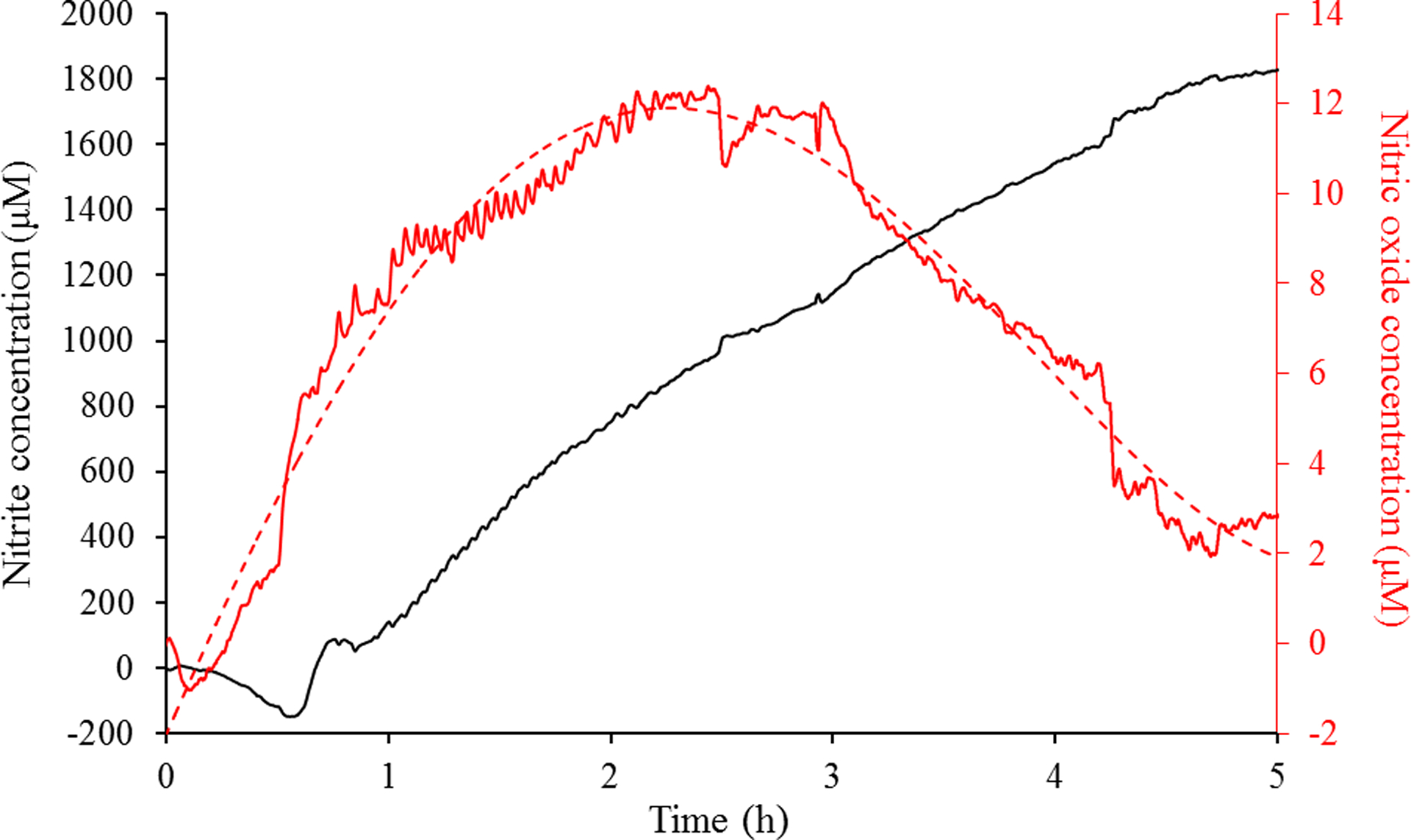
Simultaneous electrochemical detection of nitrite and NO in 10 mM PBS (pH 6.5, 37 °C) containing 1% (v/v) TSB over a 5-h exposure to 500 ppm gNO. Red trace represents NO, while the black trace represents nitrite.
To compare gNO to a macromolecular NO donor scaffold, NO-releasing chitosan (COS-EA/NO) was administered to P. aeruginosa under the identical conditions as the gNO exposure in the chamber. The MBC5h for COS-EA/NO was 0.20 mg/mL, corresponding to 0.09 μmol/mL NO over the 5-h treatment. Of note, the quantity of NO in solution required to eradicate the planktonic cultures was approximately 20x lower for COS-EA/NO compared to gNO (Table 3). Comparing the MBC5h values for the two modes of NO delivery, it is apparent that the NO-releasing chitosan achieves greater antibacterial activity against P. aeruginosa relative to gNO. We hypothesize that this disparity results from the required phase transition (i.e., from gas to solution phase) which requires constant and high gNO levels to facilitate the necessary rate of diffusion into solution. Nitric oxide-releasing chitosan releases its NO payload directly into solution over time and only once it is added to the aqueous solution, allowing for more targeted and effective treatment.
Antibacterial action of gNO versus COS-EA/NO against a Gram-positive bacterium.
While P. aeruginosa is the most prevalent pathogen in adult CF patients, Gram-positive bacteria, such as Staphylococcus aureus, are problematic in adolescents and flourish in the stagnant CF lung mucus.4 Gram-positive bacteria have been cited as requiring greater NO doses for eradication, likely a result of the thick peptidoglycan layer surrounding their outer cell membrane, thus inhibiting NO diffusion into the bacteria.46 Planktonic S. aureus was treated with either gNO or COS-EA/NO under aerobic conditions to determine how the treatment mode might influence antibacterial activity between Gram classes. As expected, the MBC5h for COS-EA/NO increased to 3.12 mg/mL (1.37 μmol/mL of NO), an order of magnitude greater than that observed for P. aeruginosa (Table 3). Treatment with gNO did not lead to S. aureus eradication at the maximum NO concentration which only reduced viability by 2 logs. Of note, the 600 ppm gNO upper limit was the greatest achievable prior to creating an anoxic environment in the exposure chamber. Logically, greater gNO levels (i.e., beyond the capability of our exposure chamber) would likely be required for S. aureus eradication. The decreased bactericidal action of NO against Gram-positive strains, compared to Gram-negative, is corroborated by previous reports that also observed increases in the MBC.59
Effect of anaerobic exposure conditions on antibacterial action.
The thick, viscous CF mucus generates an oxygen gradient, with regions near the airway epithelium that are essentially anaerobic.4 Pathogens such as P. aeruginosa are capable of surviving in such hypoxic environments by altering their metabolic pathways, which almost always reduces antibiotic activity against them.4,58 Testing the antibacterial action of a potential CF therapeutic under anaerobic conditions is essential for replicating these physiological situations. Both P. aeruginosa and S. aureus were exposed to either gNO (NO supplemented with N2 gas) or COS-EA/NO under anoxic conditions. In absence of normal oxygen levels, the antibacterial activity of COS-EA/NO against P. aeruginosa decreased significantly (0.39 mg/mL or 0.17 μmol/mL NO) relative to aerobic testing. This deviation is likely the result of altered bacterial metabolism under anaerobic conditions, upregulating denitrification enzymes including nitric oxide reductase that lessen the concentration and antibacterial activity of the exogenous NO.4 Conversely, the MBC5h for COS-EA/NO against S. aureus improved to 0.78 mg/mL (0.34 μmol/mL NO; Table 3). Anaerobic adaptation in S. aureus involves gene expression associated with nitrate respiration.60 The lack of nitrate in solution may limit the bacteria’s ability to readily adapt, leaving it more vulnerable to eradication by NO. Additionally, reduced NO oxidation by oxygen in the anaerobic solution would increase the quantity of NO able to act on the bacteria.
As provided in Table 3, the gNO treatment was considerably less potent in the absence of oxygen. At the maximum anaerobic gNO level achievable with our chamber of 800 ppm, only a 1.5-log reduction in viability for both the Gram-positive and Gram-negative bacteria was capable with gNO. Antibacterial action against P. aeruginosa in the anaerobic environment was significantly inhibited even at greater NO concentrations relative to aerobic exposure. In contrast to the burst of NO from COS-EA/NO resulting from the decomposition of the N-diazeniumdiolate, diffusion of gNO into solution is slower. Indeed, the diffusion-dependent gNO exposure did not result in effective killing in the absence of oxygen due to the inability to form reactive oxygen and nitrogen species (e.g., peroxynitrite and dinitrogen trioxide) that ultimately induce oxidative and nitrosative stress on the bacteria.25,26
Anti-biofilm action.
Pseudomonas aeruginosa biofilms in the lungs of CF patients lead to antibiotic and host immune response resistance due to the formation of an exopolysaccharide matrix surrounding the pathogens that slows drug diffusion and reduces oxygen concentrations.4,58 Biofilm killing has also been shown to require greater NO concentrations compared to planktonic bacteria.5,46 To understand the role of NO delivery mode on biofilm eradication, P. aeruginosa biofilms were grown in vitro and treated with either COS-EA/NO or gNO (500 ppm) under aerobic conditions for 18 h. Treatment with gNO did not impact the biofilms, resulting in statistically indistinguishable colony counts versus untreated controls. Negligible bactericidal action was observed with gNO, even when exposed in unbuffered saline. As anticipated, a greater quantity of COS-EA/NO was required to achieve a 5-log reduction (MBEC18h) relative to the MBC5h for the planktonic form (20 mg/ml or 8.80 μmol/mL NO versus 0.20 mg/mL or 0.09 μmol/mL NO). Additionally, the chitosan scaffold is positively charged, promoting the electrostatic interaction between COS-EA/NO and the negatively-charged biofilm and reducing NO diffusion distance.32 Both treatment methods required greater NO levels as a result of the protective external matrix and altered bacteria metabolism in biofilms.
In vitro cytotoxicity.
Nitric oxide is known to be harmful to mammals at sufficiently large concentrations, as it can induce asphyxiation via platelet inhibition.61 The concentrations of NO required for the described gNO treatment are an order of magnitude above the OSHA permissible exposure limit (25 ppm) and near the LC50 (797 ppm in rats), suggesting that systemic exposure to gNO may be harmful at 500 ppm.61 Human epithelial lung cells (A549) were cultured and treated with NO as a function of the NO delivery mode to determine cytotoxic levels. Interestingly, exposure of the A549 cells to 500 ppm aerobic gNO over 5 h was not cytotoxic (112 ± 27% viable after exposure), perhaps stemming from the low amount of NO in solution (Figure 3) and/or that gNO toxicity is rather the result of systemic asphyxiation via methemoglobinemia.
Human lung epithelial cells were likewise exposed to a range of COS-EA/NO concentrations (0.05 – 100 mg/mL) for 5 h. Negligible cytotoxicity (≥70% cell viability) was observed for all concentrations at or below 3.12 mg/mL (78 ± 6% viable; Figure 4), a level greater than or equal to the MBC5h for both P. aeruginosa and S. aureus under anaerobic and aerobic environments (Table 3). These results suggest that COS-EA/NO could successfully treat infections without significant toxicity risk. The large error at 6.25 and 12.5 mg/mL is likely the result of partial killing or temporary senescence induced during the initial burst release of NO from COS-EA/NO. As NO levels decreased, inconsistent cell growth or regained metabolic activity translate to greater variability. The cytotoxicity of COS-EA without appended NO was also tested against the A549 cells, with toxicity observed above 3.12 mg/mL, indicating that the ethanolamine-modified scaffold, not NO, is inducing the observed toxicity.
Figure 4.
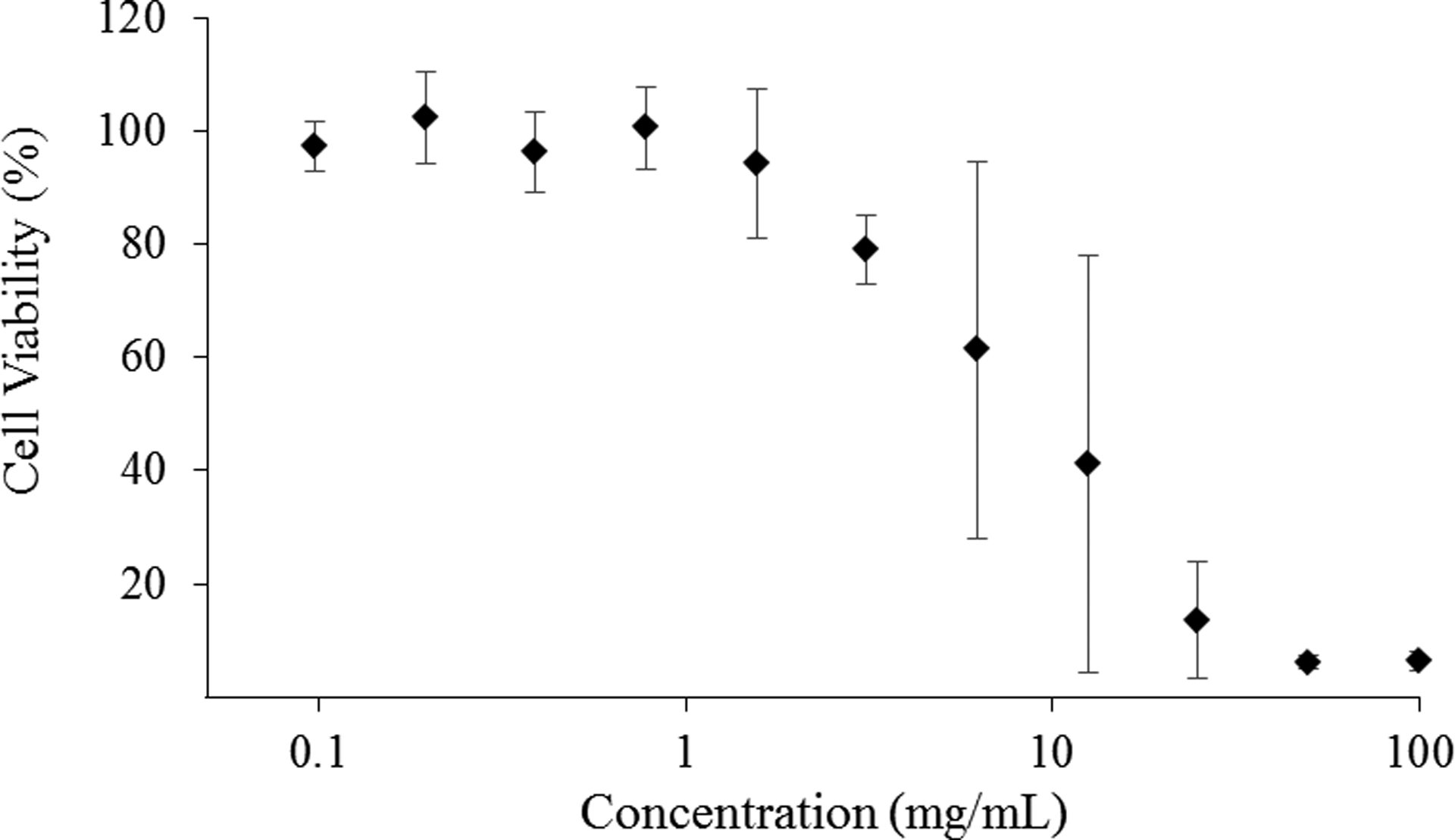
Viability of human epithelial lung cells (A549) exposed to COS-EA/NO as a function of concentration for 5 h at 37 °C. Percent cell viability was determined by the MTS assay and normalized against untreated cells to account for proliferation. n=4 for all concentrations.
Conclusions
A standardized protocol was developed to compare the antibacterial action of gNO to a water-soluble biopolymer releasing NO in solution. Exposure conditions (i.e., pH, NO concentration, and solution depth), bacteria viability versus CF-relavent bacteria under aerobic and anaerobic environments, and toxicity to mammalian cells were investigated for both NO delivery methodologies. Under all tested conditions, COS-EA/NO required significantly lower doses of NO in solution to achieve a 3-log reduction in bacterial viability compared to gNO. The NO-releasing biopolymer system allows for a more direct treatment approach, releasing the NO payload over time in solution. Such delivery necessitates shorter NO diffusion distances to bacteria, leading to reduced NO scavanging and greater antibacterial action at lower doses. Additionally, the positively-charged chitosan scaffold may provide unique targeting features by associating with the negatively-charged biofilm, further reducing the required NO diffusion distance to the bacteria. Although NO-releasing chitosan was utilized as a model biopolymer system in this study, the methodology described herein allows for comparison of gNO to any water-soluble NO donor.
Supplementary Material
Acknowledgements
Funding for this research was provided by the National Institutes of Health (DE025207) and KnowBIO, LLC.
Footnotes
Supporting Information
Detailed synthesis and NO-release profile of the NO-releasing ethanolamine-modified chitosan, bactericidal action of saline (pH 7.0 and 4.5) and PBS (pH 6.5), and the electrochemical detection of NO and nitrite at 160 ppm NO.
Conflicts of Interest
The corresponding author declares competing financial interest. Mark Schoenfisch is a co-founder, member of the board of directors, and maintains a financial interest in KnowBIO, LLC and Vast Therapeutics. Vast Therapeutics is commercializing macromolecular nitric oxide-releasing biopolymers for the treatment of respiratory infections.
References
- (1).Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; 2013.
- (2).Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; 2014. [Google Scholar]
- (3).Döring G; Flume P; Heijerman H; Elborn JS Treatment of Lung Infection in Patients with Cystic Fibrosis: Current and Future Strategies. J. Cyst. Fibros 2012, 11, 461–479. [DOI] [PubMed] [Google Scholar]
- (4).Hassett DJ; Cuppoletti J; Trapnell B; Lymar SV; Rowe JJ; Yoon S; Hilliard GM; Parvatiyar K; Kamani MC; Wozniak DJ; Hwang SH; McDernott TR; Ochsner UA Anaerobic Metabolism and Quorum Sensing by Pseudomonas Aeruginosa Biofilms in Chronically Infected Cystic Fibrosis Airways: Rethinking Antibiotic Treatment Strategies and Drug Targets. Adv. Drug Deliv. Rev 2002, 54, 1425–1443. [DOI] [PubMed] [Google Scholar]
- (5).Reighard KP; Schoenfisch MH Antibacterial Action of Nitric Oxide-Releasing Chitosan Oligosaccharides against Pseudomonas Aeruginosa under Aerobic and Anaerobic Conditions. Antimicrob. Agents Chemother. 2015, 59, 6506–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Plosker GL Aztreonam Lyine for Inhalation Solution in Cystic Fibrosis. Drugs 2010, 70, 1843–1855. [DOI] [PubMed] [Google Scholar]
- (7).Kirkby S; Novak K; McCoy K Aztreonam (for Inhalation Solution) for the Treatment of Chronic Lung Infections in Patients with Cystic Fibrosis: An Evidence-Based Review. Core Evid. 2011, 6, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Heirali AA; Workentine ML; Acosta N; Poonja A; Storey DG; Somayaji R; Rabin HR; Whelan FJ; Surette MG; Parkins MD The Effects of Inhaled Aztreonam on the Cystic Fibrosis Lung Microbiome. Microbiome 2017, 5, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Koerner-Rettberg C; Ballmann M Colistimethate Sodium for the Treatment of Chronic Pulmonary Infection in Cystic Fibrosis: An Evidence-Based Review of Its Place in Therapy. Core Evid. 2014, 9, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Westerman EM; De Boer AH; Le Brun PPH; Touw DJ; Roldaan AC; Frijlink HW; Heijerman HGM Dry Powder Inhalation of Colistin in Cystic Fibrosis Patients: A Single Dose Pilot Study. J. Cyst. Fibros 2007, 6, 284–292. [DOI] [PubMed] [Google Scholar]
- (11).Kasiakou S; Michalopoulos A; Soteriades ES; Samonis G; Sermaides GJ; Falagas ME Combination Therapy with Intravenous Colistin for Management of Infections Due to Multidrug-Resistant Gram-Negative Bacteria in Patients without Cystic Fibrosis. Antimicrob. Agents Chemother 2005, 49, 3136–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Elkins MR; Bye PT Mechanisms and Applications of Hypertonic Saline. J. R. Soc. Med 2011, 104, S2–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Enderby B; Doull I Hypertonic Saline Inhalation in Cystic Fibrosis - Salt in the Wound, or Sweet Success? Arch. Dis. Child 2007, 92, 195–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Levin MH; Sullivan S; Nielson D; Yang B; Finkbeiner WE; Verkman AS Hypertonic Saline Therapy in Cystic Fibrosis: Evidence against the Proposed Mechanism Involving Aquaporins. J. Biol. Chem 2006, 281, 25803–25812. [DOI] [PubMed] [Google Scholar]
- (15).Pai VB; Nahata MC Efficacy and Safety of Aerosolized Tobramycin in Cystic Fibrosis. Pediatr. Pulmonol 2001, 32, 314–327. [DOI] [PubMed] [Google Scholar]
- (16).VanDyke RD; McPhail GL; Huang B; Fenchel MC; Amin RS; Carle AC; Chini BA; Seid M Inhaled Tobramycin Effectively Reduces FEV1 Decline in Cystic Fibrosis: An Instrumental Variables Analysis. Ann. Am. Thorac. Soc 2013, 10, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Shteinberg M; Elborn JS Use of Inhaled Tobramycin in Cystic Fibrosis. Adv. Ther 2015, 32, 1–9. [DOI] [PubMed] [Google Scholar]
- (18).Geller DE; Nasr SZ; Piggott S; He E; Angyalosi G; Higgins M Tobramycin Inhalation Powder in Cystic Fibrosis Patients: Response by Age Group. Respir. Care 2014, 59, 388–398. [DOI] [PubMed] [Google Scholar]
- (19).Poole K Aminoglycoside Resistance in Pseudomonas Aeruginosa. Antimicrob. Agents Chemother 2005, 49, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Waters V; Ratjen F Multidrug-Resistant Organisms in Cystic Fibrosis: Management and Infection-Control Issues. Expert Rev. Anti. Infect. Ther 2006, 4, 807–819. [DOI] [PubMed] [Google Scholar]
- (21).Sanguinetti M; Ardito F; Fiscarelli E; La Sorda M; D’Argenio P; Ricciotti G; Fadda G Fatal Pulmonary Infection Due to Multidrug-Resistant Mycobacterium Abscessus in a Patient with Cystic Fibrosis. J. Clin. Microbiol 2001, 39, 816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wolcott RD; Rumbaugh KP; James G; Schultz G; Phillips P; Yang Q; Watters C; Stewart PS; Dowd SE Biofilm Maturity Studies Indicate Sharp Debridement Opens a Time-Dependent Therapeutic Window. J. Wound Care 2010, 19 , 320–328. [DOI] [PubMed] [Google Scholar]
- (23).Van Acker H; Van Dijck P; Coenye T Molecular Mechanisms of Antimicrobial Tolerance and Resistance in Bacterial and Fungal Biofilms. Trends Microbiol. 2014, 22, 326–333. [DOI] [PubMed] [Google Scholar]
- (24).Jones WL; Sutton MP; Mckittrick L; Stewart PS Chemical and Antimicrobial Treatments Change the Viscoelastic Properties of Bacterial Biofilms. Biofouling 2011, 27, 207–215. [DOI] [PubMed] [Google Scholar]
- (25).Jones ML; Ganopolsky JG; Labbé A; Wahl C; Prakash S Antimicrobial Properties of Nitric Oxide and Its Application in Antimicrobial Formulations and Medical Devices. Appl. Microbiol. Biotechnol 2010, 88, 401–407. [DOI] [PubMed] [Google Scholar]
- (26).Fang FC Mechanisms of Nitric Oxide-Related Antimicrobial Activity. J. Clin. Invest 1997, 99, 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Fang FC Antimicrobial Reactive Oxygen and Nitrogen Species: Concepts and Controversies. Nat. Rev. Microbiol 2004, 2, 820–832. [DOI] [PubMed] [Google Scholar]
- (28).Privett BJ; Broadnax AD; Bauman SJ; Riccio DA; Schoenfisch MH Examination of Bacterial Resistance to Exogenous Nitric Oxide. Nitric Oxide 2012, 26, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Stratton CW Dead Bugs Don’t Mutate: Susceptibility Issues in the Emergence of Bacterial Resistance. Emerg. Infect. Dis 2003, 9, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Carpenter AW; Schoenfisch MH Nitric Oxide Release: Part II. Therapeutic Applications. Chem. Soc. Rev 2012, 41, 3742–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Reighard KP; Hill DB; Dixon GA; Worley BV; Schoenfisch MH Disruption and Eradication of P. Aeruginosa Biofilms Using Nitric Oxide-Releasing Chitosan Oligosaccharides. Biofouling 2015, 31, 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Reighard KP; Ehre C; Rushton ZL; Ahonen MJR; Hill DB; Schoenfisch MH Role of Nitric Oxide-Releasing Chitosan Oligosaccharides on Mucus Viscoelasticity. ACS Biomater. Sci. Eng 2017, 3, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ghaffari A; Neil DH; Ardakani A; Road J; Ghahary A; Miller CC A Direct Nitric Oxide Gas Delivery System for Bacterial and Mammalian Cell Cultures. Nitric Oxide 2005, 12, 129–140. [DOI] [PubMed] [Google Scholar]
- (34).Miller C; McMullin B; Ghaffari A; Stenzler A; Pick N; Roscoe D; Ghahary A; Road J; Av-Gay Y Gaseous Nitric Oxide Bactericidal Activity Retained during Intermittent High-Dose Short Duration Exposure. Nitric Oxide 2009, 20, 16–23. [DOI] [PubMed] [Google Scholar]
- (35).McMullin BB; Chittock DR; Roscoe DL; Garcha H; Wang L; Miller CC The Antimicrobial Effect of Nitric Oxide on the Bacteria That Cause Nosocomial Pneumonia in Mechanically Ventilated Patients in the Intensive Care Unit. Respir. Care 2005, 50, 1451–1456. [PubMed] [Google Scholar]
- (36).Miller CC; Hergott CA; Rohan M; Arsenault-Mehta K; Döring G; Mehta S Inhaled Nitric Oxide Decreases the Bacterial Load in a Rat Model of Pseudomonas Aeruginosa Pneumonia. J. Cyst. Fibros 2013, 12, 817–820. [DOI] [PubMed] [Google Scholar]
- (37).Deppisch C; Herrmann G; Graepler-Mainka U; Wirtz H; Heyder S; Engel C; Marschal M; Miller CC; Riethmüller J Gaseous Nitric Oxide to Treat Antibiotic Resistant Bacterial and Fungal Lung Infections in Patients with Cystic Fibrosis: A Phase I Clinical Study. Infection 2016, 44, 513–520. [DOI] [PubMed] [Google Scholar]
- (38).Riccio DA; Schoenfisch MH Nitric Oxide Release: Part I. Macromolecular Scaffolds. Chem. Soc. Rev 2012, 41, 3731–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Yang L; Feura ES; Ahonen MJR; Schoenfisch MH Nitric Oxide – Releasing Macromolecular Scaffolds for Antibacterial Applications. Adv. Healthc. Mater 2018, 7, 1800155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sadrearhami Z; Nguyen TK; Namivandi-Zangeneh R; Jung K; Wong EHH; Boyer C Recent Advances in Nitric Oxide Delivery for Antimicrobial Applications Using Polymer-Based Systems. J. Mater. Chem. B 2018, 6, 2945–2959. [DOI] [PubMed] [Google Scholar]
- (41).Liang H; Nacharaju P; Friedman A; Friedman JM Nitric Oxide Generating/Releasing Materials. Futur. Sci. OA 2015, 1, FSO54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Cheng J; He K; Shen Z; Zhang G; Yu Y; Hu J Nitric Oxide (NO)-Releasing Macromolecules: Rational Design and Biomedical Applications. Front. Chem 2019, 7, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Lu Y; Slomberg DL; Schoenfisch MH Nitric Oxide-Releasing Chitosan Oligosaccharides as Antibacterial Agents. Biomaterials 2014, 35, 1716–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Lu Y; Shah A; Hunter RA; Soto RJ; Schoenfisch MH S-Nitrosothiol-Modified Nitric Oxide-Releasing Chitosan Oligosaccharides as Antibacterial Agents. Acta Biomater. 2015, 12, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Soto RJ; Yang L; Schoenfisch MH Functionalized Mesoporous Silica via an Aminosilane Surfactant Ion Exchange Reaction: Controlled Scaffold Design and Nitric Oxide Release. ACS Appl. Mater. Interfaces 2016, 8, 2220–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Worley BV; Schilly KM; Schoenfisch MH Anti-Biofilm Efficacy of Dual-Action Nitric Oxide-Releasing Alkyl Chain Modified Poly(Amidoamine) Dendrimers. Mol. Pharm 2015, 12, 1573–1583. [DOI] [PubMed] [Google Scholar]
- (47).Breed RS; Dotterrer WD The Number of Colonies Allowable on Satisfactory Agar Plates. J. Bacteriol 1916, 1, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Brown MD; Schoenfisch MH Nitric Oxide Permselectivity in Electropolymerized Films for Sensing Applications. ACS Sensors 2016, 1, 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Brown MD; Schoenfisch MH Selective and Sensocompatible Electrochemical Nitric Oxide Sensor with a Bilaminar Design. ACS Sensors 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Suchyta DJ; Schoenfisch MH Encapsulation of N-Diazeniumdiolates within Liposomes for Enhanced Nitric Oxide Donor Stability and Delivery. Mol. Pharm 2015, 12, 3569–3574. [DOI] [PubMed] [Google Scholar]
- (51).Thomas DD; Ridnour LA; Isenberg JS; Flores-Santana W; Switzer CH; Donzelli S; Hussain P; Vecoli C; Paolocci N; Ambs S; Colton CA; Harris CC; Roberts DD; Wink DA The Chemical Biology of Nitric Oxide: Implications in Cellular Signaling. Free Radic. Biol. Med 2008, 45, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Jordan RC; Jacobs SE The Effect of pH at Different Temperatures on the Growth of Bacterium Coli with a Constant Food Supply. Microbiology 1948, 2, 15–24. [DOI] [PubMed] [Google Scholar]
- (53).Lund P; Tramonti A; De Biase D Coping with Low pH: Molecular Strategies in Neutralophilic Bacteria. FEMS Microbiol. Rev 2014, 38, 1091–1125. [DOI] [PubMed] [Google Scholar]
- (54).Yoon SS; Coakley R; Lau GW; Lymar SV; Gaston B; Karabulut AC; Hennigan RF; Hwang S-H; Buettner G; Schurr MJ; Mortensen JE; Burns JL; Speert D; Boucher RC; Hassett DJ Anaerobic Killing of Mucoid Pseudomonas Aeruginosa by Acidified Nitrite Derivatives under Cystic Fibrosis Airway Conditions. J. Clin. Invest 2006, 116, 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Platt MD; Schurr MJ; Sauer K; Vazquez G; Kukavica-Ibrulj I; Potvin E; Levesque RC; Fedynak A; Brinkman FSL; Schurr J; Hwang SH; Lau GW; Limbach PA; Rowe JJ; Lieberman MA; Barraud N; Webb J; Kjelleberg S; Hunt DF; Hassett DJ Proteomic, Microarray, and Signature-Tagged Mutagenesis Analyses of Anaerobic Pseudomonas Aeruginosa at pH 6.5, Likely Representing Chronic, Late-Stage Cystic Fibrosis Airway Conditions. J. Bacteriol 2008, 190, 2739–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Garland AL; Walton WG; Coakley RD; Tan CD; Gilmore RC; Hobbs CA; Tripathy A; Clunes LA; Bencharit S; Stutts MJ; Betts L; Redinbo MR; Tarran R Molecular Basis for pH-Dependent Mucosal Dehydration in Cystic Fibrosis Airways. Proc. Natl. Acad. Sci 2013, 110, 15973–15978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Fukuto JM; Cho JY; Switzer CH The Chemical Properties of Nitric Oxide and Related Nitrogen Oxides In Nitric Oxide: Biology and Pathobiology; Academic Press: San Diego, CA, 2000; pp 23–40. [Google Scholar]
- (58).Hoiby N; Ciofu O; Bjarnsholt T Pseudomonas Aeruginosa Biofilms in Cystic Fibrosis. Future Microbiol. 2010, 5, 1663–1674. [DOI] [PubMed] [Google Scholar]
- (59).Ahonen MJR; Suchyta DJ; Zhu H; Schoenfisch MH Nitric Oxide-Releasing Alginates. Biomacromolecules 2018, 19, 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Fuchs S; Pané-Farré J; Kohler C; Hecker M; Engelmann S Anaerobic Gene Expression in Staphylococcus Aureus. J. Bacteriol 2007, 189, 4275–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Blaise GA; Gauvin D; Gangal M; Authier S Nitric Oxide, Cell Signaling and Cell Death. Toxicology 2005, 208, 177–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


