Abstract
Multiple myeloma (MM) is a disease of aging adults, and numerous therapeutic options are available for this growing demographic. MM treatment of older adults continues to evolve and includes novel combinations, new generations of targeted agents, immunotherapy, and increasing use of autologous stem cell transplantation (ASCT). Understanding age-related factors, independent of chronologic age itself, is an increasingly recognized factor in MM survivorship, especially in understudied populations, such as octogenarians. Octogenarians have inferior survival that cannot be explained by cytogenetic profiles alone. Incorporating assessments of geriatric factors can provide guidance on how to intensify or de-escalate therapeutic options. Functional status, using objective testing, is superior to traditional metrics of performance status and should be implemented to optimize the risk-benefit ratio of ASCT. ASCT is feasible and cost-effective, and chronologic age should not exclude ASCT eligibility. Upfront ASCT remains the standard of care, in the context of a sequential approach that includes pre-transplantation induction and post-transplantation maintenance. High-risk MM is classically defined by disease characteristics, yet shifting frameworks suggest that the high-risk designation could refer to any patient subgroup who is at risk for poorer outcomes—beyond disease-focused outcomes to patient-focused outcomes. Defining the optimal treatment of subgroups of older patients with high-risk disease on the basis of chromosomal abnormalities is unexplored. Here, we review tools to assess individual health status, explore vulnerability in octogenarians with MM, address ASCT decision-making, and examine high-risk MM to understand factors that contribute to survival disparities for older adults with MM.
INTRODUCTION
Multiple myeloma (MM) is a disease of older adults, and approximately 30,000 new occurrences will be diagnosed this year.1 The median age of patients with myeloma is 70 years. Therapy for MM continues to evolve and includes novel combination therapy, new generations of targeted agents, immunotherapy, and increasing use of autologous stem cell transplantation (ASCT).2 As a result of these advances, survival for MM has improved for most demographics, yet improvements in survival for older adults with myeloma have been modest and require ongoing investigation. Understanding the needs of the aging myeloma demographic requires research in real-world myeloma populations with diverse health status, variable functional trajectories, and pre-existing comorbidities. Prescriptive clinical trial designs often lead to highly selected MM populations, which results in queries about how to implement and optimize therapy for patients with MM who have age-related conditions that predated and may be exacerbated by a myeloma diagnosis. Numerous MM treatment combinations are available for older adults and are stratified for the individual according to the ability to tolerate ASCT and by disease risk stratification.3 Randomized trials have demonstrated the survival advantages of ASCT, yet less than 20% of all patients older than age 65 are undergoing ASCT.4 When comparing trends in the modern era, patients with MM who are younger than age 65 have improved 10-year relative survival rates (9.6% vs. 35%; p < .001); patients age 75 or older do not share the same survival advantages (7.8% vs. 9.3%; p = .3).5 MM deaths overall are highest in patients age 75 or older, and early mortality is most common in those age 70 or older.6,7 Optimizing quality of life, improving early mortality, and increasing access to ASCT for MM are active areas of investigation.8–10 Here, we examine evidence to support geriatric assessments, explore MM in octogenarians, address ASCT decision-making, and explore high-risk MM to understand factors that are attributable to survival disparities for older adults with MM.
GERIATRIC ASSESSMENT IN MM
Bridging the disciplines of geriatric oncology with MM is an important first step in the field of aging research. Understanding age-related factors, independent of chronologic age itself, is imperative in rendering a myeloma disease trajectory. Age influences all facets of myeloma disease course, including diagnosis, outcomes, and survivorship. The study of aging and life span, and the influence of myeloma development, parallels studies in geriatrics. Geriatrics is the study of health and disease in later life,11 wherein a comprehensive geriatric assessment (GA), although variably defined, is an interdisciplinary approach to identify, intervene, and create a longitudinal care plan to improve clinical outcomes for frail older adults.12,13 The term frailty “is used more frequently than it is defined” but is accepted to be a complex syndrome of physiologic decline that signifies increased vulnerability.14,15 As such, many myeloma providers have recognized the need to incorporate assessments of geriatric factors, given numerous treatment options and divergent intensities of therapeutic options. In the myeloma setting, implementing geriatric screening tools or collating assessments of geriatric domains (e.g., functional status, psychological status, cognition, socioeconomic factors, comorbidities, nutritional status, and sensory loss) have served as a diagnostic index in predicting vulnerability to adverse outcomes (Table 1). An understanding of the specific geriatric tools and domain assessments helps characterize how to best care for older adults. We aim to outline geriatric screening tools, GAs, and evidence to support use in older adults with MM.
TABLE 1.
Geriatric Variable Assessment in MM Populations
| Study | Geriatric Domains | Population | Status | Endpoints |
|---|---|---|---|---|
| IMWG frailty score 201516 | Age | NDMM age ≥ 65 years | Fit | OS |
| ADL/IADL | Median age, 74 years (range, 70–78 years) | Intermediate fit | PFS | |
| Comorbidities | Nontransplantation | Frail | Drug discontinuation | |
| Clinical trial population (n = 869) | Toxicity | |||
| Freiburg Comorbidity Index (I-MCI) 201317 | KPS | Chart audit | Fit | OS |
| Lung disease* | Symptomatic MM | Intermediate fit | ||
| Renal disease | Median age, 62 years (range, 27–90 years) | Frail | ||
| Transplantation and nontransplantation (n = 466) | ||||
| R-MCI 201718 | Age | NDMM | Fit | OS |
| KPS | Median age, 63 years (range, 21–93 years) | Intermediate fit | PFS | |
| Lung disease* | Transplantation and nontransplantation (n = 801) | Frail | ||
| Renal disease | ||||
| Frailty** | ||||
| Cytogenetics | ||||
| Comorbidities | ||||
| GAH 201519,20 | Medications | Patients with MM | Score ≤ 1 | OS |
| Gait speed | GAH original cohort | Score 2–6 | ||
| Mood | Median age, 76 years (range, 71–81 years; validation cohort; n = 60 MM/164 total) | Score > 6 | ||
| ADL | ||||
| Health status | ||||
| Nutrition | ||||
| Mental status | ||||
| Comorbidities | ||||
| Edmonton frailty, Schutz et al 201721 | Cognitive impairment | Chart audit age > 65 years | Fit | OS |
| Depression | NDMM | Vulnerable | ||
| Medications | Median age, 77 years (range, 65–98 years; n = 150) | Frail | ||
| Urinary incontinence | ||||
| Functional impairment | ||||
| Gait disturbance/falls | ||||
| Low weight | ||||
| Prior hospitalization | ||||
| Wildes et al, GA 201822 | Age | NDMM | NA | Transplantation recipient status |
| IADL | Median age, 71 years (SD, ±5.1 years) | |||
| Cognition | Transplantation and nontransplantation (n = 40) | |||
| Mental health inventory | ||||
| TUG | ||||
| Medications | ||||
| Comorbidities | ||||
| Rosko et al, GA 201823 | Nutrition | Transplantation MM | NA | Length of stay |
| KPS | Median age, 59.5 years (range, 36–75 years; n = 100) | Hospitalization | ||
| Self-report function | EFS | |||
| Fatigue | Change in deficits | |||
| Hand grip | ||||
| Gait disturbance | ||||
| Anxiety/depression | ||||
| Social support | ||||
| Cognition | ||||
| Frailty index 201824 | ADL | SEER-Medicare database age > 66 years | Cumulative deficits frailty index | OS |
| Comorbidities | NDMM | |||
| Physical function | Median age, 74 years (range, 66–110; n = 30b) | |||
| General health | ||||
| Mental health | ||||
| Murillo et al25 | IMWG frailty | Patients with MM age > 75 years | Fit/intermediate fit/frail | OS |
| IMWG vs. Fried Model26 | score Frailty** | Median age, 79 years (n = 107) | Robust/pre-frail/frail |
Abbreviations: ADL, activities of daily living; EFS, event-free survival; FEV1, forced expiratory volume; FVC, forced vital capacity; GA, geriatric assessment; GAH, geriatric assessment in hematology; IADL, instrumental ADL; I-MCI, Intial-Myeloma Comorbidity Index; IMWG, International Myeloma Working Group; KPS, Karnofsky performance status; MM, multiple myeloma; NA, not applicable; NDMM, newly diagnosed MM; OS, overall survival; PFS, progression-free survival; R-MCI, Revised Myeloma Comorbidity Index; SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results; TLC, total lung capacity; TUG, Timed up and Go.
Lung disease: dyspnea, FEV1//FVC, FEV1, TLC, respiratory insufficiency.
Frailty: weakness, poor endurance, low physical activity, slow gait speed.
Oncologists routinely assess patient vulnerability, often subjectively, to determine the likelihood of treatment tolerance, estimate treatment benefit, and predict risk of adverse complications and/or mortality. GA tools are shown to accurately assess risk of morbidity and mortality in cancer populations independent of performance status and age.27–29 Myeloma-specific studies evaluating geriatric variables are an important advance when caring and managing treatment decisions for older adults. The International Myeloma Working Group (IMWG) used a simplified GA tool based on age, comorbidities, activities of daily living (ADL), and instrumental activities of daily living (IADL) for newly diagnosed older adults enrolled into nontransplantation frontline clinical trials.16 The IMWG tool classified patients as fit, intermediate fit, and frail; scores were predictive of death, progression, treatment discontinuation, and nonhematologic toxicities. The IMWG frailty score profiles were independent of treatment, cytogenetics, or stage in the MM population.16 The Freiburg Comorbidity Index, also known as the Initial Myeloma Comorbidity Index (I-MCI), is an assessment tool based on Karnofsky performance status, lung disease, and renal disease by estimated glomerular filtration rate.17 The Freiburg Comorbidity Index is predictive of survival independent of MM stage, therapy, and age (p < .0015).26 The Revised Myeloma Comorbidity Index (R-MCI) expanded to include age, frailty, and cytogenetics; in the Engelhardt study, frailty was identified in 74 (13%) of 552 patients and the hazard ratio (HR) for overall survival among frail patients was 9.57 (CI, 6.52–14.03).18 The Geriatric Assessment in Hematology (GAH) scoring system focuses on eight geriatric dimensions (polypharmacy, gait speed, mood, ADL, health status, nutrition, mental status, comorbidities) and is brief (approximately 10–12 minutes); with this tool, increasing deficits have been associated with survival in adults older than age 65 who were newly diagnosed with hematologic malignancy.19,20 Recently, Murillo et al25 compared the IMWG frailty score with the Fried model30 and reported that frailty defined by the Fried model was significantly associated with risk of death (prefrail HR, 2.88 [95% CI, 0.80–10.41]; frail HR, 4.91 [95% CI, 1.29–18.61]; global p = .04). In contrast to the Fried model, there was no statistically significant association with risk of death by frailty category using the IMWG model.25 These results reflect either an older demographic or a more real-world analysis of patients with myeloma. Efforts to streamline GA tools for ASCT decision-making are ongoing and require further investigation; GA instruments may predict the likelihood of receiving a transplantation,22 and geriatric deficits are associated with increased length of hospital admission and event-free survival.23 Others have explored large MM data sets, creating a frailty index using geriatric domains (mental health, physical function, comorbidities, ADL, general health) whereby a 10% increase in the frailty index was associated with a 16% increased risk for death (adjusted HR, 1.159; 95% CI, 1.080–1.244; p < .001).24
Personalizing therapy on the basis of patient fitness or frailty may improve patient outcomes in older adults, but requires prospective clinical trials. Each of these assessments, whether through a survey, risk assessment profile, or clinical visit, is intended to screen and evaluate for frailty. Frailty is a state of patient vulnerability and is highly relevant for the older adult MM population. Essential for this discussion is recognizing that frailty is dynamic and that one-time screening for frailty is unlikely to result in meaningful benefit as the state of frailty may improve or decline, and therefore alter treatment decisions.
OCTOGENARIANS WITH MM
Because of the increasing lifespan, 434 million people in the world will be older than age 80 in 2050.31 The availability of MM treatment options that are not only effective but also feasible in octogenarians will lead to more rigorous screening for MM. This age-related impact was recently described in Europe and is expected to occur also in middle-income countries.32 Therefore, the incidence and prevalence of octogenarians with MM will continue to expand, so exploring treatment modalities in this demographic is increasingly relevant.
The IMWG frailty index was created to identify vulnerable patients with MM; the index reflects worse PFS and OS as well as increased incidences of grades 3 to 4 nonhematologic toxicities and discontinuation rates in frail patients. According to this index, patients older than age 80 are, by definition, frail.16 Although chronologic aging is accompanied by agerelated physiologic changes, there is a pronounced heterogeneity in physiologic and functional age.33 Therefore, it is questionable whether age of 80 years alone should be a definition of frailty in patients with MM. Patients defined as frail by the IMWG frailty index have had higher rates of functional impairments and loss of muscle mass than nonfrail patients, suggesting that the index indeed reflects biologic frailty.34 However in this study, although more frail than fit patients have died 3 years after the initiation of treatment (43% vs. 16%), 57% of frail patients survived, emphasizing the benefit of treatment among even patients deemed vulnerable.16 Therefore, there is an unmet need to identify patients older than age 80 who are expected to benefit from treatment before deciding to abstain from MM treatment and provide supportive care only.
An important consideration for weighing treatment decisions in octogenarians is life expectancy based on age in concert with general health status, because they relate to the expected survival benefit with a given MM treatment. Also, the risk of developing treatment-related mortality, but especially morbidity, must be considered. In octogenarians, morbidity not only may affect the quality of life but also might have a negative impact on independent ADLs and the ability to maintain independence. Therefore, the preferences and values of patients in life before the MM diagnosis are important to discuss in the shared decision-making model before treatment decision are made.35 For the prediction of general life expectancy, there are several tools available in which not only age but also functional and physiologic characteristics are taken into account; these were recently reviewed by Yourman et al.36,37
Whether OS will be improved by MM treatment is difficult to answer, because data on the efficacy of treatment in octogenarians are scarce. Actually, there are no separate analyses of patients older than age 80 in recent clinical trials. Data on the eldest patients with MM are limited to subanalyses of patients older than age 75. In the FIRST trial comparing lenalidomide/dexamethasone continuously (Rd) versus 18 cycles of Rd (Rd18) versus melphalan/prednisone/thalidomide (MPT), patients older than age 75 also benefited from Rd versus MPT; however, the PFS and OS decreased for this older group relative to patients age 75 years or younger (PFS, 20.3 vs. 28.1 months; OS, 52.3 vs. 60.9 months).38 In the VISTA trial comparing bortezomib/melphalan/prednisone (VMP) with melphalan/prednisone, a benefit of VMP was also found in older adults; however, again, the benefit was less pronounced in the eldest (OS, 43.3 months in patients age 75 or older vs. not reached in patients younger than age 75), data on PFS were not reported.39 In the recent ALCYONE trial, no difference in PFS was found between patients younger than age 75 and those age 75 or older in either the VMP or daratumumab/VMP arm.40 In addition, two studies were specifically developed for patients age 75 or older to investigate two- or three-drug regimens with bortezomib or dose-adjusted VMP. Both studies supported dose adjustments. However, even with dose adjustment, the outcome was still inferior compared (in a non–head-to-head manner) with younger non–transplantation eligible patients; the median PFS was only approximately 15 months in the older patients versus 20–25 months in all patients who were not eligible for transplantation (in general, age 65 and older).34,41
We performed a subanalysis based on age in three HOVON trials for non–transplantation eligible patients. In the HOVON 87 trial, melphalan/prednisone was combined with either thalidomide or lenalidomide. Both immunomodulatory drugs (IMiDs) were given until progression occured or afternine induction cycles.42 Of the population, 65 (10%) of 636 patients were older than age 80. The median PFS was similar independent of age (22 months in age 80 or older vs. 22 and 21 months in patients age 75–79 and age 75 or younger, respectively). In contrast, the median OS was worse in patients age 80 and older (41 vs. 55 and 56 months for the two younger age groups, respectively). In the HOVON 126, non–transplantation eligible patients were treated with nine induction cycles of ixazomib/thalidomide/dexamethasone followed by a random assignment to maintenance with placebo or ixazomib.43 Of the population, 11 (8%) of 143 patients were older than age 80. PFS was not significantly different in those age 80 or older (median PFS, 10 months vs. 15 and 14 months in ages 75–79 and age 75 or younger, respectively). OS at 2 years was worse (63% vs. 75% and 90% for the younger groups, respectively). The HOVON 123 was a study specifically designed for patients age 75 or older, and 101 (42%) of 238 patients were age 80 or older. The median PFS was 17 months, irrespective of age, and the OS was 29 months among octogenerians.34 Moreover, the GIMEMA and PETHEMA studies revealed results similar to the HOVON studies (personal communication, A. Larocca, February 2019; and M. V. Mateos, February 2019; respectively): Comparable PFS but inferior OS was observed in patients age 80 and older compared with the age groups of 75–79 and 75 or younger (GIMEMA MM-03–05 plus EMN 01 trial: median PFS, 24, 19, and 27 months, respectively; median OS, 39, 52, and not reached, respectively; GEM2005MAS65: median PFS, 22, 16, and 29 months, respectively; median OS, 35, 35, and 53 months, respectively).
The inferior OS in patients age 80 or older cannot be explained by a higher prevalence of adverse cytogenetic profile, which was even less pronounced in older adults because of a lower incidence of translocation (4;14). Only International Staging System (ISS) stage was higher in patients age 80 or older in all above-described subanalyses of the HOVON and GIMEMA studies. Life expectancy is shorter for octogenarians, but other relevant factors among individuals being treated for MM could include higher levels of toxicity induced during first-line treatment and more comorbidities that preclude subsequent treatment at relapse. Indeed, Bringhen and colleagues44 found that the risk of death was higher in patients who experienced severe infections, cardiac events, or gastrointestinal events and in those who required drug discontinuation because of adverse events.44 They also found a higher incidence of toxic deaths among octogenarians compared with patients younger than age 80 (12% vs. 4%).45 A subanalysis showed a slightly higher incidence of grades 3 and 4 toxicity in patients age 80 or older (21% vs. 17% in the whole patient population; personal communication, A. Larocca, February 2019). Similarly, in the HOVON studies, we found an approximately 80%–90% rate of grades 3 and 4 toxicity in patients age 80 or older versus a rate of approximately 65%–85% in patients younger than age 80. The reason for such limited differences in toxicity is probably caused by a selection bias in clinical studies. This is supported by the fact that the World Health Organization performance status and number of comorbidities were rather comparable across age groups in the GIMEMA, PETHEMA, and HOVON trials.
The demographics of hospital or population-based registries have been found to be different as well. In a Greek hospital based octogenarian population (n = 110), both disease characteristics and patient characteristics differed from what is generally observed in a clinical trial population: 61% had ISS stage 3 disease, 13% had high lactate dehydrogenase levels, 36% had an estimated glomerular filtration rate less than 30 mL/min/1.73m2, 66% had a World Health Organization performance status of 3 or 4, and 46% had a Charlson comorbidity index of 2 or greater. The median PFS was 7 months, and the OS was 21 months compared with 69 months for patients younger than age 80. Moreover, there was a high early death rate (< 2 months) of 21%.46 From the Dutch population-based registry, comparable data were found; the OS was 27 months for patients age 80–84 and was 8 months for patients age 85 or older, with accordingly high early death rates (< 3 months) of 17% and 26%, respectively (personal communication, A. Dinmohamed, February 2019). That such patients are less often eligible for studies has been observed in the MM Connect database,47 which showed that, in general, 40% of patients with MM were not eligible for participation in trials independent of octogenarian status. Those patients had worse renal function, higher ISS stages, and worse survival compared with patients who were eligible. As such, integrating a personalized approach for the aging demographic, which is at risk for high mortality and morbidity from the disease and from treatment, is imperative for the MM population.
TRANSPLANTATION IN OLDER ADULT PATIENTS WITH MM
High-dose chemotherapy followed by ASCT is currently considered a standard procedure for younger patients with newly diagnosed multiple myeloma (NDMM). Four randomized trials compared upfront ASCT with a non-ASCT, novel, agent-based approach in younger patients (< 65 years). Incorporating high-dose melphalan followed by ASCT in a novel, agent-based, frontline strategy significantly improved PFS and OS, although the OS benefit was inconsistent across the trials.48–53
Historically, the age cutoff adopted to determine ASCT eligibility in clinical trials was 65 years. This age limit prevents a notable proportion of patients with MM from undergoing ASCT, because more than two-thirds of patients with NDMM are age 65 or older. In clinical practice, however, this limit has been extended to the age of 70–75.54–56 In Europe, the European Society for Medical Oncology guidelines recommended ASCT in patients up to the age of 7057; conversely, in the United States, the NCCN guidelines did not set an age cutoff for ASCT eligibility.58
Recent population-based studies reported an increasing use of ASCT in older adult patients with MM.5,56 In a study evaluating age-related trends in the use of ASCT in 31 European countries (1991–2010), the highest increase in the rates of ASCT occurred in patients older than age 65, representing 3% of all ASCTs between 1991 and 1995 and 18% between 2006 and 2010.56 Similarly, in a study in the United States and Canada, the use of ASCT in patients older than age 65 increased from 8% (1995–1999) to 24% (2005–2010).5
Table 2 summarizes prospective and retrospective trials that evaluated the efficacy and safety of ASCT in older adult patients. Unfortunately, most of the studies are retrospective or registry based, and only two randomized trials, conducted before the introduction of lenalidomide and bortezomib, compared ASCT with a nontransplantation approach, with conflicting results.59,60
TABLE 2.
Prospective and Retrospective Studies of ASCT for Older Adult Patients With MM
| First Author | No. of Patients | Patient Population (age in years) | Median (range) Age (years) | Conditioning Regimen | ASCT | TRM (%) | ORR/CR (%) | PFS | OS | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||||
| Palumbo et al 200459 | 95 | 65–70 | NA | MEL100 | Double | 7 (early deaths) | 68/NA | Median EFS: 28 months | 3-year: 77% | |
| Facon et al 200760 | 126 | 65–75 | ≥ 70 (39% / total population) | MEL100 | Double | 9 | 65/18 | Median: 19.4 months | Median: 38.3 months | |
| Gay et al 201361 | 102 | ≤ 75 | 67 (46–74) | MEL100 | Double | < 70 years: 5 | 95/53 | 5-year: 48% | 5-year: 68% | |
| 70–75 years: 19 | ||||||||||
| Garderet et al 201662 | 56 | ≥ 65 | 67.4 (64–74) | MEL200 (64% of patients) | Single | 0 | 94/40 | 2-year: 76% | 2-year: 88% | Patients in the MEL200 group were slightly younger (68.7 vs. 66.5 years) and had fewer comorbidities (HCT-CI, 0: 79% vs. 50%) |
| MEL140 (36% of patients) | ||||||||||
| Straka et al 201663 | 434 | 60–70 | 65 (60–72) | MEL140 | Double | First-line ASCT: 1.4 | 87/12–17 | Median: 20–21.4 months | Median: 53.4–55.9 months | > 65 years: TRM, 1.1% (first ASCT) and 0% (second ASCT); 60–64 years and 65–70 years: no difference in PFS/OS |
| Second-line ASCT: 0 | ||||||||||
| Nadiminti et al 201864 | 37 | ≥ 65 | 68 (65–75) | MEL200 | Single | 2.7 | 99/47 | 3-year: 82% | 3-year: 90% | |
| Retrospective studies | ||||||||||
| Bashir et al 201265 | 84 | ≥ 70 | 72 (70–80) | MEL200: 65% of patients | NA | All patients: 3 | 85/18 | 5-year: 27% | 5-year: 67% | No difference in grade 3–4 toxicities in patients < 75 and ≥ 75 years |
| MEL140: 25% of | < 75 years: 2 | |||||||||
| patients MEL100: 10% of patients | ≥ 75 years: 6 | |||||||||
| Merz et al 201466 | 202 | ≥ 60 | 65 | MEL200 | Single: 100% | 60–64 years: 2.4 | nCR + CR: | Median: | Median: | |
| Double: 8% | 65–69 years: 1 | 60–64 years: 38 | 60–64 years: 27 months | 60–64 years: NR | ||||||
| 70–75 years: 0 | 65–69 years: 33 | 65–69 years: 23 months | 65–69 years: NR | |||||||
| 70–75 years: 31 | 70–75 years: 23 months | 70–75 years: NR | ||||||||
| Ozaki et al 201467 | 38 | ≥ 65 | 65–68 | MEL200/140 | NA | 0 | NA | Median: 26.8–35. 2 months | NR | |
| Sanchez et al 201768 | 2209 | < 65:74.7% | NA | NA | NA | < 65 years: 2.3 | NA | NA | NA | Patients > 65 years had longer in-hospital stay and higher risk of transplantation-related mortality. |
| ≥ 65: 25.3% | ≥ 65 years: 1.2 | |||||||||
| Stettler et al 201769 | 61 | ≥65 | NA | MEL200: 65–70 years | NA | 65–70 years: 0 | CR: | 2-year: | 2-year: | Patients > 70 years had longer hospitalization time (26 vs. 20 days) than patients 65–70 years |
| MEL140: > 70 years | > 70 years: 0 | 65–70 years: 45 | 65–70 years: 80% | 65–70 years: 96% | ||||||
| > 70 years: 58 | > 70 years: 53% | > 70 years: 100%. | ||||||||
| Belotti et al 201870 | 72 | 65–75 | NA | MEL200: 68% of patients | Single: 70% | 0 | NA | Median: 35.6 months | NA | |
| < MEL200: 32% of patients | Double: 30% | 65–69 years: 51.5 months; ≥ 70 years: 27.7 months | ||||||||
| Ghilardi et al 201871 | 388 | > 65 | 67.5 (65–77) | MEL200: 75.3% | Single: 88.7% | 1.5 | NA | Median: | Median: | |
| MEL70-MEL180: 24.7% | Double: 11.3% | MEL200: 1.4; MEL70-180: 2. | ≤ 70 years: 27.2 months; > 70 years: 21.5 months (p = NS) | ≤ 70 years: 82. 8 months | ||||||
| > 70 years: 56. 2 months (p = .02). | ||||||||||
| Marini et al 201972 | 29 | > 65 | 67 (66–70) | MEL200: 38% | NA | NA | 51 | Median: | NA | |
| MEL140: 62% | MEL200: 62 months; MEL100/140: 45 months | |||||||||
| Mizuno et al 201873 | 287 | ≥ 65 | 66 (65–76) | MEL200/140/100: | Single | < 65 years: 0.4 | NA | NA | 5-year: | |
| < 65 years: 95%/4%/l% of patient | ≥ 65 years: 1.2 | < 65 years: 63% | ||||||||
| ≥ 65 years: 76%/18%/6% of patients | ≥ 65 years 64% | |||||||||
| Saini et al 201874 | 9 | ≥ 80 | 81 (80–83) | MEL140 | Single | 0 | 100/45 | Median: 31.5 months | NR; 2-year 75% | |
Abbreviations: ASCT, autologous stem cell transplantation; CR, complete response; EFS, event-free survival; HCT-CI, Hematopoietic Cell Transplantation-Comorbidity Index; MEL, melphalan; NA, not applicable; nCR, near-complete response; NR, not reported; NS, nonsignificant; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TRM, treatment-related mortality.
Subsequent trials, however, showed that ASCT in older patients with MM was a feasible and effective procedure, with a low treatment-related mortality (TRM). In 2001, Badros et al75 initially reported a high TRM (16%) in patients older than age 70 receiving 200 mg/m2 of melphalan (MEL) as a conditioning regimen before ASCT. More recently, however, the TRM has been significantly lowered (< 5%) through the adoption of reduced MEL doses (140 mg/m2) and the improvement of patient selection and supportive care.75 As reported in some studies, older patients (>. 65 years) were at higher risk of prolonged hospitalization68 and post-ASCT complications (especially nonhematologic toxicities) than younger patients.69 This, however, did not translate into a higher TRM compared with younger patients, probably because of upfront dose reductions applied in most of the studies.65,66,68,69 A prospective, phase II study showed that bortezomib induction followed by tandem MEL (100 mg/m2), ASCT, lenalidomide/prednisone consolidation, and lenalidomide maintenance was highly effective and feasible in patients age 75 or younger. However, tandem transplant followed by consolidation and maintenance in patients older than age 70 experienced a significantly higher rate of TRM (19%) compared to younger patients (age ≤ 70, 5%).61
These data clearly showed that we need a careful assessment to identify patients for ASCT. All older adult patients should be evaluated for frailty with the IMWG frailty score or similar tool, as outlined in Table 1.16,70,76 In fit patients, performance status and organ function should be considered to better define the risk-benefit ratio of transplantation (Fig. 1). In the absence of prospective trials that clearly identify the optimal dose of MEL with ASCT, we cannot make definite recommendations on the dose of MEL in older adult patients. However, it seems reasonable to reduce the dose to 100–140 mg/m2 in the presence of the following: age older than 70, renal impairment, R-MCI of 4–6, or performance status less than 90% not related to MM. Novel combinations of therapy for patients with NDMM are numerous and are often adjusted on the basis of individual tolerance (Table 3).
FIGURE 1. Newly Diagnosed Patients With MM: Approach to Treatment.
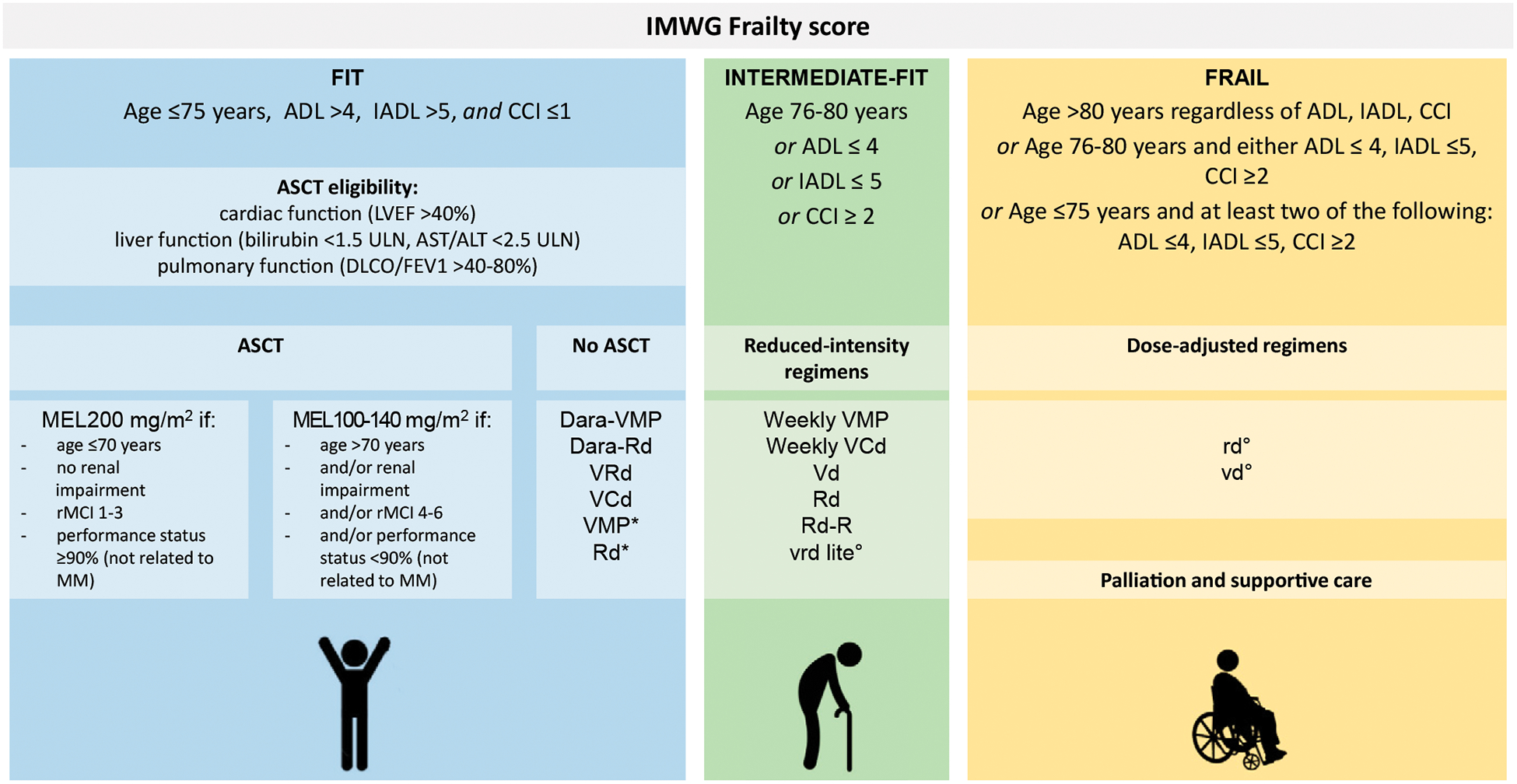
Abbreviations: ADL, activities of daily living; ALT, alanine aminotransferase; ASCT, autologous stem cell transplantation; AST, aspartate aminotransferase; CCI, Charlson comorbidity index; Dara, daratumumab; DLCO, diffusion capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; IADL, instrumental ADLs; IMWG, International Myeloma Working Group; LVEF, left ventricular ejection fraction; MEL, melphalan (with dosages in mg/m2); MM, multiple myeloma; Rd, lenalidomide and dexamethasone; Rd-R, lenalidomide and dexamethasone followed by lenalidomide maintenance; rMCI, revised myeloma comorbidity index; ULN, upper limit of normal; VCd, bortezomib, cyclophosphamide, dexamethasone; VMP, bortezomib, melphalan, and prednisone; VRd/vrd, bortezomib, lenalidomide, and dexamethasone. (*) If daratumumab-based combinations or VRd are unavailable. (°) The lowercase letter indicates a reduced dose.
TABLE 3.
Novel Combinations for the Treatment of Older, Newly Diagnosed Adult Patients With Multiple Myeloma
| Treatment | Schedule |
|---|---|
| VMP77 | Nine 6-week cycles: |
| V: 1.3 mg/m2 days 1, 4, 8, 11, 22, 25, 29, 32 (cycles 1–4); and days 1, 8, 22, 29 (cycles 5–9) | |
| M: 9 mg/m2 days 1–4 | |
| P: 60 mg/m2 days 1–4 | |
| VMP weekly78,79 | Nine 5-week cycles: |
| V: intravenously 1.3 mg/m2 days 1, 8, 15, 22 | |
| M: 9 mg/m2 orally days 1–4 | |
| P: 60 mg/m2 orally days 1–4 | |
| VCd80 | Four 4-week cycles: |
| V: 1.3 mg/m2 intravenously days 1, 4, 8, 11 | |
| C: 300 mg/m2 orally days 1, 8, 15, 22 | |
| d: 40 mg orally days 1–4, 9–12, and 17–20 | |
| Dara-VMP40,81 | Nine 6-week cycles: |
| Dara 16 mg/kg days 1, 8, 15, 22, 29, 36 of cycle 1 and days 1, 22 of cycles 2–9, then every 4 weeks thereafter | |
| V: 1.3 mg/m2 days 1, 4, 8, 11, 22, 25, 29, 32 of cycle 1 and days 1, 8, 22, 29 of cycles 2–9 | |
| M: 9 mg/m2 days 1–4 | |
| P: 60 mg/m2 days 1–4 | |
| Rd82,83 | 4-week cycles, until PD: |
| R: 25mg/day days 1–21 | |
| d: 40 mg days 1,8,15,22 | |
| Rd-R84 | Induction: nine 4-week cycles: |
| R: 25 mg/day days 1–21 | |
| d: 20 mg days 1, 8, 15, 22 | |
| Maintenance, until PD: | |
| R: 10 mg/day for 21 days until PD | |
| VRd50,51 | Induction: eight 3-week cycles: |
| V: 1.3 mg/m2 intravenously (push) days 1, 4, 8, 11 | |
| R: 25 mg/day days 1–14 | |
| d: 20 mg/day days 1, 2, 4, 5, 8, 9, 11, 12 | |
| Maintenance: 4-week cycles until PD: | |
| R: 25 mg/day days 1–21 | |
| d: days 1, 8, 15, 22 | |
| Vrd lite85 | Induction: 5-week cycle: |
| v: 1.3 mg/m2 days 1, 8, 15, 22 | |
| r: 15 mg days 1–21 | |
| d: 20 mg days 1, 2, 8, 9, 15, 16, 22, 23 for patients age ≤ 75 years; days 1, 8, 15, 22 for patients age > 75 years | |
| Consolidation: 6-week cycles: | |
| v: 1.3 mg/m2 days 1,15 | |
| r: 15 mg days 1–21 | |
| Dara-Rd86 | Until PD: |
| Dara: 16 mg/kg (intravenously) every week for cycles 1 and 2, every other week for cycles 3–6, every 4 weeks thereafter | |
| R: 25 mg (oral) daily days 1–21 | |
| d: 40 mg (oral) days 1, 8, 15, 22 |
Abbreviations: C, cyclophosphamide; d, dexamethasone; Dara, daratumumab; M, melphalan; P, prednisone; PD, progressive disease; R, lenalidomide; V, bortezomib.
Using the Surveillance, Epidemiology, and End Results-Medicare database, Shah et al87 compared the survival of and cost associated with 270 patients age 65 or older who received ASCT versus those of patients who did not. The mean overall cost of care for a patient who underwent transplantation was $299,554 versus $199,973 for a patient who did not, which was an increase of $99,581. Similarly, the mean survival was 4.94 years with ASCT compared with 3.57 years without ASCT, which meant a gain of 1.37 years with ASCT. The resultant incremental cost-effectiveness ratio was $72,852 per life-year gained. Given the commonly accepted threshold of $100,000 per life-year gained, ASCT for patients with MM who are older than 65 is cost-effective.88
The greater efficacy of the new combinations, including second-generation proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies, may still challenge the role of upfront ASCT, especially in older patients with MM.81,86,89 Two recent randomized trials showed that the addition of daratumumab to standard regimens, such as VMP or Rd, significantly halved the risk of death or progression (HR, 0.43, and HR, 0.56, respectively), inducing minimal residual disease negativity (the strongest predictor for prolonged OS) in one-fourth of patients.81,86 Similarly, preliminary results from a phase I/II study showed that carfilzomib combined with lenalidomide and dexamethasone provided a high rate of deep and durable responses (minimal residual disease negativity rate, 60%) with good tolerability. Impressively, after a median follow-up time of 30.5 months, four patients had experienced progression and none had died.90,91 Although comparisons among different settings are usually inappropriate, the preliminary results (especially in terms of response rate and PFS) achieved by older adults in these recent trials remarkably challenged those obtained by younger patients who underwent ASCT: the PFS with daratumumab/lenalidomide/dexamethasone (71% at 30 months)86 compared favorably with that of standard induction with bortezomib/lenalidomide/dexamethasone followed by ASCT.57
In conclusion, upfront ASCT still remains the standard of care in the context of a sequential approach that includes pre-transplantation induction and post-transplantation consolidation/maintenance with novel drugs in eligible patients. In older patients with MM, ASCT is feasible and cost-effective, showing that age per se should not be used as an exclusion criterion for ASCT. A thorough identification of potential ASCT-eligible patients and a careful evaluation of their fitness are essential to define the eligibility criteria for ASCT and the appropriate dose of MEL. Future clinical trials comparing ASCT-based versus non-ASCT-based approaches will help clarify whether newer regimens that combine immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies will eventually supersede ASCT in the treatment of older fit patients.
MANAGING HIGH-RISK MYELOMA IN OLDER PATIENTS
Defining High Risk
Within the myeloma literature, the term “high risk” is generally used in reference to disease biology, whereby intrinsic features of the malignant plasma cells make responses to therapy shorter, yielding shorter PFS and OS. However, viewed more broadly, high risk could refer to any patient subgroup who is at risk for poorer outcomes, even beyond disease-focused outcomes to include patient-focused outcomes (Fig. 2). In addition to disease factors, aging-associated vulnerabilities, including functional impairments and comorbidities, are associated with poorer outcomes as well.16,92 As outlined earlier, several scales have been developed, highlighting the impact of aging-associated deficits on outcomes.
FIGURE 2. Reconsidering High-Risk Myeloma: A Conceptual Framework for Older Adults.
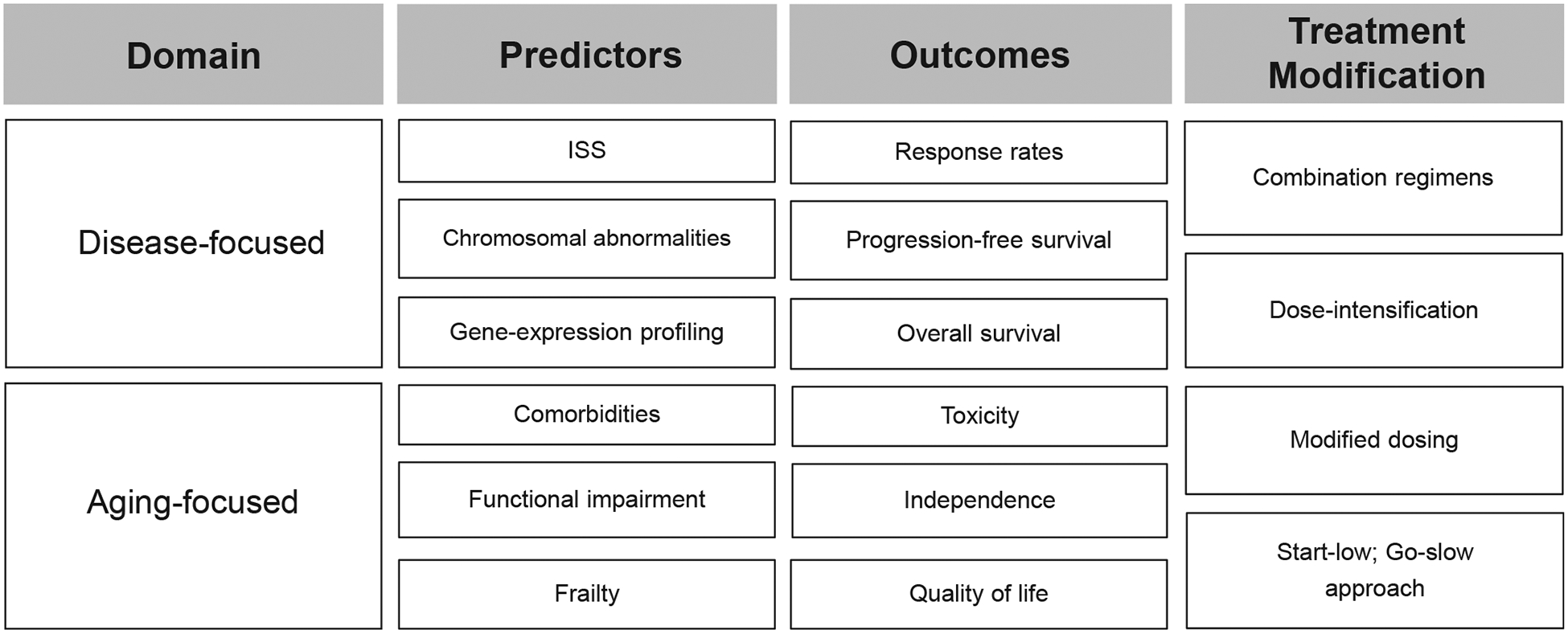
Abbreviation: ISS, international staging system.
The chromosomal abnormalities grouped to define a high-risk subgroup of MM currently refer in general to translocation (4;14), translocation (14;16), and deletion (17p), though the definition of high risk can vary by study. The prevalence of individual chromosomal abnormalities differs across the age spectrum. In a cohort of more than 1,800 older patients with myeloma, translocation (4;14) is actually less common in the oldest subgroup—present in 8.3% of patients older than age 75—compared with the group younger than age 65 (14.3%). The prevalence of deletion (17p) was consistent, at 6% across the age groups. The prevalence of translocation (14;16) was not presented in this study.93 The translocation (4;14) and deletion (17p) abnormalities were associated with poorer OS (HR 1.89; 95% CI, 1.28–2.80, p < .001; and 2.14; 95% CI, 1.39–3.28; p < .001, respectively). Numerous studies have confirmed the prognostic impact of high-risk chromosomal abnormalities in older adults with myeloma.42,82,94–96
Regarding disease-focused stratification system, the revised-ISS (R-ISS) incorporates serum albumin, β2-microglobulin, and lactate dehydrogenase levels along with high-risk chromosomal abnormalities.92,96 Although disease-related factors like theR-ISS stage and chromosomal abnormalities can influence outcomes in older adults with myeloma, so too can patient-related factors. In an analysis of 490 older patients treated on two clinical trials, investigators examined factors associated with disease-related death within 2 years of diagnosis, which occurred in 13.8% of patients.97 After deaths as a result of other factors were excluded, on multivariable analysis, four factors were associated with increased risk of early death as a result of disease progression: elevated serum lactate dehydrogenase (odds ratio, 3.8; 95% CI, 1.9–7.9), high-risk chromosomal abnormalities of translocation (4;14), translocation (14;16), and/or deletion (17p) (odds ratio, 2.6; 95% CI, 1.4–5.0), ISS stage III (odds ratio, 2.1; 95% CI, 1.2–3.9), and age older than 75 (odds ratio, 1.9; 95% CI, 1.0–3.5). As discussed earlier, age in this setting likely serves as a surrogate for aging-associated deficits. The R-MCI includes both aging-associated domains and chromosomal abnormalities, highlighting that neither exist in isolation.18 An analysis of more than 3,800 patients with myeloma showed that chromosomal abnormalities actually had a decreasing impact on outcomes across the age spectrum, whereas performance status remained of similar relevance across age subgroups.98
It is also relevant to consider what outcomes the individual at high risk is at risk for. Traditional approaches have focused on inferior PFS and OS.99 The IMWG frailty model examined toxicity and treatment discontinuation as outcomes affected by frailty.16 However, the outcomes of greatest importance to older adults may not be disease specific. Seminal work by Fried et al100 completed nearly 2 decades ago showed that older adults prioritized maintaining independence in function and preservation of cognition instead of longer survival. This work was recently confirmed in a study of older adults with malignancies, in which 58% prioritized maintaining the independence instead of survival, and 80% prioritized maintaining cognition instead of survival.101 A recent latent-class analysis of preferences in patients with myeloma (half of whom were older than age 65) showed that a subgroup prioritized avoiding peripheral neuropathy, by nearly two-fold, instead of longer PFS.102 It is of particular interest that, by definition, grade 2 and grade 3 neuropathy cause dependence in IADL and ADL, respectively. To date, myeloma studies have largely focused on survival-oriented outcomes, and these results indicate that research on functional outcomes is greatly needed.
Treatment Based on Chromosomal Abnormalities
Defining the optimal treatment of subgroups of older patients with high-risk disease on the basis of chromosomal abnormalities has been challenging because of the relative rarity of each abnormality. To date, no published clinical trial for older adults has exclusively been designed for patients with high-risk disease. Clinicians must extrapolate from subgroup analyses and observations applied from younger cohorts to determine the optimal therapy for an older patient with high-risk disease on the basis of myeloma biology.
Interest in carfilzomib as a drug of choice for patients with high-risk disease has been piqued by the efficacy of carfilzomib in these patients. The strongest evidence for this is in the relapsed setting, in studies that enrolled patients across the age spectrum rather than confined the research to older or transplantation-ineligible patients. Carfilzomib with dexamethasone was superior to bortezomib and dexamethasone in patients with high-risk relapsed/refractory myeloma (PFS: HR 0.65; 95% CI, 0.45–0.92; p = .0075).103 In the randomized trial of carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone, the triplet improved PFS compared with the doublet in patients with high-risk relapsed/refractory myeloma (HR 0.70; 95% CI, 0.43–1.16).104 However, a randomized trial of carfilzomib, melphalan, prednisone (KMP) versus VMP for initial therapy in older adults with myeloma demonstrated similar PFS times (22.3 months with KMP vs. 22.1 months with VMP).105 The overall response rate favored KMP (84.3% with KMP vs. 78.8% with VMP; odds ratio, 1.41; 95% CI, 1.09–1.97); although the rates of grade 3 or greater adverse events were similar (74.7% with KMP vs. 76.2% with VMP), renal failure, cardiac toxicity, dyspnea, and hypertension were more frequent in the KMP group. Subgroup analysis by risk group has not been reported, but the lack of PFS benefit in the overall study makes a benefit in the high-risk subset less likely. The reason for the difference in PFS outcomes between this study and those mentioned earlier with regard to the role of carfilzomib needs more clarification, so the role for carfilzomib in the initial treatment of older adults with high-risk myeloma remains to be defined.
As detailed in a recent review by Avet-Loiseau and Facon,98 no study focused on older adults has demonstrated a sustained, consistent survival benefit in patients with high-risk myeloma. Outcomes in the high-risk subgroups from selected studies of initial therapy in older adults with high-risk myeloma are presented in Table 4. Overall, the longest PFS in a high-risk subgroup was reported in S0777 with the combination of bortezomib, lenalidomide, and dexamethasone; notably, however, this study was in a substantially younger population than the other relevant studies.50 Lenalidomide, bortezomib, dexamethasone (RVD)-lite is a regimen modified to improve the tolerability of this triplet in older adults, and it has an excellent response rate (86%) and a low rates of discontinuation of therapy-associated toxicity (4%).85 However, the number of individuals with high-risk disease in the phase II study was too small to draw conclusions about the subgroup (personal communication, February 2019). An IMWG consensus panel analyzed the available evidence and concluded that patients with high-risk myeloma should be treated with a combination of a proteasome inhibitor and lenalidomide or pomalidomide along with dexamethasone. Similarly, when they focused on studies designed for older adults with myeloma, Avet-Loiseau and Facon98 concluded that older adults with high-risk cytogenetics would benefit from regimens that combine novel agents.
TABLE 4.
Outcomes in High-Risk Subgroups: Results From Selected Recent Trials for Transplantation-Ineligible Patients With Myeloma
| Study or First Author | Proportion of Entire Study Population Age 65 Years or Older (%) | Treatment Arm | Median Progression-Free Survival in High-Risk Subgroup (months) | Median Overall Survival in High-Risk Subgroup (months) |
|---|---|---|---|---|
| Mateos et al40 | 91.5 | VMP-dara | 18.0 | Not reported |
| VMP | 18.1 | Not reported | ||
| SWOG S077750 | 43 | RVD | 38 | Not reported |
| Rd | 16 | Not reported | ||
| FIRST trial79,83 | 94.3 | Rd continuous | 8.4 | 29.3 |
| Rd-18 | 17.5 | 24.3 | ||
| MPT | 14.6 | 35.5 | ||
| VISTA trial39,77 | 96.6 | VMP | Not reported | 40.0 |
| Zweegman et al42 | 33–34 (≥ age 75) | MPR-R with +(1q21) | 19 | 50 |
| MPR-R with del (17p) | 15 | 35 | ||
| MPR-R with t(4; 14) | 14 | Not reached | ||
| MPR-R with +(1q21) | 17 | 30 | ||
| MPT-T with (17p) | 15 | 41 | ||
| MPT-T with t(4; 14) | 12 | 23 |
Abbreviations: D, dexamethasone; dara, daratumumab; M, melphalan; P, prednisone; R, lenalidomide, T, thalidomide; V, bortezomib.
Treatment of Myeloma in a Vulnerable Patient
In the patient-centered view of high risk, focused on agingassociated vulnerabilities rather than on myeloma biology, adverse outcomes for which the patient may be at risk include early death, toxicity of therapy, functional decline, and other outcomes of interest in older adults. In a pooled analysis of more than 1,100 older patients enrolled in clinical trials and treated with novel agents, early death rates were lower than historical comparisons, but deaths as a result of toxicity were still nearly as likely as deaths as a result of disease progression (4.1% vs. 4.4%).45 Of toxicity-related deaths during first-line of therapy, 28% were caused by cardiac complications; 26%, infections; and 15%, vascular complications. On multivariable analysis, age and ISS stage were associated with increased risk of toxic death. Whether modifying treatment according to frailty improves outcomes is not yet known. In solid tumor oncology, there are well-validated measures to estimate the risk of toxicity of systemic therapy in older adults, and predictors include falls, hearing impairment, IADL dependence, nutritional compromise, and cognitive impairment.106–108 This approach will be tested in the MRC XIV Fitness Trial, wherein 740 patients will be randomly assigned to standard treatment with lenalidomide, ixazomib, and dexamethasone or to treatment dosing adapted to their level of frailty (NCT03720041).
Associations between chronologic age and adverse outcomes imply that age serves as a surrogate for aging-associated vulnerabilities. Efforts are underway to define which specific geriatric impairments increase an individual’s risk for treatment toxicity. In the myeloma literature, efforts are ongoing, and several recommended approaches to modifying treatment according to frailty scores, comorbidities and functional impairments have been published.76,109 In conclusion, personalized decisions based on disease-related characteristics in conjunction with age-related factors will continue to affect survivorship for older adults with MM. Scarce data exist about octogenarians with MM, but investigations demonstrate similar PFS but inferior OS compared with younger patients. ASCT use in older adults is the standard of care, is feasible, and is cost-effective. Future clinical trials focusing on older adults with high-risk myeloma is an unmet need. Whether modifying treatment according to frailty improves outcomes is not yet known, but it is clearly worth exploring.
PRACTICAL APPLICATIONS.
Evaluate metrics of frailty using geriatric variables and assessment tools for older adults with multiple myeloma, including octogenarians with multiple myeloma.
Explore autologous stem cell transplantation use, risks, and benefits in older adults with multiple myeloma.
Identify high-risk populations and review novel combinations of therapy with adjustment based on individual tolerance.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Disclosures provided by the authors and data availability statement (if applicable) are available with this article at DOI https://doi.org/10.1200/EDBK_239067.
REFERENCES
- 1.Noone AMHN, Krapcho M, Miller D, et al. SEER cancer statistics review, 1975–2015. https://seer.cancer.gov/csr/1975_2015/. Accessed February 1, 2018. [Google Scholar]
- 2.Turesson I, Velez R, Kristinsson SY, et al. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol. 2010;28:830–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajkumar SV. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93:981–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa LJ, Huang JX, Hari PN. Disparities in utilization of autologous hematopoietic cell transplantation for treatment of multiple myeloma. Biol Blood Marrow Transplant. 2015;21:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa LJ, Brill IK, Omel J, et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017; 1:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren JL, Harlan LC, Stevens J, et al. Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. J Clin Oncol. 2013;31:1984–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orme J, Go RS, Kumar SK, et al. Disparities in outcomes of patients with multiple myeloma based on geographic distance from NCI-designated cancer centers: a SEER-based analysis. Blood. 2017;130 (suppl; abstr 4689). [Google Scholar]
- 9.Vogl DT, Delforge M, Song KW, et al. Health-related quality of life over time in transplant ineligible patients with newly diagnosed multiple myeloma treated with lenalidomide and dexamethasone until progression. J Clin Oncol. 2016;34 (suppl; abstr 8047). [Google Scholar]
- 10.Wagner I, Dune BG, Jagannath S, et al. Health-related quality of life assessments predict relapse or death in patients with newly diagnosed multiple myeloma (MM): results from the Connect MM registry. Value Health. 2018;21:S6–S6. [Google Scholar]
- 11.Weinberg M Geriatrics: a definition. J Am Geriatr Soc. 1957;5:385–391. [DOI] [PubMed] [Google Scholar]
- 12.Devons CA. Comprehensive geriatric assessment: making the most of the aging years. Curr Opin Clin Nutr Metab Care. 2002;5:19–24. [DOI] [PubMed] [Google Scholar]
- 13.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockwood K and Frailty its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. [DOI] [PubMed] [Google Scholar]
- 15.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleber M, Ihorst G, Terhorst M, et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J. 2011;1:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelhardt M, Domm AS, Dold SM, et al. A concise revised myeloma comorbidity index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.dela Rubia J, Gonzalez BJ, Hernández Rivas JA, et al. GAH scale is a simple, comprehensive assessment tool in older patients with hematological malignancies that shows mortality prediction capacities. Clin Lymphoma Myeloma Leuk. 2015;15:e99. [Google Scholar]
- 20.Bonanad S, De la Rubia J, Gironella M, et al. ; GAH Group. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: the GAH scale. J Geriatr Oncol. 2015;6:353–361. [DOI] [PubMed] [Google Scholar]
- 21.Schutz N, Smietniansky M, Fantl D, et al. : Frailty and mortality in elderly patients with multiple myeloma. https://learningcenter.ehaweb.org/eha/2017/22nd/181040/natalia.schutz.frailty.and.mortality.in.elderly.patients.with.multiple.myeloma.html. [Google Scholar]
- 22.Wildes TM, Tuchman SA, Klepin HD, et al. Geriatric assessment in older adults with multiple myeloma. J Am Geriatr Soc. 2018;jgs.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosko AE, Huang Y, Benson DM, et al. Use of a comprehensive frailty assessment to predict morbidity in patients with multiple myeloma undergoing transplant. J Geriatr Oncol. 2018;S1879–4068(18)30157–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mian HS, Wildes TM, Fiala MA. Development of a Medicare health outcomes survey deficit-accumulation frailty index and its application to older patients with newly diagnosed multiple myeloma. JCO Clin Cancer Inform. 2018;2 Epub 2018 July 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murillo A, Cronin AM, Laubach JP, et al. Performance of the International Myeloma Working Group myeloma frailty score among patients 75 and older. J Geriatr Oncol. 2018;S1879–4068(18)30343–6. [DOI] [PubMed] [Google Scholar]
- 26.Kleber M, Ihorst G, Gross B, et al. Validation of the Freiburg comorbidity index in 466 multiple myeloma patients and combination with the International Staging System are highly predictive for outcome. Clin Lymphoma Myeloma Leuk. 2013;13:541–551. [DOI] [PubMed] [Google Scholar]
- 27.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010; 60:120–132. [DOI] [PubMed] [Google Scholar]
- 28.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. [DOI] [PubMed] [Google Scholar]
- 29.Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy: a systematic review. Leuk Res. 2014;38:275–283. [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 31.United Nations. Population division: world population prospects 2017. https://population.un.org/wpp/.
- 32.Vélez R, Turesson I, Landgren O, et al. Incidence of multiple myeloma in Great Britain, Sweden, and Malmö, Sweden: the impact of differences in case ascertainment on observed incidence trends. BMJ Open. 2016;6:e009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowsky DJ, Olshansky SJ, Bhattacharya J, et al. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stege CAM, Nasserinejad K, Levin M-D, et al. Geriatric impairments and low muscle mass are associated with treatment discontinuation and overall survival in newly diagnosed non-transplant eligible multiple myeloma patients (nte-NDMM) treated with dose-adjusted melphalan-prednisone-bortezomib (MPV)—results of the Dutch HOVON 123 study. Blood. 2018;132:1889–1889.30209119 [Google Scholar]
- 35.Soto-Perez-de-Celis E, Li D, Yuan Y, et al. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19:e305–e316. [DOI] [PubMed] [Google Scholar]
- 36.Yourman LC, Lee SJ, Schonberg MA, et al. Prognostic indices for older adults: a systematic review. JAMA. 2012;307:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz M, Covinsky K, Widera EW, et al. Predicting 10-year mortality for older adults. JAMA. 2013;309:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulin C, Belch A, Shustik C, et al. Updated outcomes and impact of age with lenalidomide and low-dose dexamethasone or melphalan, prednisone, and thalidomide in the randomized, phase III FIRST trial. J Clin Oncol. 2016;34:3609–3617. [DOI] [PubMed] [Google Scholar]
- 39.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–2266. [DOI] [PubMed] [Google Scholar]
- 40.Mateos MV, Dimopoulos MA, Cavo M, et al. ; ALCYONE Trial Investigators. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378:518–28. [DOI] [PubMed] [Google Scholar]
- 41.Larocca A, Bringhen S, Petrucci MT, et al. A phase 2 study of three low-dose intensity subcutaneous bortezomib regimens in elderly frail patients with untreated multiple myeloma. Leukemia. 2016;30:1320–1326. [DOI] [PubMed] [Google Scholar]
- 42.Zweegman S, van der Holt B, Mellqvist UH, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127:1109–1116. [DOI] [PubMed] [Google Scholar]
- 43.Zweegman S, Schjesvold FH, van der Holt B, et al. Ixazomib-thalidomide-low dose dexamethasone (ITd) induction followed by maintenance therapy with ixazomib or placebo in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplantation: results from the randomized phase II HOVON-126/Nmsg 21.13 trial. Blood. 2018;132:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1,435 individual patient data from 4 randomized trials. Haematologica. 2013;98:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bringhen S, Offidani M, Palmieri S, et al. Early mortality in myeloma patients treated with first-generation novel agents thalidomide, lenalidomide, bortezomib at diagnosis: a pooled analysis. Crit Rev Oncol Hematol. 2018;130:27–35. [DOI] [PubMed] [Google Scholar]
- 46.Gavriatopoulou M, Fotiou D, Koloventzou U, et al. Vulnerability variables among octogenerian myeloma patients: a single-center analysis of 110 patients. Leuk Lymphoma. Epub 2019 Jan 10. [DOI] [PubMed] [Google Scholar]
- 47.Shah JJ, Abonour R, Gasparetto C, et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin Lymphoma Myeloma Leuk. 2017;17:575–583.e2. [DOI] [PubMed] [Google Scholar]
- 48.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. [DOI] [PubMed] [Google Scholar]
- 49.Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015; 16:1617–1629. [DOI] [PubMed] [Google Scholar]
- 50.Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017; 389:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durie BG, Hoering A, Sexton R, et al. Longer term follow up of the randomized phase III Trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem transplant Presented at: 60th Annual Meeting of the American Society of Hematology San Diego, CA; 2018. Abstract 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavo M, Gay FM, Patriarca F, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/HO95 study. Blood. 2017;130:401. [Google Scholar]
- 53.Cavo M, Hajek R, Pantani L, et al. Autologous stem cell transplantation versus bortezomib-melphalan-prednisone for newly diagnosed multiple myeloma: second interim analysis of the phase 3 EMN02/HO95 study. Blood. 2017;130:397.28576879 [Google Scholar]
- 54.Morgan GJ. Transplants for the elderly in myeloma. Blood. 2013;122:1332–1334. [DOI] [PubMed] [Google Scholar]
- 55.Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125:3076–3084. [DOI] [PubMed] [Google Scholar]
- 56.Auner HW, Szydlo R, Hoek J, et al. Trends in autologous hematopoietic cell transplantation for multiple myeloma in Europe: increased use and improved outcomes in elderly patients in recent years. Bone Marrow Transplant. 2015;50:209–215. [DOI] [PubMed] [Google Scholar]
- 57.Moreau P, San Miguel J, Sonneveld P, et al. ; ESMO Guidelines Committee. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv52–iv61. [DOI] [PubMed] [Google Scholar]
- 58.Kumar SK, Callander NS, Alsina M, et al. Multiple myeloma, version 3.2017: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017; 15:230–269. [DOI] [PubMed] [Google Scholar]
- 59.Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052–3057. [DOI] [PubMed] [Google Scholar]
- 60.Facon T, Mary JY, Hulin C, et al. ; Intergroupe Francophone du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370:1209–1218. [DOI] [PubMed] [Google Scholar]
- 61.Gay F, Magarotto V, Crippa C, et al. Bortezomib induction, reduced-intensity transplantation, and lenalidomide consolidation-maintenance for myeloma: updated results. Blood. 2013;122:1376–1383. [DOI] [PubMed] [Google Scholar]
- 62.Garderet L, Beohou E, Caillot D, et al. Upfront autologous stem cell transplantation for newly diagnosed elderly multiple myeloma patients: a prospective multicenter study. Haematologica. 2016;101:1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Straka C, Liebisch P, Salwender H, et al. Autotransplant with and without induction chemotherapy in older multiple myeloma patients: long-term outcome of a randomized trial. Haematologica. 2016;101:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nadiminti K, Strouse C, Vikas P, et al. A single autologous stem cell transplant (ASCT) followed by two years of post-transplant therapy in recently diagnosed elderly multiple myeloma (MM) patients: safety and response results from the prospective phase II Trial (NCT01849783) Presented at: 60th Annual Meeting of the American Society of Hematology. San Diego, CA; 2018. Abstract 2153. [Google Scholar]
- 65.Bashir Q, Shah N, Parmar S, et al. Feasibility of autologous hematopoietic stem cell transplant in patients aged ≥ 70 years with multiple myeloma. Leuk Lymphoma. 2012;53:118–122. [DOI] [PubMed] [Google Scholar]
- 66.Merz M, Neben K, Raab MS, et al. Autologous stem cell transplantation for elderly patients with newly diagnosed multiple myeloma in the era of novel agents. Ann Oncol. 2014;25:189–195. [DOI] [PubMed] [Google Scholar]
- 67.Ozaki S, Harada T, Saitoh T, et al. ; Japanese Society of Myeloma; European Myeloma Network. Survival of multiple myeloma patients aged 65–70 years in the era of novel agents and autologous stem cell transplantation: a multicenter retrospective collaborative study of the Japanese Society of Myeloma and the European Myeloma Network. Acta Haematol. 2014;132:211–219. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez L, Sylvester M, Parrondo R, et al. In-hospital mortality and post-transplantation complications in elderly multiple myeloma patients undergoing autologous hematopoietic stem cell transplantation: a population-based study. Biol Blood Marrow Transplant. 2017;23:1203–1207. [DOI] [PubMed] [Google Scholar]
- 69.Stettler J, Novak U, Baerlocher GM, et al. Autologous stem cell transplantation in elderly patients with multiple myeloma: evaluation of its safety and efficacy. Leuk Lymphoma. 2017;58:1076–1083. [DOI] [PubMed] [Google Scholar]
- 70.Belotti A, Ribolla R, Cancelli V, et al. The IMWG frailty score and age can be more effective than clinical judgement in the selection of elderly patients with newly diagnosed multiple myeloma who can benefit from an intensive treatment approach with high dose melphalan and autologous stem cell transplantation Presented at: 60th Annual Meeting of the American Society of Hematology San Diego, CA; 2018. Abstract 2151. [Google Scholar]
- 71.Ghilardi G, Pabst T, Jeker B, et al. ; Swiss Blood Stem Cell Transplantation Registry. Melphalan dose in myeloma patients ≥ 65 years of age undergoing high-dose therapy and autologous stem cell transplantation: a multicentric observational registry study. Bone Marrow Transplant. Epub 2018 Nov 2. [DOI] [PubMed] [Google Scholar]
- 72.Marini C, Maia T, Bergantim R, et al. Real-life data on safety and efficacy of autologous stem cell transplantation in elderly patients with multiple myeloma. Ann Hematol. 2019;98:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizuno S, Kawamura K, Hanamura I, et al. Efficacy and safety of autologous stem cell transplantation in elderly patients with multiple myeloma in the era of novel agents Presented at: 60th Annual Meeting of the American Society of Hematology San Diego, CA; 2018. Abstract 3437. [Google Scholar]
- 74.Saini NY, Patel R, Varma A, et al. Melphalan-based autologous transplantation in the octogenarian multiple myeloma patient populationPresented at: 60th Annual Meeting of the American Society of Hematology San Diego, CA; 2018. Abstract 4608. [Google Scholar]
- 75.Badros A, Barlogie B, Siegel E, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol. 2001;114:600–607. [DOI] [PubMed] [Google Scholar]
- 76.Larocca A, Dold SM, Zweegman S, et al. Patient-centered practice in elderly myeloma patients: an overview and consensus from the European Myeloma Network (EMN). Leukemia. 2018;32:1697–1712. [DOI] [PubMed] [Google Scholar]
- 77.San Miguel JF, Schlag R, Khuageva NK, et al. ; VISTA Trial Investigators. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. [DOI] [PubMed] [Google Scholar]
- 78.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. [DOI] [PubMed] [Google Scholar]
- 79.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32:634–640. [DOI] [PubMed] [Google Scholar]
- 80.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dimopoulos MA, Mateos M-V, Cavo M, et al. One-year update of a phase 3 randomized study of daratumumab plus bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) in patients (pts) with transplant-ineligible newly diagnosed multiple myeloma (NDMM): ALCYONE Presented at: 60th Annual Meeting of the American Society of Hematology San Diego, CA; 2018. Abstract 156. [Google Scholar]
- 82.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. ; FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906–917. [DOI] [PubMed] [Google Scholar]
- 83.Facon T, Dimopoulos MA, Dispenzieri A, et al. : Final analysis of survival outcomes in the randomized phase 3 FIRST trial. BlooE 2018;131:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larocca A, Salvini M, De Paoli L, et al. Efficacy and feasibility of dose/schedule-adjusted Rd-R vs. continuous Rd in elderly and intermediate-fit newly diagnosed multiple myeloma (NDMM) patients: RV-MM-PI-0752 phase III randomized study Presented at: 60th Annual Meeting of the American Society of Hematology San Diego, CA; 2018. Abstract 305. [Google Scholar]
- 85.O’Donnell EK, Laubach JP, Yee AJ, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol. 2018;182:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Facon T, Kumar SK, Plesner T, et al. Phase 3 randomized study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA) Presented at: 60th Annual Meeting of the American Society of Hematology San Diego, CA; 2018. Abstract LBA-2. [Google Scholar]
- 87.Shah GL, Winn AN, Lin P-J, et al. Cost-effectiveness of autologous hematopoietic stem cell transplantation for elderly patients with multiple myeloma using the Surveillance, Epidemiology, and End Results–Medicare database. Biol Blood Marrow Transplant. 2015;21:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014; 371:796–797. [DOI] [PubMed] [Google Scholar]
- 89.Gay FM, Scalabrini DR, Belotti A, et al. Carfilzomib-lenalidomide-dexamethasone (KRd) vs carfilzomib-cyclophosphamide-dexamethasone (KCd) induction: planned interim analysis of the randomized FORTE trial in newly diagnosed multiple myeloma (NDMM). J Clin Oncol. 2017;35(suppl; abstr 8003). [Google Scholar]
- 90.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dytfeld D, Jasielec J, Griffith KA, et al. Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engelhardt M, Dold SM, Ihorst G, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101:1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the Intergroupe Francophone du Myélome experience. J Clin Oncol. 2013;31:2806–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacobus SJ, Kumar S, Uno H, et al. Impact of high-risk classification by FISH: an Eastern Cooperative Oncology Group (ECOG) study E4A03. Br J Haematol. 2011;155:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mateos MV, Oriol A, Martínez-López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934–941. [DOI] [PubMed] [Google Scholar]
- 96.Jimenez-Zepeda VH, Duggan P, Neri P, et al. Bortezomib-containing regimens (BCR) for the treatment of non-transplant eligible multiple myeloma. Ann Hematol. 2017;96:431–439. [DOI] [PubMed] [Google Scholar]
- 97.Rodríguez-Otero P, Mateos MV, Martínez-López J, et al. Early myeloma-related death in elderly patients: development of a clinical prognostic score and evaluation of response sustainability role. Leukemia. 2018;32:2427–2434. [DOI] [PubMed] [Google Scholar]
- 98.Pawlyn C, Kaiser MF, Cairns D, et al. Factors predicting poor outcomes for myeloma patients at different ages: results from 3,894 patients in the myeloma XI trial. Blood. 2017;130:3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Avet-Loiseau H, Facon T. Front-line therapies for elderly patients with transplant-ineligible multiple myeloma and high-risk cytogenetics in the era of novel agents. Leukemia. 2018;32:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. [DOI] [PubMed] [Google Scholar]
- 101.De Celis ESP, Li DN, Sun CL, et al. Patient- defined goals and preferences among older adults with cancer starting chemotherapy (CT). J Clin Oncol. 2018;36 (suppl; abstr 10009). [Google Scholar]
- 102.Auclair D, Mansfield C, Chari A, et al. Understanding the preferences of patients and caregivers for relapsed/refractory multiple myeloma treatment: a mixed-mode patient-centric approach. Blood. 2017;130:5662. [Google Scholar]
- 103.Chng WJ, Goldschmidt H, Dimopoulos MA, et al. Carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia. 2017;31:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Avet-Loiseau H, Fonseca R, Siegel D, et al. Carfilzomib significantly improves the progression-free survival of high-risk patients in multiple myeloma. Blood 2016;128:1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Facon T, Lee JH, Moreau P, et al. Phase 3 study (CLARION) of carfilzomib, melphalan, prednisone (KMP) v bortezomib, melphalan, prednisone (VMP) in newly diagnosed multiple myeloma (NDMM). Clin Lymph, Myeloma Leuk. 2017;17:e26–e27. [Google Scholar]
- 106.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016; 34:2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 109.Zweegman S, Engelhardt M, Larocca A; EHA SWG on Aging and Hematology. Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol. 2017;29:315–321. [DOI] [PubMed] [Google Scholar]


