Abstract
Background/Objectives:
We hypothesized that physical activity (PA) improves insulin sensitivity in adolescents with severe obesity beyond that attributable to metabolic bariatric surgery (MBS).
Subjects/Methods:
StepWatch™ monitors objectively measured PA in 88 participants in the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. Primary outcomes included absolute change in fasting insulin, HOMA-IR, and fasting glucose from pre-surgery (baseline) to 6, 12, 24, and 36 months post-MBS. SAS PROC TRAJ generated activity trajectories based on probability and individual participant step count trajectories. Linear regression models were used, adjusted for baseline value, visit, surgical procedure, sex, and percent change in BMI. Additional models adjusted for percent change in iliac waist circumference (IWC) or percent body fat (BF), measured by bio-impedance.
Results:
Two activity trajectories were identified: more active (MA, n=13) and less active (LA, n=75). MA baseline mean daily step count was >6 000, increasing to > 9 000 at 2 years. LA mean daily step count remained at approximately 4 000. Few participants recorded moderate step activity (cadence >80 steps/minute). Still, fasting insulin and HOMA-IR differed in association with activity trajectory. MA was associated with a greater absolute decrease in fasting insulin (−7.8μU/ml [95% CI: (−11.8, −3.7)], p =<0.001) and a greater decrease in HOMA- IR (−1.9 [95% CI: (−3.0, −0.7)], p = 0.001), when adjusted for percent change in BMI. The significant independent effect of MA remained when adjusted for percent change in IWC or percent BF. Clinically, 100% of MA trajectory participants normalized fasting insulin, HOMA-IR and fasting glucose by 6 months and normalization remained throughout the 36 month follow up. In contrast, 76.3% and 65.8% of LA trajectory participants normalized fasting insulin and HOMA-IR, respectively, by 12 months with 28.6% of both remaining normalized at 36 months.
Conclusion:
PA is independently associated with improved insulin sensitivity beyond that attributable to MBS in adolescents with severe obesity.
Keywords: physical activity, bariatric surgery, insulin sensitivity, severe obesity, adolescent
INTRODUCTION
Obesity is strongly associated with insulin resistance (1). Adolescents with severe obesity and insulin resistance are at increased risk of developing type 2 diabetes (T2D) and cardiovascular disease (CVD) (2). β-cell function declines up to 35% each year in adolescents with insulin resistance, two-to-four-times faster than in adults (2). Metabolic bariatric surgery (MBS) effectively normalizes hyperinsulinemia and induces remission of T2D in association with weight loss in adults (3) and adolescents with severe obesity (4).
Physical activity (PA) and fitness improve insulin sensitivity, while inactivity promotes insulin resistance (5). Moreover, insulin resistance can impair PA tolerance and fitness (6). Adolescents with severe obesity are more sedentary and achieve 50% fewer steps per day than adolescents of healthy weight (7, 8). While MBS is associated with improved insulin sensitivity, it is unclear whether PA further improves insulin sensitivity beyond that associated with surgery alone. We therefore dichotomized adolescents undergoing MBS by daily PA into trajectory groups of more (MA) or less (LA) daily PA, to test the hypothesis that those with higher levels of PA would demonstrate greater insulin sensitivity.
MATERIALS/SUBJECTS/METHODS
Design
This analysis examined associations between objectively measured PA and changes in clinical measures of insulin sensitivity from baseline to 6, 12, 24, and 36 months post-MBS in adolescents with severe obesity. PA was measured as daily step counts using StepWatch™ (CYMA Corp., Mountlake Terrace, Washington). The methods used for data collection have been previously described (9). Also, the influence of objectively measured PA on lipid measures and improved weight loss and weight maintenance in this population have previously been reported (10). The original Teen-LABS protocol and data and safety monitoring plans were approved by the institutional review board (IRB) at each of the five participating institutions and by the NIH appointed data and safety monitoring board. All participants and/or their legally-authorized representatives provided informed, written consent. The analysis plan was also reviewed and approved by the IRB at the University of Colorado, Anschutz Medical Campus.
Participants
Eighty-eight adolescent participants from the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study met inclusion criteria for this analysis. Inclusion criteria were: 1) StepWatch™ worn for a minimum of 3 days (2 weekdays and 1 weekend day) to objectively measure PA (9), 2) at least 6 hours of daily wear time with at least 10 steps per hour per day of recorded data, 3) available PA data at baseline and at least one postoperative time point (6 months, 1 year, 2 years or 3 years), 4) time point-matched laboratory and anthropometric data, and 5) participant underwent either vertical sleeve gastrectomy (VSG) or Roux-en-Y gastric bypass (RYGB). Participants were excluded if they reported taking metformin or insulin, or had a current or previous diagnosis of diabetes.
Outcomes of interest
The primary outcomes of interest were measures of change in insulin sensitivity, including HOMA-IR, fasting insulin, and fasting glucose, over time from baseline to 6, 12, 24, and 36 months. HOMA-IR was calculated as: fasting insulin (μU/ml) x fasting plasma glucose (mg/dl)/405. The exploratory outcome of interest was change in resting heart rate (RHR). Activity trajectory membership (i.e., more activity [MA] or less activity [LA]) was the independent variable of interest and covariates chosen a priori included the baseline value of each outcome, sex, race, visit, surgical procedure, and a measure of adiposity: iliac waist circumference (IWC), BMI, or % body fat (%BF) as measured by bioelectric impedance (Tanita Body Composition Analyzer TBF-310, Manchester, UK).
Statistical Analysis
SAS v9.4 (SAS Institute Inc., Cary, NC, USA) PROC TRAJ was used to determine the optimal number of categorical activity trajectories and their respective shape (e.g., linear vs. quadratic) based on average daily step counts at each visit, as per Andruff et al. (11). Using this model, two categories were identified that we label “less active” (LA) and “more active” (MA), that were linear and quadratic in shape, respectively. The probability of group trajectory membership for each individual was determined from the individual’s trajectory of average step counts across all available time points (10). The analyses examined insulin sensitivity outcomes and anthropometric outcomes longitudinally from baseline to 6, 12, 24 and 36 months post-MBS. Descriptive summaries included mean and standard deviation for continuous measures and count and percent of total for discrete measures. Baseline comparisons between activity categories were made using two-sample t-tests for continuous outcomes and two-sample tests of proportions for dichotomous outcomes.
Analysis of change in outcomes by activity trajectory categories used linear regression with generalized estimating equations, assuming an exchangeable working correlation structure to account for the longitudinal, correlated nature of the data to estimate the change from baseline to a measured interval or visit. Changes in outcomes from baseline were fit, adjusting for percent change from baseline to a given visit for both IWC and BMI, as independent covariates of weight loss in order to examine the impact of obesity (as BMI) and central obesity (as IWC). Additionally, body composition as percent body fat (% BF) was considered as an independent covariate of weight loss for fasting insulin and HOMA-IR. The model also included covariates for baseline value of the outcome, sex, surgical procedure type, race/ethnicity (white, non-Hispanic vs. all others), PA trajectory group, study visit number indicator, and the interaction between PA trajectory and visit indicator. To improve interpretability, reduced models were created by iteratively removing sex or race if the adjusted p>0.1, and the model refit before evaluating significance of the remaining covariates. Additionally, the interaction was removed if the adjusted p value was >0.05. Given the smaller number of More Active (MA) participants determined by the SAS trajectory procedure (PROC TRAJ) based upon the observed data over time, an additional sensitivity analysis was explored where PA was treated as a continuous variable for the primary outcomes of the association between physical activity and insulin sensitivity. Similar models were fit for change in central adiposity measure, but with percent change in the covariate excluded from the model. Graphs were created to illustrate individual and activity category trajectories, plotting average step count over time from baseline to specified visits and changes in the measures of interest over time, as well. The statistical package “R,” v3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for all other analyses and figures. (12)
RESULTS
Of the 242 adolescents originally enrolled in the Teen-LABS study, 125 provided PA data by wearing a StepWatch™ activity monitor. Eighty-eight met the criteria for inclusion in this analysis. Thirteen participants were classified as MA (mean probability of correct inclusion in the MA trajectory by SAS PROC TRAJ was 99%), and 75 were classified as LA (mean probability of correct inclusion in the LA trajectory was 98%). Of the 88 participants, 77% were female, 65% were white, non-Hispanic, 17% were Black/African American only, and 11% were Hispanic. The baseline mean (±SD) age was 16.9 (±1.6) years. The baseline BMI was 54.9 (±10.6) kg/m2. Baseline HOMA-IR was 6.6 (±4.7), fasting insulin was 28.3 (±21.4) μU/ml and fasting glucose was 94.9 (±15.6) mg/dl. Baseline comparisons between MA and LA for the means of fasting glucose, fasting insulin, HOMA-IR were significantly different, therefore these models were adjusted for baseline values. There was, however, no significant difference between MA and LA for mean 400 meter walk time measure of aerobic fitness/capacity, suggesting that fitness was not a factor in explaining the between-group differences in insulinemia (Table 1).
Table 1.
Baseline comparisons of more active and less active trajectories using two-sample t-tests for p-values (continuous) or two-sample tests of proportions (discrete).
| Covariate | More Active Mean (SD) [N=13] | Less Active Mean (SD) [N=75] | Difference [More-Less] (95% CI) | p-value |
|---|---|---|---|---|
| Fasting Glucose (mg/dL) | 84.0 (6.0) | 96.6 (16.5) | −12.6 (−17.7,−7.5) | <0.001 |
| Fasting Insulin (μU/mL) | 23.6 (11.2) | 31.9 (22.3) | −8.3 (−16.5,0.0) | 0.05 |
| HOMA-IR | 5.0 (2.5) | 7.6 (5.2) | −2.6 (−4.5,−0.7) | 0.008 |
| Triglycerides (mg/dL) | 107.5 (46.2) | 130.5 (72.8) | −22.9 (−54.6,8.7) | 0.147 |
| HDL-C (mg/dL) | 43.0 (10.9) | 38.7 (9.2) | 4.3 (−2.6,11.1) | 0.205 |
| SBP (mmHg) | 122.2 (16.4) | 127.3 (13.3) | −5.1 (−15.4,5.1) | 0.304 |
| DBP (mmHg) | 76.2 (10.1) | 75.0 (10.6) | 1.2 (−5.2,7.7) | 0.689 |
| Resting Heart Rate (bpm) | 80.5 (10.5) | 81.4 (12.8) | −0.9 (−7.9,6.0) | 0.782 |
| CRP (μM) | 0.7 (0.8) | 1.1 (1.2) | −0.3 (−0.8,0.2) | 0.237 |
| Vitamin D (ng/mL) | 24.2 (7.9) | 21.7 (8.0) | 2.6 (−2.5,7.6) | 0.296 |
| HbA1C (%) | 5.2 (0.3) | 5.3 (0.7) | −0.1 (−0.3,0.1) | 0.389 |
| 400-m Walk Time (min) | 6.6 (0.9) | 6.6 (2.2) | 0.0 (−0.8,0.7) | 0.902 |
| BMI | 50.8 (8.1) | 54.4 (9.9) | −3.6 (−8.9,1.7) | 0.17 |
| Iliac Waist Circumference (cm) | 146.1 (16.0) | 149.4 (16.9) | −3.4 (−13.6,6.9) | 0.5 |
| Age (years) | 17.2 (1.5) | 16.9 (1.6) | 0.4 (−0.6,1.3) | 0.431 |
| White, Non-Hispanic | 10 (76.9%) | 47 (62.7%) | 14.3% (−15.6%, 44.2%) | 0.497 |
| Sleeve Gastrectomy | 1 (7.7%) | 13 (17.3%) | −9.6% (−31.0%, 11.7%) | 0.641 |
The average daily step count of MA trajectory was >6 000 average steps at baseline and increased to >9 000 at 2 years. The LA trajectory maintained a daily step count of <4 000 steps per day from baseline to 3 years. (Figure 1). Cadence was slow with only one participant in the LA group recording more than 4 minutes of moderate activity cadence per day compared to eleven MA group participants (>80 steps/minute).
Figure 1.
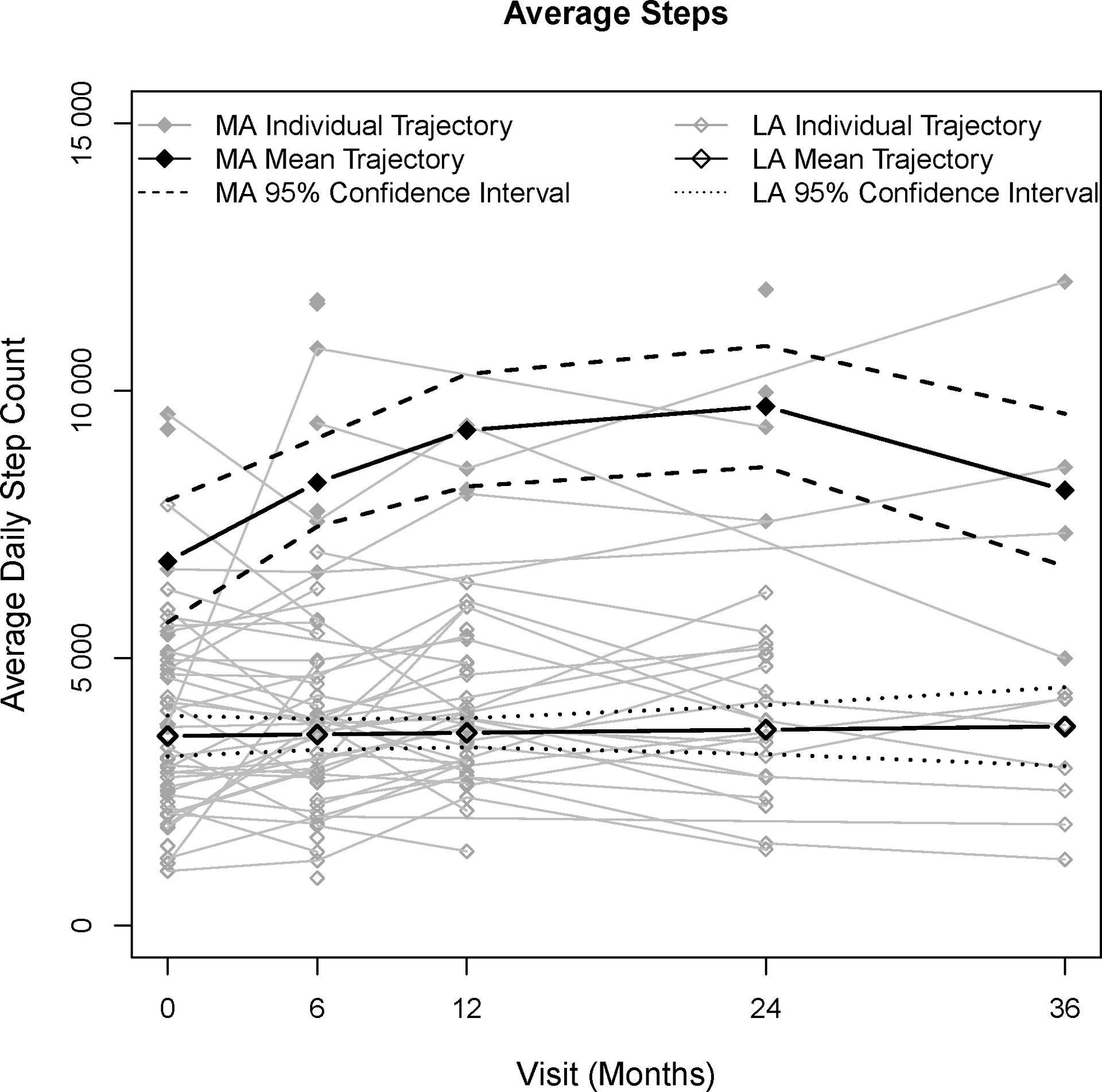
Comparison of mean trajectory for Less Active and More Active step counts over time with individual step count trajectories.
Primary outcome
MA was associated with greater decreases in fasting insulin and insulin resistance as HOMA-IR post-MBS (Figure 2). Of interest, fasting glucose was relatively stable over time and was not associated with activity trajectory. The MA trajectory was associated with greater absolute decrease in fasting insulin (−7.8 μU/ml [95% CI: (−11.8, −3.7], p =<0.001) when adjusted for baseline insulin, % change in BMI, and visit time. Likewise, a greater absolute decrease in HOMA-IR was associated with the MA trajectory, adjusting for baseline HOMA-IR, % change in BMI, and visit time (−1.9 [95% CI:(−3.0, −0.730)], p = 0.001). Importantly, the significant independent effect of more PA on both fasting insulin and HOMA-IR remained when adjusted for % change in IWC (Table 2) or % BF (not included in Table 2). Additionally, the significant effect of PA was confirmed by a sensitivity analysis examining PA as a continuous variable in association with fasting insulin and insulin resistance as HOMA-IR (Supplement S1a). The clinical significance of the independent effect of PA was demonstrated by the difference between MA and LA % of normal fasting insulin and HOMA-IR values from baseline to 36 months. All values in the MA trajectory returned to acceptable values by 6 months and remained within normal limits through-out the 36 month follow up period. The LA trajectory values improved following active weight loss at 12 months, but did not remain within acceptable values over time, decreasing to 28.6% 36 months. (Table 3).
Figure 2. HOMA-IR and fasting insulin outcomes by trajectory.
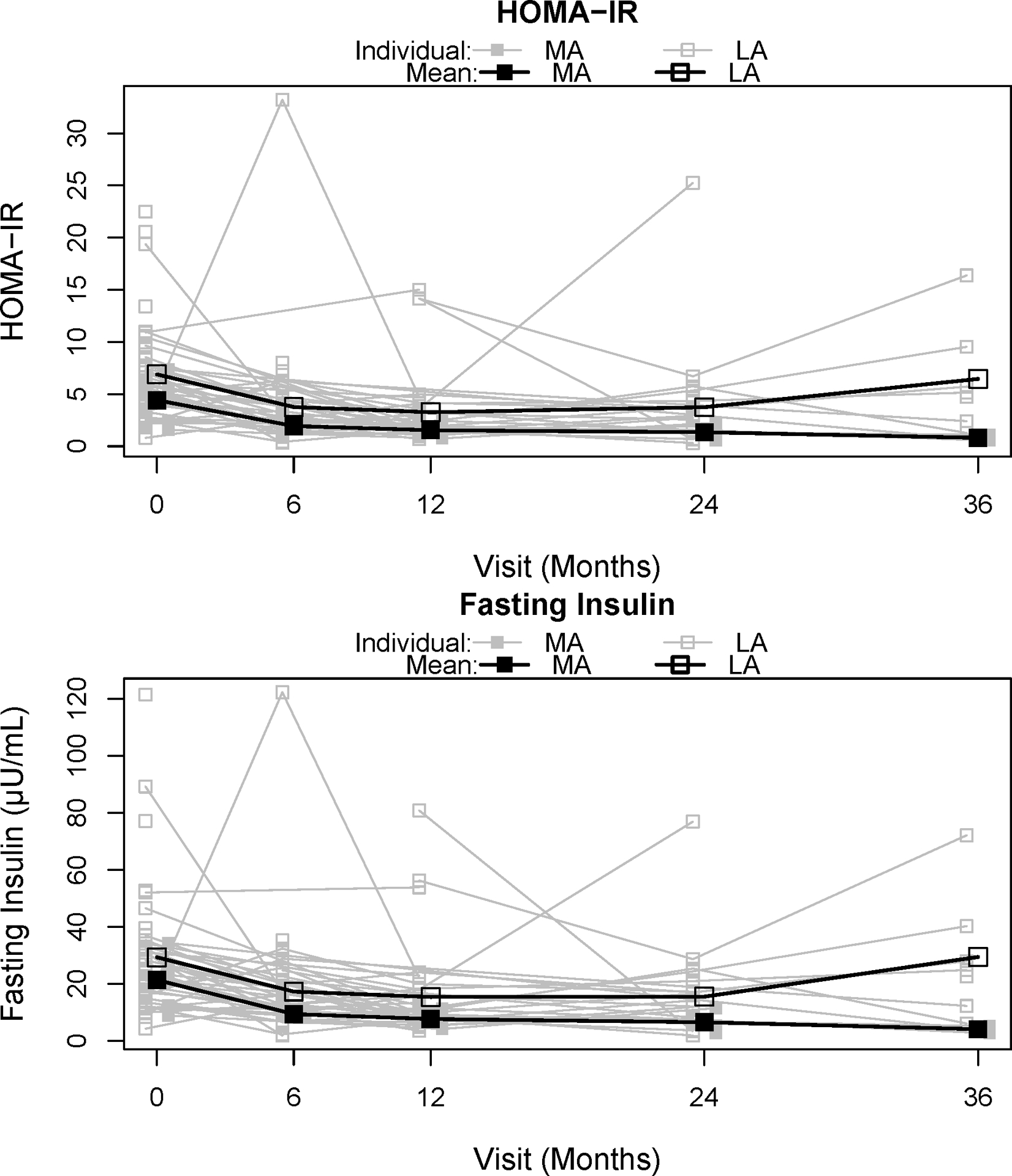
Table 2.
Coefficient table with absolute change in metabolic outcome from baseline regression output with % change in adiposity measure from baseline to 36 months by column.
| Iliac Waist Circumference | Body Mass Index | |||
|---|---|---|---|---|
| Outcome: Fasting Glucose (mg/dl) | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Intercept | 93.14 (82.67, 103.62) | <0.001 | 94.49 (84.82, 104.15) | <0.001 |
| Baseline Glucose | −1.09 (−1.17, −1.01) | <0.001 | −1.08 (−1.16, −1.01) | <0.001 |
| White (vs. Other) | 5.37 (2.01, 8.73) | 0.002 | 5.49 (2.45, 8.54) | <0.001 |
| % Ad Change from Baseline | 0.25 (0.05, 0.45) | 0.014 | 0.24 (0.09, 0.39) | 0.002 |
| MA (vs. LA) | −4.41 (−8.91, 0.09) | 0.055 | −3.39 (−7.72, 0.93) | 0.124 |
| Baseline to 1 Year | 1.12 (−1.93, 4.16) | 0.473 | 1.18 (−1.94, 4.30) | 0.459 |
| Baseline to 2 Years | 4.42 (−0.62, 9.46) | 0.086 | 4.52 (−0.51, 9.55) | 0.078 |
| Baseline to 3 Years | 1.53 (−2.12, 5.19) | 0.411 | 1.62 (−1.63, 4.86) | 0.329 |
| Outcome: Fasting Insulin (μU/ml) | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Intercept | 29.59 (18.73, 40.45) | <0.001 | 30.81 (19.18, 42.45) | <0.001 |
| Baseline Insulin | −1.00 (−1.14, −0.86) | <0.001 | −0.95 (−1.09, −0.81) | <0.001 |
| % Ad Change from Baseline | 0.65 (0.35, 0.96) | <0.001 | 0.55 (0.25, 0.86) | <0.001 |
| MA (vs. LA) | −9.77 (−14.43, −5.11) | <0.001 | −7.75 (−11.77, −3.73) | <0.001 |
| Baseline to 1 Year | −1.02 (−7.54, 5.50) | 0.758 | −0.51 (−7.08, 6.05) | 0.878 |
| Baseline to 2 Years | −2.68 (−9.82, 4.47) | 0.463 | −2.41 (−9.48, 4.67) | 0.505 |
| Baseline to 3 Years | 4.87 (−4.62, 14.36) | 0.315 | 5.25 (−3.25, 13.76) | 0.226 |
| Outcome: HOMA-IR | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Intercept | 6.79 (3.92, 9.67) | <0.001 | 6.91 (3.91, 9.91) | <0.001 |
| Baseline HOMA-IR | −1.02 (−1.15, −0.89) | <0.001 | −0.97 (−1.09, −0.84) | <0.001 |
| % Ad Change from Baseline | 0.15 (0.08, 0.23) | <0.001 | 0.12 (0.05, 0.19) | <0.001 |
| MA (vs. LA) | −2.34 (−3.63, −1.04) | <0.001 | −1.86 (−2.98, −0.73) | 0.001 |
| Baseline to 1 Year | −0.30 (−1.93, 1.32) | 0.714 | −0.22 (−1.85, 1.40) | 0.789 |
| Baseline to 2 Years | −0.21 (−2.38, 1.95) | 0.846 | −0.19 (−2.34, 1.96) | 0.861 |
| Baseline to 3 Years | 1.15 (−1.10, 3.40) | 0.317 | 1.21 (−0.84, 3.25) | 0.247 |
Table 3.
Counts (%) of those with normal fasting insulin (<17μU/mL) and HOMA-IR (<3) at each time point by activity trajectory.
| Fasting Insulin | MA Trajectory | LA Trajectory |
|---|---|---|
| Baseline | 2/6 (33.3%) | 13/43 (30.2%) |
| Month 6 | 7/7 (100.0%) | 28/44 (63.6%) |
| Year 1 | 4/4 (100.0%) | 29/38 (76.3%) |
| Year 2 | 3/3 (100.0%) | 15/21 (71.4%) |
| Year 3 | 4/4 (100.0%) | 2/7 (28.6%) |
| HOMA-IR | MA Trajectory | LA Trajectory |
| Baseline | 2/6 (33.3%) | 9/43 (20.9%) |
| Month 6 | 7/7 (100.0%) | 23/44 (52.3%) |
| Year 1 | 4/4 (100.0%) | 25/38 (65.8%) |
| Year 2 | 3/3 (100.0%) | 13/21 (61.9%) |
| Year 3 | 4/4 (100.0%) | 2/7 (28.6%) |
Secondary outcome
The MA trajectory was associated with a greater decrease in absolute RHR (−8.4 bpm [95% CI:(−15.6, −1.2)], p=0.022) at 6 months. (Table 4.) However, the independent relationship between PA and HOMA and insulin remained significant when RHR was added to the adjusted model.
Table 4.
Coefficient table with absolute change in resting heart rate from baseline regression output.
| Iliac Waist Circumference | Body Mass Index | |||
|---|---|---|---|---|
| Outcome: Resting Heart Rate (bpm) | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Intercept | 42.48 (24.76, 60.20) | <0.001 | 46.24 (29.20, 63.27) | <0.001 |
| Baseline Resting Heart Rate | −0.61 (−0.80, −0.41) | <0.001 | −0.63 (−0.82, −0.44) | <0.001 |
| % Ad Change from Baseline | 0.00 (−0.30, 0.31) | 0.977 | 0.07 (−0.16, 0.31) | 0.542 |
| MA (vs. LA) | −8.33 (−15.62, −1.03) | 0.025 | −8.40 (−15.56, −1.23) | 0.022 |
| Baseline to 1 Year | −4.27 (−9.39, 0.84) | 0.101 | −4.31 (−9.26, 0.64) | 0.088 |
| Baseline to 2 Years | −0.62 (−7.14, 5.90) | 0.852 | −0.78 (−7.31, 5.74) | 0.814 |
| Baseline to 3 Years | 5.04 (−2.10, 12.17) | 0.166 | 2.21 (−5.54, 9.96) | 0.577 |
| Active Trajectory x 1 Year Visit | 2.74 (−4.92, 10.40) | 0.483 | 3.36 (−3.97, 10.70) | 0.369 |
| Active Trajectory x 2 Year Visit | 13.52 (2.27, 24.77) | 0.019 | 14.09 (2.65, 25.54) | 0.016 |
| Active Trajectory x 3 Year Visit | 3.95 (−5.85, 13.74) | 0.43 | 7.62 (−3.34, 18.58) | 0.173 |
Unfortunately, limited RHR data beyond 6 months restricted the opportunity to make extended longitudinal comparisons of the secondary outcome. There were no differences noted by surgical procedure in this outcome of interest.
DISCUSSION
These results demonstrate a positive association between objectively measured PA and improvement in clinical measures of insulin sensitivity, beyond the active weight loss period and independent of body fat reduction in adolescents with severe obesity, post-MBS. Although recorded PA of both MA and LA trajectories was well below the recommended moderate-to-vigorous PA daily for adolescents (13, 14), or adults (13, 15), MA was associated with greater improvement in absolute change in HOMA-IR and fasting insulin, as well as lower baseline values. Additionally, the improvement in measures of insulin sensitivity was clinically, as well as statistically significant with early and sustained normalization of HOMA-IR and fasting insulin associated with MA. LA trajectory participants failed to maintain the benefit of improvement in HOMA-IR and fasting insulin beyond the active weight-loss period of baseline to 12 months, post-MBS. This is important because improving and maintaining insulin sensitivity is an important goal of MBS in adolescents with severe obesity, as β-cell function declines up to 20–35% each year in adolescents with insulin resistance.
Importantly, MA was also associated with greater improvement in RHR, a valuable measure of cardiovascular health, at 6 months post-MBS, independent of weight loss. A greater percentage of MA participants achieved clinically significant improvement in RHR measures over time when adjusted for baseline value of the outcome, sex, race, visit, surgical procedure, and % change in BMI, IWC or body fat as a measure of adiposity.
Many studies have demonstrated the benefit of MBS in reducing comorbidities of severe obesity (4, 16–19), but little is known regarding potential additional benefit of PA as adjunctive therapy in combination with MBS. PA has been shown to improve health, promote weight loss, assist with weight loss maintenance, and reduce CVD risk in a dose response manner with increasing benefits as activity increases (13). Evidence is growing that suggests that PA may provide metabolic and health benefits at lower doses following MBS in adolescents with severe obesity, beyond that attributable to weight/adiposity loss (10). Our study results provide additional evidence that more PA independently improves insulin sensitivity beyond that provided by MBS alone, which likely predicts a further reduction of the sequelae of insulin resistance and overall CVD risks over time.
This is an important observation because the prevalence of insulin resistance and T2D have increased in parallel with rising obesity rates in youth and adults (20–23) and are difficult to treat and reverse. In adolescents, the progression from insulin resistance to beta cell failure (24) which leads to T2D and greatly increases CVD risk (25) occurs more rapidly than in adulthood (26). PA has previously been established to improve insulin sensitivity (27) and has been described as a “robust approach” (28) for effectively reducing insulin resistance (29). Both moderate to vigorous PA interventions alone (30–32) and MBS alone (33) have been shown to improve insulin sensitivity. Our study adds evidence that even small increases in PA will improve insulin sensitivity. While it is well recognized that insulin resistance relates to excess adiposity (24) and improves with change in body composition or weight loss, this study further demonstrates that more PA independently improves insulin sensitivity in adolescents with severe obesity beyond that associated with a loss of body fat post- MBS. Additionally, our study demonstrates the added value of more PA in extending the benefit of improved insulin metabolism following MBS. More activity was associated with a greater proportion of participants achieving acceptable fasting insulin and HOMA-IR values at an earlier time point and maintaining those values across time. Because adolescents with severe obesity who receive MBS in the second decade of life may have many years of life ahead, sustained insulin sensitivity would likely translate into prolonged improvement of health and quality of life.
Additionally, more PA was associated with a greater reduction in resting heart rate. Resting heart rate (RHR) is classically used as a simple clinical indicator of fitness and is a valued measure of response to a variety of therapies. Heart rate negatively correlates with cardiopulmonary fitness (34). Therefore, the greater reduction in resting heart rate may reflect improved cardiopulmonary fitness even with small increases in PA post-MBS. Changes in RHR and fitness related to PA have not been well demonstrated in populations with severe obesity. This clinically significant finding may offer evidence that even small increases in PA can improve cardiopulmonary fitness following MBS in adolescents with severe obesity. Cardiopulmonary fitness has not been found to improve as a result of MBS, but rather to improve only in those adults who participated in a moderate to vigorous physical training intervention (35).
Overall, these results uniquely demonstrate the value of PA to independently improve and prolong positive changes in cardiometabolic outcomes in adolescents with severe obesity, from baseline to 3 years post-MBS. The use of longitudinal data and the extended data collection over 3 years pre- and post-MBS strengthens the study results. The use of objectively measured PA and the analysis of individual activity trajectories and group trajectories instead of median or group means better demonstrates the variability of outcomes in association with less and more PA. Addressing the influence of co-variates and baseline values, accounting for sex, race, procedure, and percent change in multiple adiposity measures, as well as baseline values, also strengthens the study results. However, limitations also exist, including variable but limited PA participation pre- and post-MBS among the study population, limited StepWatch™ data and availability of some outcomes of interest, and a lack of individual dietary data. While individual dietary intake data were not available in the Teen-LABS study, pre- and post-MBS dietary instructions were consistent for all participants. Also, participant self-selection may have influenced participation initially and over time.
In summary, PA appears to further improve measures of insulin sensitivity and may reduce RHR and CVD risk in adolescents with severe obesity, post-MBS. These findings support the need for additional studies of the benefits of PA in those undergoing MBS and the need for PA support as part of the chronic care plan for all adolescents with severe obesity, post-MBS. Additional research is needed to evaluate the long term benefit of PA on cardiopulmonary fitness in adolescents with severe obesity. There remains a need for clarity regarding frequency, intensity and duration of PA necessary to modify specific cardiometabolic outcomes over time in adolescents. Additionally, improving PA participation and adherence to treatment recommendations among adolescents remains poorly understood (28). There is a need to develop sustainable strategies to motivate adherence to PA recommendations in this unique population with limited PA experience and poor participation. Finally, identification of effective PA strategies to extend the benefits of MBS through decades of future life in adolescents is an urgent need.
Supplementary Material
FUNDING:
The Teen-LABS consortium is funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), through grants: UM1DK072493 (PI, Dr. Thomas Inge, University of Colorado, Denver, CO), and UM1DK095710 (PI, Dr. Changchun Xie, University of Cincinnati). Dr. Paula Holland Price was supported by the National Heart, Lung and Blood Institute (NHLBI): 5T32HL116276-05.
Footnotes
DISCLOSURES:
Paula Holland Price, Alexander M. Kaizer, Thomas H. Inge and Robert H. Eckel declare no conflicts of interest.
Supplementary information is available at International Journal of Obesity’s website
References
- 1.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. The Journal of Pediatrics. 1996;128(5):608–15. [DOI] [PubMed] [Google Scholar]
- 2.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining beta-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care. 2011;34(9):2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL. Metabolic Surgery: Weight Loss, Diabetes, and Beyond. J Am Coll Cardiol. 2018;71(6): p 670–687. [DOI] [PubMed] [Google Scholar]
- 4.Michalsky MP, Inge TH, Jenkins TM, Xie C, Courcoulas A, Helmrath M, et al. Cardiovascular Risk Factors After Adolescent Bariatric Surgery. Pediatrics. 2018;141(2) e20172485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011;12(3):163–72. [DOI] [PubMed] [Google Scholar]
- 6.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berglind D, Willmer M, Tynelius P, Ghaderi A, Naslund E, Rasmussen F. Women undergoing Roux-en-Y Gastric Bypass surgery: Family resemblance in pre- to postsurgery physical activity and sedentary behavior in children and spouses. Surg Obes Relat Dis. 2015;11(3):690–6. [DOI] [PubMed] [Google Scholar]
- 8.Lent MR, Bailey-Davis L, Irving BA, Wood GC, Cook AM, Hirsch AG, et al. Bariatric Surgery Patients and Their Families: Health, Physical Activity, and Social Support. Obes Surg. 2016;26(12):2981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inge TH, Zeller M, Harmon C, Helmrath M, Bean J, Modi A, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42(11):1969–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price PH, Kaizer AM, Daniels SR, Jenkins TM, Inge TH, Eckel RH. Physical Activity Improves Lipid and Weight‐Loss Outcomes After Metabolic Bariatric Surgery in Adolescents with Severe Obesity. Obesity. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent Class Growth Modelling: A tutorial. Tutorials in Quantitative Methods for Psychology. 2009; Vol 5(1): p11–24. [Google Scholar]
- 12.R: A language and environment for statistical computingR Foundation for Statistical Computing Vienna, Austria2018 [cited 2018. Available from: https://www.R-project.org/.
- 13.Services UDoHaH. Physical Activity Guidelines for Americans, 2nd edition. 2nd ed Washington, DC: US Department of Health and Human Services; 2018. [Google Scholar]
- 14.Tudor-Locke C, Craig CL, Beets MW, Belton S, Cardon GM, Duncan S, et al. How many steps/day are enough? For children and adolescents. Int J Behav Nutr Phys Act. 2011;8 p78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, et al. How many steps/day are enough? for adults. International Journal of Behavioral Nutrition and Physical Activity. 2011;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xanthakos SA, Jenkins TM, Kleiner DE, Boyce TW, Mourya R, Karns R, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Adolescents Undergoing Bariatric Surgery. Gastroenterology. 2015;149(3):623–34.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Algooneh A, Almazeedi S, Al-Sabah S, Ahmed M, Othman F. Non-alcoholic fatty liver disease resolution following sleeve gastrectomy. Surg Endosc. 2016;30(5):1983–7. [DOI] [PubMed] [Google Scholar]
- 18.da Cruz MRR, Branco-Filho AJ, Zaparolli MR, Wagner NF, de Paula Pinto JS, Campos ACL, et al. Predictors of Success in Bariatric Surgery: the Role of BMI and Pre-operative Comorbidities. Obes Surg. 2018;28(5):1335–41. [DOI] [PubMed] [Google Scholar]
- 19.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374(2):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118(9):2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care. 2011;34 Suppl 2:S161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473–80. [DOI] [PubMed] [Google Scholar]
- 23.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caprio S, Perry R, Kursawe R. Adolescent Obesity and Insulin Resistance: Roles of Ectopic Fat Accumulation and Adipose Inflammation. Gastroenterology. 2017;152(7):1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JD, Mills E, Carlisle SE. Treatment of Pediatric Type 2 Diabetes. Ann Pharmacother. 2016;50(9):768–77. [DOI] [PubMed] [Google Scholar]
- 26.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015;13531(1):113–37. [DOI] [PubMed] [Google Scholar]
- 27.Berman LJ, Weigensberg MJ, Spruijt-Metz D. Physical activity is related to insulin sensitivity in children and adolescents, independent of adiposity: a review of the literature. Diabetes Metab Res Rev. 2012;28(5):395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi H, Kotani K, Tanaka K, Egucih Y, Anzai K. Therapeutic Approaches to Nonalcoholic Fatty Liver Disease: Exercise Intervention and Related Mechanisms. Front Endocrinol (Lausanne). 2018;9:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordenave S, Brandou F, Manetta J, Fédou C, Mercier J, Brun JF. Effects of acute exercise on insulin sensitivity, glucose effectiveness and disposition index in type 2 diabetic patients. Diabetes Metab. 2008;34(3):250–7. [DOI] [PubMed] [Google Scholar]
- 30.Aadland E, Robertson L. Physical activity is associated with weight loss and increased cardiorespiratory fitness in severely obese men and women undergoing lifestyle treatment. J Obes. 2012;2012:810594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Look ARG. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: The look AHEAD study. Obesity. 2015;23(3):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman KM, Carver TE, Christou NV, Andersen RE. Keeping the weight off: physical activity, sitting time, and weight loss maintenance in bariatric surgery patients 2 to 16 years postsurgery. Obes Surg. 2014;24(7):1064–72. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes RA, Vaz Ronque ER, Venturini D, Barbosa DS, Silva DP, Cogo CT, et al. Resting heart rate: its correlations and potential for screening metabolic dysfunctions in adolescents. BMC Pediatr. 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onofre T, Carlos R, Oliver N, Felismino A, Fialho D, Corte R, et al. Effects of a Physical Activity Program on Cardiorespiratory Fitness and Pulmonary Function in Obese Women after Bariatric Surgery: a Pilot Study. Obes Surg. 2017;27(8):2026–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


