Abstract
We have limited knowledge of the patterns, causes, and prevalence of elevational migration despite observations of seasonal movements of animals along elevational gradients in montane systems worldwide. While a third of extant Hawaiian landbird species are estimated to be elevational migrants this assumption is based primarily on early naturalist’s observations with limited empirical evidence. In this study, we compared stable hydrogen isotopes (δ2H) of metabolically inert (feathers) and active (blood plasma, red blood cells) tissues collected from the same individual to determine if present day populations of Hawaiian honeycreepers undergo elevational movements to track areas of seasonally high flower bloom that constitute significant food resources. We also measured stable carbon isotopes (δ13C) and stable nitrogen isotopes (δ15N) to examine potential changes in diet between time periods. We found that the majority of ‘apapane (Himatione sanguinea) and Hawaiʻi ʻamakihi (Chlorodrepanis virens) captured at high elevation, high bloom flowering sites in the fall were not year-round residents at the capture locations, but had molted their feathers at lower elevations presumably in the summer after breeding. δ2H values of feathers for all individuals sampled were higher than blood plasma isotope values after accounting for differences in tissue-specific discrimination. We did not find a difference in the propensity of elevational movement between ‘apapane and Hawaiʻi ‘amakihi, even though the ‘amakihi is considered more sedentary. However, consistent with a more generalist diet, δ15N values indicated that Hawaiʻi ʻamakihi had a more diverse diet across trophic levels than ʻapapane, and a greater reliance on nectar in the fall. We demonstrate that collecting multiple tissue samples, which grow at different rates or time periods, from a single individual can provide insights into elevational movements of Hawaiian honeycreepers over an extended time period.
Introduction
The movement of animals in response to seasonal fluctuations in climate and availability of resources is a widespread and taxonomically diverse behavior occurring in animals such as birds, bats, insects, and ungulates [1, 2]. However, our understanding of ecological factors shaping migratory behavior has primarily been shaped by studies of long-distance migration across latitudinal scales. In contrast, we have limited knowledge of the patterns, causes, and prevalence of elevational migration despite observations of seasonal movements of animals along elevational gradients in montane systems worldwide [3, 4]. Unlike obligate, long-distance migration (i.e., every year the entire population migrates), elevational migration typically occurs over short distances and is facultative such that individuals may adapt their behavior in response to environmental conditions that vary from year to year [3]. Seasonal migration across elevations may be driven by ecological factors such as spatial and temporal variation in food resources, weather events, or predation risks that vary at different elevations [4]. For example, the abundance of some frugivorous and nectarivorous bird species, birds that primarily eat fruit and nectar, respectively, in tropical montane systems has been shown to vary across elevations associated with seasonal changes in fruit and flower availability [5, 6]. Alternatively, elevational migration can be in response to storms, such as seen with white-ruffed manakins (Corapipo altera) in montane wet forests in Central America that migrate to lower elevations following severe storm events as a result of reduced foraging opportunities at high elevations [7].
While elevational migration has been well documented for Neotropical frugivore and nectarivore bird species, many nectarivores outside of the Neotropics, along with other feeding guilds (e.g., insectivores, granivores), are also thought to engage in elevational migrations [3]. For example, a third of extant Hawaiian landbird species are estimated to be elevational migrants [3]. However, for many Hawaiian species this assumption is primarily based on early naturalist’s observations of Hawaiian forest birds making long, high flights over the forest canopy, seasonal changes in the abundance of birds at different elevations [8–11], and observations of birds in low-elevation habitat following large storms [8]. Early naturalists hypothesized that nectarivorous birds made seasonal movements across elevations as they tracked the timing of flowering ʻōhiʻa (Metrosideros polymorpha). ʻŌhiʻa is the dominant tree from sea level to tree line in Hawaiian wet tropical forests, accounting for more than 80% of the biomass of native forests [12], and is the primary nectar resource utilized by Hawaiʻi’s native nectarivorous birds [13]. Differences in the timing of ʻōhiʻa bloom by ʻōhiʻa varieties that occur at different elevations creates spatially and temporaly variable distributions of bloom [14, 15] that may drive elevational migration. However, this “Elevational-Migration Hypothesis” is based on limited empirical evidence of seasonal movements of Hawaiian forest birds across elevations (but see [16]) given the challenges in following birds across Hawaiʻi’s rugged and remote tropical forests. Moreover, recent correlative studies examining the seasonal abundance of Hawaiian forest birds at different elevations have not found strong synchrony between bird abundances and the flowering phenology of ʻōhiʻa [17, 18]. The lack of correspondence between flower and bird densities indicates that elevational movements may not be as prevalent as early naturalists thought or that present day movement strategies of native Hawaiian forest birds are potentially changing because of factors such as the loss and fragmentation of forests across the landscape [19], reduced competition for nectar resources as populations decline or become extinct [20], or higher disease prevalence of introduced diseases (e.g., avian malaria, pox, mange) at low elevations [21].
Limitations in following individuals through time in steep mountainous terrains, particularly small species that are too light to carry tracking devices [22], has hindered our ability to understand elevational movements in birds worldwide. The use of stable isotopes has been one approach to documenting movement of small animals across large geographic areas given that stable isotopes vary predictably across the landscape and are incorporated into animal tissues through biochemical processes [23]. Stable hydrogen isotopes (δ2H) in particular have become a well-established technique for studying long-distance movements of birds at continental scales (reviewed in [23]), but only a few studies in comparison have used δ2H to study elevational migration [24–28]. However, δ2H values vary predictably with not only latitude but also elevation, with approximately a 1‰ to 4‰ decrease of δ2H in precipitation with every 100 m increase in elevation [29]. Depletion of H with altitude results from Rayleigh distillation and depletion of precipitation as an air mass rises over a mountain range and loses moisture to rainfall and decreasing temperatures [29, 30]. Patterns of δ2H in precipitation are correlated with animal tissues as a result of biochemical processes [23], and thus animal tissues are expected to reflect the isotopic signatures of the elevation of feeding where the tissue was grown.
Stable carbon isotopes (δ13C) can also be used to understand elevational movement, although the gradient of change across elevations is much smaller, and thus, δ13C are often not as informative as δ2H for small elevational gradients. For example, the rate of increase in δ13C values of bird feathers was only ~1.3 to 1.5‰ per 1,000 m for adult male black-throated blue warblers (Setophaga caerulescens) and multiple hummingbird species collected across elevational gradients in the Appalachian and Andean mountains, respectively [24, 31]. Therefore, in areas with small elevational gradients, δ13C, along with stable nitrogen isotopes (δ15N), are better for providing an understanding of the diet niche of a species than movement [23].
Animal tissues incorporate local isotopic signatures over different time periods, from days to years [32–34], and thus at a single point in time different tissues can provide different time frames associated with the movement of animals. For example, feathers are metabolically inert after formation, and reflect the diet and water inputs of the bird only during the discrete period of feather growth (but see [35]). In contrast, metabolically active tissues like blood, liver, and muscle continuously incorporate the isotopic signature of their environment at varying rates. Blood plasma quickly incorporates isotopic signatures of the local environment with an average residency time of only ~3 to 5 days, while red blood cells (RBCs) and muscle have slower isotopic incorporation rates and integrate local isotopic signatures over longer timescales ranging from 1 to 2 months [32, 33, 36, 37]. However, inter-tissue differences in stable isotope values can not only be a function of differences in the residency time of isotopes, but also physiological mechanisms that control isotopic discrimination among different tissues resulting in tissue-specific discrimination [34]. Field and laboratory studies of birds have shown that feathers are more enriched in 2H than other tissues (e.g., plasma, RBCs, muscle), while isotopic discrimination of 2H between RBCs and blood plasma was not shown to differ [32, 33, 38, 39]. While controlled laboratory studies are beginning to shed light on the patterns of tissue-specific discrimination of stable isotopes, the processes that determine these patterns (e.g., differences in protein synthesis and nutrient routing between tissues) are still not completely understood [32]. Thus, comparisons of multiple tissues collected from the same individual at one point of time can provide insights into shifts in elevational movement and diet for different time periods of the annual cycle, as long as comparisons are made while also incorporating tissue-specific discrimination factors.
We tested the elevational-migration hypothesis in nectarivorous birds on the east side of Hawaiʻi Island in a wet tropical forest to determine if present day populations undergo elevational movements to access ʻōhiʻa flower blooms that form significant food resources. We captured birds at high elevation sites near the upper limits of the forest that had heavy ʻōhiʻa bloom. Birds were captured in the fall after peak molt (i.e., summer) and peak breeding (i.e., January to May) seasons were complete. To assess the propensity of movement, we compared δ2H of metabolically inert (feathers) and active (blood plasma, RBC) tissues collected from the same individual. Based on the elevational-migration hypothesis, we predicted that birds making elevational migrations upslope to high bloom areas would have lower blood plasma δ2H values, representative of the high elevation capture location, compared to their feathers that were grown at lower elevation breeding and molting sites. In contrast, if birds captured at high elevation, high bloom areas were resident breeders, we would expect similar stable isotope values among all tissue types, after accounting for differences in tissue-specific discrimination. We tested these predictions for two Hawaiian honeycreeper species, ‘apapane (Himatione sanguinea) and Hawaiʻi ʻamakihi (Chlorodrepanis virens), that vary in their degree of nectarivory and hypothesized propensity for movement. ‘Apapane are nectarivores, primarily feeding from ʻōhiʻa flowers, but are also known to consume foliage arthropods during the breeding season [13, 40]. In contrast, Hawaiʻi ‘amakihi are generalists that eat foliage arthropods but also consume large quantities of nectar when available [41]. Hawaiʻi ‘amakihi are thought to be more sedentary than ‘apapane given differences in their foraging strategies and greater genetic structure [42], and thus we predicted that a greater proportion of ‘apapane captured at high elevation, high bloom areas would be elevational migrants making long-distance movements in search of ʻōhiʻa nectar. To examine potential changes in diet between time periods of the annual cycle we also measured δ13C and δ15N from feathers and RBCs of target birds. We predicted that Hawaiʻi ‘amakihi would have greater inter-tissue variation for both δ13C and δ15N given their more generalist diet compared to ʻapapane. However, we did not predict large differences in δ13C associated with elevational movements given the small elevational range (~500 to 1500 m) of movements expected by ‘apapane and Hawaiʻi ‘amakihi.
Methods
Ethics statement
The research conducted for this study was carried out in accordance with the Ornithological Council’s guidelines for the use of wild birds in research and was approved by The University of Hawaiʻi at Hilo’s Institutional Animal Care and Use Committee (protocol # UH 12–1315). Other permits were from the United States Department of the Interior bird banding laboratory (permit # 23064), Hawaii State Protected Wildlife Research Permit (WL 13–07), and National Park Service Research Permit (HAVO-2012-SCI-0041).
Study species and area
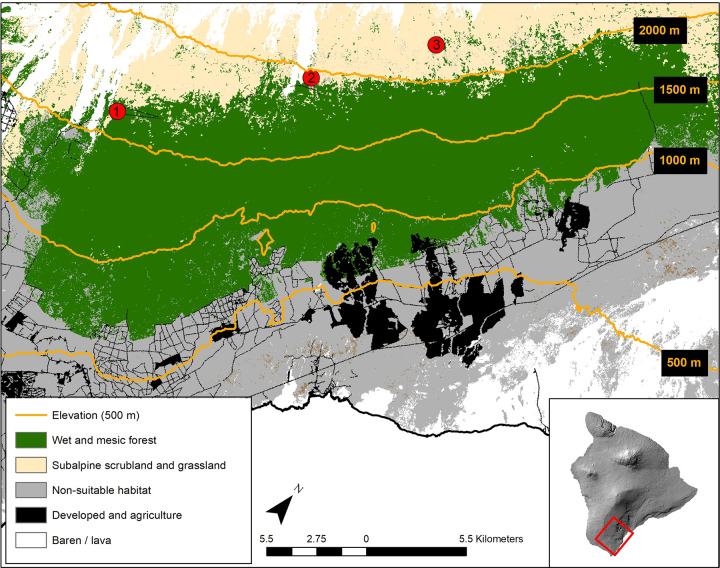
We sampled ʻapapane and Hawaiʻi ʻamakihi at Hawaiʻi Volcanoes National Park in the upper Kaʻū Forest at three sites ranging in elevation from 1615 to 2191 m (Fig 1). Both species are Hawaiian honeycreepers (Fringillidea) that are locally abundant and widely distributed within the Kaʻū Forest and have been detected through surveys during the breeding season at elevations ranging from tree line (~2200m) to 700 m, with moderately high densities below 1500 m where mosquitoes that vector avian malaria are present [43]. The Kaʻū Forest is one of the largest intact native tropical wet forests on Hawaiʻi Island located on the southeast windward slopes of Mauna Loa Volcano. The forest is comprised primarily of mature ‘ōhiʻa and varying amounts of koa (Acacia koa) with a predominantly native understory. Southern regions of the Kaʻū Forest have been used for cattle ranching over the last 150 years and consist of forested pastures with isolated patches of ʻōhiʻa-koa forests and understory grasses [43]. All three capture sites in this study were near tree line in low stature scrubby ‘ōhiʻa patches that had heavy ʻōhiʻa bloom, immediately above the high stature mixed ʻōhiʻa-koa forest. We also attempted to capture birds in the high stature mixed ʻōhiʻa-koa forest which had little to no ʻōhiʻa bloom, however, we did not catch any birds most likely because densities of birds were so low. The climate of the Kaʻū Forest is affected by Mauna Loa Volcano as winds are driven around and upward creating three rainfall patterns: trade wind and thermally driven sea breeze cycles dominate rainfall patterns from Pāhala to Nāʻālehu, a rain-shadow is present in the area southwest of Kīlauea summit, and high elevation areas that are above the trade wind inversion zone have rainfall only during storms [44].
Fig 1. Map of the Kaʻū Forest on Hawaiʻi Island displaying the capture sites at high elevations (depicted by contour lines).
Habitat layers were obtained from the Landfire program (https://www.landfire.gov).
Sample collection
We captured birds via passive mist netting at three high elevation sites that were experiencing high ʻōhiʻa bloom during the months of September and October in 2012. Upon capture, birds were banded with a U.S. Geological Survey (USGS) aluminum numerical band, weighed to nearest 0.1g with an electronic scale, aged and sexed based on plumage, breeding characteristics, and size (as described in [45]), and assessed for active molt. Additionally, we pulled the two outer most tail feathers and collected a blood sample via brachial vein for δ2H (plasma and RBCs) and δ13C and δ15N (RBCs only) analysis. For most birds, the amount of blood collected was enough for δ2H and δ13C and δ15N analysis. However, we prioritize blood for δ2H analysis when the blood volume was too low for both analyses to be conducted. We stored blood samples on ice until the plasma and RBCs could be seperated at the end of the day, and then placed on dry ice until they could be transfered to a -20°C freezer.
We used plasma samples to represent the isotopic signature of the capture location given the short residency time of blood plasma, while feather samples represented the location of molt. Molt typically occurs following the breeding season during primarily the summer and early fall, and is believed to occur largely on the breeding grounds [46, 47]. While Hawaiian honeycreepers can breed anytime between October to May, depending on weather conditions, their peak breeding season occurs between January to May [46]. RBCs represented the time period in between breeding and capture in the fall.
Stable isotope analysis
Feather and blood samples were prepared for stable isotope analysis at the University of Oklahoma using the protocols outlined in [48]. Briefly, feather samples were cleaned with a 2:1 chloroform methanol solution as well as a phosphate-free detergent and rinsed in deionized water before drying for 24–36 hours under a fume hood. Plasma and RBCs were freeze-dried and powdered. Lipids were not extracted from blood samples prior to freeze drying given the low concentration of lipids in bird blood [49]. Feather material from the distal end of the sample, powdered RBC samples, and powdered plasma samples were weighed (δ2H: 140 to 160 μg, δ13C and δ15N: 350 μg) and wrapped in a silver (δ2H) or tin (δ13C and δ15N) capsule.
Samples were analyzed for stable hydrogen isotope ratios at the Colorado Plateau Stable Isotope laboratory (CPSIL; Flagstaff, Arizona, USA) using a comparative equilibrium approach with calibrated keratin standards to correct for uncontrolled isotope exchange between non-carbon-bound hydrogen in feathers and ambient water vapor [50]. The three calibrated isotope keratin standards analyzed with feather samples included: Cow Hoof (CBS; δ2H = -197‰); Kudi Horn (KHS; δ2H = -54.1‰); Spectrum Keratin Powder Lot SJ (SKP; δ2H = -121.6‰). Stable hydrogen isotope ratios were determined with a Thermo Scientific Delta Plus isotope ratio mass spectrometer connected to a Thermo Scientific TC/EA elemental analyzer and configure through a Thermo Scientific CONFLO IV for automated continuous-flow analysis. Samples were analyzed for stable carbon and nitrogen isotope ratios at the University of Arkansas using a Thermo-Finnigan DeltaPlus isotope ratio mass spectrometer connected to an Carlo Erba elemental analyzer. Two standards analyzed with feather and RBC samples included USGS40 and BHCO (powdered brown-headed cowbird (Molothrus ater) feather) used extensively as a lab standard as documented by Kelly et al. [51]. Stable isotope ratios are expressed in standard notation, where δ2H, δ13C, and δ15N = [(isotope ratiosample/isotope ratiostandard)– 1] x 1000. Consequently, δ2H, δ13C, and δ15N are expressed in parts per thousand (‰) deviation from a standard (δ2H: Vienna Standard Mean Ocean Water, δ13C: Vienna Pee Dee Belemnite, δ15N:Air). Measurement of the three keratin reference materials corrected for linear instrumental drift were both accurate and precise with mean δ2H ± standard deviation of -198.0 ± 0.4‰ (CBS), -55.8 ± 0.7‰ (KHS), and -120 ± 1.8‰ (SKP). Likewise, repeated analysis of δ13C and δ15N standards were -26.2 ± 0.3‰ (δ13C USGS40), -4.2 ± 0.3‰ (δ15N USGS40), -15.7 ± 0.1‰ (δ13C BHCO), and 7.6 ± 0.1‰ (δ15N BHCO). We ran standards and a replicate sample every 10th sample and flagged any replicate sample that differed by > 6‰.
Statistical analysis
We conducted all statistical analyses in R version 3.6.0 (R Core Team 2019) using the packages lme4 [52], lmerTest [53], and lsmeans [54]. We used general linear mixed models (GLMM) to separately examine differences in δ2H, δ13C, and δ15N values among tissue types (δ2H: feathers, RBC, plasma; δ13C, δ15N: feathers, RBC) and species (‘apapane, Hawaiʻi ‘amakihi). Individual was included as a random effect in each model to account for multiple tissues collected from the same individual. We assumed statistical significance at alpha ≤ 0.05, but with multiple comparisons we conducted a Tukey’s post-hoc analysis of least squared means to determine differences among significant factors. Prior to running GLMMs we adjusted δ2H feather values by -19.8‰ to account for tissue-specific discrimination between feathers and blood [34]. We adjusted δ2H feather values based on the average difference in tissue discrimination factors of feathers and blood plasma (-18.6‰±4.4‰) and feathers and RBCs (-21‰±1.5‰) calculated from an experiment with house sparrows (Passer domesticus), the species most closely related to Hawaiian honeycreepers with tissue-specific discrimination values [38]. RBCs and blood plasma do not differ in isotopic discrimination of 2H; therefore, we did not adjust these tissue types [32, 33, 38, 39]. We did not adjust δ13C and δ15N feather values prior to analysis because tissue-specific discrimination factors for δ13C and δ15N are highly sensitive to diet [36, 55] and discrimination factors have not been established for a nectarivore. In addition, published discrimination factors have primarily been calculated for feathers and whole blood, but not RBCs [36, 55, 56]. Instead, similar to the approach of Podlesak et al. [36] we examined only general changes in diet by considering differences in δ13C and δ15N values between feathers and RBCs greater than 2‰ to be indicative of a change in diet.
We estimated the elevation of feather growth for feather samples collected at our study sites based on the relation between elevation and stable hydrogen isotopes in precipitation (δ2Hp) for wet tropical forests on the east side of Hawaiʻi Island, δ2Hp = -0.018(elevation) -11.25. We derived the relationship between δ2Hp and elevation using published volume-weighted average δ2Hp values collected from 50 locations sampled across east Hawaiʻi Island, including sample locations within the Kaʻū Forest, and ranging in elevation from 6 to 4000 meters ([57] Appendix 1). Scholl et al. [57] calculated volume-weighted average δ2Hp values for each location based on rainfall samples collected at 6-month intervals between August 1991 to August 1994. Because all three rain patterns (i.e., trade winds, rain shadow, high elevation) are present in the Kaʻū Forest we used all sampling locations representing these rainfall patterns to establish the relationship between δ2Hp and elevation for the Kaʻū Forest. Prior to estimating elevation, we adjusted stable hydrogen isotopes in feathers (δ2Hf) to reflect δ2Hp utilizing a conversion equation from a species in the same foraging guild as our study species, Rufous hummingbird (Selasphorus rufus). As nectarivores, hummingbirds and Hawaiian honeycreepers receive most of their hydrogen from plant-derived water and plant-derived carbohydrates through consumption of large quantities of nectar. Thus, in the absence of a species-specific conversion equation for our species [58], hummingbirds likely provide the best approximation of the relation between feather and precipitation δ2H values for Hawaiian honeycreepers. The equation, δ2Hp = 1.15(δ2Hf) + 29.01, was calculated based on Rufous hummingbird feathers grown at known locations across North America [59]. The same relation between δ2Hf and δ2Hp was also found for Ruby-throated hummingbirds (Archilochus colubris) [60], indicating a consistent relation between δ2Hf and δ2Hp for nectarivorous birds.
Results
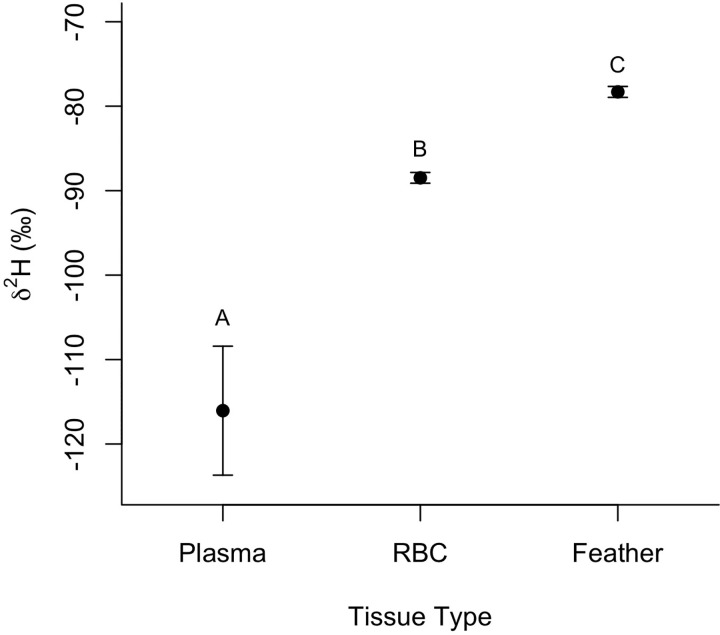
We captured a total of 102 birds during the 2-month time period (δ2H analysis: ‘apapane = 60, ‘amakihi = 42; δ13C and δ15N analysis: ‘apapane = 51, ‘amakihi = 36). We found significant differences in δ2H values among tissues taking into account individual variability (F2,118.3 = 172.5, p<0.001) with patterns not differing between species (F1,99.7 = 1.6, p = 0.21). Feathers had the highest mean δ2H values followed by RBCs, and then blood plasma (Fig 2). The average difference in δ2H values between feathers and blood plasma collected from the same individual was 40.7‰ (range 24.0–62.5‰), suggesting feathers were grown at lower elevations than the capture location represented by the blood plasma sample. The average difference in δ2H values between RBCs and blood plasma of the same individual was 32.3‰ (range 23.9–41.4‰).
Fig 2. Stable hydrogen isotope (δ2H) values (± SE) for plasma, red blood cells (RBC), and feathers adjusted for tissue discrimination collected from ‘apapane and Hawaiʻi ‘amakihi in the upper Kaʻū Forest.
Letters above bars indicate tissue types that are significantly different from one another based on a Tukey’s post-hoc analysis.
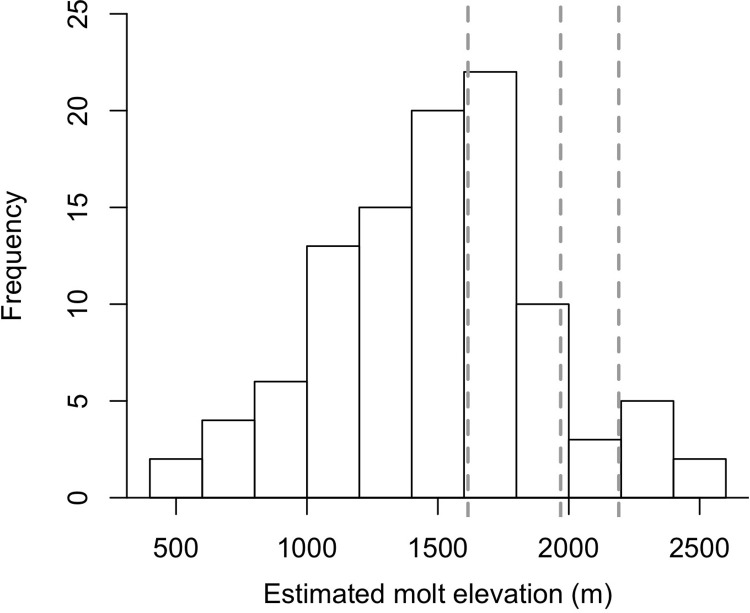
Based on the relationship between δ2Hp and elevation on the east side of Hawaiʻi Island we found the average estimated elevation of feather growth (1501 m, range 505 to 2528 m) was lower than the elevation of all three capture locations (1615 m, 1968 m, 2191 m) (Fig 3). The average distance between the elevation of a birdʻs capture location and estimated elevation of feather growth was 562 m (range 5–1686 m) with the distance between capture and molt elevation increasing with increasing elevation of the capture location (Table 1). All of the honeycreepers captured at our highest elevation site were estimated to have grown their feathers at lower elevations with on average 1008 m (range 193–1686 m) between the capture site and the estimated molt location. In contrast, 83% and 45% of honeycreepers captured at the other two sites (1968 m, 1615 m) had an estimated molt location lower than their capture location.
Fig 3. Estimated elevation of feather growth for Hawaiʻi ‘amakihi and ‘apapane captured during the fall in upper Kaʻū Forest.
Dashed lines represent the elevations of the three capture sites.
Table 1. For each capture location, the sample size (n) and mean, minimum, and maximum difference in elevation between a birdʻs capture location and their estimated molt location.
Site numbers refer to the locations indicated in Fig 1.
| Difference in elevation of capture and estimated molt location | |||||
|---|---|---|---|---|---|
| Site | Elevation (m) | n | Mean (m) | Minimum (m) | Maximum (m) |
| 1 | 1615 | 33 | 231 | 9 | 913 |
| 2 | 1968 | 35 | 442 | 5 | 1248 |
| 3 | 2191 | 34 | 1008 | 193 | 1686 |
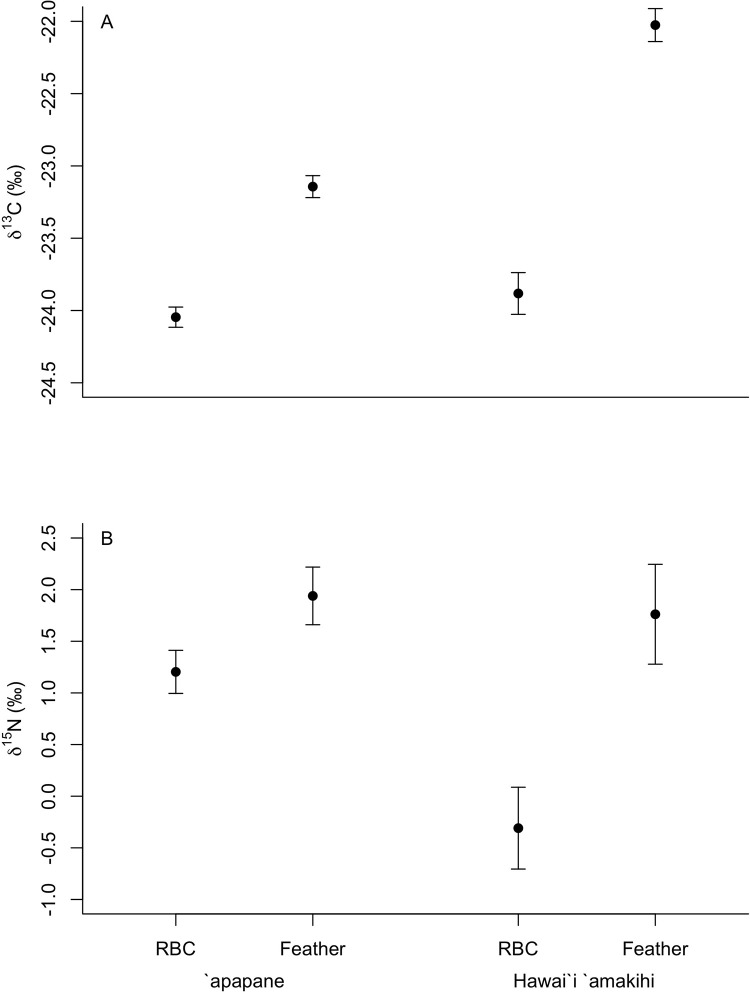
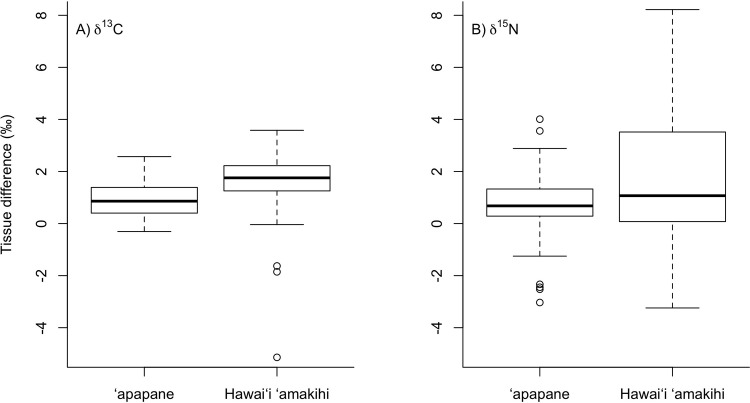
We found significantly higher δ13C and δ15N values of feathers compared to RBC values (δ13C: F1,86 = 161.4, p<0.001, δ15N: F1,86 = 30.3, p<0.001) with overall higher δ13C and δ15N values for Hawaiʻi ‘amakihi compared to ‘apapane (δ13C: F1,85 = 36.5, p<0.001, δ15N: F1,85 = 4.2, p = 0.04) (Fig 4). However, for ʻapapane the average difference in isotope values between feathers and RBCs collected from the same individual was less than 2‰ (δ13C: 0.9‰, range -0.3 to 2.6‰, δ15N: 1.3‰, range -3.0 to 4.0‰) (Fig 5). In contrast, for Hawaiʻi ʻamakihi almost half (δ13C: 42%, δ15N: 44%) of the individuals captured had differences in isotope values between feathers and RBCs greater than 2‰ (δ13C: 2.0‰, range -1.9 to 3.6‰, δ15N: 2.6‰, range -3.2 to 8.2‰) (Fig 5).
Fig 4.
(A) Stable carbon isotope (δ13C) and (B) stable nitrogen isotope (δ15N) values (± SE) for red blood cells (RBC) and feathers collected for ‘apapane and Hawaiʻi ‘amakihi in the upper Kaʻū Forest.
Fig 5.
Boxplots showing the difference in (A) stable carbon isotope (δ13C) and (B) stable nitrogen isotope (δ15N) values between feathers and red blood cells collected from ‘apapane and Hawaiʻi ‘amakihi in the upper Kaʻū Forest. Box plot whiskers depict the 10th and 90th percentiles and boxes show the 25th and 75th percentiles with the median value indicated; circles represent outliers.
Discussion
We demonstrate that collecting multiple tissue samples, which grow at different rates or time periods, from a single individual can provide insights into elevational movements over an extended time period. By examining stable hydrogen isotope values from a feather, RBCs, and blood plasma collected from the same individual during the fall, we documented elevational movements for ‘apapane and Hawaiʻi ‘amakihi between the summer, the period of peak feather molt following breeding, and the time around capture in the fall, represented by the blood plasma sample. RBCs provide information on movement between the two periods given the longer residency time of isotopes in RBCs (6 to 8 weeks) compared to plasma (~3 to 5 days) [32, 33, 37].
Consistent with our predictions based on the elevational-migration hypothesis we found that the majority of ‘apapane and Hawaiʻi ‘amakihi captured at high elevation sites in the fall where not year-round residents at the capture locations, but had molted their feathers at lower elevations presumably in the summer after breeding. δ2H values of feathers adjusted for tissue-specific discrimination for all ‘apapane and Hawaiʻi ‘amakihi sampled were higher than blood plasma isotope values, and the direction and magnitude of difference between δ2H values of feathers and blood plasma indicates that feathers were grown at lower elevations than their capture location. Likewise, estimations of the elevation of feather growth based on the relationship between δ2Hp and elevation on the east side of Hawaiʻi Island indicated that the average elevation of feather growth was around 1500m, which is below all three capture locations. Seventy-five percent of the birds captured had an estimated elevation of feather growth below their capture location, with primarily only birds captured at the lowest elevation capture site deviating from this pattern. The molting of feathers at lower elevations than the high elevation capture sites is also consistent with survey data during the breeding season showing the highest densities of ‘apapane and Hawaiʻi ‘amakihi in the Kaʻū Forest around 1500m [43]. Moreover, the closer proximity of δ2H values of RBCs to adjusted δ2H of feathers (average difference = 10.1‰) compared to plasma (average difference 32.3‰), indicates that birds may have 1) recently migrated to higher elevations, and still retain isotopes from lower elevations among their RBCs, 2) that birds are making daily long-distance movements to forage within both high and low elevation areas, and the isotope signature of RBCs represents an integration of both locations, or 3) some combination of the two scenarios. Visual observations of large numbers of birds making morning flights above the canopy from lower elevations forests, that were not in bloom, to flowering ʻōhiʻa trees at the high elevation capture sites in the fall (E. Paxton personal observations) is consistent with the second scenario. Collectively, the isotope results from multiple tissues provides empirical evidence for seasonal elevational migrations of both ‘apapane and Hawaiʻi ‘amakihi after the breeding season to high elevation sites within the Kaʻū Forest that have heavy ʻōhiʻa bloom.
Surprisingly, we did not find a difference in the propensity of elevational movement between ‘apapane and Hawaiʻi ‘amakihi. Given differences in the foraging strategies of ‘apapane, a nectivore, and Hawaiʻi ‘amakihi, a generalist, we predicted that ‘apapane would be more likely to make elevational movements in search of seasonally variable nectar resources while Hawaiʻi ʻamakihi would be more likely to switch diets when ʻōhiʻa bloom is scarce. However, our results, along with other studies [9, 61, 62] indicate that there may be more overlap in the foraging strategies of these two species, particularly at times when resources are highly concentrated. ʻŌhiʻa accounts for 90% of the trees and shrubs producing nectar in Hawaiian wet forests from sea level to tree line [12, 17], but the bloom of ʻōhiʻa is not uniform in space and time and the timing of peak flowering varies depending on a siteʻs elevation, substrate age, and genetic variation of ʻōhiʻa varieties present [14, 15, 17]. High elevation ʻōhiʻa varieties polymorpha and incana bloom in fall and winter, whereas lower elevation varieties such as glaberrima have peak bloom primarily in spring [15, 17, 46]. Differences in the timing of bloom by variety and elevation creates spatially and temporaly variable distributions of bloom that may drive elevational migration. Indeed, the mature stature ʻōhiʻa forest below our capture sites had virtually no bloom and the forest was quiet with little bird activity detected. In contrast, the low stature ʻōhiʻa patches where we captured birds in the fall had heavy bloom and high densities of birds, which were evident by both sight and auditory detection (E. Paxton personal observations). The high energy content of nectar compared to arthropods [63], may make long distant flights to track nectar resources across the landscape beneficial from an energetic consideration (e.g., [11]) for not only true nectivores like ʻapapane and ʻiʻiwi (Drepanis coccinea), but also Hawaiʻi ʻamakihi, especially when high volumes of nectar are concentrated in a particular area like the high elevation capture sites in this study. ‘Apapane are conspicous when moving long distances, flying above the canopy, whereas Hawaiʻi ‘amakihi are rarely seen flying above the canopy and likely move within or below the forest canopies (E. Paxton personal observations). Differences in the conspicuousness of the two speciesʻ flight patterns potentially associated with different foraging strategies may account for the perception that Hawaiʻi ‘amakihi do not move as much as ‘apapane. A better understanding of the consistency of bloom patterns across time within the Kaʻū Forest and other forests in Hawaiʻi would help to shed light on the mechanisms underlying the patterns found in this study.
The incorporation of carbon and nitrogen stable isotopes provides further evidence for the use of nectar resources by Hawaiʻi ʻamakihi at the high elevation capture sites in the fall. Hawaiʻi ʻamakihi and ‘apapane both had significant differences between δ13C and δ15N values of feathers and RBCs. However, the average difference between tissue types for ‘apapane was less than 2‰, indicating that for the majority of ‘apapane the change in isotope values between feathers and RBCs most likely represents only differences in tissue discrimination of isotopes [36, 55, 56], and not a shift in diet between seasons. In contrast, the majority of Hawaiʻi ʻamakihi had differences in isotope values between feathers and RBCs greater than 2‰, suggesting differences in isotope values between feathers and RBCs most likely represents a shift in diet between seasons. The high variability in δ13C and δ15N values of Hawaiʻi ʻamakihi also indicated that they had a more diverse diet across trophic levels than ʻapapane, particularly during the post-breeding period of feather molt. However, the greater depletion of N in RBC samples compared to feather samples of Hawaiʻi ʻamakihi indicated a greater reliance in the fall on nectar which is more depleted in N [34, 64]. The incorporation of δ13C and δ15N from plasma or breath samples in future studies would help to elicudate the role of nectar in the diet at the time of capture. In addition, tissue-specific discrimination factors for our study species or a comparable nectarivore species would allow for a more precise understanding of changes in diet between seasons.
The Hawaiian Archipelago, and particularly Hawaiʻi Island, is ideal for studying elevational movements with δ2H because of large elevational gradients (e.g., 0–4000 m) that occur across small geographic areas, resulting in a strong gradient of δ2Hp values that are driven by changes in elevation and not latitude. The rate of change in δ2Hp across elevations in Hawaiʻi (~1.8‰ per 100m) is consistent with other mountainous systems (e.g. Appalachian and Ecuadorian Andes Mountains) [24, 28, 31] and global patterns of precipitation (e.g., IAEA value for Hilo; [57]). However, unlike other tropical systems that have large seasonality in rainfall patterns (e.g., wet and dry seasons), which can result in different isotopic values between seasons [27], rainfall patterns on the Hawaiian Islands are largely driven by trade-winds resulting in consistent annual and seasonal δ2H values across elevations [44, 65]. While storm systems during the winter months, when trade-winds are less frequent, can sometimes result in lower δ2Hp values than expected, there was not an increase in storm events (>50mm rainfall in one event; definition given by [57]) during the time period of the study (NOAA National Climate Data Center for Hilo, Hawaiʻi, Network ID: GHCND:USW00021504).
Mobile animals such as birds can move across the landscape irespective of jurisdictional boundaries, which creates unique managment and conservation problems. Much of Ka’ū Forest is managed by Hawaiʻi Division of Forestry and Wildlife; however, the upper portion of the forest, where our study sites were located, is within the boundaries of Hawaiʻi Volcanoes National Park. Our study indicates that birds in the Ka’ū Forest move between the two reserves, and may be dependent on two different management entities, highlighting the importance of understaning movement of birds across the landscape, and how that movement connects different spatial areas over time. Ultimately, conservation of Hawaiian forest birds such as the ‘apapane and Hawaiʻi ‘amakihi may depend on the joint-management of lands under different owerships to ensure that habitat quality and protection is sufficient for the birds across the annual cycle.
Acknowledgments
Darcy Hu (National Park Service) was a key supporter of the project, and helped shape study design. Field work was conducted by Nolan Lancaster, Sonia Levitz, and Keith Burnett. We thank Hawaiʻi Volcanoes National Park for land access.
Data Availability
All data is available at a USGS data repository called ScienceBase-Catalog with the following citation and url: Paxton KL, Kelly JF, Pletchet SM, Paxton EH. 2020. Hawaii Volcanoes National Park stable isotope values from Hawaii forest birds 2012. U.S. Geological Survey data release: https://doi.org/10.5066/P98I4EP7.
Funding Statement
Funding for this study was provided to EHP through a U.S. Geological Survey Natural Resource Preservation Project grant. KLP was supported by a National Science Foundation (NSF) Centers for Research Excellence in Science and Technology (CREST) grant (0833211). JFK was supported by NSF grant EF-1840230. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of NSF. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alerstam T, Hedenström A, Åkesson S. Long distance migration: evolution and determinants. Oikos. 2003;103(2):247–60. [Google Scholar]
- 2.Dingle H, Drake VA. What is migration? Bioscience. 2007;57(2):113–21. [Google Scholar]
- 3.Boyle WA. Altitudinal bird migration in North America. The Auk. 2017;134(2):443–65. 10.1642/auk-16-228.1 [DOI] [Google Scholar]
- 4.Hsiung AC, Boyle WA, Cooper RJ, Chandler RB. Altitudinal migration: ecological drivers, knowledge gaps, and conservation implications. Biol Rev Camb Philos Soc. 2018;93(4):2049–70. 10.1111/brv.12435 [DOI] [PubMed] [Google Scholar]
- 5.Loiselle BA, Blake JG. Temporal variation in birds and fruits along an elevational gradient in Costa Rica. Ecology. 1991;72(1):180–93. [Google Scholar]
- 6.Levey DJ, Stiles FG. Evolutionary precursors of long-distance migration: resource availability and movement patterns in Neotropical landbirds. The American Naturalist. 1992;140(3):447–76. [Google Scholar]
- 7.Boyle WA, Norris DR, Guglielmo CG. Storms drive altitudinal migration in a tropical bird. Proceedings of Royal Society B. 2010;277(1693):2511–9. 10.1098/rspb.2010.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins RCL. Vertebrata In: Sharp D, editor. Fauna Hawaiiensis. 1 Cambridge, United Kingdom: University Press; 1903. p. 365–466. [Google Scholar]
- 9.Baldwin PH. Annual cycle, environment and evolution in the Hawaiian honeycreepers (Aves: Drepaniidae). Univ Calif Publ Zool; 1953;52:285–398. [Google Scholar]
- 10.Warner RE. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. The Condor. 1968;70(2):101–20. [Google Scholar]
- 11.MacMillen RE, Carpenter FL. Evening roosting flights of the honeycreepers Himatione sanguinea and Vestiaria coccinea on Hawaii. The Auk. 1980;97(1):28–37. [Google Scholar]
- 12.Mueller-Dombois D, Fosberg FR. Vegetation of the tropical Pacific islands: Springer Science & Business Media; 2013. [Google Scholar]
- 13.van Dyk KN, Paxton KL, Hart PJ, Paxton EH. Seasonality and prevalence of pollen collected from Hawaiian nectarivorous birds. Pacific Science. 2019;73(2):187–97. [Google Scholar]
- 14.Cordell S, Goldstein G, Meinzer FC, Handley LL. Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and δ13C along an altitudinal gradient. Functional Ecology. 1999;13(6):811–8. [Google Scholar]
- 15.Berlin KE, Simon JC, Pratt TK, Kowalsky JR, Hatfield JS. Akohekohe response to flower availability: seasonal abundance, foraging, breeding, and molt. Stud Avian Biol. 2001;22:202–12. [Google Scholar]
- 16.Guillaumet A, Kuntz WA, Samuel MD, Paxton EH. Altitudinal migration and the future of an iconic Hawaiian honeycreeper in response to climate change and management. Ecological Monographs. 2017;87(3):410–28. [Google Scholar]
- 17.Hart PJ, Woodworth BL, Camp RJ, Turner K, McClure K, Goodall K, et al. Temporal variation in bird and resource abundance across an elevational gradient in Hawaii. The Auk. 2011;128(1):113–26. [Google Scholar]
- 18.Ralph CJ, Fancy SG. Demography and movements of Apapane and Iiwi in Hawaii. The Condor. 1995;97(3):729–42. [Google Scholar]
- 19.Pratt LW, Jacobi JD. Loss, degradation, and persistence of habitats In: Pratt TK, Atkinson CT, Banko PC, J.D. J, Woodworth BL, editors. Conservation Biology of Hawaiian Forest Birds New Haven, CT: Yale University Press; 2009. p. 137–58. [Google Scholar]
- 20.Banko WE, Banko PC. Historic decline and extinction In: Pratt TK, Atkinson CT, Banko PC, J.D. J, Woodworth BL, editors. Conservation Biology of Hawaiian Forest Birds. New Haven, CT: Yale University Press; 2009. p. 25–58. [Google Scholar]
- 21.Atkinson CT, LaPointe DA. Introduced avian diseases, climate change, and the future of Hawaiian honeycreepers. J Avian Med Surg. 2009;23(1):53–63. 10.1647/2008-059.1 [DOI] [PubMed] [Google Scholar]
- 22.Bridge ES, Thorup K, Bowlin MS, Chilson PB, Diehl RH, Fléron RW, et al. Technology on the move: recent and forthcoming innovations for tracking migratory birds. Bioscience. 2011;61(9):689–98. [Google Scholar]
- 23.Hobson KA. Application of isotopic methods to tracking animal movements In: Hobson KA, Wassenaar LI, editors. Tracking Animal Migration with Stable Isotopes: Elsevier; 2019. p. 85–115. [Google Scholar]
- 24.Hobson KA, Wassenaar LI, Mila B, Lovette I, Dingle C, Smith TB. Stable isotopes as indicators of altitudinal distributions and movements in an Ecuadorean hummingbird community. Oecologia. 2003;136(2):302–8. 10.1007/s00442-003-1271-y [DOI] [PubMed] [Google Scholar]
- 25.Boyle WA, Guglielmo CG, Hobson KA, Norris DR. Lekking birds in a tropical forest forego sex for migration. Biol Lett. 2011;7(5):661–3. 10.1098/rsbl.2011.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newsome SD, Sabat P, Wolf N, Rader JA, Martinez Del Rio C. Multi-tissue d2H analysis reveals altitudinal migration and tissue-specific discrimination patterns in Cinclodes. Ecosphere. 2015;6(11). [Google Scholar]
- 27.Villega M, Newsome SD, Blake JG. Seasonal patterns in δ2H values of multiple tissues from Andean birds provide insights into elevational migration. Ecological Applications. 2016;26(8):2381–7. 10.1002/eap.1456 [DOI] [PubMed] [Google Scholar]
- 28.Hardesty JL, Fraser KC. Using deuterium to examine altitudinal migration by Andean birds. Journal of Field Ornithology. 2010;81(1):83–91. 10.1111/j.1557-9263.2009.00264.x [DOI] [Google Scholar]
- 29.Poage MA, Chamberlain CP. Empirical relationships between elevation and the stable isotope composition of precipitation and surface waters: considerations for studies of paleoelevation change. American Journal of Science. 2001;301(1):1–15. [Google Scholar]
- 30.Bowen G, Wilkinson B. Spatial distribution of d18O in meteoric precipitation. Geology 30, 315e318 2002. [Google Scholar]
- 31.Graves GR, Romanek CS, Navarro AR. Stable isotope signature of philopatry and dispersal in a migratory songbird. Proceedings of the National Academy of Sciences. 2002;99(12):8096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf N, Newsome SD, Fogel ML, Martinez Del Rio C. An experimental exploration of the incorporation of hydrogen isotopes from dietary sources into avian tissues. J Exp Biol. 2012;215(11):1915–22. 10.1242/jeb.065219 [DOI] [PubMed] [Google Scholar]
- 33.Storm-Suke A, Norris DR, Wassenaar LI, Chin E, Nol E. Factors influencing the turnover and net isotopic discrimination of hydrogen isotopes in proteinaceous tissue: experimental results using Japanese quail. Physiol Biochem Zool. 2012;85(4):376–84. 10.1086/666476 [DOI] [PubMed] [Google Scholar]
- 34.Martínez del Rio C, Wolf N, Carleton SA, Gannes LZ. Isotopic ecology ten years after a call for more laboratory experiments. Biological Reviews. 2009;84(1):91–111. 10.1111/j.1469-185X.2008.00064.x [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain C, Blum J, Holmes RT, Feng X, Sherry T, Graves GR. The use of isotope tracers for identifying populations of migratory birds. Oecologia. 1997;109(1):132–41. [DOI] [PubMed] [Google Scholar]
- 36.Podlesak DW, McWilliams SR, Hatch KA. Stable isotopes in breath, blood, feces and feathers can indicate intra-individual changes in the diet of migratory songbirds. Oecologia. 2005;142(4):501–10. 10.1007/s00442-004-1737-6 [DOI] [PubMed] [Google Scholar]
- 37.McKinnon EA, Fraser KC, Diamond AW, Rimmer CC, Townsend JM. Stable-hydrogen isotope turnover in red blood cells of two migratory thrushes: application to studies of connectivity and carry-over effects. Journal of Field Ornithology. 2012;83(3):306–14. 10.1111/j.1557-9263.2012.00380.x [DOI] [Google Scholar]
- 38.Wolf N, Bowen GJ, Del Rio CM. The influence of drinking water on the dD and d18O values of house sparrow plasma, blood and feathers. J Exp Biol. 2011;214(Pt 1):98–103. 10.1242/jeb.050211 [DOI] [PubMed] [Google Scholar]
- 39.Wolf N, Newsome SD, Fogel ML, Del Rio MC. The relationship between drinking water and the hydrogen and oxygen stable isotope values of tissues in Japanese quail (Cortunix japonica). The Auk. 2013;130(2):323–30. [Google Scholar]
- 40.Fancy SG, Ralph CJ. Apapane (Himatione sanguinea) In: Poole A, Gill F, editors. In The Birds of North America, No 296 Ithaca, NY: Cornell Lab of Ornithology; 1997. [Google Scholar]
- 41.Lindsey GD, VanderWerf EA, Baker H, P.E. B. Hawaii Amakihi (Chlorodrepanis virens) In: Poole A, editor. The Birds of North America. Ithaca, NY: Cornell Lab of Ornithology; 1998. [Google Scholar]
- 42.Eggert LS, Terwilliger LA, Woodworth BL, Hart PJ, Palmer D, Fleischer RC. Genetic structure along an elevational gradient in Hawaiian honeycreepers reveals contrasting evolutionary responses to avian malaria. BMC Evol Biol. 2008;8:315 10.1186/1471-2148-8-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorresen PM, Camp RJ, Pratt TK. Forest bird distribution, density and trends in the Ka`ū region of Hawai`i Island. 2007. US Geological Survey Open-File Report. [Google Scholar]
- 44.Scholl MA, Ingebritsen SE, Janik CJ, Kauahikaua JP. Use of precipitation and groundwater isotopes to interpret regional hydrologyon a tropical volcanic island: Kilauea volcano area, Hawaii. Water Resources Research 1996;32(12):3525–37. [Google Scholar]
- 45.Paxton EH, McLaughlin R, Levins S, Vanderwerf E, Lancaster N. Aging and sexing guide to the forest birds of Hawai ‘i Island. Hilo, HI: University of Hawai'i at Hilo, 2016. [Google Scholar]
- 46.Ralph CJ, Fancy SG. Timing of breeding and molting in six species of Hawaiian honeycreepers. Condor. 1994;96:151–61. [Google Scholar]
- 47.Freed LA, Cann RL. Changes in timing, duration, and symmetry of molt of Hawaiian forest birds. PLoS ONE. 2012;7(1):e29834 10.1371/journal.pone.0029834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chew B, Kelly J, Contina A. Stable isotopes in avian research: a step by step protocol to feather sample preparation for stable isotope analysis of carbon (δ13C), nitrogen (δ15N), and hydrogen (δ2H). Version 1.1. protocolsio. 2019. dx.doi.org/10.17504/protocols.io.z2uf8ew. [Google Scholar]
- 49.Bearhop S, Waldron S, Votier SC, Furness RW. Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiological and Biochemical Zoology. 2002;75(5):451–8. 10.1086/342800 [DOI] [PubMed] [Google Scholar]
- 50.Wassenaar L, Hobson K. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes in Environmental and Health Studies. 2003;39(3):211–7. 10.1080/1025601031000096781 [DOI] [PubMed] [Google Scholar]
- 51.Kelly JF, Bridge ES, Fudickar AM, Wassenaar LI. A test of comparative equilibration for determining non-exchangeable stable hydrogen isotope values in complex organic materials. Rapid Communications in Mass Spectrometry. 2009;23(15):2316–20. 10.1002/rcm.4150 [DOI] [PubMed] [Google Scholar]
- 52.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 53.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software. 2017;82(13):1–26. 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 54.Lenth R, Lenth MR. Package ‘lsmeans’. The American Statistician. 2018;34(4):216–21. [Google Scholar]
- 55.Pearson SF, Levey DJ, Greenberg CH, Martinez Del Rio C. Effects of elemental composition on the incorporation of dietary nitrogen and carbon isotopic signatures in an omnivorous songbird. Oecologia. 2003;135(4):516–23. 10.1007/s00442-003-1221-8 [DOI] [PubMed] [Google Scholar]
- 56.Hobson KA, Bairlein F. Isotopic fractionation and turnover in captive Garden Warblers (Sylvia borin): implications for delineating dietary and migratory associations in wild passerines. Can J Zool. 2003;81(9):1630–5. 10.1139/z03-140. [Google Scholar]
- 57.Scholl MA, Ingebritsen SE, Janik CJ, Kauahikaua JP. An Isotope hydrology study of the Kilauea volcano area, Hawaii U.S. Geological Survey, 1995. [Google Scholar]
- 58.Nordell CJ, Hache S, Bayne EM, Solymos P, Foster KR, Godwin CM, et al. Within-Site Variation in Feather Stable Hydrogen Isotope (delta2Hf) Values of Boreal Songbirds: Implications for Assignment to Molt Origin. PLoS ONE. 2016;11(11):e0163957 10.1371/journal.pone.0163957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moran JA, Wassenaar LI, Finlay JC, Hutcheson C, Isaac LA, Wethington SM. An exploration of migratory connectivity of the Rufous Hummingbird (Selasphorus rufus), using feather deuterium. J Ornithol. 2012;154(2):423–30. 10.1007/s10336-012-0906-3 [DOI] [Google Scholar]
- 60.Hutcheson CA, Hendrix L, Moran JA. An isotopic analysis of migratory connectivity in Ruby-throated Hummingbirds. N Am Bird Bander. 2010;35:5–11. [Google Scholar]
- 61.Carpenter FL. Food abundance and territoriality: to defend or not to defend? American Zoologist. 1987;27(2):387–99. [Google Scholar]
- 62.Pimm SL, Pimm JW. Resource use, competition, and resource availability in Hawaiian honeycreepers. Ecology. 1982;63(5):1468–80. [Google Scholar]
- 63.Ford HA, Paton DC. The value of insects and nectar to honeyeaters. Emu. 1976;76(2):83–4. [Google Scholar]
- 64.Symes CT, McKechnie AE, Nicolson SW, Woodborne SM. The nutritional significance of a winterterrs. Ecology. 1982;63(5):1468-80.tic avian nectarivores. Ibis. 2011;153(1):110–21. [Google Scholar]
- 65.Scholl MA, Gingerich SB, Tribble GW. The influence of microclimates and fog on stable isotope signatures used in interpretation of regional hydrology: East Maui, Hawaii. Journal of Hydrology. 2002;264:1–4. [Google Scholar]