INTRODUCTION
Osmotic Demyelination Syndrome (ODS) occurs following rapid overcorrection of hyponatremia.1 It is characterized by destruction of neuronal myelin sheaths in either the central area of the pons or in other susceptible areas causing severe irreversible neurologic deficits. Methods described in the literature suggested varying sodium correction rates which lead to different definitions of overcorrection of hyponatremia.2 The incidence of ODS consequently would be expected to increase. However, no general consensus exists regarding the optimal treatment regimen for this disease. Nevertheless, evidence-based management is essential for those who are diagnosed. We present a case of ODS treated with plasmapheresis (PP), intravenous immunoglobulins (IVIG), and intravenous methylprednisolone with significant recovery of neurologic function.
CASE REPORT
A 49-year-old male with a history of peptic ulcer disease and newly diagnosed hypertension was transferred to our facility for management of acute neurological deficits. He was also an alcoholic and had been drinking eight beers daily for the past 10 years. Three weeks prior to arrival to our hospital, the patient’s primary care provider (PCP) started him on hydrochlorothiazide for new-onset hypertension. A renal panel was drawn during the office visit and revealed a sodium level of 135 mEq/L. Two weeks following the initiation of the thiazide drug, a renal panel revealed a sodium level of 128 mEq/L. Two days later, he started having acute behavioral changes which prompted his wife to take him to the nearest hospital in their vicinity.
The patient was admitted to the peripheral hospital for severe symptomatic hypovolemic hyponatremia with a sodium level of 102 mEq/L. Per the transfer notes, he was treated with three boluses of 1 liter of normal saline followed by 900 ml of hypertonic saline (3%) at a rate of 100 ml/hour, which increased his sodium level to 140 mEq/L. The patient did not exhibit any neurologic deficits during the admission and upon discharge. He was sent home the following day with instructions to discontinue hydrochlorothiazide.
Follow-up laboratory testing were obtained at the PCP’s clinic the day he was discharged and revealed a sodium level of 138 mEq/L. However, the patient’s wife noticed that her husband started developing bilateral tremors, rigidity, and dysarthria. She took him back to the facility from which he was discharged for evaluation. He was re-admitted there for treatment of sodium overcorrection. He was given 5% dextrose in 0.45% normal saline and 2 mcg of desmopressin, then transferred to our hospital for a higher level of care.
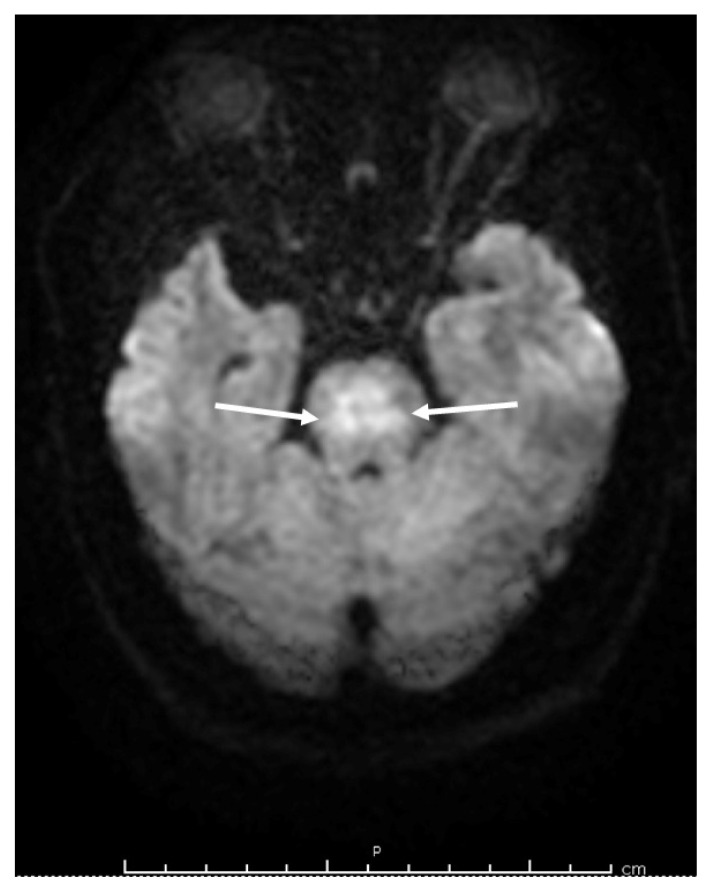
Upon arrival to our facility, his sodium level was 141 mEq/L. His physical examination was remarkable for lethargy, diaphoresis, and decorticate posture. He was arousable to voice commands and was aphasic. His pupils were pinpoint, equal, and reactive. Bilateral upper and lower extremities were stiff and tremulous, while reflexes were difficult to assess. Magnetic resonance imaging (MRI) of the brain revealed pathologic diffusion restriction within the central pons with hyperintense signals in the basal ganglia (Figure 1). Electroencephalogram (EEG) did not show any epileptiform discharges.
Figure 1.
Magnetic resonance imaging of the brain showing diffusion restriction within the pontine region sparing the descending tracts (arrows) which is typically seen in osmotic demyelination syndrome.
The patient’s hyponatremia was corrected from 102 to 140 mEq/L within less than 24 hours during his first admission. He then started developing acute neurological symptoms concerning for brainstem dysfunction. MRI of his brain ruled out stroke and revealed features pathognomonic of central pontine myelinolysis. EEG ruled out status epilepticus or any seizure activity. The patient’s clinical picture was most consistent with osmotic demyelination syndrome.
The nephrology team initiated 2 mcg of desmopressin every six hours and 5% dextrose in water with a goal to lower sodium to 120 mEq at a rate of 6 – 8 mEq per day. Treatment options and prognosis were discussed with the patient’s wife. PP, IVIG, and steroids were initiated in an attempt to recover his neurologic function as much as possible. He received plasmapheresis every other day for a total of six treatments. He was given 125 mg of intravenous methylprednisolone every eight hours for three days followed by dose tapering and 25 g of IVIG daily for five days. The neurology team started levetiracetam 500 mg twice a day for seizure prophylaxis. Sodium level was maintained around 120 mEq during the first five days and gradually increased by 4 mEq daily thereafter.
During the course of his admission, he required intubation due to his altered mental status and inability to protect his airways. Following extubation, he required a percutaneous endoscopic gastrostomy (PEG) tube for feeding. He worked daily with a multidisciplinary team, including speech, physical, and occupational therapy. Eleven weeks following admission, the patient was 30% weight-bearing and was able to speak in short sentences. The PEG tube was removed and he was able to swallow pureed food and thickened liquids. He was able to use utensils for feeding, comb his hair, and wash his face. He required some assistance during bathing and dressing. He was discharged to a skilled nursing facility for inpatient rehabilitation services. Six months following his discharge, he was able to walk using a cane, perform activities of daily living without assistance, and regained 60% of his strength.
DISCUSSION
ODS is characterized by severe irreversible neurological deficits. The spectrum of symptoms is wide and affected individuals can exhibit one or a combination of encephalopathy, extrapyramidal symptoms, seizures, dystonia, gaze palsy, quadriparesis, dysarthria, dysphagia, and/or locked-in syndrome.3,4 The most common inciting event is rapid correction of hyponatremia. Additional risk factors include concurrent electrolyte imbalances, a sodium level less than 105 mEq/L, chronic alcoholism, diuretics use, liver transplant, malnutrition, and endocrinopathies.3
Thiazide diuretics, in particular, are known to cause serious interactions when mixed with alcohol.5 This combination was probably the main culprit behind his profound hyponatremia. Prognosis is very poor and small case studies have shown that a third of the patients die after one year.3,6 Among those that survive, the rate and degree of recovery of neurologic function is unpredictable. The pathogenesis of ODS is poorly understood making its management challenging and uncertain. During the treatment of hyponatremia, the serum osmolality increases with the increasing sodium concentration. Water shifts out of the brain cells to equilibrate between intracellular and extracellular solute concentration. Organic osmolytes are necessary for the protection of astrocytes and oligodendrocytes. When shrinking of glial cells occurs at a faster rate than repletion of organic osmolytes, most commonly during rapid correction of hyponatremia, programmed cell death is activated in these myelin producing cells leading to disruption of the blood brain barrier and release of inflammatory cytokines.7,8
In an attempt to minimize the incidence of ODS, the American Society of Nephrology has set forth guidelines regarding therapeutic goals and limits for the management of hyponatremia.9 The recommended rate of sodium correction in acute hyponatremia is 4 to 6 mEq/L within the first two hours, with a limit of 10 mEq/L within 24 hours and 8 mEq/L per day thereafter. For chronic hyponatremia, the recommended rate is 4 to 6 mEq/L within the first 12 hours and 8 mEq/L per day afterwards.9 However, Woodfine et al.2 showed in their cohort study that ODS may occur in patients regardless of the rate of sodium correction.
Multiple treatment options have been proposed for ODS including intravenous methylprednisolone, IVIG, and PP. However, these recommendations are based on small case series. Atchaneeyasakul et al.10 theorized that PP reduces the myelinotoxic products released following the osmotic stress. Five cases treated with PP resulted in near complete recovery of neurological symptoms.11–13 Murase et al.8 demonstrated that ODS can have an autoimmune component as well, antibodies are released following the osmotic insult into the circulation. Six patients had significant recovery following treatment with IVIG.14–17 Treatment with both PP and IVIG has been trialed as well with significant improvement in four patients.10,18,19 The use of intravenous methylprednisolone has been described as well, however, further studies are needed to establish the true efficacy of the use of steroids in this setting.3,20
Case reports usually describe regimens with successful outcomes. Patients who did not benefit from specific treatments are not documented in the literature, making it difficult to evaluate the efficacy and success rate associated with every treatment available. No clinical trials have been initiated for the treatment of ODS. In an attempt to provide our patient with the highest chance of recovery possible, he was treated with PP, IVIG, and steroids. Recovery was achieved gradually with the support of a multidisciplinary team (physical, occupational, and speech therapy).
The aim of our case report was to provide data regarding the outcome following triple therapy and to invite more physicians to report positive and negative progress seen in patients treated as such. To the best of our knowledge, this is the first case of ODS treated with PP, IVIG, and intravenous methylprednisolone concurrently.
REFERENCES
- 1.Lambeck J, Hieber M, Dreßing A, Niesen WD. Central pontine myelinosis and osmotic demyelination syndrome. Dtsch Arztebl Int. 2019;116(35–36):600–606. doi: 10.3238/arztebl.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodfine JD, van Walraven C. Criteria for hyponatremic overcorrection: Systematic review and cohort study of emergently ill patients. J Gen Intern Med. 2020;35(1):315–321. doi: 10.1007/s11606-019-05286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallakatta RN, Radhakrishnan A, Fayaz RK, Unnikrishnan JP, Kesavadas C, Sarma SP. Clinical and functional outcome and factors predicting prognosis in osmotic demyelination syndrome (central pontine and/or extrapontine myelinolysis) in 25 patients. J Neurol Neurosurg Psychiatry. 2011;82(3):326–331. doi: 10.1136/jnnp.2009.201764. [DOI] [PubMed] [Google Scholar]
- 4.Odier C, Nguyen DK, Panisset M. Central pontine and extrapontine myelinolysis: From epileptic and other manifestations to cognitive prognosis. J Neurol. 2010;257(7):1176–1180. doi: 10.1007/s00415-010-5486-7. [DOI] [PubMed] [Google Scholar]
- 5.Holton AE, Gallagher PJ, Ryan C, Fahey T, Cousins G. Consensus validation of the POSAMINO (POtentially Serious Alcohol–Medication INteractions in Older adults) criteria. BMJ Open. 2017;7(11):e017453. doi: 10.1136/bmjopen-2017-017453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis G, Megarbane B, Lavoué S, et al. Long-term outcome of patients hospitalized in intensive care units with central or extrapontine myelinolysis*. Crit Care Med. 2012;40(3):970–972. doi: 10.1097/CCM.0b013e318236f152. [DOI] [PubMed] [Google Scholar]
- 7.Lien YH, Shapiro JI, Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J Clin Invest. 1991;88(1):303–309. doi: 10.1172/JCI115292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murase T, Sugimura Y, Takefuji S, Oiso Y, Murata Y. Mechanisms and therapy of osmotic demyelination. Am J Med. 2006;119(7 Suppl 1):S69–73. doi: 10.1016/j.amjmed.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Sterns RH. Treatment of severe hyponatremia. Clin J Am Soc Nephrol. 2018;13(4):641–649. doi: 10.2215/CJN.10440917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atchaneeyasakul K, Tipirneni A, Gloria S, Berry AC, Shah K, Yavagal DR. Osmotic demyelination syndrome: Plasmapheresis versus intravenous immunoglobulin? Intern Emerg Med. 2017;12(1):123–126. doi: 10.1007/s11739-016-1452-4. [DOI] [PubMed] [Google Scholar]
- 11.Bibl D, Lampl C, Gabriel C, Jüngling G, Brock H, Köstler G. Treatment of central pontine myelinolysis with therapeutic plasmapheresis. Lancet. 1999;353(9159):1155. doi: 10.1016/S0140-6736(99)01145-9. [DOI] [PubMed] [Google Scholar]
- 12.Grimaldi D, Cavalleri F, Vallone S, Milanti G, Cortelli P. Plasmapheresis improves the outcome of central pontine myelinolysis. J Neurol. 2005;252(6):734–735. doi: 10.1007/s00415-005-0738-7. [DOI] [PubMed] [Google Scholar]
- 13.Chang KY, Lee IH, Kim GJ, Cho K, Park HS, Kim HW. Plasma exchange successfully treats central pontine myelinolysis after acute hypernatremia from intravenous sodium bicarbonate therapy. BMC Nephrol. 2014;15(1):56. doi: 10.1186/1471-2369-15-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deleu D, Salim K, Mesraoua B, El Siddig A, Al Hail H, Hanssens Y. “Man-in-the-barrel” syndrome as delayed manifestation of extrapontine and central pontine myelinolysis: Beneficial effect of intravenous immunoglobulin”. J Neurol Sci. 2005;237(1–2):103–106. doi: 10.1016/j.jns.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Finsterer J, Engelmayer E, Trnka E, Stiskal M. Immunoglobulins are effective in pontine myelinolysis. Clin Neuropharmacol. 2000;23(2):110–113. doi: 10.1097/00002826-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Murthy SB, Izadyar S, Dhamne M, Kass JS, Goldsmith CE. Osmotic demyelination syndrome: Variable clinical and radiologic response to intravenous immunoglobulin therapy. Neurol Sci. 2013;34(4):581–584. doi: 10.1007/s10072-012-1027-8. [DOI] [PubMed] [Google Scholar]
- 17.Thirunavukarasu S, Biswas A, Furruqh F, Gnanavelan A. Response to IV immunoglobulin in a case of osmotic demyelination syndrome. BMJ Case Rep. 20152015 doi: 10.1136/bcr-2015-212985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig KP, Thiesset HF, Gayowski TJ, Schwartz JJ. Plasmapheresis and intravenous immune globulin improve neurologic outcome of central pontine myelinolysis occurring post orthotopic liver transplant. Ann Pharmacother. 2011;45(2):e10. doi: 10.1345/aph.1P371. [DOI] [PubMed] [Google Scholar]
- 19.Saner FH, Koeppen S, Meyer M, et al. Treatment of central pontine myelinolysis with plasmapheresis and immunoglobulins in liver transplant patient. Transpl Int. 2008;21(4):390–391. doi: 10.1111/j.1432-2277.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto E, Hagiwara D, Morishita Y, Tsukiyama K, Kondo K, Yamamoto M. [Complete recovery of central pontine myelinolysis by high dose pulse therapy with methylprednisolone]. Nippon Naika Gakkai Zasshi. 2007;96(10):2291–2293. doi: 10.2169/naika.96.2291. [DOI] [PubMed] [Google Scholar]