Abstract
After the advent and the subsequent gradual disuse of laser technology in gynaecological surgery, in recent years, thanks to technical improvements, this technology is progressively reaffirming itself in various areas of minimally invasive gynaecological surgery ranging from laparoscopy to robotic surgery and hysteroscopy.
This paper, through a SWOT (strengths, weaknesses, opportunities and threats) analysis, shows positives and negatives of this technology with particular attention to present and future applications.
Keywords: Laser, laparoscopy, hysteroscopy, endometriosis
Background
Laser (Light Amplification by Stimulated Emission of Radiation) is a useful energy source available for gynaecological endoscopy. Laser technology is based on the amplification of a specific light wavelength that generates the emission of a beam of photons with a high degree of spatial and temporal coherence. The contact of a laser beam with organic tissue generates molecular vibration, inducing the disruption of chemical bonds and the production of heat. Tissue effect is the result of laser beam absorption, refraction and reflection and can be modulated by adapting exposure time and power density (Law et al., 2014).
The selective tissue absorption characteristics of different kind of lasers can be exploited in surgery. The CO2 laser is excellent for tissue vaporisation because it is absorbed by water but is not effective in tissue desiccation or coagulation; while the Nd:YAG laser could be selectively absorbed by pathologically coloured tissues containing haemoglobin such as endometriotic tissue (Lomano, 1987).
Initially, the use of the laser was promoted as a response to some of electrosurgery complications. Specifically, the laser offered the possibility of more selective tissue effect through minimal lateral thermal damage when compared to classical electrosurgery.
In this regard, the following SWOT analyses will focus on positives and negatives of this technology with particular attention to present and future applications (Figure 1).
Figure 1.
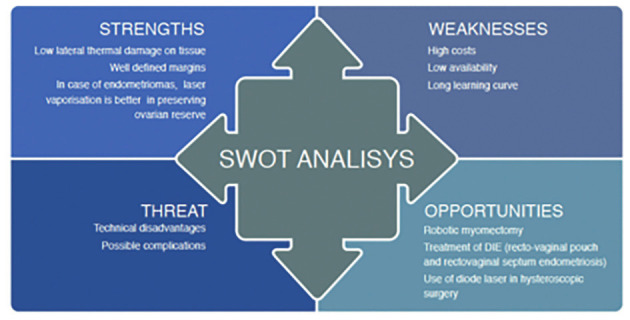
— SWOT Analysis on lasers in gynaecology.
DIE: Deeply infiltaring endometriosis.
Strengths
Several studies have compared different types of energy employed in gynaecological surgery and their collateral tissue effects. Laser energy inflicts the least amount of damage to the surrounding tissues both in human and animal models and it shows greater surgical precision than monopolar electrosurgery, both in terms of cut and coagulation modes.
Bailey et al. (2014) have shown, in animal models, how monopolar energy causes a greater collateral thermal damage on the myometrium than the flexible CO2 laser fibre. This lateral thermal damage increases in a linear way with monopolar power settings. Conversely increasing the power setting of the laser (in the range from 5 to 15 W) affects only the depth of incision and dissection capacity without increasing collateral thermal damage.
Also in a microscopic comparison of the shape of the incision, the Nd:YAG laser produced the smoothest lesions with well-defined margins compared to the monopolar energy that was more often associated with irregular and fissured margins (Schurr et al., 1994).
Concerning endometriotic cyst management, laparoscopic endometrioma excision was considered the gold standard of care being more effective than drainage or vaporisation in terms of pelvic pain control (Saito et al., 2014), recurrence and pregnancy rate. The pregnancy rate increases after laparoscopic cystectomy, varying from 30% to 67%, with an average of approximately 50% (Vercellini et al., 2009).
In a randomised trial of 90 women comparing ovarian cystectomy and CO2 laser vaporisation of the internal wall of the endometrioma, Carmona et al. (2011) found that the laser group was affected by an earlier time of recurrence (7.5 vs 18.1 months, P < 0.003) and a significantly higher rate of recurrence at 12 months’ follow-up (11% vs 31%, P = 0.04).
Conversely, the absence of a clear cleavage plan during stripping determines an involuntary removal of healthy ovarian cortex that is proportional to the size of the cyst itself (Saito et al., 2018).
Several studies show how adverse changes in ovarian vascularisation after stripping (Li et al., 2009; Suksompong et al., 2012) inducing ischemic vascular damage to the residual ovary, can be a consequence of the attempt to reach an accurate haemostasis via bipolar coagulation or through the application of haemostatic stitches on the ovary (La Torre et al., 1998). It has been widely accepted that only the internal lining of the cyst wall should be destroyed with a depth of ablation not exceeding 1.0–1.5 mm, due to endometriotic cells being localised only on the surface of the cyst capsule (Saridogan et al., 2017).
Taking this perspective makes the use of laser ablation of endometriotic cysts interesting: CO2 laser ablation was demonstrated to be effective in the treatment of endometriomas in three different settings: the “three stages technique”, the “stripping and ablation combined technique” and the “pure ablative technique”. Tsolakidis et al. (2010) and Pados et al. (2010) performed a randomised controlled trial comparing the stripping technique with the so-called “three-stage technique”. This last procedure consists of: 1) Laparoscopic fenestration and drainage of the endometriotic cyst; 2) Gonadotrophin-releasing hormone agonist (GnRHa) therapy for 3 months 3) second laparoscopy in which CO2 laser ablation of the cyst wall is performed. They concluded that ovarian vascularisation and volumes were comparable between the two laparoscopic techniques, but the follicular reserve, determined by AFC (antral follicle count) at six months, was significantly higher in patients subjected to the “three-step procedure” compared to the other group. Furthermore, although the ovarian reserve did not improve in either group, the AMH level is less diminished after the three-step procedure compared with cystectomy.
Donnez et al. (2010) and Nappi et al. (2016) proposed a combined technique, stripping most of the cyst wall and vaporising the remaining 10–20% of endometrioma wall close to the hilum, using the CO2 laser or DWLS (new diode laser). Donnez et al. (2010) found a pregnancy rate of 41% at 8.3 months and a recurrence rate of only 2%.
Recently, Munrós et al. (2019) confirmed how ablative techniques, such as CO2 laser vaporisation, are better in preserving ovarian reserve compared to laparoscopic stripping by reducing the accidental removal of healthy tissue with a subsequent lower post-surgical inflammatory and procoagulant state whose marker is the curve of microparticles. The increase in the microparticles level is observed only after stripping but not after laser vaporisation, highlighting how this procedure determines a minimal inflammatory response that doesn’t negatively affect the ovarian reserve in terms of AMH and AFC at six months after surgery.
Finally, laser vaporisation shows similar pregnancy rates to cystectomy at long- term follow-up (1 and 5 years): this data has been confirmed by a recent meta-analysis (Dan et al., 2013).
Weaknesses
Lasers were introduced into gynaecological surgery almost forty years ago; the CO2 laser was the first one developed by Patel and his colleagues in 1964 (Bell Laboratories in California) (Sutton et al., 2013).
Laser utilisation experienced the acme in the 1980’s and its use was clinically validated in 1994 with a randomised, double-blind controlled trial for the treatment of pelvic pain associated with endometriosis (Sutton et al., 1994). This period was followed by a progressive decline due to the advent of laparoscopy. In the laparoscopic setting, lasers become a less useful and unwieldy tool due to the limitations in terms of manoeuvrability imposed by the traditional line-of-sight systems that displayed a great difficulty in obtaining sufficient anatomical exposure. Engineering advancements, associated with lower costs, facilitated the reaffirmation of the electrosurgery in laparoscopy and has relegated the laser to limited utilisation in specialised centers (Choussein et al., 2015).
In the recent years, the use of the laser in gynaecological laparoscopy was limited by high cost, low availability and long learning curves (Law et al., 2014).
Opportunities
In recent years, technological advances and technical improvements are increasing the number of procedures in minimally invasive gynaecology that can exploit laser technology again. Robotic myomectomy, thanks to the advent of a flexible, fully articulated CO2 laser delivery system is demonstrated to be feasible and safer than other alternatives such as electrosurgery or ultrasonic scalpel (Sutton et al., 1994).
Lasers delivered by flexible fibres assure both higher incising efficiency and superior wound- healing effects on the uterus than monopolar electrosurgery (Choussein et al., 2015), leading to better obstetrical outcomes in terms of the risk of uterine rupture during pregnancy (Parker et al., 2010).
The higher costs of fully articulated CO2 laser delivery systems find their potential justification in the reduction of direct and indirect costs by lowering complication rates and shortening hospital stay.
The CO2 laser could also be used to treat women affected by deep infiltrating endometriosis (DIE) in different settings. Meuleman et al. (2011) demonstrated the achievement of low complication rates and a good clinical outcome within 2 years of surgery in a subgroup of women with DIE and colorectal wall invasion treated by a multidisciplinary laparoscopic surgery including CO2 laser and consisting of radical endometriosis excision with segmental bowel resection and reanastomosis. Kristensen et al. (2007) confirmed that the laser could be effectively used to treat patients with recto-vaginal pouch and rectovaginal septum endometriosis, with a significant statistical difference between preoperative and postoperative pain scores and quality of life.
Over the years, the laser has also been used in other fields, such as in hysteroscopic surgery. In this field, different kinds of lasers have been used; the Nd-Yag laser, KTP or the Argon laser. The diode laser represents the most significant novelty and is able to produce two wavelengths between 980 and 1470 nm. These wavelengths allow simultaneous cutting and coagulation generating haemostasis significantly higher than the CO2 laser. In a pilot study of 18 patients, Nappi et al. (2016) used the diode laser for hysteroscopic metroplasty, demonstrating how the procedure was safe, feasible, potentially preventing the formation of intrauterine adhesions and reducing the risk of recurrences. The same group also demonstrated the safety and effectiveness of the diode-laser hysteroscopic endometrial polypectomy in a prospective study on 300 women (Nappi et al., 2017). A randomised clinical trial has demonstrated how polypectomy with the diode laser resulted in fewer relapses and a higher procedure satisfaction rate compared to the bipolar energy system (Lara- Domínguez et al., 2016). Other recent studies also proposed office hysteroscopic laser enucleation of submucous myomas with good results (Haimovich et al., 2015).
Threats
The laser showed several technical disadvantages; it can only be used with a rigid lens system, it can be reflected by surgical instruments leading to injury of nontargeted tissues, it also hides the risk of igniting flammable materials or causing eye damage and, particularly with the CO2 laser, it is difficult to use in presence of a haemorrhagic field because it is absorbed by water and other fluids (Bailey et al., 2014).
Concluding Remarks
The laser in gynaecology is indicated for a growing range of minimally invasive surgery procedures. It appears that laparoscopic laser vaporisation is a good option for the treatment of ovarian endometriomas as it reduces the damage to ovarian tissue compared to laparoscopic stripping (Munrós et al., 2019). Moreover, it can be useful in the treatment of DIE in patients with recto-vaginal pouch and rectovaginal septum endometriosis (Kristensen et al., 2007).
The laser has also been proposed for robotic myomectomy, thanks to the advent of a flexible, fully- articulated CO2 laser delivery system. In addition, the diode laser has been widely used in hysteroscopic surgery: in case of endometrial polyps it is safe and effective (Nappi et al., 2017); it can be used for metroplasty (Nappi et al., 2016); in recent studies the diode laser has been proposed to treat submucous myomas.
On the other hand, the use of the laser in gynaecological endoscopy has been limited, over the years, by high costs, a low availability and long learning curves (Law et al., 2014).
Technological advancement and cost reduction are necessary to further extend its application in minimally invasive gynaecological surgery.
Contributor Information
ESGE Special Interest Group ‘Innovations’ Working Group:
U Catena, A Rosati, MM Ianieri, I Romito, N Vlahos, A Daniilidis, G Scambia, and S Becker
References
- 1.Bailey AP, Lancerotto L, Gridley C, et al. Greater surgical precision of a flexible carbon dioxide laser fiber compared to monopolar electrosurgery in porcine myometrium. J Minim Invasive Gynecol. 2014;21:1103–1109. doi: 10.1016/j.jmig.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Carmona F, Martínez-Zamora MA, Rabanal A, et al. Ovarian cystectomy versus laser vaporization in the treatment of ovarian endometriomas: a randomized clinical trial with a five-year follow-up. Fertil Steril. 2011;96:251–254. doi: 10.1016/j.fertnstert.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 3.Choussein S, Srouji SS, Farland LV, et al. Flexible Carbon Dioxide Laser Fiber Versus Ultrasonic Scalpel in Robot-Assisted Laparoscopic Myomectomy. J Minim Invasive Gynecol. 2015;22:1183–1190. doi: 10.1016/j.jmig.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Dan H, Limin F. Laparoscopic ovarian cystectomy versus fenestration/coagulation or laser vaporization for the treatment of endometriomas: A meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;76:75–82. doi: 10.1159/000351165. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Lousse JC, Jadoul P, et al. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94:28–32. doi: 10.1016/j.fertnstert.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 6.Haimovich S, Lopez-Yarto M, Urresta Avila J, et al. Office Hysteroscopic Laser Enucleation of Submucous Myomas without Mass Extraction: A Case Series Study. Biomed Res Int. 2015;2015:905204. doi: 10.1155/2015/905204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen J, Kjer JJ. Laparoscopic laser resection of rectovaginal pouch and rectovaginal septum endometriosis: the impact on pelvic pain and quality of life. Acta Obstet Gynecol Scand. 2007;86:1467–1471. doi: 10.1080/00016340701645006. [DOI] [PubMed] [Google Scholar]
- 8.Lara-Domínguez MD, Arjona-Berral JE, Dios-Palomares R, et al. Outpatient hysteroscopic polypectomy: bipolar energy system (Versapoint ® ) versus diode laser – randomized clinical trial. Gynecol Endocrinol. 2016;32:196–200. doi: 10.3109/09513590.2015.1105209. [DOI] [PubMed] [Google Scholar]
- 9.La Torre R, Montanino-Oliva M, Marchiani E, et al. Ovarian blood flow before and after conservative laparoscopic treatment for endometrioma. Clin Exp Obstet Gynecol. 1998;25:12–14. [PubMed] [Google Scholar]
- 10.Law KS, Abbott JA, Lyons SD. Energy sources for gynecologic laparoscopic surgery: a review of the literature. Obstet Gynecol Surv. 2014;69:763–776. doi: 10.1097/OGX.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 11.Li CZ, Liu B, Wen ZQ, et al. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cysts: a prospective clinical study of 191 patients. Fertil Steril. 2009;92:1428–1435. doi: 10.1016/j.fertnstert.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Lomano JM. Nd: YAG laser ablation of early pelvic endometriosis: a report of 61 cases. Lasers Surg Med. 1987;7:56–60. doi: 10.1002/lsm.1900070110. [DOI] [PubMed] [Google Scholar]
- 13.Meuleman C, Tomassetti C, D’Hoore A, et al. Clinical outcome after CO 2 laser laparoscopic radical excision of endometriosis with colorectal wall invasion combined with laparoscopic segmental bowel resection and reanastomosis. Hum Reprod. 2011;26:2336–2343. doi: 10.1093/humrep/der231. [DOI] [PubMed] [Google Scholar]
- 14.Munrós J, Martínez-Zamora MA, Tàssies D, et al. Total Circulating Microparticle Levels After Laparoscopic Surgical Treatment for Endometrioma: A Pilot, Prospective, Randomized Study Comparing Stripping with CO2 Laser Vaporization. J Minim Invasive Gynecol. 2019;26:450–455. doi: 10.1016/j.jmig.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Nappi L, Angioni S, Sorrentino F, et al. Anti-Mullerian hormone trend evaluation after laparoscopic surgery of monolateral endometrioma using a new dual wavelengths laser system (DWLS) for hemostasis. Gynecol Endocrinol. 2016a;32:34–37. doi: 10.3109/09513590.2015.1068754. [DOI] [PubMed] [Google Scholar]
- 16.Nappi L, Pontis A, Sorrentino F, et al. Hysteroscopic metroplasty for the septate uterus with diode laser: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2016b;206:32–35. doi: 10.1016/j.ejogrb.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Nappi L, Sorrentino F, Angioni S, et al. Feasibility of hysteroscopic endometrial polypectomy using a new dual wavelengths laser system (DWLS): preliminary results of a pilot study. Arch Gynecol Obstet. 2017;295:3–7. doi: 10.1007/s00404-016-4232-5. [DOI] [PubMed] [Google Scholar]
- 18.Pados G, Tsolakidis D, Assimakopoulos E, et al. Sonographic changes after laparoscopic cystectomy compared with three-stage management in patients with ovarian endometriomas: a prospective randomized study. Hum Reprod. 2010;25:672–677. doi: 10.1093/humrep/dep448. [DOI] [PubMed] [Google Scholar]
- 19.Parker WH, Einarsson J, Istre O, et al. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;7:551–554. doi: 10.1016/j.jmig.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Saito N, Okuda K, Yuguchi H, et al. J Minim Invasive Gynecol. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve. 2014;21:804–810. doi: 10.1016/j.jmig.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Saito N, Yamashita Y, Okuda K, et al. Comparison of the impact of laparoscopic endometriotic cystectomy and vaporization on postoperative serum anti-Mullerian hormone levels. Asian J Endosc Surg. 2018;11:23–29. doi: 10.1111/ases.12412. [DOI] [PubMed] [Google Scholar]
- 22.Saridogan E, Becker CM, Feki A, et al. (Working group of ESGE, ESHRE and WES) Recommendations for the surgical treatment of endometriosis – part 1: ovarian endometrioma. Gynecol Surg. 2017;14:27. doi: 10.1186/s10397-017-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schurr MO, Wehrmann M, Kunert W, et al. Histologic effects of different technologies for dissection in endoscopic surgery: Nd:YAG laser, high frequency and water-jet. Endosc Surg Allied Technol. 1994;2:195–201. [PubMed] [Google Scholar]
- 24.Suksompong S, Dejarkom S, Petyim S, et al. Ovarian reserve evaluation by anti-mullerian hormone in women undergoing laparoscopic cystectomy of endometrioma. J Med Assoc Thai. 2012;95:1389–1395. [PubMed] [Google Scholar]
- 25.Sutton C, Abbott J. History of power sources in endoscopic surgery. J Minim Invasive Gynecol. 2013;20:271–278. doi: 10.1016/j.jmig.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Sutton CJ, Ewen SP, Whitelaw N, et al. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- 27.Tsolakidis D, Pados G, Vavilis D, et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94:71–77. doi: 10.1016/j.fertnstert.2009.01.138. [DOI] [PubMed] [Google Scholar]
- 28.Vercellini P, Carmignani L, Rubino T, et al. Surgery for deep endometriosis: A pathogenesis-oriented approach. Gynecol Obstet Invest. 2009;68:88–103. doi: 10.1159/000219946. [DOI] [PubMed] [Google Scholar]


