Abstract
Laboratory research with Pseudomonas aeruginosa commonly involves the prototype strain PAO1. There is continued concern that PAO1 sublines maintained and propagated in the same laboratory or different laboratories exhibit genetic and phenotypic variability that may affect the reproducibility and validity of research. Whole-genome sequencing and other research identified the mexT locus as a mutational hotspot, but the explication of the diverse mutations present in the various sublines and consequences remained rather cursory. Here we present evidence that MexT sequence diversity is a predictor of PAO1 lineage integrity and define the protein’s prototype sequence.
Keywords: Pseudomonas aeruginosa, strain PAO1, lineages, MexT diversity
Impact Statement
Our studies confirmed that mexT is indeed a mutational hotspot and that sequence information can be utilized to quickly assess the integrity of the PAO1 lineage that a laboratory possesses, propagates and employs in experiments. MexT is a local and global transcriptional regulator of diverse physiological and pathogenic processes in Pseudomonas aeruginosa and serendipitous use of mexT mutant strains may negatively affect research outcomes, reproducibility and the interpretation of results.
Introduction
Pseudomonas aeruginosa is a medically significant opportunistic pathogen that mostly affects compromised individuals. Because of widespread multidrug resistance (MDR) the United States Centers for Disease Control and Prevention (CDC) listed P. aeruginosa as ‘serious’ among the top 18 bacterial and fungal threats to human health in 2019 [1]. Of more than 50 000 healthcare-associated infections in the USA, 13 % (or 6 000) are caused by MDR P. aeruginosa , and approximately 400 of these infections are fatal (www.cdc.gov). On a global scale, carbapenem-resistant P. aeruginosa were listed in 2018 as ‘Priority 1: Critical’ in the World Health Organization’s (WHO’s) Priority List for R & D of New Antibiotics [2].
For over four decades laboratory research with P. aeruginosa has been dominated by strain PAO1. This strain arose in Bruce Holloway’s laboratory as a spontaneous chloramphenicol-resistant derivative of the original PAO strain that was isolated in 1954 from a wound infection in Melbourne, Australia [3]. Over the ensuing decades PAO1 (formerly PAO1c [3, 4]) was distributed and adopted worldwide as the prototype P. aeruginosa strain for laboratory research [3, 4]. Almost 20 years ago a PAO1 subline (PAO1-UW) maintained at the University of Washington became the first fully sequenced strain [5]. A concern raised over the years is that PAO1 sublines maintained and propagated in the same laboratory or different laboratories exhibit variability in genetic and phenotypic properties, which may affect reproducibility in research [5–9]. Analyses of 12 different PAO1 whole-genome sequences have been published [8, 10], including ATCC15692, also known as PAO1c or PAO1 [3, 4], that was deposited in the ATCC by Bruce Holloway (www.atcc.org/Products/All/15692; no information on year of deposition is provided but the ATCC description is mentioned in Holloway’s 1969 publication [3, 4, 11]). Ten of the sequenced isolates are from various US laboratories, the ATCC strain is an Australian isolate, and one is an isolate maintained in a German strain collection.
It has been noted that the mexT gene is a hotspot for mutations in the PAO1 genome [7–10, 12–15]. This gene encodes MexT, a LysR-type transcriptional regulator (LTTR) that was originally described as an activator of MexEF-OprN multidrug efflux pump expression and repressor of outer membrane porin OprD expression [16]. Subsequent studies showed that MexT regulates multiple other genes, including diverse virulence traits, in either a MexEF-OprN-dependent or MexEF-OprN-independent manner. These include the type three secretion system and homoserine lactone-dependent virulence traits, such as pyocyanin, protease and rhamnolipid production [17–19]. Of note in this context is the transition of PAO1 with prototype MexT to a more virulent P2 phenotype in intestinal tissues and likely other environments, and that the emergence of this phenotype involves diverse mexT mutations [13, 14]. Recent studies showed that evolved populations with a LasR-independent RhlI–RhlR quorum-sensing system contain mutations in mexT [20, 21].
While mexT single-nucleotide polymorphisms (SNPs) and other mutations have been described before in clinical [12, 15, 22, 23], animal infection [13] and laboratory [7, 8, 14, 18, 20, 21] isolates, only a few publications – and among these to our knowledge only one whole-genome sequencing (WGS) paper – discuss the presence of an 8 bp insertion present in some PAO1 strains, including the PAO1-UW reference strain [7, 9, 14, 19, 24, 25]. Furthermore, some mutants in the two-allele transposon library created in the PAO1 strain, MPAO1, lack mexT altogether [9, 26]. This is rather surprising given the profound implications for the interpretation of data obtained when conducting research with such strains. Here, we present an updated mexT nucleotide and MexT amino acid sequence analysis to address some of the shortcomings of recent WGS analyses, and provide evidence that focused mexT gene sequencing can be used as a simple means to rapidly assess which PAO1 lineage(s) any given laboratory may acquire, possess and propagate.
Methods
mexT amplification and sequencing
The mexT coding sequences were PCR-amplified from purified genomic DNA (Wizard Genomic DNA Purification kit, Promega, Madison, WI, USA) using Q5 High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA). The primers used were P3649 (5′-GCAAGGCTTGACGGCGAGC), P3650 (5′-TTCCCGTTGCGACGCCTC),
P3651 (5′-TCGAGTTCGGCCTGTTGC) and P3652 (5′-CGGCATCAATAGATAGTTGGC). From the vast majority of strains tested, P3649 and P3650 amplify the entire mexT gene on a 1 016 bp fragment (mexT plus 61 bp upstream and 40 bp downstream sequences); P3649 and P3652 amplify a 487 bp fragment containing 426 bp amino-terminal mexT and 61 bp upstream sequences; and P3651 and P3650 amplify a 627 bp fragment containing 587 bp carboxy-terminal mexT and 40 bp downstream sequences. The 487 and 627 bp fragments overlap by 98 bp. Fragment sizes are affected by inserted or deleted sequences. The amplified fragments were subjected to Sanger sequencing (Genewiz, South Plainfield, NJ, USA or Eurofins, Louisville, KY, USA) using the primers employed for PCR.
Sequence analysis
DNA and protein sequences were analysed and aligned using SnapGene software version 4.3.9 (GSL Biotech, Chicago, IL, USA) or online Clustal Omega software [27] on EMBL-EBI [28] (https://www.ebi.ac.uk/Tools/msa/clustalo/). Protein domain assignments were made using online InterPro Protein sequence analysis and classification software (https://www.ebi.ac.uk/interpro). Helix–turn–helix (HTH) DNA-binding domains and HTH probability scores were predicted by the Rhone-Alpes Bioinformatic Pole Gerland Site (https://npsa-prabi.ibcp.fr) [29].
Results and discussion
Analysis of published mexT sequences
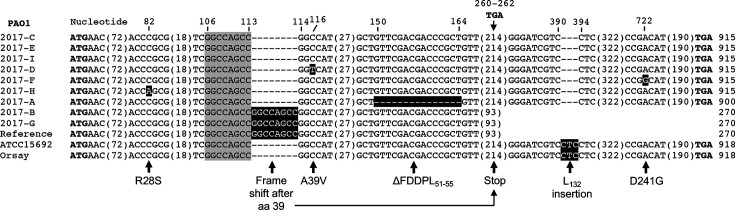
The mexT sequences of the 12 PAO1 strains for which genome sequences were published in peer-reviewed journals were aligned using Clustal Omega software (Fig. 1). It is evident that only three of the aligned mexT sequences are identical. In comparison to these three, the other nine sequences contain insertions, deletions or single nucleotide substitutions. These result in four different mexT open reading frame (ORF) sizes, ranging from 270 to 918 bp.
Fig. 1.
clustal alignment of MexT from 12 sequenced PAO1 sublines. Sequence differences – deletions, insertions and single nucleotide substitutions – are indicated by black highlights. PAO1 strain identifiers are the same as in Table 1 of [8, 10]. The 8 nucleotide (nt) GGCCAGCC insertion found in the PAO1 reference strain (also known as PAO1-UW) and sublines PAO1-2017-B and PAO1-2017-G results from duplication of the sequence highlighted in grey. (Note: instead of the insertion sequence indicated in this figure, blast alignments indicate an 8 nt CGGCCAGC insertion between nt 104 and 112 that is the result of duplication of the same sequence found immediately downstream and consistent with what has previously been reported [7, 14, 25]. Insertion and deletion of either sequence results in the same outcome, i.e. the same frame shift in the insertion mutant or restoration of wild-type amino acid sequence upon deletion.) The nt numbering at the top and in parentheses indicates the respective positions in the 915 bp mexT sequences containing no insertions or deletions. Numbers on the right indicate the lengths of mexT genes in the respective strains encompassed by the bolded ATG start and TGA stop codons. Note that the frame-shifted mexT ORFs in the PAO1 reference strain and sublines PAO1-2017-B and PAO1-2017-G terminate prematurely at a TGA that corresponds to bp 268–270 in the 915 mexT sequences. MexT changes at the amino acid level as a consequence of deletions, insertions and single nucleotide substitutions are indicated at the bottom (see text for details). The origins of PAO1 sublines and their genomic sequence GenBank accession numbers are as follows: PAO1-2017-A, R. Hancock, Univ. of British Columbia (QZFW00000000); PAO1-2017-B, J. Burns, Univ. of Washington (QZFX00000000); PAO1-2017-C, B. Iglewski (via E. P. Greenberg, Univ. of Washington) (QZFY00000000); PAO1-2017-D, B. Holloway (via D. Ohman, Virginia Commonwealth Univ.) (QZFZ00000000); PAO1-2017-E and PAO1-2017-I, MPAO1, C. Manoil, Univ. of Washington (QZGA00000000 and QZGE00000000, respectively); PAO1-2017-F, PAO1 V, J. Goldberg, Emory Univ. (QZGB00000000); PAO1-2017-G, H. Nikaido, Univ. of California, Berkeley (QZGC00000000); PAO1-2017-H, A. Prince, Columbia Univ. (QZGD00000000) [10]; ATCC15692, PAO1, B. Holloway (NZ_CP017149.1); PAO1-Orsay, C. Pourcel, Univ. Paris-Sud (NZ_LN871187.1) [41]; reference PAO1 or PAO1-UW (NC_002516.2) [5].
Functional MexT, including the one originally characterized in the PAO1-Geneva strain (GenBank accession number AJ007825.1) [7, 16], is encoded by a 915 bp gene and translated from the start codon shown in Fig. 1 [nucleotides (nt) 2 807 590–2 807 592 of the reference sequence; GenBank accession number NC_002516.2] and terminates at the stop codon indicated in Fig. 1 for all full-length mexT genes (nt 2 808,510–2 808 512 of the reference sequence).
The first sequenced PAO1 strain, the PAO1-UW reference strain, contains an 8 bp insertion in mexT [PA2492]. This insertion (nt 2 807 703–2 807 710) is the result of a duplication of the preceding 8 bp GGCCAGCC sequence (nt 2 807 695–2 807 702). When translated from this start codon, the PAO1-UW mexT gene terminates prematurely at a stop codon specified by nt 2 807 857–2 807 859. The resulting 267 bp ORF encodes a MexT polypeptide of 89 amino acids (aa), which is frame-shifted after residue 39. This truncated MexT lacks the last 6 aa of the predicted 22 aa MexT HTH DNA-binding domain specified by residues 24–45 (RSVTRAAEKLFLGQPAISAALS) [29] that is present in all strains lacking the 8 bp insertion. The PAO1-2017-B and PAO1-2017-G sublines also contain the 8 bp insertion and thus encode the same truncated MexT polypeptide (Fig. 1). It is known that PAO1 strains containing the 8 bp insertion are unable to express MexEF-OprN due to a non-functional MexT [7]. When this insertion is cleanly excised, e.g. in spontaneous norfloxacin-resistant derivatives of such PAO1 sublines, it leads to the restoration of functional MexT and thus MexEF-OprN expression [7].
Of concern is that the start codon of the mexT gene in the PAO1-UW reference sequence was misannotated as nt 2 807 469–2 807 471 and is thus located 121 nt upstream of the correct ATG. The resulting ‘mexT’ terminates at the same stop codon indicated in Fig. 1 for all full-length mexT genes (nt 2 808 510–2 808 512). The 1041 nt ORF of PAO1-UW ‘MexT’ would encode a 347 aa protein. This hypothetical protein consists of the same 269 carboxy-terminal aa that are present in strains without the 8 bp insertion and 78 erroneous aa at the amino terminus. The resulting hybrid protein only contains the 10 distal residues of the HTH domain contributed by the 269 aa carboxy-terminal MexT sequences and thus lacks a functional HTH DNA-binding domain.
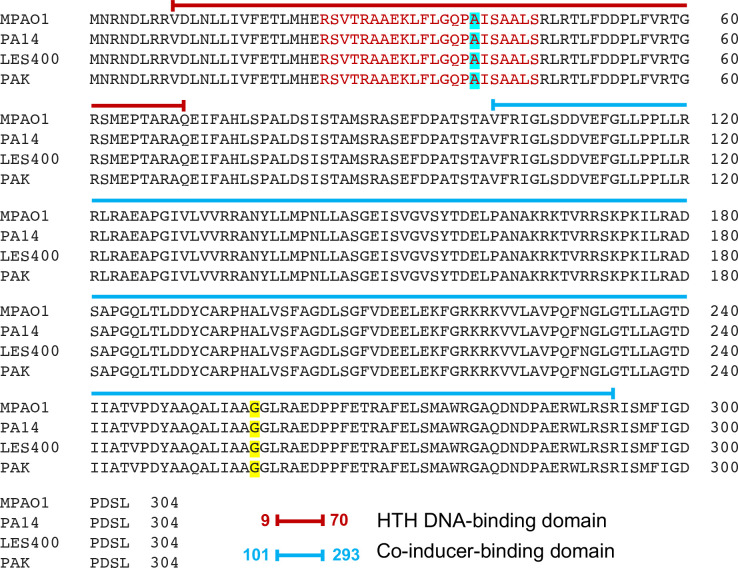
When compared to other PAO1 lineages, the PAO1 ATCC15692 and PAO1-Orsay mexT sequences contain three extra nucleotides (CTC), which results in the insertion of a leucine residue after valine 131 of MexT. A separate laboratory research strain, P. aeruginosa strain PA14, which is a highly pathogenic clinical burn wound isolate from the USA [30–32], has a mexT DNA sequence that diverges from PAO1, although its aa sequence is identical to that from several PAO1 strains without the additional leucine, PAO1-2017-C, PAO1-2017-E and PAO1-2017-I, as well as other P. aeruginosa isolates, including PAK [31, 33] and the Liverpool endemic strain LES400 (Fig. 2) [31, 34]. Thus, we surmise that PAO1 sublines ATCC15692 and PAO1-Orsay likely acquired the extra leucine codon rather than the other PAO1 sublines being derived from a more ancestral strain having lost this codon. The presence of the CTC codon in ATCC15692 and its absence from other PAO1 lineages strains was verified by PCR amplification of the mexT region and Sanger sequencing (see below).
Fig. 2.
Amino acid sequences and LTTR domains of prototype MexT from four distinct P. aeruginosa lineages. Amino acid sequences from strains MPAO1 (GenBank accession number QZGA00000000), PA14 (CP000438), PAK (LR657304) and LES400 (CP006982.1) were aligned using online Clustal Omega. Domain assignments were made using online InterPro Protein sequence analysis and classification software. The DNA-binding domain, including the HTH specifying amino acids 24–45 (red letters), was predicted using the Dodd and Eagan algorithm [29]. The putative co-inducer-binding domain extends from residues 101–293. Mutation of the cyan-shaded alanine 39 to a valine results in loss of MexT function. Conversely, mutational change of the yellow-shaded glycine 257 to either alanine or serine results in constitutive MexT activation.
Strain PAO1-2017-A contains a 15 bp deletion that results in loss of amino acids 51–55 from the 300 aa MexT protein expressed in this strain. This deletion shortens the predicted DNA-binding region, but it is unknown what effect this deletion has on MexT activity.
Several of the PAO1 sublines contain single nucleotide substitutions in mexT, e.g. PAO1-2017-B, PAO1-2017-D, PAO1-2017-F and PAO1-2017-H. Even if they are located within a known functional LTTR domain, either the HTH DNA-binding domain or the co-inducer-binding domain (Fig. 2), it is difficult to predict what effects these substitutions have on MexT function. For instance, the two PAO1 sublines PAO1-2017-D and PAO1-2017-H contain substitutions, R28S and A39V, respectively, that are located within the predicted MexT HTH DNA-binding domain (Fig. 2). The PAO1-2017-H mutation A39V is known to be non-functional [18], while it is not known if the PAO1-2017-D R28S is affected. However, MexT proteins with aa changes affecting the HTH domain are not necessarily inactive, since functional PAO1-Geneva MexT contains a leucine instead of a valine at position 26 [7, 16]. HTH probability scores can be used to estimate the potential impact of mutations in this domain. For instance, PAO1-Geneva MexT (V26L) has a higher score (90%) than its wild-type counterparts with V26 (71 %). The MexT A39V (non-functional) and R28S scores are 50 and 50 %, respectively. Lastly, strain 2017-F contains a D241G substitution in the carboxy-terminal half of MexT. This area of LTTR proteins contains the co-inducer-binding domain [35]. It is not known what effect the D241G substitution has on MexT function, but mutations in this domain have been shown to confer co-inducer-independent constitutive activation of the LTTR target gene(s) in diverse bacteria, including Salmonella enterica serovar Typhimurium [36, 37], Acinetobacter baylyi [38] and Burkholderia pseudomallei [39, 40]. In B. pseudomallei , carboxy-terminal mutations in the MexT homologue BpeT allow for co-inducer-independent expression of the P. aeruginosa MexEF-OprN efflux pump homologue BpeEF-OprC [39, 40]. A recent study with non-clonal clinical P. aeruginosa isolates showed that G257A and G257S amino acid substitutions led to constitutive activation of MexT due to co-inducer-independent oligomerization [15].
MexT analysis as predictor of PAO1 lineage
To ascertain whether a targeted mexT sequence analysis rather than WGS can be used to rapidly assess which PAO1 lineage(s) any given laboratory might possess and propagate, we PCR-amplified and sequenced the mexT coding sequences of nine PAO1 sublines maintained in our laboratory.
These analyses (Table 1) revealed that the nine PAO1 strains our laboratory possesses constitute eight different PAO1 sublines: (1) two (PAO1-89 and PAO1-00) contain the identical 8 bp insertion and frame-shifted mexT as PAO1-UW; (2) one (PAO1-06) contains a novel mexT frame-shift mutation; (3) one (PAO1-05) contains a MexT P195T amino acid substitution; (4) one (PAO1-96) contains MexT P195T and I129F amino acid substitutions; (5) one (PAO1-99) contains a MexT A202P amino acid substitution; (6) two (MPAO1 and PAO1-Caliper) possess the same DNA and amino acid sequences as MPAO1 lineages (PAO1-2017-E, PAO1-2017-I and PAO1-2017-C); (7) and (8) interestingly, we found that ATCC15692 has two separate sublines. We discovered upon initial plating that the culture vial we obtained contained a heterogeneous mixture of P. aeruginosa colony phenotypes. We therefore extracted genomic DNA from cultures of 10 randomly selected single colonies and sequenced PCR-amplified mexT. These analyses showed that only DNA from 2 of the 10 picked yielded the expected published sequence, i.e. the presence of an additional CTC codon, which results in the insertion of a leucine residue after valine 131 of MexT (Fig. 1). The DNA preparations from the other eight colonies contained a mexT gene that is identical to PAO1-Geneva (GenBank accession AJ007825.1) [7, 16]. The mexT gene from these isolates contained a single nucleotide substitution (G76C) that resulted in the aforementioned V26L amino acid change. Once single colony-purified, the respective mexT sequence types were stable, indicating that we started with a mixed culture. We can exclude cross-contamination in our laboratory, since we possessed neither ATCC15692 nor PAO1-Geneva prior to acquisition of ATCC15692 in May 2019.
Table 1.
PAO1 sublines inventoried in Schweizer laboratory
|
Subline |
Acquired |
Source |
mexT open reading frame*† |
|---|---|---|---|
|
PAO1-89 |
1989 |
P. Phibbs, East Carolina Univ. |
Same as PAO1-UW reference (NC_002516.2); mexT gene 270 bp; frame shift after aa A39; termination after aa 89 |
|
PAO1-96 |
1996 |
M. Vasil, Univ. of Colorado HSC |
nt A385T>I129F and nt C583A>aa P195T (MN646028) |
|
PAO1-99 |
1999 |
K. Poole, Queen’s Univ. |
nt G604C>aa A202P (MN646029) |
|
PAO1-00 |
2000 |
W. Bitter, Utrecht Univ. |
Same as PAO1-UW reference |
|
MPAO1 |
2003 |
M. Jacobs, Univ. of Washington |
Same as MPAO1 [10] |
|
PAO1-05 |
2005 |
D. Hassett, Univ. of Cincinnati |
nt C583A>aa P195T (MN646030) |
|
PAO1-06 |
2006 |
T. Tomofusa, Okayama Univ. |
Insertion of T after nt 353; frame shift after aa L118; +1 reading frame until termination at aa 305 (MN646031) |
|
PAO1-Caliper |
2011 |
Caliper Life Sciences‡ |
Same as MPAO1 [10] |
|
ATCC15692 |
2019 |
ATCC |
2/10 isolates tested same as GenBank NZ_CP017149.1 8/10 tested same as GenBank AJ007825.1 |
*Changes are relative to the MPAO1 suggested prototype sequence.
†GenBank accession numbers in parentheses.
‡Now Perkin Elmer.
Conclusions
In conclusion, our studies confirmed that PAO1 mexT is indeed a mutational hotspot and that sequence information can be utilized to quickly assess the integrity of the PAO1 lineage that a laboratory possesses, propagates and employs in experiments. This is important because MexT is a local and global transcriptional regulator of diverse physiological and pathogenic processes in P. aeruginosa and serendipitous use of mexT mutant strains may negatively affect the interpretation of research results and reproducibility. The mexT sequence variety established by diverse studies also raises the question of what a prototypic MexT sequence looks like. MexT of P. aeruginosa strains PA14, PAK and LES400 have an identical amino acid sequence to MexT from several PAO1 sublines, e.g. PAO1-2017-C, MPAO1 and PAO1-Caliper (Fig. 2). Lastly, using this presumed MexT prototype sequence as the subject we performed two separate DIAMOND blastp queries against either completed P. aeruginosa genomes or against all P. aeruginosa sequences within the Pseudomonas Genome Database (version 18.1, 12 March 2019, www.pseudomonas.com). In analyses performed 27 August 2019, 125/148 (or 84.5 %) of the completed genomes contained the prototype MexT sequence. Of all MexT proteins in the database, 2321/2659 (or 87.2 %) were identical to this prototype MexT. In both categories, MexT proteins that were not identical to the prototype mostly had various single amino acid substitutions, with a few having deletions or insertions. The evidence overwhelmingly shows that the MexT sequences shown in Fig. 2 specify the prototype sequence. To further ascertain mexT’s unique suitability as a target for assessing the integrity of PAO1, we used blastn to analyse the 12 published P. aeruginosa genomes listed in Fig. 1 and its legend for: (1) mutations in 4 genes – mexZ, lasR, mexA and mexS – that, like mexT, are frequently mutated in clinical isolates, especially strains from chronically infected cystic fibrosis (CF) patients [12], and (2) mutations in mexF, a MexT-regulated gene that, like mexT, is involved in the emergence of the so-called P2 phenotype [14]. Unlike mexT, for which only 3 of the 12 aligned mexT nucleotide sequences are identical, which resulted in 7 different MexT protein sequences, there were no nucleotide sequence changes in mexZ, lasR, mexA, mexF and mexS and thus no amino acid changes in the resulting proteins. These data instil further confidence in our conclusion that mexT sequence information can be utilized to quickly assess the integrity of the PAO1 lineage(s) that a laboratory possesses. And, based on these findings, it is recommended that laboratories occasionally, for instance after genetic manipulations, either confirm that their strains carry the MexT prototype amino acid sequence or perform MexT functionality testing (e.g. by assessing mexE expression by RT-qPCR [14]).
Funding information
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI136803. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
We thank all collaborators who graciously provided P. aeruginosa strains over the years.
Author contributions
E. D. L., conceived experiments, performed laboratory work and data analyses, and contributed to writing the manuscript. H. P. S., secured funding, conceived experiments, performed data analyses and wrote the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: aa, amino acid(s); CDC, United States Centers for Disease Control and Prevention; HTH, helix-turn-helix; LTTR, LysR-type transcriptional regulator; MDR, multidrug resistance; nt, nucleotide(s); ORF, open reading frame; RT-qPCR, reverse transcription-quantitive real-time polymerase chain reaction; SNP, single-nucleotide polymorphism; WGS, whole-genome sequencing; WHO, World Health Organization.
References
- 1.Centers for Disease Control and Prevention Antibiotic resistance threats in the United States. 2019 https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- 2.World Health Organization Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2018 https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf
- 3.Holloway BW. Genetic recombination in Pseudomonas aeruginosa . Microbiology. 1955;13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- 4.Holloway BW. Genetics of Pseudomonas . Bacteriol Rev. 1969;33:419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 6.Preston MJ, Fleiszig SM, Zaidi TS, Goldberg JB, Shortridge VD, et al. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun. 1995;63:3497–3501. doi: 10.1128/iai.63.9.3497-3501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maseda H, Saito K, Nakajima A, Nakae T. Variation of the mexT gene, a regulator of the MexEF-OprN efflux pump expression in wild-type strains of Pseudomonas aeruginosa . FEMS Microbiol Lett. 2000;192:107–112. doi: 10.1111/j.1574-6968.2000.tb09367.x. [DOI] [PubMed] [Google Scholar]
- 8.Klockgether J, Munder A, Neugebauer J, Davenport CF, Stanke F, et al. Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J Bacteriol. 2010;192:1113–1121. doi: 10.1128/JB.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidorenko J, Jatsenko T, Kivisaar M. Ongoing evolution of Pseudomonas aeruginosa PAO1 sublines complicates studies of DNA damage repair and tolerance. Mutat Res. 2017;797-799:26–37. doi: 10.1016/j.mrfmmm.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Chandler CE, Horspool AM, Hill PJ, Wozniak DJ, Schertzer JW, et al. Genomic and Phenotypic diversity among ten laboratory isolates of Pseudomonas aeruginosa PAO1. J Bacteriol. 2019;201:e00595–18. doi: 10.1128/JB.00595-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holloway BW, Morgan AF. Genome organization in Pseudomonas . Annu Rev Microbiol. 1986;40:79–105. doi: 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- 12.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One. 2012;7:e44326. doi: 10.1371/journal.pone.0044326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luong PM, Shogan BD, Zaborin A, Belogortseva N, Shrout JD, et al. Emergence of the P2 phenotype in Pseudomonas aeruginosa PAO1 strains involves various mutations in mexT or mexF . J Bacteriol. 2014;196:504–513. doi: 10.1128/JB.01050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juarez P, Broutin I, Bordi C, Plésiat P, Llanes C. Constitutive activation of MexT by amino acid substitutions results in MexEF-OprN overproduction in clinical isolates of Pseudomonas aeruginosa . Antimicrob Agents Chemother. 2018;62:e02445–17. doi: 10.1128/AAC.02445-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehler T, Epp SF, Curty LK, Pechere JC. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa . J Bacteriol. 1999;181:6300–6305. doi: 10.1128/jb.181.20.6300-6305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian Z-X, Mac Aogáin M, O'Connor HF, Fargier E, Mooij MJ, et al. MexT modulates virulence determinants in Pseudomonas aeruginosa independent of the MexEF-OprN efflux pump. Microb Pathog. 2009;47:237–241. doi: 10.1016/j.micpath.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Tian Z-X, Fargier E, Mac Aogáin M, Adams C, Wang Y-P, et al. Transcriptome profiling defines a novel regulon modulated by the LysR-type transcriptional regulator MexT in Pseudomonas aeruginosa . Nucleic Acids Res. 2009;37:7546–7559. doi: 10.1093/nar/gkp828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Yang H, Qiao M, Jin S. MexT regulates the type III secretion system through MexS and PtrC in Pseudomonas aeruginosa . J Bacteriol. 2011;193:399–410. doi: 10.1128/JB.01079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oshri RD, Zrihen KS, Shner I, Omer Bendori S, Eldar A. Selection for increased quorum-sensing cooperation in Pseudomonas aeruginosa through the shut-down of a drug resistance pump. Isme J. 2018;12:2458–2469. doi: 10.1038/s41396-018-0205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostylev M, Kim DY, Smalley NE, Salukhe I, Greenberg EP, et al. Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc Natl Acad Sci USA. 2019;116:7027–7032. doi: 10.1073/pnas.1819796116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klockgether J, Miethke N, Kubesch P, Bohn Y-S, Brockhausen I, et al. Intraclonal diversity of the Pseudomonas aeruginosa cystic fibrosis airway isolates TBCF10839 and TBCF121838: distinct signatures of transcriptome, proteome, metabolome, adherence and pathogenicity despite an almost identical genome sequence. Environ Microbiol. 2013;15:191–210. doi: 10.1111/j.1462-2920.2012.02842.x. [DOI] [PubMed] [Google Scholar]
- 23.Clark ST, Diaz Caballero J, Cheang M, Coburn B, Wang PW, et al. Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis. Sci Rep. 2015;5:10932. doi: 10.1038/srep10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Schweizer HP. Evidence of MexT-independent overexpression of MexEF-OprN multidrug efflux pump of Pseudomonas aeruginosa in presence of metabolic stress. PLoS One. 2011;6:e26520. doi: 10.1371/journal.pone.0026520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawalek A, Kotecka K, Modrzejewska M, Jagura-Burdzy G, Bartosik AA. Genome sequence of Pseudomonas aeruginosa PAO1161, a PAO1 derivative with the ICEFP2 integrative and conjugative element. bioRxiv. 2018 doi: 10.1186/s12864-019-6378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa . Proc Natl Acad Sci USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, et al. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madeira F, Park YM, Lee J, Buso N, Gur T, et al. The EMBL-EBI search and sequence analysis tools Apis in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodd IB, Egan JB. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroth MN, Cho JJ, Green SK, Kominos SD. Epidemiology of Pseudomonas aeruginosa in agricultural areas. In: VM Y, editor. Pseudomonas aeruginosa: Ecological Aspects and Patient Colonization. New York: Raven Press; 1977. pp. 1–29. editor. [Google Scholar]
- 31.De Soyza A, Hall AJ, Mahenthiralingam E, Drevinek P, Kaca W, et al. Developing an international Pseudomonas aeruginosa reference panel. Microbiologyopen. 2013;2:1010–1023. doi: 10.1002/mbo3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahme L, Stevens E, Wolfort S, Shao J, Tompkins R, et al. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 33.Strom MS, Lory S. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli . J Bacteriol. 1986;165:367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salunkhe P, Smart CHM, Morgan JAW, Panagea S, Walshaw MJ, et al. A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J Bacteriol. 2005;187:4908–4920. doi: 10.1128/JB.187.14.4908-4920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maddocks SE, Oyston PCF. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology. 2008;154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 36.Colyer TE, Kredich NM. Residue threonine-149 of the Salmonella typhimurium CysB transcription activator: mutations causing constitutive expression of positively regulated genes of the cysteine regulon. Mol Microbiol. 1994;13:797–805. doi: 10.1111/j.1365-2958.1994.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 37.Colyer TE, Kredich NM. In vitro characterization of constitutive CysB proteins from Salmonella typhimurium . Mol Microbiol. 1996;21:247–256. doi: 10.1046/j.1365-2958.1996.6301347.x. [DOI] [PubMed] [Google Scholar]
- 38.Craven SH, Ezezika OC, Haddad S, Hall RA, Momany C, et al. Inducer responses of BenM, a LysR-type transcriptional regulator from Acinetobacter baylyi ADP1. Mol Microbiol. 2009;72:881–894. doi: 10.1111/j.1365-2958.2009.06686.x. [DOI] [PubMed] [Google Scholar]
- 39.Podnecky NL, Rhodes KA, Mima T, Drew HR, Chirakul S, et al. Mechanisms of Resistance to Folate Pathway Inhibitors in Burkholderia pseudomallei : Deviation from the Norm. MBio. 2017;8:e01357–01317. doi: 10.1128/mBio.01357-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhodes KA, Somprasong N, Podnecky NL, Mima T, Chirakul S, et al. Molecular determinants of Burkholderia pseudomallei BpeEF-OprC efflux pump expression. Microbiology. 2018;164:1156–1167. doi: 10.1099/mic.0.000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latino L, Midoux C, Hauck Y, Vergnaud G, Pourcel C. Pseudolysogeny and sequential mutations build multiresistance to virulent bacteriophages in Pseudomonas aeruginosa . Microbiology. 2016;162:748–763. doi: 10.1099/mic.0.000263. [DOI] [PubMed] [Google Scholar]