Abstract
Background
Molecular point-of-care testing (POCT) for influenza in primary care could influence clinical care and patient outcomes.
Aim
To assess the feasibility of incorporating influenza POCT into general practice in England.
Design and setting
A mixed-methods study conducted in six general practices that had not previously participated in respiratory virology sampling, which are part of the Royal College of General Practitioners Research and Surveillance Centre English sentinel surveillance network, from February 2019 to May 2019.
Method
A sociotechnical perspective was adopted using the Public Health England POCT implementation toolkit and business process modelling notation to inform qualitative analysis. Quantitative data were collected about the number of samples taken, their representativeness, and the virology results obtained, comparing them with the rest of the sentinel system over the same weeks.
Results
A total of 312 POCTs were performed; 276 were used for quantitative analysis, of which 60 were positive for influenza and 216 were negative. The average swabbing rate was 0.4 per 1000 population and swab positivity was between 16.7% (n = 14/84) and 41.4% (n = 12/29). Given a positive influenza POCT result, the odds ratio of receiving an antiviral was 14.1 (95% confidence intervals [CI] = 2.9 to 70.0, P<0.001) and of receiving an antibiotic was 0.4 (95% CI = 0.2 to 0.8, P = 0.01), compared with patients with a negative result. Qualitative analysis showed that it was feasible for practices to implement POCT, but there is considerable variation in the processes used.
Conclusion
Testing for influenza using POCT is feasible in primary care and may improve antimicrobial use. However, further evidence from randomised trials of influenza POCT in general practice is needed.
Keywords: antibiotic, antiviral, general practice, influenza, medical record systems, point-of-care systems
INTRODUCTION
Influenza is associated with high levels of morbidity and mortality.1 In the UK, influenza is estimated to account for 11.5% of all episodes of respiratory infection, with approximately 67 000 patients admitted to hospital and 2000 deaths per year.2 Vaccination is suboptimally effective at preventing influenza,3 and antivirals may improve clinical outcome, especially when administered early in the course of disease.4,5
Current antiviral treatment for influenza needs to be administered within 48 hours from onset of symptoms for optimal efficacy.6,7 A novel antiviral, baloxavir, has been shown to improve the time to resolution of symptoms and reduce complications in high-risk patients with influenza.8 Use of these new agents, once approved in the UK, will likely be restricted to patients with microbiologically confirmed diagnosis given their cost.9
In the last few years, highly accurate rapid molecular test platforms for influenza have become available.10 Point-of-care testing (POCT) for influenza has the potential to improve clinical decisions and patient outcomes as a result of a more appropriate use of antibiotics, antivirals, and infection control measures.11 Additionally, POCT could provide information to enhance influenza disease surveillance and clinical research, particularly providing data to compare vaccines in clinically important subgroups, and antiviral therapy in real-world trials.12
Public Health England (PHE) has produced advice for institutions interested in using influenza POCT (Supplementary Box S1).13
The aim of this study was to assess the feasibility of incorporating influenza POCT into general practice workflows and to explore its potential impact on antimicrobial use.
METHOD
A mixed-methods, multi-site cohort study was used to investigate the implementation of influenza POCT in primary care workflows. The protocol for this study has previously been published.14
Study setting and population
The study took place between February 2019 and May 2019, during the influenza season as defined by PHE. It was nested in the English national sentinel surveillance network run by the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC), one of the longest established primary care sentinel networks in Europe.15,16 Previous work has shown that the age and sex distribution of patients in the sentinel network is broadly similar to the English national census distribution.15
How this fits in
| Highly accurate rapid molecular test platforms for influenza have been evaluated in secondary care settings by Public Health England, but no data on their use in primary care settings in the UK have been published to date. This study, nested in six general practices that are part of the English national sentinel surveillance network, explored the feasibility and impact of implementing molecular point-of-care testing (POCT) in primary care. The study showed an impact on antibiotic and antiviral use: patients with a positive POCT test were significantly less likely to be prescribed an antibiotic and significantly more likely to be prescribed an antiviral medication. The study results are helpful for healthcare providers, commissioners, and policymakers interested in the use of POCT to monitor influenza in primary care and its impact on the clinical care of patients. |
Six practices with a registered population of approximately 78 500 patients took part in the study. These practices had not previously participated in respiratory virology swabbing, according to RCGP RSC records, and were considered as ‘naive’ to respiratory virus sampling. The Abbott ID Now POCT machine was used for this study. Its diagnostic accuracy has previously been assessed: in a systematic review published in 2017, Merckx et al 10 reported a sensitivity in adults of 80.3% (95% confidence intervals [CI] = 63.7 to 90.8%) and 68.5% (95% CI = 40.2 to 87.2%) for influenza A and B, respectively. Vos et al 17 reported a pooled sensitivity of 81.6% (95% CI = 75.4 to 87.9%) for respiratory viruses and pooled specificity of 94.0% (95% CI = 86.0 to 100%). This compares with a pooled sensitivity of all rapid molecular respiratory viral tests of 90.9% (95% CI = 88.7 to 93.1%) and pooled specificity of 96.1% (95% CI = 94.2 to 97.9%).17 At the time of the study, key advantages to using the Abbott ID Now test over alternatives (such as the Roche Cobas Liat test) in primary care were that the consumables could be stored at room temperature and did not require additional cold storage space, and that the test was quicker, which is important in a busy primary care clinic setting. Another key advantage of the Abbott test system is the overall cost of machine and consumables, which are substantially less than the Roche test system, at the time of the study. Clinicians in the practices were encouraged to undertake influenza POCT swabs from consented patients presenting with an acute influenza-like illness and acute respiratory illness, during the weeks when the sentinel network suggested that influenza was circulating. Practices were provided with a leaflet explaining the study to staff and eligible patients, and were encouraged to display a poster about the study in their waiting areas.
Data collection and analysis
A sequential explanatory approach was taken to data collection in this study,18 with quantitative data analysis followed by qualitative interviews with the study’s practice participants.
The primary quantitative outcome was the number of valid influenza swabs taken and tested by the study practices. These data were collected from POCT machines. The virology results obtained from study practices and the representativeness of swabbed populations were compared with the rest of the sentinel system over the same weeks.
Invalid or voided swabs were those swabs that did not provide a negative or positive influenza result or were swabs that had been used for the purposes of quality assurance or staff training. For the purposes of this study, swabs that were performed on patients who had registered to opt out of data sharing as per the national data opt-out policy,19 with no medical record being made available to anyone outside their direct patient care, were also considered void.
Secondary quantitative outcomes, including age, sex, socioeconomic status, ethnicity, chronic conditions, influenza vaccination status, antibiotic prescribing, influenza antiviral prescribing, and influenza vaccine effectiveness, were obtained by linking the results from POCT machines to details from the patient’s electronic medical record, as outlined in a previously published protocol.14 Socioeconomic status was measured using the Index of Multiple Deprivation (IMD) score.20
Secondary qualitative outcomes on the implementation of the POCT machines were collected using a semi-structured questionnaire survey of practice staff (Supplementary Box S2). Responses from the questionnaires were reviewed to elicit common themes related to the following domains, which have previously been shown to be important determinants of implementation of influenza POCT in clinical practice: performance of the POCT platform; clinical pathways and staff training; result reporting; clinical governance; costs; and monitoring of effectiveness.13
A sociotechnical perspective informed the assessment of qualitative information from the questionnaires. This research outlook focuses on the interdependence and inextricable linkages between people and technological systems.21 The authors compared business process models within each practice to assess which were successful at integrating POCT into their workflows, thus able to jointly optimise their sociological and technological systems to produce positive outcomes. Business process models are graphical representations of the commercial and organisational workflow processes within an organisation. This is helpful to model collaborations and business transactions within health systems. Business processes were modelled using the Business Process Modelling Notation (BPMN).22 BPMN can be used to depict the end-to-end flow of a business process. The notation has been specifically designed to coordinate the sequence of processes and the messages that flow between different process participants in a related set of business activities.23
RESULTS
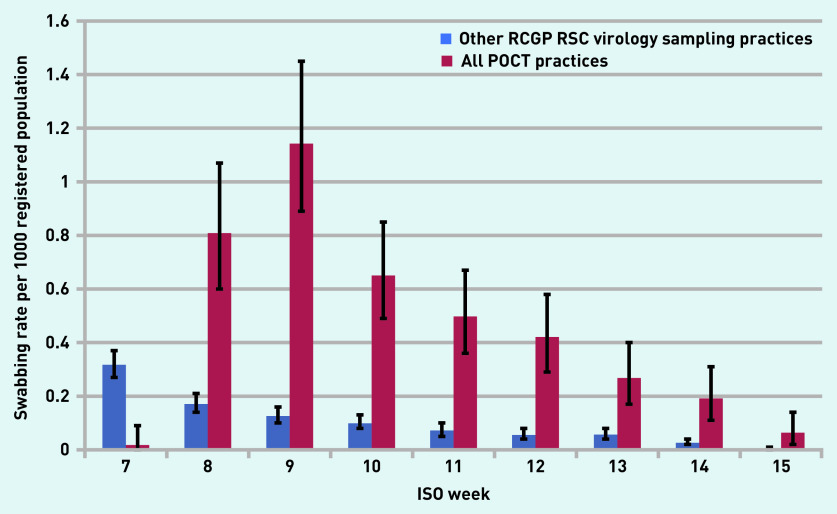
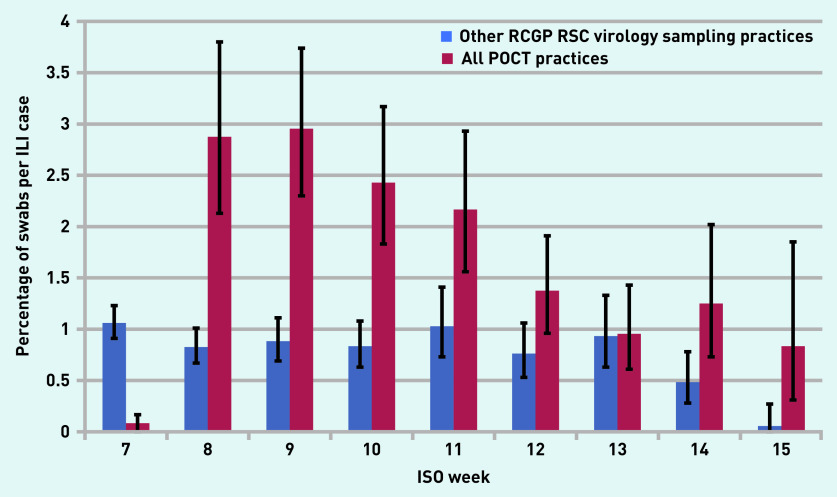
In total, 312 tests were recorded by the POCT machines. This equates to approximately six swabs per practice per week over the duration of the study. This compares favourably with the rate of influenza swabbing in the rest of the sentinel network of just under one swab per practice per week. Figures 1 and 2 show that POCT practices performed more swabs than the other RCGP RSC virology sampling practices when their practice population size and influenza-like illness rates were taken into account. The average swabbing rate for POCT practices was 0.4 compared with 0.1 per 1000 population for other RCGP RSC virology sampling practices (P = 0.15).
Figure 1.
Comparison of swabbing rates per 1000 registered population in all POCT practices versus other RCGP RSC virology sampling practices. The POCT practices conducted tests in-house while the RCGP RSC sentinel practices sent samples to the Public Health England reference laboratory. ISO = International Organization for Standardization. POCT = point-of-care test. RCGP RSC = Royal College of General Practitioners Research and Surveillance Centre.
Figure 2.
Comparison of swabbing rates and percentage of ILI cases in all POCT practices compared with the RCGP RSC sentinel virology sampling practices. ILI = influenza-like illness. ISO = International Organization for Standardization. POCT = point-of-care test. RCGP RSC = Royal College of General Practitioners Research and Surveillance Centre.
After cross-checking data from the POCT machines with electronic medical records from the practices, 36 tests were deemed to be void. In total, 276 swab results were used for the quantitative analysis, of which 60 were positive for influenza and 216 were negative. There was substantial variation in the number of swabs taken between practices over the course of the study (Supplementary Figures S1–S3).
Practice F had the highest swabbing rates of 2.8 per 1000 population taking into account its practice size. However, taking into account the number of influenza-like illness case presentations, practice A had the highest swabbing rate with 15 swabs per influenza-like illness case presentation in International Organization for Standardization week 9.
Of the six study practices, 59.8% (n = 165/276) of swabs taken were in females (Supplementary Figure S4), this compares with data from other RCGP RSC virology sampling practices where 58.0% of swabs were taken in females. More swabs were undertaken in patients <20 years of age, although individual practices varied in the age distribution of the patients that they swabbed. The proportion swabbed from each age group compared favourably with the swabbing rate by age for other RCGP RSC virology sampling practices (Supplementary Figure S5). More swabs were undertaken in patients in the most deprived quintiles (Supplementary Figure S6). A total of 25 of 276 (9.1%) swabs were taken from patients of black or ethnic minority origin (Supplementary Figure S7), which is slightly lower than the proportion of patients of black or ethnic minority origin registered with study practices or presenting with influenza-like illness (approximately 13.1%; data not shown).
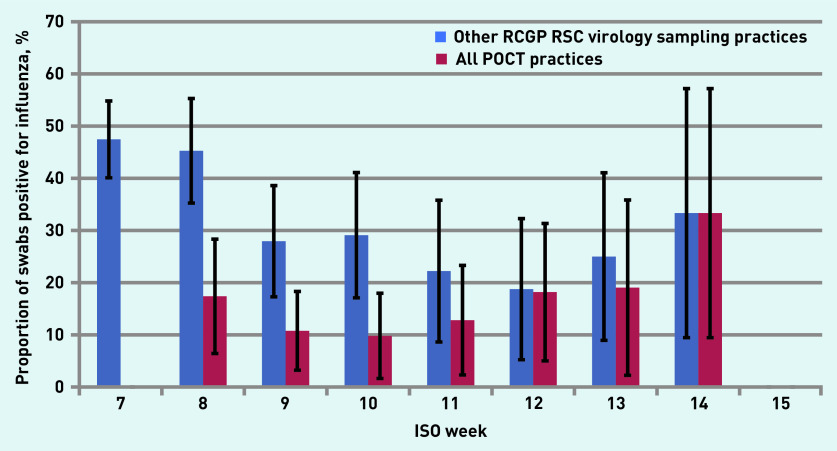
Practices varied two-fold, between 20.0% (n = 1/5) and 54.8% (n = 17/31), in the proportion of swabs taken from people with existing risk factors for influenza infection, as defined by the English Chief Medical Officer (Supplementary Figure S8).24 The overall positivity rate for swabs for influenza varied from 16.7% (n = 14/84) to 41.4% (n = 12/29) between practices (Supplementary Figure S9). Considered as a whole, the swab influenza positivity rate for practices that used POCT compared favourably with the positivity rate of swabs collected by other RCGP RSC virology sampling practices (Figure 3).
Figure 3.
Swab positivity rate for influenza in POCT practices versus other RCGP RSC virology sampling practices. No positive swabs were taken in ISO week 15. ISO = International Organization for Standardization. POCT = point-of-care test. RCGP RSC = Royal College of General Practitioners Research and Surveillance Centre.
The proportion of invalid test results was low across all practices, with an average of 5.4% (n = 15/276) of tests yielding an invalid result (Supplementary Figures S9 and S10).
Using data about vaccination status of patients who were swabbed showed that the odds ratio (OR)25 for influenza vaccine effectiveness was 1.1 (95% CI = 0.6 to 2.0) and the risk ratio for influenza in those who have been vaccinated was 1.1 (95% CI = 0.7 to 1.7), although the small sample size makes the interpretation of results difficult (Supplementary Table S1).
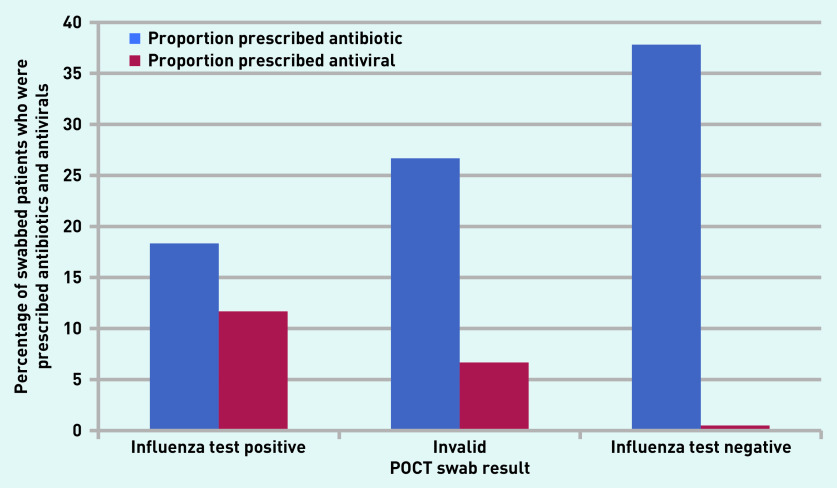
Using data about antibiotic and antiviral prescribing for patients who were tested showed that 37.8% (n = 76/201) of patients received antibiotics following a negative influenza POCT result (Figure 4). The OR for being prescribed an antibiotic given a positive result was 0.4 (95% CI = 0.2 to 0.8; P = 0.01) compared with a negative test (Supplementary Table S2). Conversely, the OR for receiving an antiviral given a positive result was 14.1 (95% CI = 2.9 to 70.0; P<0.001) compared with a negative test. The OR for being prescribed an antiviral in those testing positive for influenza and with an existing condition identified by the Chief Medical Officer as at high risk of complications from influenza infection was 16.0 (95% CI = 2.9 to 88.9; P<0.001) (Supplementary Tables S3 and S4).24
Figure 4.
Proportion of swabbed patients who received antibiotics or antivirals following influenza point-of-care testing. POCT = point-of-care test.
Qualitative analysis showed considerable variation in implementing POCT processes in the participating practices (Table 1). At a high level, common sub-processes that could be mapped to the PHE POCT implementation checklist were identified (Figure 5). The largest difference was observed between practices in the methods used to systematically identify patients eligible for swabbing and in who managed the POCT machines. These variations were primarily influenced by the clinical governance (Domain [D]2) and clinical pathway domains (D3) (Supplementary Figure S11 and S12).
Table 1.
Information extracted from the interviews mapped onto the domains considered for analysis
| Domain | Evaluation measure | Practice A | Practice B | Practice C | Practice D | Practice E | Practice F |
|---|---|---|---|---|---|---|---|
| POCT platform | Platform location | Mobile trolley | Dedicated room | Dedicated room | Dedicated room | Mobile trolley | Desk in reception area |
|
| |||||||
| Clinical pathway and staff training | Training | Training provided by study investigator. Additional training organised locally by practice lead for study | Training provided by study investigator. Additional training organised locally by practice lead for study | Training provided by study investigator. Additional training organised locally by practice lead for study | Training provided by study investigator. Additional training organised locally by practice lead for study | Training provided by study investigator. Additional training organised locally by practice lead for study | Training provided by study investigator. Additional training organised locally by practice lead for study |
| Clinical algorithm used for patient identification | Triaged and swab taken by nurse practitioners | Candidates identified by clinician and swabbed in consultation room. Swab taken to POCT room within 2 hours | Candidates selected during regular appointments | EMIS template developed locally that opens when eligible patient present | No specific method used | Candidates selected during regular appointments | |
| Dissemination of clinical algorithm | No specific dissemination | During practice clinical meeting | Email/face-to-face | Dissemination by being incorporated into the clinical system | Introduction email/weekly email reminders | Daily email reminders | |
| Home visits considered? | Yes | No | Yes | Yes | No | Yes | |
| Consent | Nurse practitioner | Clinician | Nurse practitioner/clinician | Clinician | Nurse manager | Clinician | |
|
| |||||||
| Result reporting | Action of the results | Results coded in the medical record by nurse practitioner | POCT results coded in the medical record by nurse practitioner | Results coded in the medical record by clinician/nurse practitioner | POCT results coded in the medical record by HCA | POCT results coded in the medical record by HCA. The lead has created a protocol with codes to standardised recording | POCT results coded in the medical record by HCA |
| Results communication to clinician | POCT results are shared with the clinician | POCT results are shared with the clinician | POCT results are shared with the clinician | POCT results are shared with the clinician | POCT results are shared with the clinician | POCT results are shared with the clinician | |
|
| |||||||
| Clinical governance | Role of practice lead for study | Nurse practitioner | Clinician | Clinician | Research administrator | Nurse manager | Practice manager |
| Role of machine operator | Nurse | HCA | HCA | Research administrator | Practice manager | Practice manager | |
| Quality control | Nurse | HCA | HCA | Research administrator | Practice manager | Practice manager | |
| Stock supply management | Stock replenishment by machine operator | Stock replenishment by machine operator | Stock replenishment by machine operator | Stock replenishment by machine operator | Stock replenishment by machine operator | Stock replenishment by machine operator | |
|
| |||||||
| Costs | Domain not assessed | ||||||
|
| |||||||
| Monitoring of effectiveness | Planned to conduct local audit of POCT use | No plans for monitoring effectiveness | No plans for monitoring effectiveness | No plans for monitoring effectiveness | No plans for monitoring effectiveness | No plans for monitoring effectiveness | |
EMIS = Egton Medical Information Systems. HCA = healthcare assistant. POCT = point-of-care testing.
Figure 5.
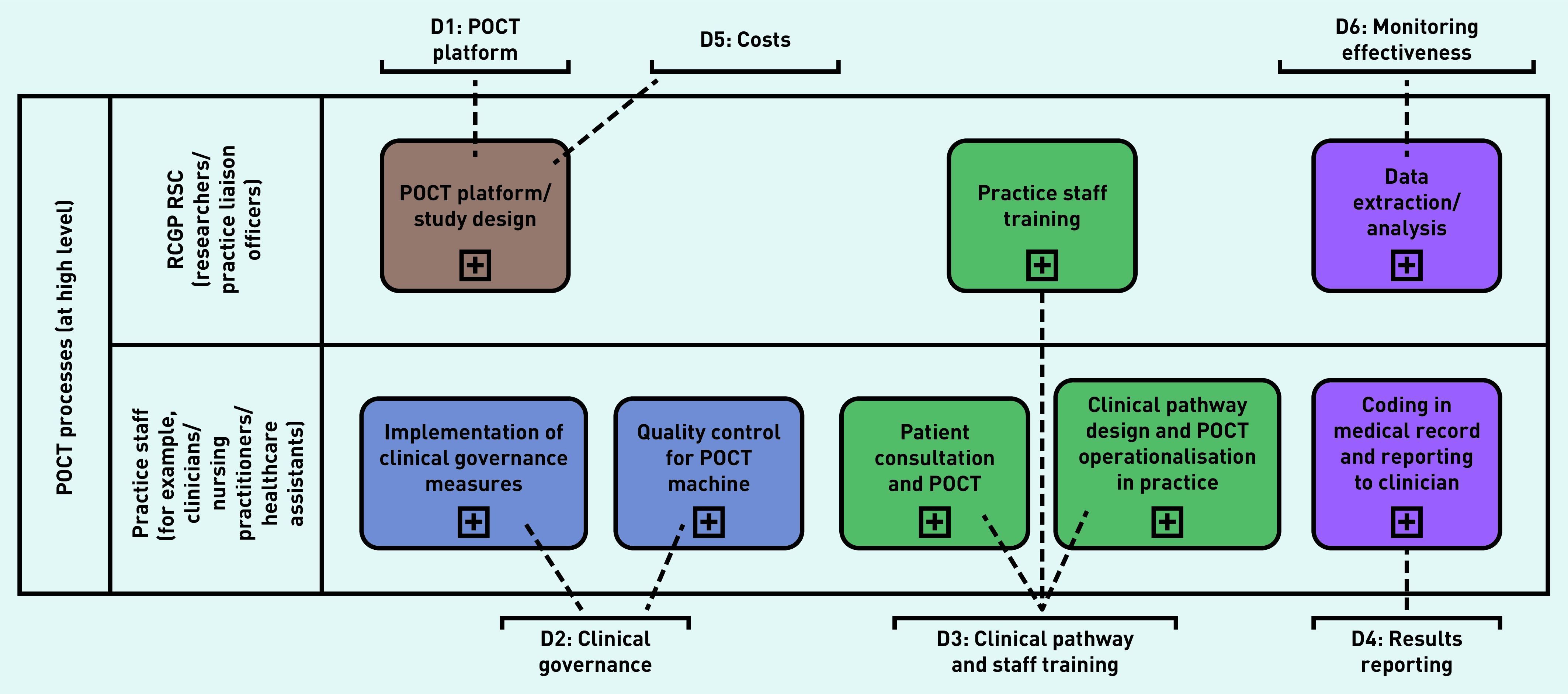
Sub-processes identified during the qualitative evaluation mapped to the six domains of the Public Health England POCT implementation checklist. D = domain. POCT = point-of-care test. RCGP RSC = Royal College of General Practitioners Research and Surveillance Centre.
DISCUSSION
Summary
To the authors’ knowledge, this is the first time POCT for influenza has been robustly evaluated in general practice in the UK. This evaluation has shown that it is feasible to use the Abbott ID Now POCT machine to test for influenza in a sentinel network in primary care, with comparable overall swabbing and positivity rates for practices using POCT versus practices that participate in the usual virology sampling programme. However, there was a wide variation in swabbing rates between POCT practices.
The results of POCT also influenced clinical prescribing practices, with patients who received positive tests in primary care being significantly more likely to receive antivirals on the day of the swab result, especially if they were in the Chief Medical Officer’s risk groups for influenza,24 and those who received positive tests being significantly less likely to receive antibiotic prescriptions on the day of the swab result.
Qualitative analysis suggests that variations in implementation of the POCT platform in primary care were influenced by domains related to clinical governance of the machine and clinical pathways to testing. Higher swabbing rates were seen in practices with a clearly identified study lead or clinical champion with responsibility for the new technology. In addition, practices performed well if they had systematic methods for identifying suitable patients for swabbing, such as an electronic template mated to the patient’s electronic medical record.
Strengths and limitations
Limitations of the study include the non-randomised nature of the study design, its short duration, and small sample size, because it was only possible to initiate the study halfway through the 2018/2019 influenza season. The POCT machines used did not include information about influenza subtype, which restricted interpretation of the results, especially those pertaining to the effectiveness of the seasonal influenza vaccine. Additionally, it was not possible to evaluate the full potential that POCT machines might have had if they had been used throughout the season when practice staff may have gained more experience with using the machines. Finally, information about the duration of respiratory illness was not collected before swab testing, thus this limits the conclusions regarding the appropriate use of antiviral medications following POCT.
Strengths of the study included that it was nested in the RCGP RSC English sentinel surveillance network, which allowed a comparison of the performance of practices using POCT for influenza testing versus practices that participate in the usual virology sampling programme conducted by PHE. The mixed methodology enabled the use of qualitative data from interviews with frontline staff to provide an understanding of some of the reasons for variations in the implementation of POCT between practices.
Comparison with existing literature
The results of the current study are similar to those of a previous study, which suggested that clinicians are more likely to perform rapid testing for influenza compared with clinicians in control clinics using conventional centralised laboratory testing.26 The current study found that POCT practices performed up to six times more tests, although, after taking into account differences in influenza-like illness rates between practices, POCT practices swabbed at approximately double the rate of other RCGP RSC virology sampling practices. Gren et al 26 found that increased near-patient testing would result in alerts 9 days earlier than surveillance alerts via traditional systems. However, the small sample size and the late initiation of the current study preclude the ability to study this.
The results regarding the clinical impact of POCT contrast with a 2019 systematic review and meta-analysis of influenza POCT in ambulatory care, which suggested that POCTs had no effect on antibiotic prescribing rates (relative risk [RR] = 0.97, 95% CI = 0.82 to 1.15; I2 = 70%).27 However, in common with the study by Lee et al,27 the current study showed increased prescribing of appropriate antivirals for influenza (RR = 2.65; 95% CI = 1.95 to 3.60; I2 = 0%). The differences in the effects on antibiotic prescribing between the studies may relate to differences in study characteristics between the current study and the studies included in the meta-analysis, which were mainly randomised trials performed in paediatric emergency departments. Of the non-randomised studies that reported on antibiotic prescribing, four out of five reported significant reductions, although there was strong evidence of statistical heterogeneity, possibly as a result of the pooling of results from primary care and emergency departments. It is also of note that all studies included in the meta-analysis used antigen-based POCT, whereas the current study used molecular POCT.
Implications for research
To the authors’ knowledge, this study provides the first evidence for the use of POCT to monitor influenza in primary care in the UK; however, further robust evidence is required, especially from randomised trials of POCT in general practice. Further work is required to study the impact of POCT on important public health tasks for sentinel surveillance, including influenza notification as well as infection control, and to study the cost–benefits of POCT in UK general practice.28
Acknowledgments
The authors would like to thank the participating practices and patients for providing the data for this study.
Funding
This study is part of Development of Robust and Innovative Vaccine Effectiveness (DRIVE), an EU-funded project, and part of the Innovative Medicines Initiative project (grant agreement number: 777363).
Ethical approval
The study was approved by the English National Research Ethics Committees (REC) (Integrated Research Application System ref: 252081; REC ref: 19/WM/0015).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Simon de Lusignan receives research funding from Eli Lilly Co., GlaxoSmithKline, Takeda, AstraZeneca, and Novo Nordisk Ltd. Tristan Clark has received speaker fees, honoraria, and equipment and consumables free of charge for the purposes of independent research from BioFire LLC and BioMerieux. Tristan Clark has also taken part in advisory board meetings for Roche and Janssen, and is a member of independent data-monitoring committees for trials sponsored by Roche. All other authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Iuliano A, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger CE, Blacker BF, Khalil IA, et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez A, Godoy P, Torner N. The effectiveness of influenza vaccination in different groups. Expert Rev Vaccines. 2016;15(6):751–764. doi: 10.1586/14760584.2016.1142878. [DOI] [PubMed] [Google Scholar]
- 4.Public Health England . PHE guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza, Version 8.0. London: Public Health England; 2017. [Google Scholar]
- 5.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD008965.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nie S, Roth RB, Stiles J, et al. Evaluation of Alere i Influenza A&B for rapid detection of influenza viruses A and B. J Clin Microbiol. 2014;52(9):3339–3344. doi: 10.1128/JCM.01132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young S, Illescas P, Nicasio J, Sickler JJ. Diagnostic accuracy of the real-time PCR cobas® Liat® Influenza A/B assay and the Alere i Influenza A&B NEAR isothermal nucleic acid amplification assay for the detection of influenza using adult nasopharyngeal specimens. J Clin Virol. 2017;94:86–90. doi: 10.1016/j.jcv.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Ison MG, Portsmouth S, Yoshida Y, et al. LB16. Phase 3 trial of baloxavir marboxil in high-risk influenza patients (CAPSTONE-2 Study) Open Forum Infect Dis. 2018;5(Suppl 1):S764–S765. [Google Scholar]
- 9.Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 10.Merckx J, Wali R, Schiller I, et al. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med. 2017;167(6):394–409. doi: 10.7326/M17-0848. [DOI] [PubMed] [Google Scholar]
- 11.Fleming DM, Durnall H. Ten lessons for the next influenza pandemic — an English perspective: a personal reflection based on community surveillance data. Hum Vaccin Immunother. 2012;8(1):138–145. doi: 10.4161/hv.8.1.18808. [DOI] [PubMed] [Google Scholar]
- 12.Pebody RG, Warburton F, Andrews N, et al. Uptake and effectiveness of influenza vaccine in those aged 65 years and older in the United Kingdom, influenza seasons 2010/11 to 2016/17. Euro Surveill. 2018. [DOI] [PMC free article] [PubMed]
- 13.Public Health England . Point of care tests for influenza and other respiratory viruses. London: Public Health England; 2018. [Google Scholar]
- 14.de Lusignan S, Hoang U, Liyanage H, et al. Feasibility of point-of-care testing for influenza within a national primary care sentinel surveillance network in England: protocol for a mixed methods study. JMIR Res Protoc. 2019;8(11):e14186. doi: 10.2196/14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa A, Hinton W, McGovern A, et al. Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC) sentinel network: a cohort profile. BMJ Open. 2016 doi: 10.1136/bmjopen-2016-011092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lusignan S, Correa A, Smith GE, et al. RCGP Research and Surveillance Centre: 50 years’ surveillance of influenza, infections, and respiratory conditions. Br J Gen Pract. 2017 doi: 10.3399/bjgp17X692645. [DOI] [PMC free article] [PubMed]
- 17.Vos LM, Bruning AH, Reitsma JB, et al. Rapid molecular tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review of diagnostic accuracy and clinical impact studies. Clin Infect Dis. 2019;69(7):1243–1253. doi: 10.1093/cid/ciz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 2nd edn. Thousand Oaks, CA: SAGE Publications; 2003. [Google Scholar]
- 19.Digital NHS. National data opt-out. 2019 https://digital.nhs.uk/services/national-data-opt-out (accessed 3 Jun 2020). [Google Scholar]
- 20.Payne RA, Abel GA. UK indices of multiple deprivation — a way to make comparisons across constituent countries easier. Health Stat Q. 2012;53:1–16. [Google Scholar]
- 21.Long S, editor. Socioanalytic methods: discovering the hidden in organisations and social systems. New York, NY: Routledge; 2018. [Google Scholar]
- 22.Silver B. BPMN method and style, with BPMN implementer’s guide: a structured approach for business process modeling and implementation using BPMN 2.0. 2nd edn. Aptos, CA: Cody-Cassidy Press; 2011. [Google Scholar]
- 23.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma — summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Chief Medical Officer . Annual national flu programme letter 2018. London: Department of Health; 2018. [Google Scholar]
- 25.O’Connor AM. Interpretation of odds and risk ratios. J Vet Intern Med. 2013;27(3):600–603. doi: 10.1111/jvim.12057. [DOI] [PubMed] [Google Scholar]
- 26.Gren LH, Porucznik CA, Joy EA, et al. Point-of-care testing as an influenza surveillance tool: methodology and lessons learned from implementation. Influenza Res Treat. 2013;2013:242970. doi: 10.1155/2013/242970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JJ, Verbakel JY, Goyder CR, et al. The clinical utility of point-of-care tests for influenza in ambulatory care: a systematic review and meta-analysis. Clin Infect Dis. 2019;69(1):24–33. doi: 10.1093/cid/ciy837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schols AM, Dinant GJ, Cals JW. Point-of-care testing in general practice: just what the doctor ordered? Br J Gen Pract. 2018 doi: 10.3399/bjgp18X698033. [DOI] [PMC free article] [PubMed]