Abstract
Long-term culture of the human lung adenocarcinoma cell line A549 promotes the differentiation of these cells toward an alveolar type II cell phenotype. Here, we evaluated the susceptibility of long-term cultured A549 cells to human influenza viruses. A549 cells were cultured continuously for 25 days (D25-A549) or 1 day (D1-A549) in Ham’s F12K medium. Six human influenza A viruses grew much faster in D25-A549 cells than in D1-A549 cells; however, two influenza B viruses replicated poorly in both cell types. Two avian influenza viruses replicated efficiently in both cell types, with similar titres. Expression levels of human virus receptors were higher in D25-A549 cells than in D1-A549 cells. D25-A549 cells thus more efficiently support the replication of human influenza A viruses compared with D1-A549 cells. Our data suggest that long-term cultured A549 cells will be useful for influenza A virus research.
Keywords: Human influenza A viruses, A549 cells, long-term culture, replication
Influenza viruses, which belong to the family Orthomyxoviridae, contain a segmented genome of negative-strand RNA molecules enclosed in a lipid envelope. They are classified into four different types: A, B, C and D [1, 2]. Among these, influenza A and B viruses are responsible for the severe morbidity and mortality worldwide during annual influenza epidemics. In addition, influenza A viruses cause influenza pandemics that have devastating effects on global health and economies. The adenocarcinoma human alveolar basal epithelial cell line A549 has been used widely for in vitro studies of influenza A virus replication. However, this cell line has been shown to be less permissive for the productive infection of some influenza A strains [3, 4]. Recently, Cooper et al. reported that long-term culture of A549 cells leads to the differentiation of these cells toward an alveolar type II cell phenotype [5]. Here, we evaluated the susceptibility of long-term cultured A549 cells to human influenza viruses.
A previous study showed that long-term culture of A549 cells in Ham’s F12 nutrient medium (Ham’s F12K) promoted a differentiated alveolar type II cell phenotype [5]. Therefore, we cultured A549 cells in Ham’s F12K containing 10 % FCS to prepare differentiated A549 cells. Cells seeded onto 12-well plates at 1.5–2×104 cells cm− 2 were cultured continuously for 25 days at 37 °C with 5 % CO2, and the media were changed every 2–4 days, as described previously [5]. A549 cells cultured for 25 days (designated D25-A549 cells) or 1 day (designated D1-A549) in Ham’s F12K medium were used for all experiments. We did not subculture D25-A549 cells, because trypsinized D25-A549 cells showed low viability. Alveolar type II pneumocytes contain characteristic electron-dense multilamellar bodies, and Cooper et al. showed that long-term culture of A549 cells promotes the formation of these bodies [5]. To confirm the expression of multilamellar bodies in cultured D25-A549 cells, we observed D25-A549 cells by thin-section electron microcopy. Confluent monolayer cultures of D25-A549 cells were pre-fixed with 2.5 % glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) on ice for 1 h (Fig. 1a). After being washed with the same buffer, the cells were post-fixed with 2 % osmium tetroxide on ice for 1 h. The samples were then en bloc stained on ice for 30 min in a solution of 1 % uranyl acetate dissolved in distilled water, dehydrated with a series of ethanol gradients followed by propylene oxide, and embedded in epoxy resin (Epon 812; TAAB). Thin sections were stained with uranyl acetate and lead citrate and examined by using a Tecnai F20 electron microscope (FEI) at 200 kV. Transmission electron micrographs revealed that the D25-A549 cells contained many multilamellar bodies (Fig. 1b). In contrast, few multilamellar bodies were found in A549 cells cultured for 1 day in Ham’s F12K (designated D1-A549 cells) as control cells.
Fig. 1.
Morphological characterization of long-term cultured A549 cells. A549 cells were cultured for 25 days (D25-A549) or 1 day (D1-A549) in Ham’s F12K medium. (a) Phase contrast images of D25-A549 and D1-A549 cells. Bar, 200 µm. (b) Representative transmission electron micrograph of D25-A549 and D1-A549 cells. Nuclei (n) and multilamellar bodies (MLB) are shown. Bar, 1 µm.
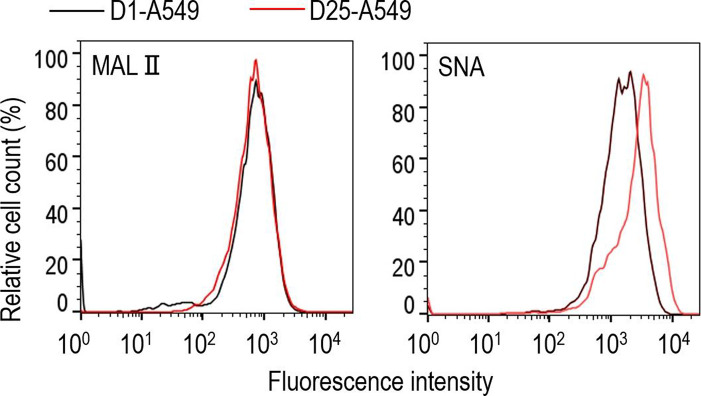
Influenza virus binds to sialic-acid-containing glycans on glycoproteins and glycolipids on the surface of epithelial cells and initiates infection. Human influenza viruses prefer to bind to glycans that end with sialic acid linked to galactose by α2,6-linkages, whereas avian viruses preferentially bind to glycans that terminate with sialic acid linked to galactose by α2,3-linkages [6–8]. We compared the cell surface expression levels of sialic acids on D25-A549 and D1-A549 cells by using the Maackia amurensis lectin II (MAL II) (Vector), which is specific for α2,3-linked sialic acids, and the Sambucus nigra agglutinin (SNA) lectin (Vector), which is specific for α2,6-linked sialic acids. Cells were detached by incubation for 10 min in PBS with 0.125 % Trypsin–20 mM EDTA. After being washed with PBS, the cells were blocked with Carbo-Free Blocking Solution (Vector) at 4 °C for 15 min. The cells were then incubated with either biotinylated MAL II or SNA at 4 °C for 30 min, then rinsed with PBS before being incubated with Alexa 488-conjugated streptavidin for 30 min at 4 °C (Invitrogen). Fluorescence was measured using a FACS Verse system (Becton Dickinson) and analysed using FlowJo software (Becton Dickinson). Flow cytometric analysis revealed that there were no differences in the expression level of α2,3-linked sialic acids between D25-A549 and D1-A549 cells (Fig. 2). However, the expression level of α2,6-linked sialic acids was markedly higher in D25-A549 cells compared with D1-A549 cells.
Fig. 2.
Flow cytometric analysis of the cell surface expression of α2,6- and α2,3-linked sialic acids. D1-A549 cells (black line profiles) and D25-A549 cells (red line profiles) were incubated with biotinylated Maackia amurensis lectin II (MAL II) (specific for α2,3-linked sialic acids; left panel) or Sambucus nigra agglutinin (SNA) lectin (specific for α2,6-linked sialic acids; right panel), followed by Alexa 488-conjugated streptavidin, and then analysed by flow cytometry.
To determine whether long-term culture of A549 cells could enhance the replication of human influenza viruses in these cells, we examined the growth kinetics of diverse virus strains [one A/H1N1, two A/H1N1 2009 pandemic (A/H1N1pdm), three A/H3N2 and two type B] in D25-A549 cells. D25-A549 and D1-A549 cells (1.7–1.9×106 cells per well) in a 12-well plate were infected with virus at an m.o.i. of 0.01. The inoculum was removed after 30 min of incubation at 37 °C, and the cells were further incubated at 37 °C in MEM containing 0.3 % BSA and 1 µg ml−1 l-1-tosylamide-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin. Virus titres in the cell culture supernatant were determined by means of plaque assays in MDCK cells. For A/Tokyo/UT-DA30/2018 (H3N2) virus, the virus titres were determined by use of plaque assays in hCK cells [9]. All six human influenza A viruses grew much faster and to higher titres (0.62–2.59 log units higher at 48 h post-infection) in D25-A549 cells than in D1-A549 cells (Fig. 3a). In contrast, the two influenza B viruses grew poorly in both D25-A549 and D1-A549 cells (Fig. 3b). We also compared the growth kinetics of two avian influenza viruses isolated from humans (one A/H5N1 and one A/H7N9) in D25-A549 and D1-A549 cells. D25-A549 and D1-A549 cells were infected with virus at an m.o.i. of 0.001, and virus titres were determined by means of plaque assays in MDCK cells. These avian viruses replicated efficiently in D25-A549 and D1-A549 cells, and no substantial differences in titres were observed (Fig. 3c). These results suggest that long-term culture of A549 cells more efficiently supports the replication of human influenza A viruses than does short-term culture of A549 cells.
Fig. 3.
Growth kinetics of influenza viruses in D25-A549 and D1-A549 cells. D1-A549 and D25-A549 cells were infected with viruses at an m.o.i. of 0.01 (a, b) or 0.001 (c). The supernatants of the infected cells were harvested at the indicated times, and virus titres were determined by means of plaque assays on MDCK or hCK cells. Data are shown as the mean (±sd) of three independent experiments. Mean values were compared by using a two-way ANOVA, followed by Dunnett’s test (**P<0.01, ***P<0.001). Asterisks indicate statistically significant differences between D1-A549 and D25-A549 cells.
Our flow cytometric analysis suggests that the higher surface density of human-type receptors (i.e. α2,6 receptors) on the cell surface of D25-A549 cells may enhance the strength of the binding interaction between virions and receptors required for virus entry, leading to the higher sensitivity of D25-A549 cells to human influenza A viruses compared with that of D1-A549 cells. In addition, a large number of genes have been shown to be up-regulated or down-regulated in long-term cultured A549 cells compared with in short-term cultured A549 cells [5]. This suggests the possibility that various host factors may contribute to the increased viral titrs in D25-A549 cells. For example, the expression of LAMP3 (lysosome-associated membrane protein 3) is upregulated in the former compared with in the latter [5], and this host factor plays an important role in the replication of influenza A viruses [10]. We quantified the expression level of the LAMP3 gene in D25-A549 and D1-A549 cells by using real-time PCR. Total RNA was extracted from D25-A549 and D1-A549 cells by using the Isogen RNA extraction kit (Nippongene) according to the manufacturer’s instructions. Oligo (dT) primer and SuperScriptⅢ Reverse Transcriptase (Invitrogen) were used for cDNA synthesis. Amplification and detection by real-time PCR were performed with the Thunderbird SYBR qPCR mix (Toyobo) and the 7900HT Fast Real-Time PCR System (Applied Biosystems) according to the manufacturers’ instructions. To normalize the amount of cDNA across samples, 18S rRNA was used as a housekeeping control gene. The specific primers for PCR were: forward (5′-ACCGATGTCCAACTTCAAGC) and reverse (5′-TGACACCTTAGGCGGATTTT). Relative fold changes in expression were calculated by using the 2−ΔΔCt method. Real-time PCR revealed that D25-A549 cells expressed higher LAMP3 mRNA levels compared with D1-A549 cells (Fig. 4). Thus, higher LAMP3 expression in D25-A549 cells might confer enhanced replication to human influenza A viruses. Further investigations are required to determine which host factors are involved in the enhanced replication of human influenza A viruses in D25-A549 cells.
Fig. 4.

Relative ratios of LAMP3 gene expression for D25-A549 and D1-A549 cells. RNA was extracted from A549 cells and reverse transcribed, and the resultant cDNA was quantified by real-time PCR with primers directed to the LAMP3 gene. Levels of 18S rRNA were used to normalize the relative expression levels of LAMP3 mRNAs in D25-A549 and D1-A549 cells. Data are shown shown as means±sd.
In conclusion, our data suggest that long-term cultured A549 cells will be useful for influenza virus research, particularly for studies involving human influenza A viruses.
Funding information
This research was supported by Leading Advanced Projects for medical innovation (LEAP) from the Japan Agency for Medical Research and Development (AMED) (JP18am001007), by Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan (Nos. 16H06429, 16K21723, and 16H06434), by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from AMED (JP19fm0108006), by a Research Program on Emerging and Re-emerging Infectious Diseases from AMED (JP19fk0108031 and JP19fk0108056), and by the NIAID-funded Center for Research on Influenza Pathogenesis (CRIP, HHSN272201400008C).
Acknowledgements
We thank Yuelong Shu for A/Anhui/1/2013 (H7N9) and Ron A. M. Fouchier for B/Netherlands/1000/76. In addition, we thank Susan Watson for scientific editing. Flow cytometry was performed in the IMSUT FACS Core laboratory; we acknowledge the IMSUT FACS Core laboratory for assistance with the flow cytometric analysis.
Conflicts of interest
M.U., K.T., M.K., Y,S.-T., M. Ito, K.N., S.W. and M. Imai have no competing interests. Y.K. has received speaker’s honoraria from Toyama Chemical and Astellas, Inc.; grant support from Daiichi Sankyo Pharmaceutical, Toyama Chemical, Shionogi and Co., Ltd, and Kyoritsu Seiyaku; and is a founder of FluGen.
Footnotes
Abbreviations: MAL II, Maackia Amurensis lectin II; MDCK, Madin-Darby canine kidney; SNA, Sambucus Nigra agglutinin; TPCK, L-1-tosylamide-2-phenylethyl chloromethyl ketone;
References
- 1.Hause BM, Ducatez M, Collin EA, Ran Z, Liu R, et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013;9:e1003176. doi: 10.1371/journal.ppat.1003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hause BM, Collin EA, Liu R, Huang B, Sheng Z, et al. Characterization of a novel influenza virus in cattle and swine: proposal for a new genus in the Orthomyxoviridae family. MBio. 2014;5:e00031-14. doi: 10.1128/mBio.00031-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knepper J, Schierhorn KL, Becher A, Budt M, Tönnies M, et al. The novel human influenza A(H7N9) virus is naturally adapted to efficient growth in human lung tissue. MBio. 2013;4:e00601–00613. doi: 10.1128/mBio.00601-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumari K, Gulati S, Smith DF, Gulati U, Cummings RD, et al. Receptor binding specificity of recent human H3N2 influenza viruses. Virol J. 2007;4:42. doi: 10.1186/1743-422X-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper JR, Abdullatif MB, Burnett EC, Kempsell KE, Conforti F, et al. Long term culture of the A549 cancer cell line promotes multilamellar body formation and differentiation towards an alveolar type II pneumocyte phenotype. PLoS One. 2016;11:e0164438. doi: 10.1371/journal.pone.0164438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, et al. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 7.Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 8.Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 9.Takada K, Kawakami C, Fan S, Chiba S, Zhong G, et al. A humanized MDCK cell line for the efficient isolation and propagation of human influenza viruses. Nat Microbiol. 2019;4:1268–1273. doi: 10.1038/s41564-019-0433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Xue Q, Wan Y, Yang Y, Wang J, et al. Lysosome-Associated membrane glycoprotein 3 is involved in influenza A virus replication in human lung epithelial (A549) cells. Virol J. 2011;8:384. doi: 10.1186/1743-422X-8-384. [DOI] [PMC free article] [PubMed] [Google Scholar]