Abstract
Purpose of review
The present coronavirus disease 2019 (COVID-19) pandemic has created additional challenges with an increased number of presumed healthy, full-term newborns being discharged at 24 h after delivery. Short lengths of stay raise the possibility of mother–infant dyads being less ready for discharge, defined as at least one of the three informants (i.e., mother, pediatrician, and obstetrician) believing that either the mother and/or infant should stay longer than the proposed time of discharge. This public health crisis has reduced the number of in-person well child visits, negatively impacting vaccine receipt, and anticipatory guidance.
Recent findings
Extra precautions should be taken during the transition period between postpartum discharge and follow-up in the ambulatory setting to ensure the safety of all patients and practice team members. This should include restructuring office flow by visit type and location, limiting in-person visits during well infant exams, instituting proper procedures for personal protective equipment and for cleaning of the office, expanding telehealth capabilities for care and education, and prioritizing universal vaccinations and routine well child screenings.
Summary
Based on current limited evidence, this report provides guidance for the postdischarge management of newborns born to mothers with confirmed or suspected disease in the ambulatory setting as well as prioritizing universal immunizations and routine well child screenings during the COVID-19 pandemic.
Keywords: ambulatory practice management, COVID-19, coronavirus disease 2019, newborns, postpartum discharge, severe acute respiratory syndrome coronavirus 2, well child visits
INTRODUCTION
Since 1996, the Newborns’ and Mothers’ Health Protection Act has suggested hospital lengths of stay of 48-h after a healthy full-term vaginal delivery or 96-h post-C-section delivery [1]. Shorter lengths of stay (currently recommended by American College of Obstetricians and Gynecologists (ACOG) as 1 day after uncomplicated vaginal delivery and 2 days after cesarean birth [2]) raise the possibility of mother–infant dyads being less ready at discharge, which may have a variety of potential physical and psychological consequences. Infants who are not ready for discharge have a higher risk of being readmitted to the hospital for hyperbilirubinemia, feeding difficulties (dehydration, excessive weight loss) and sepsis [3]. New mothers may experience postpartum mood disorders or complications after giving birth that are not often evident within 24 h of delivery. In a study of 4300 mother–infant dyads, 16–17% met the condition for maternal or infant ‘unreadiness’ based on the perceptions of the obstetrician, pediatrician, and/or mother. Of the ‘unready’ dyads, most met the criteria based on maternal perception alone. Often, mothers who said they were ‘unready’ for discharge expressed concerns about their own physical condition and their baby's feeding patterns, which ideally should be addressed in the hospital or with clear postdischarge services in place [1].
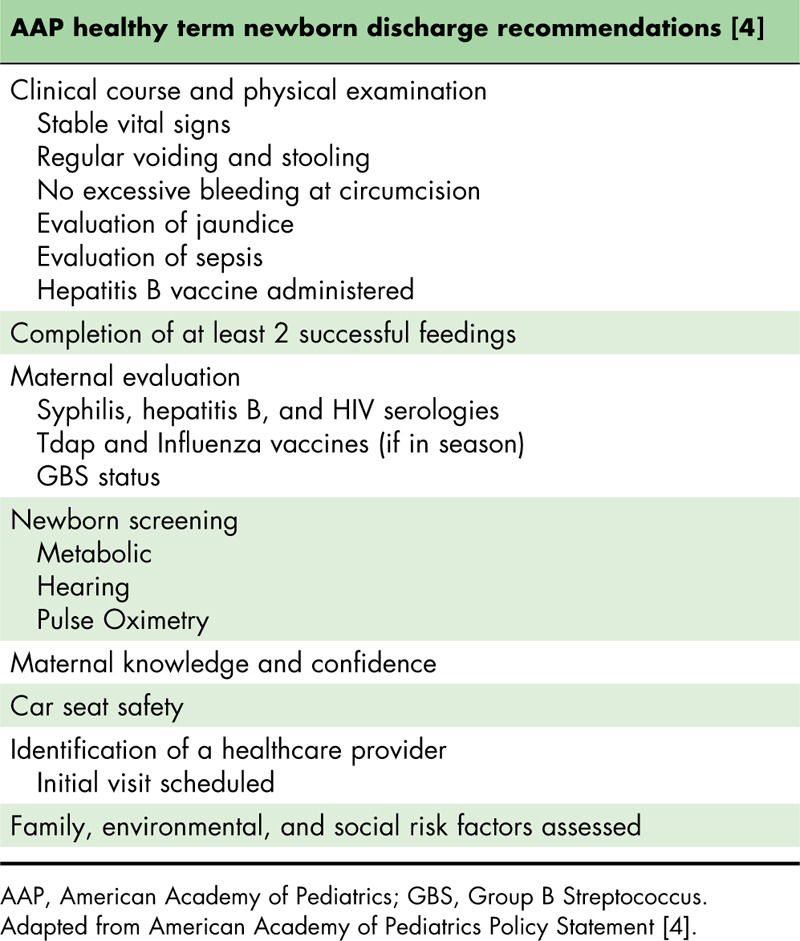
Given the challenges created by current coronavirus disease 2019 (COVID-19) pandemic, an increased number of presumed healthy, full-term newborns are being discharged at 24 h after delivery to limit potential exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the hospital setting. American Academy of Pediatrics (AAP) clinical guidelines suggest 17 criteria be met before the discharge of healthy term newborns (Table 1) [4]. These criteria include checklists for both the mother and her baby before leaving the hospital, and span physiological and social conditions. Therefore, assessing all of these factors is important to increase maternal readiness and reduce odds of readmission (e.g., for jaundice or dehydration), which has high medical, emotional, and financial implications [5,6]. In the end, early discharge decisions should be made as a consensus among all three stakeholders (i.e., mother, pediatrician, and obstetrician).
Table 1.
List of criteria to be met before discharge of all healthy term newborns
In addition, screening mothers routinely in their birth hospital (and the newborns of SARS-CoV-2 positive mothers) should be considered. A number of birth hospitals have already instituted universal screening for SARS-CoV-2 in women admitted for delivery. In one report from Columbia University, 84.6% of women were SARS-CoV-2 negative, 13.5% were asymptomatic SARS-CoV-2 positive, and only 1.9% were symptomatic SARS-CoV-2 positive [7▪]. Such testing strategy presents new objective concerns for the mother–infant dyad, as it is unclear at what rate an asymptomatic mother with SARS-CoV-2 may infect her newborn. Children, of course, are susceptible to COVID-19, and while symptoms are typically less severe than those of adults, infants are at higher risk of severe disease within the pediatric population [8▪▪]. Although only 1.7% of laboratory-confirmed cases occurred in children less than 18 years of age, children less than 1 year of age accounted for the highest percentage (range 15–62%) of hospitalizations among pediatric patients with COVID-19 [9].
Hence, women and newborns with confirmed or suspected COVID-19 require additional guidelines that should be followed to ensure disease containment and to prevent the dissemination of the virus [10▪▪]. Data suggest that the virus spreads rapidly among asymptomatic individuals [11], prompting the need to assume every patient may be an asymptomatic carrier where there is community spread. Extra precautions should be taken during the transition period between postpartum discharge and close follow-up in the ambulatory setting for all families to ensure the safety of all patients and practice team members. This should include restructuring office flow by visit type and location (e.g., COVID-19 positive vs. negative), limiting in-person visits during well infant exams, instituting proper procedures for personal protective equipment (PPE) and for cleaning of the office, expanding telehealth capabilities for care and education, and prioritizing universal vaccinations and routine well child screenings.
Box 1.

no caption available
THE TRANSITION PERIOD BETWEEN POSTPARTUM DISCHARGE AND CLOSE FOLLOW-UP FOR WOMEN AND NEWBORNS WITH CONFIRMED OR SUSPECTED DISEASE
If a mother has confirmed or suspected COVID-19, discharge of the infant to the care of a designated healthy (noninfected) caregiver should be considered, based upon shared clinical decision-making [12]. When the mother is in the same household, she should maintain 6-ft. separation from the infant or any other caregiver, as reasonably possible, to minimize the risk of postnatal infant infection and transmission from maternal respiratory secretions. This shared clinical decision-making conversation should take place between the provider and the parents on an individual basis, as each family's needs and resources differ. In the event that there is not a noninfected caregiver, the mother should continue to maintain separation as much as possible. She should make sure to wash hands before and after touching the baby, and to practice respiratory hygiene by wearing a mask or cloth face covering around the infant to cover the nose and mouth and maintaining cough etiquette.
Aerosolizability of COVID-19 has been examined and varies based upon the surface, so the family should routinely clean and disinfect surfaces [13]. Skin-to-skin contact should be minimized unless the mother wishes to breastfeed rather than express human milk. This approach should continue for any COVID-19 positive or suspected caregiver until either the caregiver has been afebrile for 72 h without use of antipyretics, and at least 10 days have passed since symptoms first appeared; or that individual has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 h apart [14]. Due to variable resource availability in different communities, safely collecting specimens and result turn-around time pose additional challenges. Acknowledging these limits, the pediatrician should attempt to follow these de-isoloation criteria while remaining cognizant of their community's resources.
Out of an abundance of caution and given scarce data regarding postpartum transmission, neonates born to women with COVID-19, as well as neonates born to women with testing for COVID-19 pending at the time of delivery, should be considered as persons under investigation (PUIs) for infection. Neonates born to mothers with confirmed or suspected COVID-19 should be tested at approximately 24 h of life, with repeat testing at 48 h of life. Infants who have not been tested should be treated as if they are positive for the virus for the 14-day observation period. Testing of the infant in the ambulatory setting should be considered. The timing of subsequent testing are currently unknown. Recent data show pediatric patients are a small subset of the total number of patients diagnosed with COVID-19. Of those testing positive, the majority of children and infants have mild upper respiratory or asymptomatic disease [9]. It should be noted, however, infants are more at risk for severe disease than any other pediatric age group [8▪▪]. Of those infants that do present symptomatically, data show that the source of infection is likely maternal in origin [15▪▪]. Infants determined to be infected by molecular testing (or whose status cannot be determined due to lack of available testing), but with no symptoms of COVID-19, may be discharged home on a case-by-case basis with appropriate precautions and plans in place for frequent outpatient follow-up contacts (either by phone, telemedicine, or in-office) through 14 days after birth. Data show that asymptomatic carriers may present with symptoms days after testing positive (median incubation period of 5.1 days), which prompts the necessity of close follow-up of these newborns [11,16,17▪]. Presymptomatic patients have been noted to have the highest viral load at or before symptom onset, inferring a peak of infectiousness at this timepoint [18].
Precautions should be followed to prevent household spread from infant to caregivers. Specific guidance regarding use of masks, gloves and hand hygiene should be provided to all caregivers – see https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/index.html. Due to increased risk of infection and severity of disease in elderly patients, noninfected individuals older than 60 years of age and those with comorbid conditions should not provide care if possible. Information about COVID-19 should be provided to all caregivers and include written as well as verbal education in person, via telephone or virtually, utilizing interpreter services when appropriate. In addition, regardless of COVID-19 status, the discharging pediatrician must be timely in communicating directly with the accepting pediatrician to assure that safe and appropriate follow-up can be accomplished.
Consideration should be given to further ensure that all families, regardless of the COVID-19 status of mother and infant, have the means to prevent the contraction and/or dissemination of this virus once discharged [19]. Although an exciting time for the new family with individuals eager to see the newborn, family gatherings are discouraged to minimize exposure to mother and infant and mitigate any rapid community transmission [20,21]. Proper hand hygiene should be practiced by every member of the household, including other children. This includes washing hands with soap and water for at least 20 s or using an alcohol-based hand sanitizer that contains at least 60% alcohol when soap and water is not available. For further information on hand hygiene: https://www.cdc.gov/healthywater/hygiene/hand/handwashing.html.
From discharge, all families should be equipped with telehealth. They should have access to cell phones, laptops, Internet, and should be aware of how to log onto webinars and download video conferencing platforms. Specifics regarding accessing the telehealth platform of the pediatrician who will be assuming postdischarge follow-up should be arranged as part of the discharge planning process. Where this is not possible, alternative means to establish regular contact between families and pediatricians are critical for safe care.
Breastfeeding guidelines for all mother–infant dyads
The AAP unequivocally promotes the health, neurodevelopmental and even economic benefits of breastfeeding newborns [22]. In addition to the known benefits of breastfeeding, mother's milk may provide protective factors to the infant after maternal COVID-19 infection. No study to date has demonstrated the presence of SARS-CoV-2 in breast milk [23]. Mothers with confirmed or suspected COVID-19 may express breast milk (after appropriate breast and hand hygiene) and this milk may be fed to the infant by designated caregivers. Detailed policies and instructions specific to COVID-19 can be found here: https://www.cdc.gov/coronavirus/2019-ncov/hcp/care-for-breastfeeding-women.html.
Breast pumps and components should be thoroughly cleaned in between pumping sessions and washing pump attachments with hot, soapy water, and using disinfectant wipes if the mother uses a shared breast pump. Further information on breast pump cleaning can be found on this Centers for Disease Control and Prevention (CDC) page: https://www.cdc.gov/healthywater/hygiene/healthychildcare/infantfeeding/breastpump.html.
If there is not a noninfected caregiver available, mother herself may feed the baby while practicing respiratory hygiene, wearing a mask, washing hands before and after touching the baby, and routinely cleaning and disinfecting surfaces. If the mother wants to directly breastfeed, she should comply with strict preventive precautions, including the use of masks and meticulous breast and hand hygiene [24▪]. Current data prove that cotton or surgical masks do not completely prevent the dissemination of COVID-19 [25]. However, masks may shorten the travel distance of the particles when coughing; thus, they may provide an additional barrier between mother and baby. Additional information on homemade masks for the public, in addition to easy-to-understand benefits, can be found on this JAMA Patient Page: https://jamanetwork.com/journals/jama/fullarticle/2764955.
For more guidance on breastfeeding for mothers or infants with confirmed or suspected COVID-19: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/breastfeeding-guidance-posthospital-discharge/.
RESTRUCTURING OFFICE FLOW BY VISIT TYPE AND LOCATION (e.g., CORONAVIRUS DISEASE 2019 POSITIVE VS. NEGATIVE)
As a result of early discharge from hospitals during the COVID-19 pandemic, there is an increased priority placed on the newborn follow-up visit to assess mother and baby. It is extremely important that this initial visit is done in-person wherever possible within 24–48 h after discharge, especially since this early well baby visit has been shown to reduce rates of readmission for newborns. The early well baby visit should ensure that all screenings meant to be completed in the hospital are complete and any gaps in knowledge or care resulting from early discharge are addressed. Communication with the family prior to the appointment should encourage only one caregiver or guardian with the patient at the visit with the option of video-chatting or speaking with additional caregivers during the encounter for proper education [26].
When confirming with a family before an in-person visit, it is important to relay the office policies and instructions specific to COVID-19. Posting signage at areas in the office regarding these policies, in addition to updating social media and the practice website, can also be beneficial. Sharing helpful instructions and videos regarding hand hygiene, respiratory hygiene, and cough etiquette provide another way to interact with patients to ensure safe practices at the office and in the community.
If it is feasible, the practice should physically and temporally separate sick and well visits to reduce potential exposure. The practice may choose to schedule only well visits in the morning, prioritizing newborns earliest in the day, accessing the practice through a designated, separate physical entrance, if possible. The practice can also delegate which physicians see sick patients, leaving the well visits to be seen by physicians who may have underlying health risks. Dedicate separate rooms for sick and well visits, if achievable; or consider using one office location to see all well visits for practices with more than one site. Time in the waiting room should be eliminated if at all possible, with families being escorted immediately to an appropriate exam room upon arrival. In addition, appointments should be scheduled to minimize the use of the waiting room and to allow for disinfecting of exam rooms in between appointments.
LIMITING IN-PERSON CONTACT DURING WELL INFANT EXAMS
For all in-person visits, the practice should confirm that patients and the accompanying parent do not have a fever or any symptoms of COVID-19. Reminders may be sent the day before the visit to emphasize calling prior to coming when anyone is unwell. If patients do have symptoms, extra precautions should be taken, and appropriate accommodations should be made. The patient and their family should be given a mask in this situation. If possible, only one person or caregiver should attend the visit with the newborn. Other caregivers should be encouraged to virtually attend the visit by allowing the parent/caregiver in the office to connect via cell phone speaker.
During the newborn visit, pediatricians should follow the measures outlined in the periodicity table of the American Academy of Pediatrics with recommendations for typical well visits during the first month of life [27]. Timing of circumcision of male infants who are PUI may be delayed until 14 days have elapsed from birth, provided the infant has remained asymptomatic.
In-person well visits
The COVID-19 pandemic has not only posed these challenges discussed, it also has led to the reduction of in-person well child visits. The benefit of attending a well visit and receiving necessary immunizations and screenings should be prioritized for all pediatric patients whenever possible [19,28,29]. Vaccines should be administered in as few appointments as possible to limit in-person appointments and telehealth can be used for other elements of the visit [30]. Attempting to adhere to the recommended universal schedule of immunizations is critically important as this schedule is optimized for development of immunity and epidemiology of disease. Both the AAP and the CDC endorse the importance and value of maintaining immunization rates during the COVID-19 pandemic:
During the visit, physicians should maintain distance when possible and limit physical contact with the newborn, with the exception of the physical exam and vaccinations. Further, well visits should be scheduled during a reserved time of the day with sick visits scheduled separately to reduce potential exposure and spread.
Follow-up visits
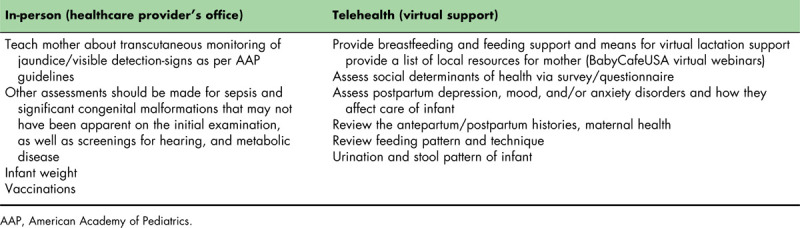
Table 2 demonstrates which guidelines might be completed in person and what can be accomplished via telehealth for all mothers, regardless of COVID-19 status, upon discharge of their infant. If possible, in-person visits should be limited to those scheduled visits at which vaccines, comprehensive physical exams, essential lab testing, and/or weight checks are necessary. If families have particular nonurgent concerns, optimally, exposure in the emergency department or in the office should be avoided, and telehealth utilized, whenever possible. Practices should obtain consent and ensure that families are equipped with telehealth capability and are confident in using it. Physician schedules should also be adapted to include time for telemedicine appointments and follow-up.
Table 2.
Guidelines that can be completed in-person and via telehealth for newborns and mothers
INSTITUTING PROPER PROCEDURES FOR PERSONAL PROTECTIVE EQUIPMENT AND FOR CLEANING OF THE OFFICE
Per current CDC recommendations, procedural masks: (a medical mask with ear loops commonly used in clean or sterile settings to decrease the risk of respiratory secretion transfer from user to others) should be worn by all staff in patient care areas for all routine patient care. Patients 2 years and older and their guardians should also wear a procedural mask while in the office. PPE should be used for clinical evaluation and for the collection of samples from anyone who is a suspected case of COVID-19 or a PUI. This includes an isolation gown, a face shield or goggles, gloves, and a procedural mask. Precautions will vary depending on the types of interactions [31]. It is recommended that an N95 respirator be worn while obtaining diagnostic respiratory specimens by swabbing the nasopharynx or oropharynx, or through washes or aspirates of nasal secretions. However, if shortages of N95 respirators exist, the healthcare provider should use a procedural mask while preserving N95 respirators for procedures at higher risk of producing infectious aerosols. Importantly, the patient's mouth should be covered with a procedural mask during nasal swabbing. N95 respirators should be used for direct, prolonged exposure with a suspected or confirmed COVID-19 patient. For further, in-depth information on PPE: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html.
Coronavirus disease 2019 testing in the ambulatory setting
When indicated, COVID-19 testing should be done in a ‘drive-through’ style. This minimizes contact with other patients coming in for care. When testing is performed in office, it should be done with appropriate PPE (i.e., procedural mask, goggles or face shield, gloves, and isolation gown). It is recommended that an N95 respirator be worn while obtaining nasopharyngeal or oropharyngeal swabbing for testing. Those individuals who experience respiratory symptoms concerning for COVID-19 should call and speak with office staff or a healthcare provider to receive specific information regarding when and where they should obtain testing. For more information on testing: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html.
Data suggest that COVID-19 has the ability to remain viable and infectious on surfaces for days and in aerosols for hours, such as 24 h on cardboard, 48 h on stainless steel, and 72 h on plastic surfaces [13]. Therefore, there should be established routine cleaning and disinfection procedures in patient care areas, especially in which aerosol-generating procedures are performed. The same protocol should also be applied to waiting rooms, or any areas and/or surfaces where patients are present. Environmental Protection Agency (EPA)-approved products can be found here: www.americanchemistry.com/Novel-Coronavirus-Fighting-Products-List.pdf.
PPE should be worn while cleaning and removed and discarded upon exit. Hands should be washed after cleaning. The room may be used immediately after a routine cleaning and disinfection, unless an aerosol-generating procedure has been done in that room. If the latter occurs, the room should be closed off to patient entry for 1–2 h (depending on the room's air exchanges per hour). Staff may enter the room during that time to clean and turn over the room, provided they are wearing PPE (including N95) during that time. For further information on cleaning facilities, see this CDC page: https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html.
EXPANSION OF TELEHEALTH CAPABILITIES FOR CARE AND EDUCATION
During all telehealth visits, providers should make sure to educate families on signs of general newborn wellness and illness, as well as reassure families that the ambulatory providers are available to support them, especially in these challenging times. Parents who may need to be physically distant from their children or newborns should be aware of access to appropriately trained mental health and psychosocial support [20].
Anticipatory guidance can be provided via telehealth including safety education, and guidance related to development, behavior, and oral health. A key area of support that should be emphasized during this period of time is nutrition. It is imperative newborns and mothers have appropriate nutrition, and access to resources to replenish feedings, if prior resources are no longer available because of COVID-19. It is important that screening for postpartum depression happens regularly with each contact (Edinburgh Scale). Connections to address social determinants of health can also be provided (e.g., community support or specialty care).
Lactation support
No study to date has demonstrated the presence of SARS-CoV-2 in breast milk, so breastfeeding promotion and education are still of the utmost importance [23]. Pediatricians should continue to emphasize the health and neurodevelopmental benefits of breastfeeding to mothers. Families should be made aware of online support groups for breastfeeding (e.g., Baby Cafe USA) and any telephone-based warm lines that provide breastfeeding tips. Board-certified lactation consultants International Board-certified lactation consultants (IBCLC) and other lactation professionals and lactation professionals in the office should be equipped with telehealth. Mothers can reach out to their local Women, Infants, and Children (WIC) programs to receive additional support. Discharge plans should include the contact information for lactation support in case the mother runs into any breastfeeding issues postpartum. Ultimately, it is the choice of the mother whether or not to breastfeed. It is recommended that mothers who are COVID-19 presumed or positive express their breastmilk to be fed to the infant by another caregiver and care taken to clean the breast pump carefully after each feed [24▪]. Of course, COVID-19 positive mothers may breastfeed their babies, as long as they follow proper hand-washing techniques, wear a mask while breastfeeding, and use good breast hygiene. For further information on breastfeeding during this COVID-19 pandemic: https://www.cdc.gov/coronavirus/2019-ncov/hcp/care-for-breastfeeding-women.html.
Education about coronavirus disease 2019 in infants
New parents should be particularly aware of the common concerns in newborns such as signs of jaundice, feeding patterns, and sleeping patterns [4,10▪▪,27]. Emergent, non-COVID-19 issues in an infant (e.g., jaundice, dehydration, pyloric stenosis) still need to be observed by parents and the pediatrician now more than ever, as there may be an increased morbidity due to delayed medical care. In addition, parents now must be aware of symptoms consistent with infant COVID-19 infection.
Data show that pediatric patients present with mild respiratory symptoms, fever, dry cough, sore throat, and fatigue [32▪▪]. Simultaneously, current evidence also suggests children do not consistently present with these symptoms and may commonly be asymptomatic [9]. Nonetheless, parents should still recognize this presentation and contact their pediatrician accordingly. Fever in infants, even if COVID-19 is suspected, must be treated in the same manner as it would prepandemic with appropriate evaluation and hospitalization, when indicated, given the existing significant risk of bacterial infections in this age group. Respiratory distress also necessitates emergent care and should be recognized by caregivers.
Providers should make sure that families are comfortable contacting them, especially in emergencies, and should obtain consent for receiving telehealth. For further guidance on providing pediatric ambulatory services via telehealth during COVID-19, see: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/guidance-on-providing-pediatric-ambulatory-services-via-telehealth-during-covid-19/.
PRIORITIZING UNIVERSAL IMMUNIZATIONS AND SCREENINGS THROUGHOUT ALL PEDIATRIC AGE RANGES
The present COVID-19 pandemic has created a public health crisis because of efforts to decrease transmission of community-acquired COVID-19. This has reduced the number of in-person well child visits, negatively impacting vaccine receipt, and anticipatory guidance. Delays in vaccinations my certainly result in secondary outbreaks of these vaccine-preventable illnesses. Well child visits are being cancelled due to fear of contracting the virus, lack of safe transportation, inability to maintain a normal work schedule for the parents, and/or loss of health insurance due to unemployment. In addition, ambulatory pediatric offices may have a reduced clinic staff and/or have physicians redeployed to work in other areas of healthcare, which also may limit access. Some practices have had to scale back their practice activities for financial reasons.
The benefits of attending well visits and receiving necessary immunizations and screenings should be prioritized throughout all pediatric age ranges whenever possible. In addition to the importance of vaccinations within this age range, physical, psychosocial, and behavioral development, as well as necessary well child screenings (e.g., Modified Checklist for Autism in Toddlers), are also critical. Therefore, many elements of this document can be applied to a wider age range of pediatric patients. Both the AAP and the CDC endorse the importance and value of maintaining immunization rates during the COVID-19 pandemic:
These guidelines are intended to provide a clear roadmap for conducting safe office visits in pediatric-focused ambulatory settings during the current pandemic. Working together to innovate and shape healthcare around this COVID-19 pandemic, while also protecting and caring for infants, children, and their families in the community, should remain a priority for all child health professionals.
CONCLUSION
The global COVID-19 pandemic has created innumerable challenges for our healthcare system. It is crucial that new mother–infant dyads are adequately assessed and prepared for early discharge under limited lengths of hospital stay. Throughout this ‘new normal’ of physical distancing and sheltering in place, it is especially important to maintain appropriate screening for any medical complications, social support at home, barriers to accessing healthcare, and mental health. Dyads who show signs of needing additional support should be referred to the appropriate healthcare professionals or social services at the earliest convenience, and proper follow-up care should be established and maintained.
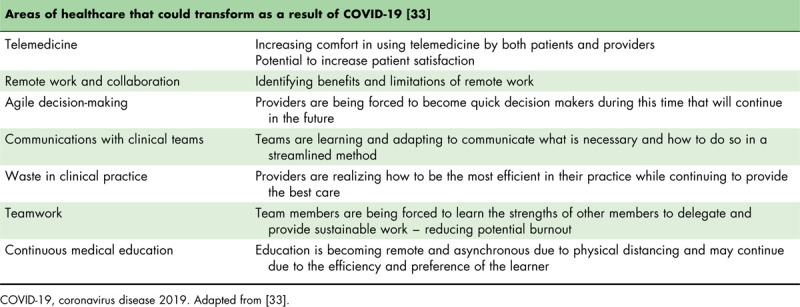
These new guidelines bring along a change in the way we practice pediatric medicine and educate our patients’ families on a daily basis. With these adaptations come growing pains, such as learning new technology and deciding what is appropriate to do through telehealth vs. face-to-face. Some of these changes, however, may become part of our future reality and permanently change the way we practice medicine. Dr. Victor Herrera and colleagues identified seven areas of healthcare that may be transformed due to the COVID-19 pandemic (Table 3) [33]. Regardless of how we continue to adapt as general pediatricians, pediatric specialists, and pediatric trainees during this pandemic, we will continue to remain steadfast in our mission to deliver the best pediatric care to newborns and children.
Table 3.
Potential areas of healthcare that could experience accelerated innovation and transformation due to coronavirus disease 2019
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Bernstein HH, Spino C, Finch S, et al. Decision-making for postpartum discharge of 4300 mothers and their healthy infants: the Life Around Newborn Discharge study. Pediatrics 2007; 120:e391–e400. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Obstetric Practice and the American Academy of Pediatrics’ Council on Environmental Health. Committee opinion no. 726: hospital disaster preparedness for obstetricians and facilities providing maternity care. Obstet Gynecol 2017; 130:e291–e297. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein HH, Spino C, Lalama CM, et al. Unreadiness for postpartum discharge following healthy term pregnancy: impact on healthcare use and outcomes. Acad Pediatr 2013; 13:27–39. [DOI] [PubMed] [Google Scholar]
- 4.Benitz WE. Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics 2015; 135:948–953. [DOI] [PubMed] [Google Scholar]
- 5.Byrd RS, Hoekelman RA, Auinger P. Adherence to AAP guidelines for well child care under managed care. American Academy of Pediatrics. Pediatrics 1999; 104 (3 Pt 1):536–540. [DOI] [PubMed] [Google Scholar]
- 6.Shakib J, Buchi K, Smith E, et al. Timing of initial well child visit and readmissions of newborns. Pediatrics 2015; 135:469–474. [DOI] [PubMed] [Google Scholar]
- 7▪.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study highlights the percentage of asymptomatic, laboring women with coronavirus disease 2019 (COVID-19) that could potentially transmit the virus.
- 8▪▪.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020; [Epub ahead of print]. [Google Scholar]; The article characterizes COVID-19 in pediatric patients.
- 9.CDC COVID-19 Response Team. Coronavirus disease 2019 in children – United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪▪.FAQs: Management of Infants Born to Mothers with Suspected or Confirmed COVID-19. American Academy of Pediatrics (AAP). https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/. Published 2020. Accessed June 14, 2020. [Google Scholar]
- 11.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Advisory Committee on Immunization Practcies (ACIP). ACIP shared clinical decision-making recommendations. 2020; Atlanta, Georgia: US Centers for Disease Control and Prevention (CDC), Available from: https://www.cdc.gov/vaccines/acip/acip-scdm-faqs.html. [Accessed 28 May 2020]. [Google Scholar]
- 13.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020; 382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Centers for Disease Control and Prevention (CDC). Criteria for return to work for healthcare personnel with suspected or confirmed COVID-19 (interim guidance). 2020; Atlanta, Georgia: US Centers for Disease Control and Prevention (CDC), Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. [Accessed 28 May 2020]. [Google Scholar]
- 15▪▪.Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr 2020. e200878. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article examines and highlights the transimission of COVID-19 from mother to her neonate and its associated symptoms.
- 16.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Wang Y, Liu Y, Liu L, et al. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis 2020; 221:1770–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article follows the progression of disease in asymptomatic patients.
- 18.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–675. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics. Guidance on providing pediatric well care during COVID-19. 2020; Itasca, Illinois: American Academy of Pediatrics, Available from: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/guidance-on-providing-pediatric-well care-during-covid-19/. [Accessed 28 May 2020]. [Google Scholar]
- 20.Public Health Agency of Canada. Pregnancy, childbirth and caring for newborns: advice for mothers during COVID-19: Government of Canada. 2020; Ottawa, Ontario: Public Health Agency of Canada, Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/pregnancy-advise-mothers.html. [Accessed 28 May 2020]. [Google Scholar]
- 21.Ghinai I, Woods S, Ritger KA, et al. Community transmission of SARS-CoV-2 at two family gatherings – Chicago, Illinois, February–March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012; 129:e827–e841. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.US Centers for Disease Control and Prevention (CDC). Coronavirus disease (COVID-19) and breastfeeding. 2020; Atlanta, Georgia: CDC, Available from: https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/maternal-or-infant-illnesses/covid-19-and-breastfeeding.html. [Accessed 28 May 2020]. [Google Scholar]; The guidance outlines the continued importance of breastfeeding while advising how to breastfeed safely during the COVID-19 pandemic.
- 25.Bae S, Kim MC, Kim JY, et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: a controlled comparison in 4 patients. Ann Intern Med 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Rodriguez JA, Clark CR, Bates DW. Digital health equity as a necessity in the 21st century cures act era. JAMA 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Pediatrics. Engaging patients and families: periodicity schedule. Itasca, Illinois: American Academy of Pediatrics; 2020. [Google Scholar]
- 28.US Centers for Disease Control and Prevention (CDC). Information for pediatric healthcare providers: maintaining childhood immunizations during COVID-19 pandemic. 2020; Atlanta, Georgia: CDC, Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html. [Accessed 28 May 2020]. [Google Scholar]
- 29.MMWR Morb Mortal Wkly Rep. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration — United States, 2020. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6919e2.htm. [Accessed 28 May 2020]. [Google Scholar]
- 30.US Centers for Disease Control and Prevention (CDC). Immunization schedules: Table 1. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2020. 2020; Atlanta, Georgia: CDC, Available from: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html#note-hepb. [Accessed 28 May 2020]. [Google Scholar]
- 31.US Centers for Disease Control and Prevention (CDC). Coronavirus disease 2019 (COVID-19): using personal protective equipment (PPE). 2020; Atlanta, Georgia: CDC, Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html. [Accessed 28 May 2020]. [Google Scholar]
- 32▪▪.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This is a systematic review of articles that characterize COVID-19 in pediatric patients.
- 33.Herrera V, Finkler N, Vincent J. Innovation and transformation in the response to COVID-19: seven areas where clinicians need to lead. NEJM Catalyst 2020; 1: [Google Scholar]


