Supplemental Digital Content is available in the text
Keywords: Biomarker, Hydrogen Sulfide, Interleukin-6, Mortality, SARS- CoV-2
ABSTRACT
Background:
The pneumonia of COVID-19 illness has often a subtle initial presentation making mandatory the use of biomarkers for evaluation of severity and prediction of final patient disposition. We evaluated the use of hydrogen sulfide (H2S) for the outcome of COVID-19 pneumonia.
Materials & Methods:
We studied 74 patients with COVID-19. Clinical data were collected, and survival predictors were calculated. Blood was collected within 24 hours after admission (day 1) and on day 7. H2S was measured in sera by monobromobimane derivation (MBB) followed by high performance liquid chromatography and correlated to other markers like procalcitonin (PCT) and C- reactive protein (CRP). Tumor necrosis factor alpha (TNFα) and interleukin (IL)-6 were also measured in serum.
Results:
Survivors had significantly higher H2S levels on day 1 and 7 after admission. A cut-off point of 150.44 μM could discriminate survivors from non-survivors with 80% sensitivity, 73.4% specificity and negative predictive value 95.9%. Mortality after 28 days was 32% with admission levels lower or equal to 150.44 μΜ and 4.1% with levels above 150.44 μΜ (p: 0.0008). Mortality was significantly greater among patients with a decrease of H2S levels from day 1 to day 7 greater or equal to 36% (p: 0.0005). Serum H2S on day 1 was negatively correlated with IL- 6 and CRP and positively correlated with the absolute lymphocyte count in peripheral blood.
Conclusion:
It is concluded that H2S is a potential marker for severity and final outcome of pneumonia by the SARS-CoV-2 coronavirus. Its correlation with IL- 6 suggests anti-inflammatory properties.
INTRODUCTION
Since December 2019, humanity is experiencing a novel pandemic by the novel SARS-CoV-2 coronavirus-19 that is causing the disease known as Covid-19 (1). As of April 17, 2020; 2,265,271 confirmed cases were reported worldwide, causing 154,900 deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). The main reason for death is severe respiratory failure (SRF) developing in the field of community-acquired pneumonia. It seems that derangement of the lung endothelium is the hallmark of disease pathogenesis. This is hypothesized by published evidence showing elevated levels of d-dimers and vascular endothelial growth factor in these patients (2, 3).
Hydrogen sulfide (H2S), long thought of solely as an environmental toxicant, is now known to be an endothelial product that drives angiogenesis, promotes vasorelaxation, reduces atherosclerosis, and prevents ischemia-reperfusion injury (4, 5). In spite of being considered having anti-inflammatory properties through inhibition of nuclear factor-kB (6), a recent animal model showed complex interaction with angiotensin-2 that is the receptor SARS-CoV-2 is using to invade host epithelia (7). In light of these observations suggesting a pivotal role of H2S in the pathogenesis of Covid-19, we studied the serum levels of H2S and its association with final outcome in a cohort of patients with Covid-19 pneumonia. Due to the described anti-inflammatory properties, we hypothesize that elevated levels of H2S in serum are associated with a favorable outcome of Covid-19 pneumonia.
PATIENTS AND METHODS
This study was conducted in eight departments participating in the Hellenic Sepsis Study Group from beginning of March 2020. The study protocol was approved by the Ethics Committees of the participating hospitals. Patients were included after written informed consent was provided by themselves or by first-degree relatives in case of patients unable to consent. We enrolled patients admitted with lower respiratory infection as diagnosed by the presence of infiltrates in chest X-ray or in computed tomography of the lung and who were tested positive upon admission by molecular testing of respiratory secretions for SARS-CoV-2. Blood was sampled within the first 24 h of hospital admission and repeated after 7 days. Exclusion criteria were: HIV-1 infection and neutropenia defined as less than 1,000 neutrophils/mm3. SRF was defined as any ratio of partial oxygen pressure to fraction of inspired oxygen below 200 necessitating mechanical ventilation.
The following variables were recorded: demographics; vital signs; admission Acute Physiology and Chronic Health Evaluation (APACHE) II score, Charlson Comorbidity Index (CCI), Sequential Organ Failure Assessment (SOFA) score (8), and Pneumonia Severity Index (PSI) (9); absolute blood cell counts and biochemistry on admission and follow-up; and 28-day survival.
Immediately after sampling, blood was collected into sterile and pyrogen-free tubes and transported ice-cold for centrifugation within less than 10 min. H2S was measured in patient serum by using monobromobimane (MBB) derivation followed by high-performance liquid chromatography as it has previously been described (10, 11). MBB, monosodium phosphate, disodium phosphate, and 5-sulfosalicylic acid (SSA) were purchased from Sigma-Aldrich (St. Lewis, Mo). Sodium sulfide (Na2S), diethylenetriaminepentaacetic acid (DTPA), and trifluoroacetic acid (TFA) were purchased from Alfa Aesar (Erlenbachweg, Germany). Tris-HCl buffer (0.1 M pH 9.5) was purchased from AlterChem (Athens, Greece). For the preparation of the derivatization buffer (Tris-HCl 0.1 M pH 9.5, 0.1 mM DTPA) DTPA was dissolved in tris-HCl. All solvents and buffers, as well as the tubes used for the derivatization reaction, were deoxygenated by using nitrogen gas flow (10 and 30 s, respectively). The solutions for the sulfide standard curve were prepared by dissolving Na2S in phosphate buffer, to final concentrations of 4 μM to 250 μM and the quantification limit was 10 μM. The MBB 10 mM derivatization solution was prepared by dissolving MBB in acetonitrile and was then aliquoted in dark containers and kept at −20oC. The SSA 200 mM stop solution was freshly prepared before each measurement by dissolving SSA in distilled water. All sample preparations were conducted under dim room lighting. After deoxygenation, 30 μl of serum sample or standard, 70 μl Tris-HCl 0.1 M pH 9.5 0.1 mM DTPA, and 50 μl MBB 10 mM were added in the tubes. The mixture was incubated at hypoxic conditions (1% O2) at 37°C for 60 min. The derivatization reaction was stopped by adding 50 μl of 200 mM SSA, followed by vortexing for 10 s. The vials were then left on ice for 10 min and centrifuged at 12,000 rpm, 4°C for another 10 min. Finally, 100 μL of the supernatant were transferred at darkened HPLC vials and kept at 4°C. Analysis was done by an Agilent 1,100 HPLC system (Agilent, Waldbronn, Germany) using one LiChroCART Reverse-Phase (RP) C18 4.6 × 250 mm, 5 μm analytical column, with a Purospher RP-18E 4 × 4 mm, 5 μM guard column (Merck, Darmstadt, Germany). Analysis was performed at 25°C, using gradient elution. The mobile phases consisted of acetonitrile (0.1% TFA, v/v) and water (0.1% TFA), v/v), at a 0.6 ml/min flow rate. All measurements were carried out at excitation and emission wavelengths of 390 nm and 475 nm respectively. The retention time of the derivatization product was 12.7 min.
Serum concentrations of tumor necrosis factor alpha (TNFα) and interleukin (IL)-6 were measured in duplicate by an enzyme immunoassay (R&D, Minneapolis, USA). The lowest limit of detection was 40 pg/ml for TNFα and 10 pg/ml for IL-6. Procalcitonin (PCT) was measured by a time-resolved amplified cryptate emission technology assay according to the manufacturer's instructions (Kryptor, Brahms, Hennigsdorf, Germany). The lower detection limit was 0.06 ng/mL. C-reactive protein (CRP) was estimated in duplicate by a nephelometric assay (Behring, Berlin, Germany). The lowest limit of detection was 0.2 mg/dL.
The association between H2S levels on day 1 and 28-day survival was the primary study endpoint. The association between change in H2S levels between day 1 and 7 and 28-day survival was the secondary study endpoint.
Statistics
Categorical data were presented as frequencies and quantitative variables as mean ± SE. Comparisons between groups were done using the Fisher exact test for categorical data, the two-sided Student t test or Mann–Whitney U test for quantitative data. Correlations were performed using the Spearman rank of order. Survival was compared by the log-rank test. Odds ratios and 95% confidence intervals (CIs) were calculated by the Mantel and Haenszel statistics. Receiver operating characteristic (ROC) curves were analyzed for outcome prediction; the best cutoff was selected using the Youden index. Stepwise forwards Cox regression analysis with hazard ratios and CIs was used to investigate independent variable associated with 28-day outcome. Any P value below 0.05 was considered statistically significant.
RESULTS
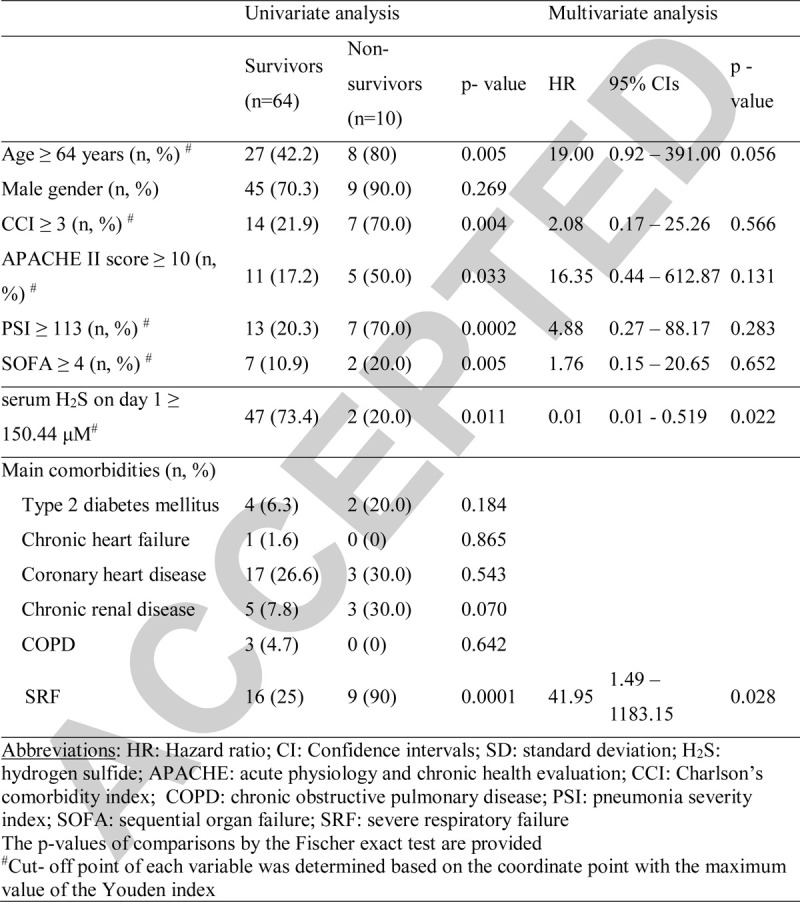
Seventy-four patients were enrolled. Their demographics in association to 28-day outcome are shown in Table 1 and in supplementary Table 1, http://links.lww.com/SHK/B52. Two patients died before day; five patients were discharged before day 7, and four patients denied blood sampling on day 7; therefore measurements on day 7 were run in 63 patients.
Table 1.
Baseline clinical and laboratory characteristics of patients with pneumonia by SARS-CoV-2 coronavirus and step-wise forward Cox regression analysis of parameters associated with unfavorable outcome
Serum H2S of days 1 and 7 was significantly higher among 28-day survivors. IL-6 and PCT of days 1 and 7 were higher among non-survivors whereas CRP of day 7 was higher among non-survivors. H2S was negatively associated with IL-6, PCT, and CRP (Fig. 1).
Fig. 1.
Hydrogen sulfide (H2S) as predictor for outcome of pneumonia by the SARS-CoV-2 coronavirus.
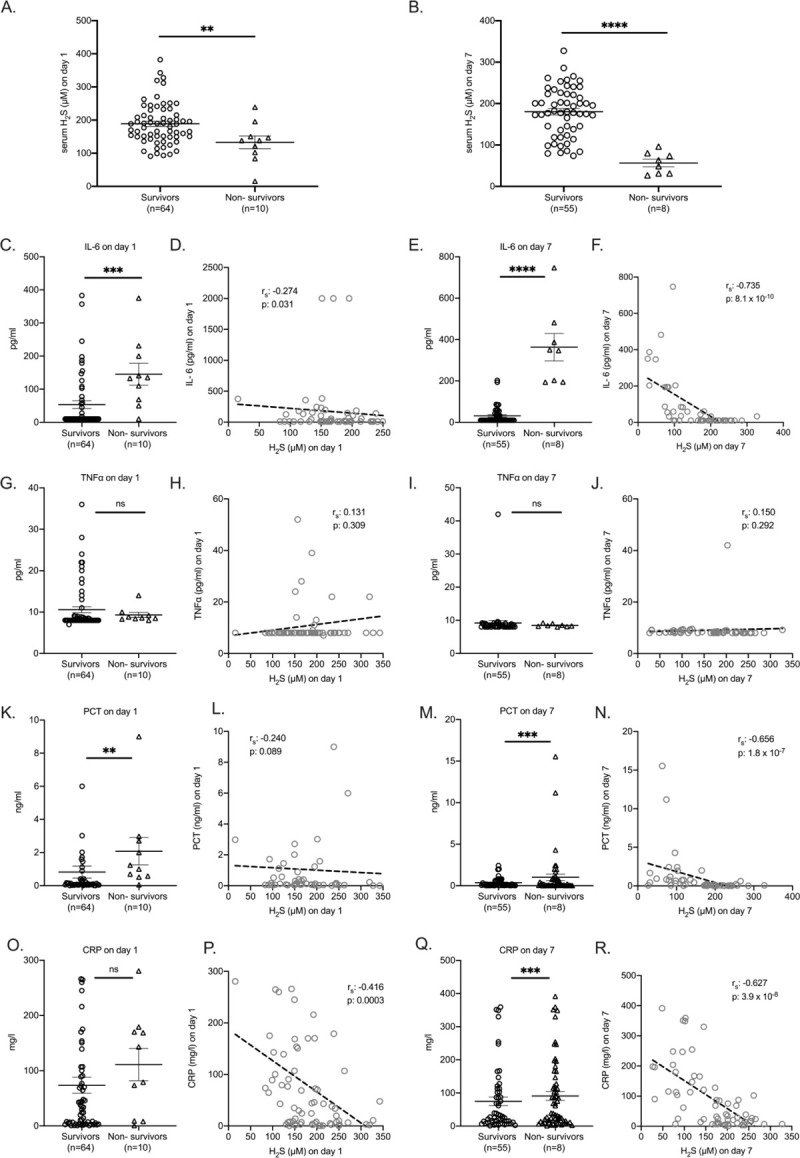
Serum levels of H2S in serum of patients on day 1 (A) and 7 (B) after hospital admission. Comparison by the Mann–Whitney U test; ∗∗P < 0.01; ∗∗∗∗P < 0.0001. Levels of interleukin (IL)-6 (C, E), tumor necrosis factor alpha (TNFα) (G, I), procalcitonin (PCT) (K, M), and C-reactive protein (CRP) (O, Q) in serum of patients on days 1 and 7 after hospital admission. Comparison by the Mann–Whitney U test; ∗∗P < 0.01; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001; ns indicates non-significant. Correlation of IL-6 (D, F), TNFα (H, J), PCT (L, N), and CRP (P, R) with H2S on days 1 and 7 after hospital admission. Spearman rank correlation coefficients (rs), interpolation lines, and P values are provided.
Non-survivors had higher absolute neutrophil counts and significantly lower lymphocytes which are consistent with already-reported characteristics of COVID-19 patients (12). Serum H2S was negatively correlated with the absolute neutrophil count; a positive correlation with the absolute lymphocyte count was found (Fig. 2).
Fig. 2.
Association of hydrogen sulfide (H2S) on days 1 and 7 and white blood cell counts.
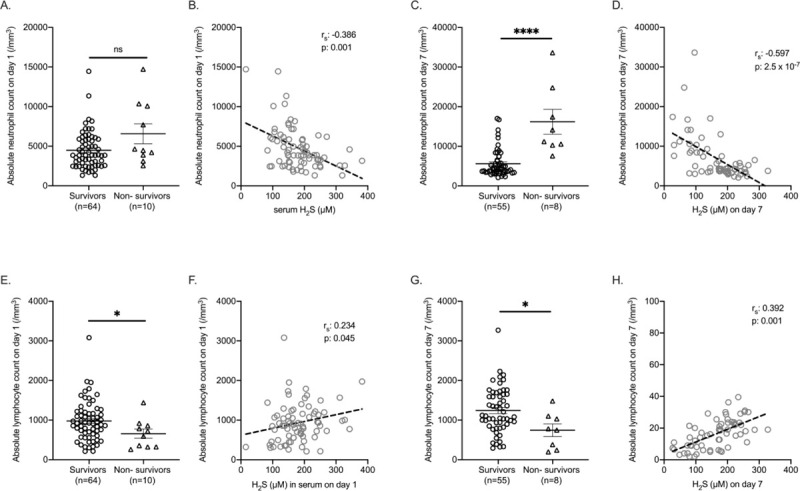
Absolute neutrophil and lymphocytes counts on days 1 and 7 between survivors and non-survivors are shown on (A, C, E, G); comparison by the Mann–Whitney U test is shown; ∗P < 0.05; ∗∗∗∗P < 0.0001; ns indicates non-significant. Correlations between absolute neutrophil and lymphocytes counts on days 1 and 7 and serum H2S are shown on (B, D, F, H). Spearman rank correlation coefficients (rs), interpolation lines, and P values are provided.
The above-mentioned results led to further evaluation of admission H2S as a marker of survival. Following ROC curve analysis, it was found that serum levels of H2S on day 1 lower than 150.44 μM had the best tradeoff for sensitivity and specificity for death (Fig. 3 A and B). In total, 49 patients had less than or equal and 25 patients had more than 150.44 μM H2S in serum on day 1. Mortality after 28 days was 32% and 4.1% respectively (Fig. 3C). The OR for death was 11.11 (95% CI: 2.13–5.88, P: 0.001).
Fig. 3.
Hydrogen sulfide (H2S) as prognostic biomarker for pneumonia by the SARS-CoV-2 coronavirus.
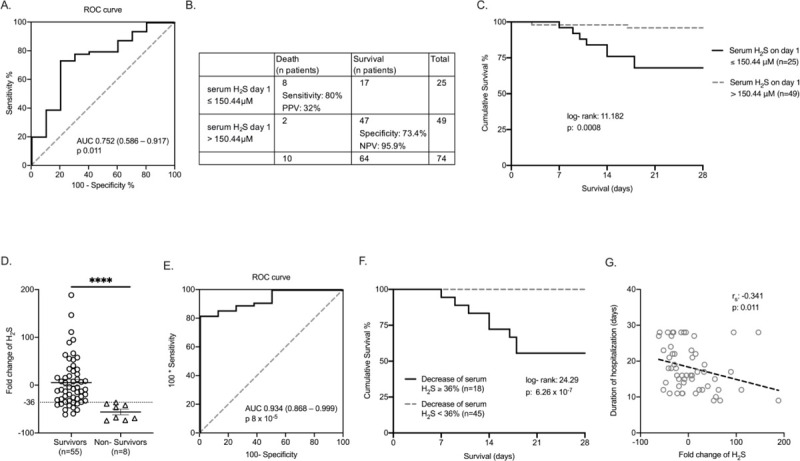
A, ROC curve of serum H2S on day 1 for 28-day survival of pneumonia by SARS-CoV-2, AUC area under the curve, 95% confidence intervals (CIs), and P value are given. B, Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of serum H2S on day 1 for 28-day mortality. C, Kaplan–Meier analysis of 28-day survival in association with serum H2S admission levels; the log-rank test and P value are given. D, Fold-changes of H2S between days 1 and 7 according to outcome; the dotted line refers to the 36% cutoff. Comparison by Mann–Whitney U test; ∗∗∗∗P < 0.0001. E, ROC curve of serum H2S fold change for 28-day outcome. F, Kaplan–Meier analysis for 28-day outcome in association with over-time change of serum H2S; log-rank test and the P value are given. F, Correlation of fold-change of H2S between days 1 and 7 after hospital admission with the duration of hospitalization. rs indicates interpolation lines; ROC, receiver operating characteristic. P values are provided.
ROC curve analysis revealed the following baseline values to be associated with unfavorable outcome: CCI greater than or equal to 3, APACHE II score greater than or equal to 10, PSI greater than or equal to 113, and SOFA score greater than or equal to 4. Forward stepwise Cox regression analysis showed that serum H2S on day 1 above 150.44 μM is an independent protective factor for unfavorable outcome of COVID-19 even in the presence of severity scores (Table 1).
We further investigated the association between over-time change of serum H2S and outcome. ROC curve analysis showed a cutoff decrease of 36% of H2S by day 7 as the best discriminator for death (Fig. 3, D and E). Survival of these patients was prolonged (Fig. 3F). Moreover, the change of serum H2S between days 1 and 7 was negatively associated with the duration of hospitalization (Fig. 3G).
DISCUSSION
This study suggests the gasotransmitter H2S as a potentially predictive variable of the outcome of pneumonia by SARS-CoV-2 mainly due to the high negative predictive value.
Although one interpretation of the presented findings is for the use of serum H2S as a biomarker, we do feel that the intrinsic value is for H2S as a reflection of the endothelial function of the body vasculature. The kinetics in the circulation reveal that 28-day survivors are those who consume less of this gas. Disturbed bioavailability of H2S has been suggested as an indicator of enhanced pro-inflammatory responses and of endothelial dysfunction (13, 14). Both these conditions often accompany severe COVID-19.
The negative association between serum H2S and IL-6 is interesting. IL-6 has been proposed as the principle pro-inflammatory cytokine involved in the cytokine storm that leads to severe lung injury, respiratory failure, and death by COVID-19 (15). This has even led to the evaluation of therapeutic strategies of modulation of IL-6 production. Tocilizumab, a blocker of IL-6R, which can effectively block IL-6 signal transduction pathway, is currently being evaluated in several clinical studies in COVID-19 patients worldwide (16). Our results postulate that H2S is an endogenous down-regulator of IL-6, the consumption of which is a driver to unfavorable outcome. It may also lead to considerations for exogenous H2S supplementation as treatment strategy. Indeed, inhaled H2S has been shown to reduce pro-inflammatory cytokines, among which IL-6, and increase the survival of mice after experimental endotoxemia (17).
Additionally, serum H2S was positively correlated with the lymphocyte count. Lymphopenia is a key characteristic of COVID-19 patients (12) and is considered a predictor of mortality (15). The negative association between endogenous H2S and the lymphocyte count may be consistent with in vitro data supporting a role of H2S as T cell activator (18).
The significant role of H2S for evaluating COVID-19 patients further relies on serial measurements. This study has shown that all patients who sustain elevated levels after 7 days had no risk of an unfavorable outcome. This finding could suggest that serial measurements of serum H2S could be utilized as an adjunctive criterion, together with other established biomarkers such as CRP and PCT, for decision making. However, these are data coming from a small cohort and mandate validation in larger cohorts of patients.
Footnotes
Georgios Renieris, Konstantina Katrini, and Christina Damoulari authors contributed equally
Sources of funding: G. Renieris is funded from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement grant European Sepsis Academy (agreement No 676129).
The study is funded by the Hellenic Sepsis Study Group.
Competing interests: E.J. Giamarellos-Bourboulis has received honoraria from AbbVie USA, Abbott CH, InflaRx GmbH, MSD Greece, XBiotech Inc. and Angelini Italy; independent educational grants from AbbVie, Abbott, Astellas Pharma Europe, AxisShield, bioMérieux Inc, InflaRx GmbH, and XBiotech Inc.; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis).
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal's Web site (www.shockjournal.com).
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382 (8):727–733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 95 (10229):1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 214:108393, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo C, Papapetropoulos A. International union of basic and clinical pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol Rev 69 (4):497–564, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy B, Bhattacharya R, Mukherjee P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J 33 (12):13098–13125, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedetti F, Davinelli S, Krishnan S, Gallo RC, Scapagnini G, Zella D, Curreli S. Sulfur compounds block MCP-1 production by Mycoplasma fermentans-infected macrophages through NF-κB inhibition. J Transl Med 12 (1):145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Zeng H, Gao L, Gu T, Wang C, Zhang H. Hydrogen sulfide attenuates atherosclerosis in a partially ligated carotid artery mouse model via regulating angiotensin converting enzyme 2 expression. Front Physiol 8:782, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao K-C, Hsieh M-J, Lin S-W, Chuang L-P, Chang C-H, Hu H-C, Wang C-H, Li L-F, Huang C-C, Wu H-P. Survival predictors in elderly patients with acute respiratory distress syndrome: a prospective observational cohort study. Sci Rep 8 (1):13459, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim M-A, Park JS, Lee CW, Choi W-I. Pneumonia severity index in viral community acquired pneumonia in adults. PLoS One 14 (3):e0210102, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike S, Kawamura K, Kimura Y, Shibuya N, Kimura H, Ogasawara Y. Analysis of endogenous H2S and H2Sn in mouse brain by high-performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radic Biol Med 113 (12):355–362, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50 (9):1021–1031, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Szabo C, Ichinose F, Ahmed A, Whiteman M, Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci 36 (9):568–578, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Schechter M, Taddei S, et al. The assessment of endothelial function: from research into clinical practice. Circulation 126 (6):753–767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020; 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokuda K, Kida K, Marutani E, Crimi E, Bougaki M, Khatri A, Kimura H, Ichinose F. Inhaled Hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal 17 (1):11–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller TW, Wang EA, Gould S, Stein EV, Kaur S, Lim L, Amarnath S, Fowler DH, Roberts DD. Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem 287 (6):4211–4221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


