Summary
Background
Factor (F) IX/IXa inactivation by plasmin has been studied; however, whether plasmin converts FIXa to a fibrinolytic enhancer is not known.
Objective
Investigate plasmin proteolysis site(s) in FIXa that inactivates and transforms it into a fibrinolytic enhancer.
Methods
NH2-terminal sequencing, mass spec analysis and functional assays.
Results
Plasmin in the presence of Ca2+/phospholipid (PL) rapidly cleaved FIXaβ at Lys316↓Gly317 to yield FIXaγ followed by a slow cleavage at Lys413↓Leu414 to yield FIXaδ. FIXaγ/FIXaδ migrated indistinguishably from FIXaβ in nondenaturing gel system indicating that C-terminal residues 317–415/317–413 of heavy chain remain noncovalently associated with FIXaγ/FIXaδ. However, as compared to FIXaβ, FIXaγ or FIXaγ/FIXaδ (25–75 mixture, 8-hour/24-hour incubation analysis by Mass Spec) was impaired ~10-fold in hydrolyzing synthetic substrate CBS 31.39 (CH3-SO2-D-Leu-Gly-Arg-pNA), ~30-fold (~5-fold higher Km, ~6-fold lower kcat) in activating FX in a system containing Ca2+/PL, and ~650-fold in a system containing Ca2+/PL and FVIIIa. Further, FIXaγ or FIXaγ/FIXaδ bound FVIIIa with ~60-fold reduced affinity as compared to FIXaβ. Additionally, in ligand blots, plasminogen or Diisopropylfluorophosphate-inhibited plasmin (DIP-plasmin) bound FIXaγ and FIXaδ but not FIXaβ. This interaction was prevented by ε-aminocaproic acid or carboxypeptidase B treatment suggesting that plasminogen/DIP-plasmin binds to FIXaγ/FIXaδ through newly generated C-terminal Lys316 and Lys413. Importantly, FIXaγ/FIXaδ mixture but not FIXaγ enhanced tissue plasminogen activator (tPA)-mediated plasminogen activation in a concentration dependent manner. Similarly, FIXaγ/FIXaδ mixture but not FIXaγ enhanced tPA-induced clot lysis in FIX-depleted plasma.
Conclusion
Plasmin cleavage at Lys316↓Gly317 abrogates FIXaβ coagulant activity, whereas additional cleavage at Lys413↓Leu414 converts it into a fibrinolytic enhancer.
Keywords: Factor IXa, Far-Western Blot, Mass Spectrometry, Proteolysis, Tissue Plasminogen Activator
Introduction
Factor (F) IX is a single chain vitamin K-dependent glycoprotein that circulates in blood with Mr ~57,000 [1,2]. It plays a crucial role in blood coagulation since its deficiency results in a hemorrhagic disorder, known as hemophilia B. During hemostasis, FXIa/Ca2+ or FVIIa/tissue factor (TF)/Ca2+ cleaves FIX at the Arg145-Ala146 and Arg180-Val181 peptide bonds with resultant formation of a serine protease, FIXaβ, and release of a 35-residue activation peptide [2–4]. FIXaβ is comprised of a light chain (residues 1–145) and a heavy chain (residues 181–415) that are held together by a disulfide bond [5]. The light chain contains the N-terminal γ-carboxyglutamic acid domain (Gla) followed by two epidermal growth factor (EGF)-like domains; whereas, the heavy chain contains the C-terminal serine protease domain, which features the catalytic triad, His221 (c57 in chymotrypsin), Asp269 (c102 in chymotrypsin), and Ser365 (c195 in chymotrypsin). In the coagulation cascade, FIXaβ activates factor X (FX) to FXa in the presence of factor VIIIa (FVIIIa), Ca2+, and phospholipid (PL) provided by platelets [5].
Plasmin is a fibrinolytic protease comprised of a heavy chain with five Kringle domains and a light chain containing the catalytic domain [6,7]. Plasmin localizes to the fibrin clot site using its Kringle domains to bind to the exposed lysine residues to carry out fibrinolysis [6,7]. Notably, several studies have shown that plasmin proteolytically inactivates several coagulation proteins including FX [8,9], FXa [9–13], FIX [14], FVa [15,16] and FVIIIa [17,18]; during this process in many instances new C-terminal lysine residues are generated. These studies suggest that plasmin not only inactivates the clotting proteins, but also creates additional lysine binding sites for its Kringle domains at the clot site to enhance fibrinolysis.
Plasmin cleavage of FX/FXa has been extensively studied [8–13,19]. Circulating FX consists of a light chain (residues 1–139) and a heavy chain (residues 143–448) linked by a single disulfide bond; the tripeptide Arg-Lys-Arg that connects the light and heavy chains is proteolytically removed during the biosynthetic process [20]. Activation of FX by FIXa/Ca2+ or FVIIa/TF/Ca2+ involves cleavage at Arg194-Ile195 peptide bond releasing a 52-residue peptide and formation of FXaα. In contrast to autoproteolysis of FXaα that generates FXaβ with C-terminal Arg327 in the heavy chain, the proteolysis by plasmin in the presence of Ca2+/PL generates C-terminal Lys435 in FXaβ [10]. FXaβ generated by autoproteolysis or by plasmin has normal coagulant activity. Additional plasmin cleavage at Lys330↓Gly331 in the plasmin generated FXaβ or intact FXaα with C-terminal Lys448 yields FXaγ, which acts as a fibrinolytic enhancer [13]. Thus, FXaγ with C-terminal Lys330 and Lys435 or Lys448 facilitates fibrinolysis [13].
Samis et al. studied plasmin-mediated cleavage of FIX in the absence of PL and found that plasmin cleaved FIX at Arg145 and Arg180, which are the sites for FIX activation [14]. These authors also found that plasmin cleaved FIX at three additional sites Lys43, Lys316 (c148), and Arg318 (c150) generating an inactive molecule [14]. However, Samis et al. did not examine whether the inactive FIX molecule also enhanced fibrinolysis. In this study, we investigated whether plasmin cleavage of FIXaβ in the presence of Ca2+/PL downregulates coagulation and enhances fibrinolysis. The extensive data presented here reveal that plasmin cleaves FIXaβ rapidly at Lys316-Gly317 (c148-c149) to yield FIXaγ, which is devoid of coagulant activity; FIXaγ is then cleaved at Lys413-Leu414 to yield FIXaδ, which promotes fibrinolysis.
Materials and methods
Reagents
Benzoyl-Ile-Glu-Gly-Arg-p-nitroanilide (S-2222), H-D-Ile-Pro-Arg-p-nitoanilide (S-2288), and H-D-Val-Leu-Arg-p-nitroanilide (S-2251) were from DiaPharma (West Chester, Ohio). CH3-SO2-D-Leu-Gly-Arg-p-nitroanilide (CBS 31.39) was from Diagnostica Stago. Dansyl-Glu-Gly-Arg-chloromethyl ketone (dEGR-ck) and diisopropylfluorophosphate (DFP) were from Calbiochem (San Diego, CA). Carboxypeptidase B (CpB) was from Roche and ε-aminocaproic acid (EACA) from ICN. Phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), recombinant hirudin, polyoxyethylenesorbitan monolaurate (Tween 20), and fatty acid free bovine serum albumin (BSA) were from Sigma (St. Louis, MO). PL vesicles containing 60% PC, 20% PS and 20% PE were prepared as described [21]. Enhanced chemiluminescence (ECL) detection reagents were from Amersham Pharmacia Biotech Inc. Normal pooled plasma (NPP) and congenital FIX-deficient plasma used in coagulation assays were from George King Bio-Medical, Inc. (Overland Park, KS) and activated partial thromboplastin time reagent was from Baxter Diagnostics, Inc. (Deerfield, IL). Human immunochemically-depleted FIX plasma used in clot fibrinolysis assays was from Haemotologic Technologies (Essex Junction, Vermont).
Proteins
Purified human FX, FXIa, Glu-plasminogen, Russel viper venom (RVV-X) and α-thrombin were purchased from Enzyme Research Laboratories (South Bend, IN). Plasmin and goat anti-human plasminogen were from Haemotologic Technologies. The two-chain tissue plasminogen activator (tPA, Alteplase) was obtained from Genentech (South San Francisco). Donkey anti-goat IgG conjugated to peroxidase was from Sigma. Wild-type recombinant FIX was expressed in HEK 293 cells and purified using a FIX A-7 mAb column followed by a Mono-Q column as described [22,23]. The FIX had ∼12 Gla residues/mol as measured by the procedure of Price et al [24] and appeared homogeneous on both reduced and non-reduced SDS-PAGE with Mr ∼57,000. FVIII was activated to FVIIIa using 1 nM human α-thrombin in the presence of 0.1% (w/v) BSA and 5 mM CaCl2 in TBS pH 7.5 (50 mM Tris–HCl, 150 mM NaCl, pH 7.5) at 37°C for two minutes as described [22,23,25] followed by the addition of recombinant hirudin to inactivate thrombin.
Characterization of plasmin cleaved FIXaβ in presence of calcium and PL
FIX was activated to FIXaβ as described in the supplementary material. FIXaβ (1 ml, 100 μg/ml) samples were then incubated with 3 μg/ml plasmin, 240 μM PL (60%PC/20%PS/20%PE) and 5 mM CaCl2 in TBS pH 7.5 at 37°C for various time points (1,1.5,2,3,4,5,10,15 minutes and 0.5,1,2,4,8,12,24 hours). SDS-PAGE (Supplementary material) was performed to determine proteolysis in FIXaβ. Plasmin in each reaction mixture was inactivated using 1 mM DFP and Ca2+ was chelated using 10 mM EDTA. Plasmin cleaved FIXaβ was then put over a Centricon100 concentrator to remove PL, and concentrated using a Centricon30 concentrator. Each sample was then subjected to NH2-terminal amino acid sequencing and mass spec analysis.
Amino acid sequencing
FIXaβ before and after proteolysis were run on 12% reduced SDS-PAGE and blotted to Immobilon-P polyvinylidene difluoride (PVDF) membrane in Towbin buffer (25 mM Tris-base, 192 mM glycine, 20% methanol). Membrane was stained with 0.1% Coomassie brilliant blue R in 50% methanol, 7% acetic acid, and destained in 50% methanol, 7% acetic acid. The membrane was then submitted to Midwest Analytical (St. Louis, MO) for NH2-terminal amino acid sequencing of the bands.
Intact protein mass spectrometry
Forty μg of FIXaβ and plasmin cleaved-FIXaβ were denatured using SDS-PAGE loading buffer. Proteins were recovered from SDS-PAGE loading buffer using chloroform/methanol precipitation [26] and dissolved in 70% formic acid prior to immediate LC-MS analysis. LC-MS used a polymeric stationary phase (PLRP/S at 40˚C) as described previously [27] in buffers containing 0.1 % trifluoroacetic acid. Positive ion electrospray-ionization mass spectrometry was performed using an ion-trap mass spectrometer (LTQ; Thermo, San Jose). Data were analyzed using qualbrowser software (Thermo, San Jose) and MagTran freeware. The data (Results section) indicate that 1,1.5,2,3,4,5,10 and 15 min incubations yield FIXaβ cleaved only at Lys316-Gly317 at 3 μg/mL plasmin; this molecule is termed FIXaγ. The FIXaγ is further cleaved at Lys413-Leu414 (~20% at 1 hour, 30% at 2 hour, ~40% at 4 hour and ~75% at 8, 12 and 24 hour); the molecule cleaved at Lys316-Gly317 and at Lys413-Leu414 is termed FIXaδ. The second cleavage was only observed in ~75% of the FIXaγ molecules as determined by mass spec analysis and was used in all comparative analyses. A higher concentration of plasmin increased the rate of FIXaδ formation but maximum conversion was still ~75% (see results). Resolution of intact mass spectrometry procedure used to determine the ratio of FIXaγ and FIXaδ was >90%.
Measurement of FX activation
FX activation by FIXaβ, FIXaγ or FIXaγ/FIXaδ mixture was measured under two conditions: One, in the presence of Ca2+/PL; and two, in the presence of Ca2+/PL and FVIIIa [23,25]. In the absence of FVIIIa, each reaction contained 20 nM FIXaβ, 25 μM PL, 5 mM CaCl2, and FX ranging from 25–6400 nM. The activations were carried out for 2.5–20 minutes. The Km and kcat values were obtained using the program GraFit from Erithacus Software. Since plasmin cleaved-FIXaβ is essentially devoid of clotting activity, only one condition for activation of FX was used in the presence of FVIIIa. Four identical reaction mixtures each containing 0.05 nM FIXa (FIXaβ or FIXaγ or FIXaγ/FIXaδ mixture), 10 μM PL, 5 mM CaCl2, 0.07 nM FVIIIa and 15 nM FX were setup and activation was carried out at 37 °C for 0.5, 1.0, 1.5 or 2 min. The buffer used in all experiments (plus/minus FVIIIa) was TBS/BSA pH 7.5 and the reaction volume was 50 μl. The reactions were carried out at 37 °C for different lengths of time, after which 2 μl of 0.5 M EDTA was added to stop the reaction. Then 100 μl of TBS/BSA pH 7.5 and 10 μl of S-2222 (final concentration 100 μM) were added. From this mixture, 150 μl was placed in a well on an Immulon 4 HBX flat-bottom 96-well microtiter plate and the p-nitroaniline release was measured (ΔA405/minute) for up to 30 minutes. FXa generated was calculated from a standard curve constructed using FXa prepared by insolubilized RVV-X.
Binding of FIXaβ, FIXaγ and FIXaγ/FIXaδ to FVIIIa
Apparent Kd (Kdapp) binding values for FIXaβ, FIXaγ or FIXaγ/FIXaδ mixture to FVIIIa were determined by the ability of dEGR-IXaβ, dEGR-IXaγ or dEGR-FIXaγ/dEGR-FIXaδ mixture to inhibit FVIIIa potentiation of FX activation by FIXaβ [23,25]. Reaction mixtures contained 0.2 nM FIXaβ, 0.07 nM FVIIIa, 480 nM FX, 10 μM PL, and various concentrations of dEGR-IXaβ, dEGR-IXaγ or dEGR-FIXaγ/dEGR-FIXaδ mixture in TBS/BSA pH 7.5 containing 5 mM CaCl2. The IC50 (concentration of inhibitor required for 50% inhibition) was determined by fitting the data to IC50 four-parameter logistic equation of Halfman [28]: where y is the rate
| (Eq. 1) |
of FXa generation in the presence of a given concentration of dEGR-IXaβ, dEGR-IXaγ or dEGR-FIXaγ/dEGR-FIXaδ mixture represented by x, a is the maximum rate of FXa formation in the absence of dEGR-IXa species, and s is the slope factor. Each point was weighted equally and the data were fitted to equation 1 using the non-linear regression analysis program GraFit from Erithcus Software. To obtain Kdapp values for the interaction of dEGR-IXa proteins with FVIIIa, we used the following equation as described by Cheng and Prusoff [29] and further elaborated by Craig [30]:
| (Eq. 2) |
where A is the concentration of FIXaβ and EC50 is the concentration of FIXaβ that gives a 50% maximum response in the absence of the competitor at a specified concentration of FX used in the experiment.
Plasminogen/plasmin binding to FIXaβ, FIXaγ or FIXaγ/FIXaδ mixture using ligand binding Western blots
Nonreduced samples were run on 12% SDS-PAGE and blotted to PVDF membranes in Towbin buffer. The membranes were blocked using 5% milk in TBS pH 7.5, 0.05% Tween 20 at room temperature for ~3 hours. The membranes were then washed two times in TBS pH 7.5, 0.01% Tween 20 and once in TBS pH 7.5. In one experiment, the membrane was incubated with Glu-plasminogen or DIP-Plasmin (3 μg/mL) in TBS pH 7.5, 1% milk over night at 4° C. In a second experiment, the PVDF membrane was incubated with 100 U/mL CpB in TBS pH 7.5 for 30 minutes at 37°C and washed in TBS pH 7.5 prior to incubation with DIP-plasmin in TBS pH 7.5, 1% milk over night at 4° C. In a third experiment, the PVDF membrane was incubated with DIP-Plasmin (3 μg/mL) and 2 mg/mL EACA in TBS pH 7.5, 1% milk at room temperature for 30 minutes followed by incubation overnight at 4°C. The membranes were then washed two times in TBS pH 7.5, and incubated with polyclonal goat anti-human plasminogen antibody (15 μg/mL) in TBS pH 7.5, 0.03% BSA for two hours at room temperature. The membranes were then washed one time with TBS pH 7.5, 0.01% Tween 20 and one time with TBS pH 7.5 and incubated with peroxidase-conjugated donkey anti-goat IgG (1:200) in TBS pH 7.5, 0.03% BSA for two hours at room temperature. Membranes were then washed once with TBS, 0.01% Tween 20 followed by once with TBS pH 7.5 and developed with the Amersham ECL kit.
Fibrinolysis (Clot Lysis) Assay
The method of Sperzel and Huetter [31] was followed with minor modifications as outlined earlier [32]. In initial experiments, RVV-X was used to initiate fibrin formation in immunochemically-depleted FIX plasma, and lysis of the formed clot was induced by simultaneous addition of tPA. Briefly, 225 μL FIX-depleted plasma was mixed with 12.5 μL of PL (60%PC/20%PS/20%PE, 100 μM) and 2.5 μL of TBS/BSA. Ten μL of each test compound (FIXaβ, FIXaγ and FIXaγ/FIXaδ mixture) or saline control was added to 240 μL of above FIX-depleted plasma mixture. Two hundred-twenty five μL of this mixture was then added to 25 μL of RVV-X and tPA in TBS/BSA containing 100 mM CaCl2. In the 250 μL final volume, concentration of RVV-X was 10 ng/mL and tPA was 0.25 μg/mL. Next, RVV-X was used to initiate fibrin formation using NPP. Here, 225 μL of NPP was mixed with 12.5 μL of PL and 12.5 μL of TBS/BSA. Two hundred-twenty five μL of this mixture was added to 25 μL of RVV-X and tPA in TBS/BSA containing 100 mM CaCl2. In the 250 μL final volume, the concentration of RVV-X was 10 ng/mL and that of tPA was 0.25 μg/mL or 0.5 μg/mL. In additional experiments, FXIa was used to initiate fibrin formation using NPP and mixtures of NPP and FIX-depleted plasma (50:50 and 20:80). Here, 225 μL of plasma was mixed with 12.5 μL of PL and 12.5 μL of TBS/BSA. Two hundred-twenty five μL of this mixture was added to 25 μL of FXIa and tPA in TBS/BSA containing 100 mM CaCl2. In the 250 μL final volume, the concentration of FXIa was 64 ng/mL and tPA was 0.5 μg/mL. Clot formation and lysis in each experiment was monitored with a Molecular Devices microplate reader (SpectraMAX 190) measuring the optical density at 405 nm. Under control conditions (zero tPA), OD405 increased immediately indicating clotting, followed by an extremely slow decrease representing fibrinolysis. Because clotting was almost complete between 5 to 8 minutes, fibrinolysis induced by tPA was evaluated as a relative decrease of OD405 up to 120 min.
Results
Plasmin cleavage of FIXaβ
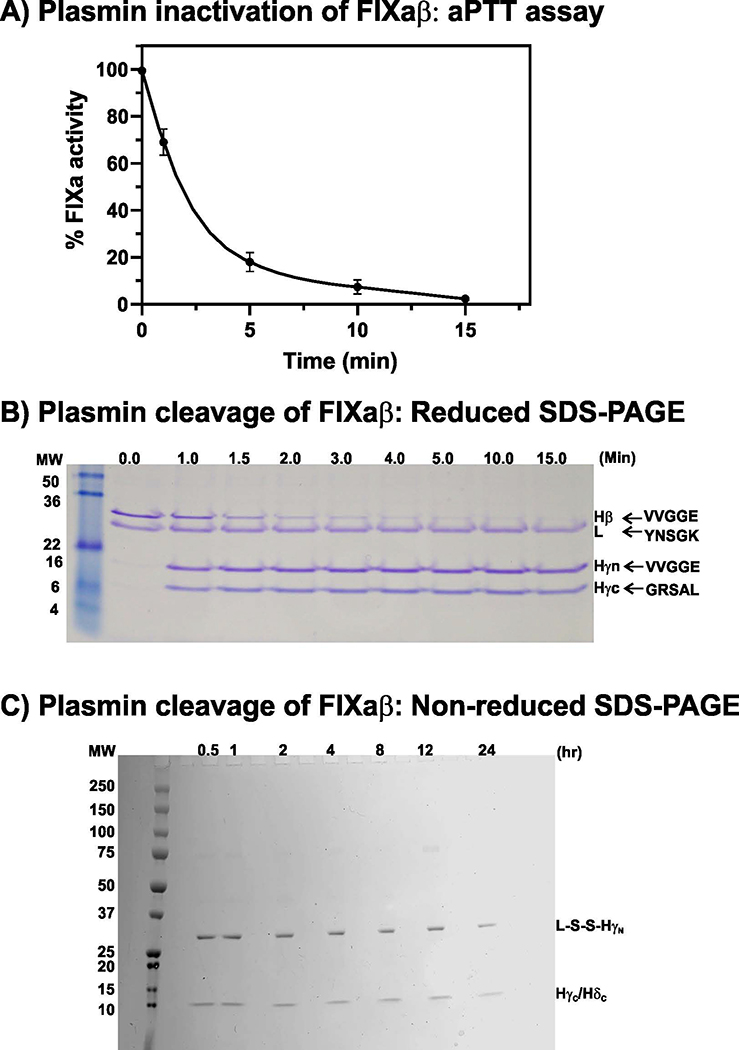
Plasmin-mediated cleavage of FIXaβ in the presence of Ca2+/PL was analyzed by FIXa activity, SDS-PAGE, NH2-terminal amino acid sequencing and mass spec analysis. After 10 minutes, only ~5% of the original clotting activity remained and after 15 minutes, <2% of the FIXaβ activity remained (Fig. 1A). Based upon the NH2-terminal amino acid sequencing data (see below), the loss of clotting activity corresponded to the generation of two fragments termed HγN and HγC on reduced SDS-PAGE (Fig. 1B). Based upon the mass spec data presented later in the manuscript, continued incubation of FIXaβ with plasmin resulted in cleavage of HγC at Lys413↓Leu414 to yield HδC (Fig. 1C). Note that HγC (Gly317-Thr415) and HδC (Gly317-Lys413) migrate indistinguishably in non-reduced SDS-PAGE.
Fig 1. Plasmin cleavage of FIXaβ.
A, Loss of FIXa clotting activity. FIXaβ (100 μg/ml) was incubated with plasmin (3 μg/ml) in the presence of 5 mM Ca2+ and 240 μM PL at 37°C. Samples were removed at indicated times, diluted 50 to 10,000-fold and measured for FIXa clotting activity as described in Materials and methods. Percent FIXa clotting activity remaining at different time points is depicted. B, Reduced SDS-PAGE analysis. Samples were removed at indicated times from the reaction mixture outlined in ′A′ above and 5μg of protein was loaded in each lane. Based upon NH2-terminal sequence and the mass spec data, the heavy (Hβ) and light chains (L) of FIXaβ as well as the heavy chain cleavage fragments of FIXaγ (HγN and HγC) up to 15 min are indicated. Molecular weight (MW) markers as well as the N-terminal sequence of each band is provided. C, Non-reduced SDS-PAGE analysis. Samples were removed between 30 min to 24 hour from the reaction mixture outlined in ′A′ above and 5 μg of protein was loaded in each lane. Two discrete bands were visualized, one corresponding to light chain disulfide linked to HγN (L-S-S-HγN) and the second corresponding to HγC/HδC (based upon mass spec analysis).
NH2-terminal amino acid sequencing of plasmin cleaved FIXaβ
FIXaβ was incubated with plasmin in the presence of Ca2+ and PL for 1,1.5,2,3,4,5,10,15 minutes and 0.5,1,2,4,8,12,24 hours at 37°C. Each sample was run on reduced SDS-PAGE, transferred to the PVDF membrane and subjected to NH2-terminal amino acid sequencing as outlined in Materials and methods. The sequencing data revealed that plasmin cleaved FIXaβ heavy chain at Lys316↓Gly317. Two new bands were visualized following cleavage of the heavy chain. One band with the NH2-terminal sequence VVGGE, which is the NH2-terminal fragment of the heavy chain, termed HγN. The other band with the NH2-terminal sequence GRSAL, which is the C-terminal fragment of the heavy chain, termed HγC (Fig. 1B). Thus, based upon the NH2-terminal sequencing and SDS-PAGE, cleavage of the FIXaβ heavy chain only at the Lys316↓Gly317 peptide bond was observed (Fig. 1B).
Mass spec analysis of plasmin cleaved FIXaβ at different time points
Plasmin cleavage at Lys316↓Gly317 in FIXaβ heavy chain yields FIXaγ with HγC fragment (Gly317-Thr415) that has an average theoretical molecular mass of 11031.63 Da (or 11027.6 Da containing the C336-C350 and C361-C389 disulfide bonds), which corresponds to sequence GRSALVLQYLRVPLVDRATCLRSTKFTIYNNMFCAGFHEGGRDSCQGDSGGPHVTEVEGTSFLTGIISWGEECAMKGKYGIYTKVSRYVNWIKEKTKLT. In the mass spec analysis of samples up to 15 minutes of incubation, the HγC of FIXaγ heavy chain was “Instrument Reported” as a molecular ion at m/z = 1839.3, the 6-charge ion that is dominant for this peptide with measured average mass of 11029.8 Da. These data are consistent with absence of any additional proteolysis in the HγC up to 15 minutes of incubation.
After removal of the two carboxy-terminal residues (Leu314 and Thr415) by subsequent cleavage of FIXaγ at Lys413↓Leu314, the resulting HδC fragment (Gly317-Lys413) has the theoretical molecular mass of 10813.3 Da with the two disulfide bonds intact. This ion was “Instrument Reported” as a molecular ion at m/z = 1803.1 Da, the 6-charge ion that is dominant for this peptide with measured average mass of 10812.6 Da. This quantification assumes that the two species (HγC and HδC) have the same ionization efficiency, which is reasonable especially considering that there is very little change in the charge-state distribution between the two species. Fraction of the second species (HδC) was ~20% at 1 hour, 30% at 2 hour, ~40% at 4 hour and ~75% at 8, 12 and 24 hour. The “Instrument Reported” data at 8 hour time point is shown in Fig. 2A. Based upon the NH2-terminal sequencing and the mass spec data, it would appear that formation of FIXaγ (cleavage at Lys316↓Gly317 in FIXaβ) is rapid followed by a slow cleavage at Lys413↓Leu414 in FIXaγ to yield FIXaδ. Since proteolysis at Lys413-Leu414 of FIXaγ with 3 μg/mL (34 nM) plasmin was slow, we performed additional experiments using 6μg/mL (68 nM) plasmin. Under these conditions FIXaγ was cleaved faster at Lys413-Leu414 (~12% at 15 min, ~20% at 30 min, ~35% at 1hour, ~45% at 2 hour,~70% at 4 hour and ~75% at 8,12 and 24 hour). As expected, the rate of FIXaδ formation was increased ~2-fold when plasmin concentration was doubled. However, at each plasmin concentration, the maximum FIXaδ formed at 24-hour was ~75% indicating strong product inhibition, which prevents complete conversion to FIXaδ. These cleavage sites are schematically depicted in Fig. 2B. Notably, both species (FIXaγ and FIXaδ) are devoid of coagulant activity.
Fig 2. Plasmin cleavage sites in FIXaβ and their influence on native gel electrophoresis.
A. Mass spec data of the HγC chain upon extended incubation with plasmin. Shown are the extracted ion chromatograms for the uncleaved and cleaved HγC incubated with plasmin for 8 hours. The uncleaved HγC of FIXaγ molecule (average mass 11029.8 Da reported by 6-charge ion at m/z = 1839.3) versus the cleaved HγC of FIXaδ (average mass 11812.6 Da reported by the 6-charge ion at m/z 1803.1) are depicted. At this data point, it was estimated that 1.53 e3 of the uncleaved product remains compared to 4.8 e3 of the cleaved species. The y-axis has arbitrary units and is dependent upon the detector response. Based on the mass spec data, the HγC is cleaved between residues Lys413 and Leu414. B. Schematic representation of the cleavage sites in FIXaβ heavy chain (Hβ) observed in the present study. Light chain (L) containing γ-carboxy glutamic acid (Gla) domain, two epidermal growth factor (EGF) like domains and the heavy chain (Hβ) containing the protease domain is depicted. The two cleavage sites are marked by black arrows at Lys316-Gly317 (312RVFHK↓GR318) and at Lys413-Leu414 (409KEKTK↓LT415). C, Native gel electrophoresis of FIX, FIXaβ and FIXaγ/δ (8-hour incubation time point). Gels were run according to Williams and Reisfeld [33] and 4 μg of protein was loaded in each lane.
Nondenaturing gel electrophoresis of FIX and FIXa
Nondenaturing gel electrophoresis (Supplementary material) of FIX, FIXaβ and FIXaγ/FIXaδ mixture at 8-hour time point is shown in Fig. 2C. FIX migrated slower than FIXaβ or FIXaγ/FIXaδ. Importantly, FIXaβ and FIXaγ/FIXaδ mixture migrated similarly. These data indicate that the activation peptide does not stay associated with FIXaβ and is released upon activation of FIX. However, the 317–415 or 317–413 fragments generated by plasmin cleavage in FIXaβ likely remain noncovalently associated with FIXaγ or FIXaδ.
CBS 31.39 amidolytic activity of FIXaβ, FIXaγ and FIXaγ/FIXaδ mixture
Next, we compared hydrolysis of synthetic substrate CBS 31.39 by FIXβ, FIXaγ and FIXaγ/FIXaδ (25/75) mixture. Method details are provided in the supplementary material and the data are presented in Table 1. Addition of Ca2+ to FIXaβ resulted in ~6.7-fold increase in Km and ~6.4-fold increase in kcat, whereas essentially no effect on the Km or kcat was observed for FIXaγ or FIXaγ/FIXaδ mixture. Overall, FIXaγ or FIXaγ/FIXaδ mixture had a decreased kcat in the presence and absence of Ca2+ and ~10-fold reduced kcat/Km as compared to FIXaβ. Since kcat/Km values for FIXaγ and FIXaγ/FIXaδ mixture are similar, it is safe to conclude that FIXaγ and FIXaδ hydrolyze CBS 31.39 with comparable efficiency.
Table 1.
Kinetic parameters for the hydrolysis of CBS 31.39 by different FIXa species
| Protein | Km (mM) | kcat (min−1) | kcat/Km (min−1 mM−1) |
|---|---|---|---|
| FIXaβ (+Ca2+) | 4.31±0.35 | 189±12 | 43.9 |
| FIXaβ (−Ca2+) | 0.64±0.07 | 29.4±2.2 | 45.9 |
| FIXaγ (+Ca2+) | 2.94±0.32 | 11.3±1.1 | 3.8 |
| FIXaγ (−Ca2+) | 2.55±0.26 | 10.5±1.0 | 4.1 |
| FIXaγ/δ (+Ca2+) | 3.01±0.37 | 11.5±1.3 | 3.8 |
| FIXaγ/δ (−Ca2+) | 3.25±0.42 | 11.9±1.4 | 3.6 |
Concentration of each reagent in the reaction mixture was: 107 nM FIXa with either 5 mM CaCl2 or 1 mM EDTA and varying concentrations of CBS 31.39.
FX activation in the presence and absence of FVIIIa
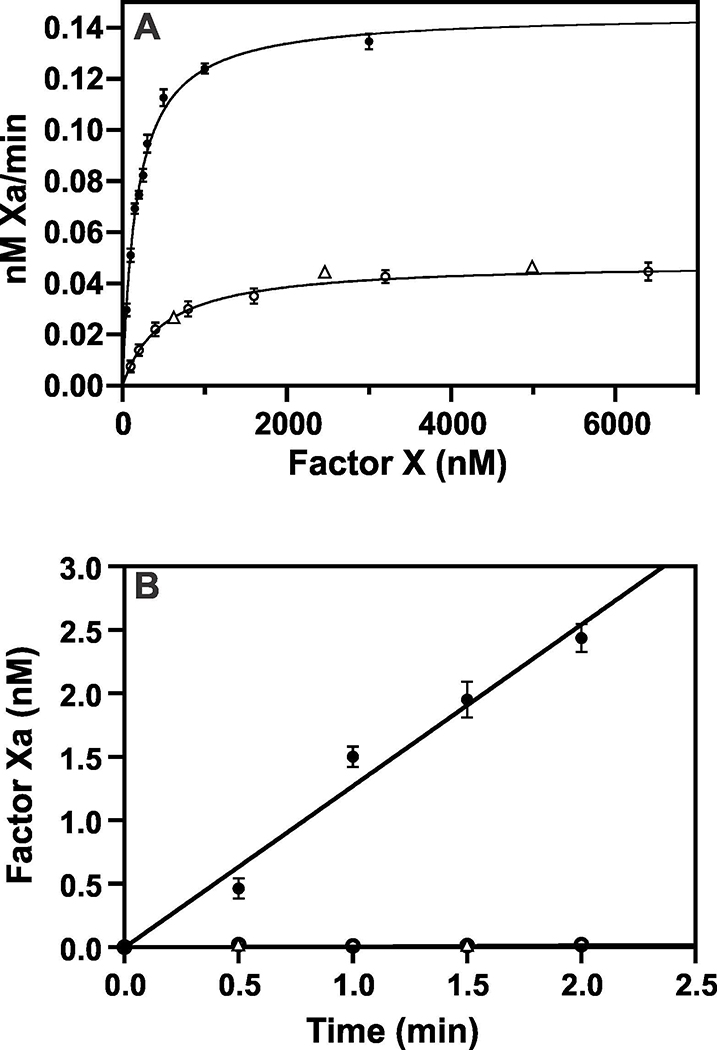
Next, we investigated activation of FX by FIXaγ or FIXaγ/FIXaδ (25/75) mixture. In the presence of Ca2+/PL only, FIXaγ activated FX with Km ~467 nM as compared to ~96 nM for FIXaβ (Fig. 3A). FIXaγ also had ~6-fold reduced kcat (1.9±0.2 × 10−3 min−1) as compared to FIXaβ (11.1±0.9 × 10−3 min−1) in activating FX. Limited data presented using FIXaγ/FIXaδ behaved similar to FIXaγ in activating FX in the presence of Ca2+/PL (Fig. 3, triangles). Thus, as compared FIXaβ, FIXaγ or FIXaδ had ~30-fold decrease in kcat/Km in activation of FX in the absence of FVIIIa. In the presence of FVIIIa, under the kinetic condition used, FIXaγ or FIXaγ/FIXaδ mixture was ~650–fold less active than FIXaβ (Fig. 3B). Thus, FIXaγ or FIXaδ is impaired in hydrolyzing the synthetic substrate as well as the physiologic substrate FX.
Fig 3. FX activation by FIXaβ, FIXaγ and FIXaγ/FIXaδ (25/75) mixture in the absence and presence of FVIIIa.
A, Activation of FX in the absence of FVIIIa. Each reaction mixture contained 20 nM FIXaβ or FIXaγ or FIXaγ/FIXaδ (25/75) mixture, 25 μM PL, 5 mM CaCl2 and various concentrations of FX. Activation was carried out at 37°C and FXa activity was measured by hydrolysis of synthetic substrate S-2222. Km and kcat were determined as described in Materials and methods. B, Activation of FX in the presence of FVIIIa. Each reaction mixture contained 0.05 nM of FIXa, 10 μM PL, 0.07 nM FVIIIa, 5 mM CaCl2 and 15 nM FX. Activation was carried out at 37°C from 0.5 to 2 min, and FXa activity was measured by hydrolysis of synthetic substrate S-2222 as described in Materials and methods. In both A and B, closed circles represent FIXaβ, open circles represent FIXaγ and triangles represent FIXaγ/FIXaδ (25/75) mixture. The data for FIXaβ or FIXaγ represent average of two experiments. The limited data for the FIXaγ/FIXaδ mixture are presented to verify that FIXaδ behaves similar to FIXaγ.
Binding of FVIIIa to FIXaγ/FIXaδ
FIXaγ/FIXaδ are defective in FX activation in the presence of Ca2+/PL, and addition of FVIIIa in the system leads to further impairment in FX activation. To explore these observations further, we evaluated Kd of interaction of FIXaγ and FIXaγ/FIXaδ mixture with FVIIIa. In this competition-based assay, dEGR-IXaβ and dEGR-IXaγ or dEGR-IXγ/dEGR-IXaδ were used to compete for FIXa binding to FVIIIa and inhibit FXa generation. Kdapp obtained under these conditions for dEGR-IXaβ was 0.7 nM and for dEGR-IXaγ it was 56 nM (Fig. 4). Limited data presented with dEGR-IXaγ/dEGR-IXaδ mixture support that dEGR-IXaδ behaves similar to dEGR-IXaγ. Thus, dEGR-IXaγ or dEGR-IXaδ is ~80-fold defective in binding to FVIIIa suggesting that the FVIIIa binding site is affected by plasmin cleavage primarily at the Lys316-Gly317 peptide bond.
Fig 4. Inhibition of FXa generation by dEGR-IXaβ and dEGR-IXaγ or dEGR-IXaγ/dEGR-IXaδ (25/75) mixture in the presence of FVIIIa.
Each reaction mixture contained 0.2 nM FIXa, 0.07 nM FVIIIa, 480 nM FX, 10 μM PL, 5 mM CaCl2, and varying concentrations of dEGR-IXa proteins (dEGR-IXaβ or dEGR-IXaγ or dEGR-IXaγ/dEGR-IXaδ mixture) in TBS/BSA, pH 7.5. The percent of FXa generated at various concentrations of the competitor—dEGR-IXaβ or dEGR-IXaγ or dEGR-IXaγ/dEGR-IXaδ mixture is depicted. The curves represent best fit to the IC50 four-parameter logistic equation (Eq. 1). Closed circles represent dEGR-IXaβ, open circles represent dEGR-FIXaγ and triangles represent dEGR-IXaγ/dEGR-IXaδ mixture. The data for dEGR-IXaβ or dEGR-IXaγ represent average of two experiments. Limited data for the dEGR-IXaγ/dEGR-IXaδ mixture are presented to verify that dEGR-IXaδ behaves similar to dEGR-IXaγ.
Plasmin binding to FIXaγ and FIXaδ using ligand blots
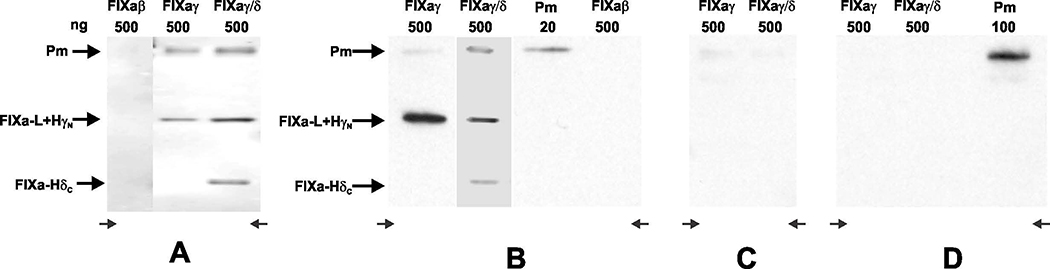
Ligand binding blots were performed in which plasmin, FIXaβ, FIXaγ and FIXaγ/FIXaδ mixture were first run on non-reduced SDS-PAGE and transferred to Immobilon-P PVDF membrane. The membrane was incubated with Glu-plasminogen or DIP-plasmin and bound plasminogen or plasmin was detected using anti-plasminogen polyclonal antibody. In this assay, Glu-plasminogen and DIP-plasmin bound to HγN of FIXaγ and to HδN (same fragment as HγN) as well as to HδC of FIXaδ but not to FIXaβ or HγC of FIXaγ (Figs. 5A and 5B). In addition, when FIXaγ/FIXaδ blot was incubated with 100 units/mL CpB prior to incubation with DIP-Plasmin, the binding of DIP-Plasmin to HγN of FIXaγ or FIXaδ and HδC of FIXaδ was abrogated (Fig. 5C). Further, when FIXaγ/FIXaδ blot was incubated with the DIP-Plasmin in the presence of EACA, DIP-Plasmin binding to FIXaγ/FIXaδ was not observed (Fig. 5D). These results suggest that plasminogen/plasmin, through its Kringle domain(s), binds to the C-terminal Lys316 of FIXaγ/FIXaδ and C-terminal Lys413 of FIXaδ. Removal of these C-terminal lysine residues by CpB abolishes plasmin binding, as does the presence of EACA, a lysine analog.
Fig 5. Ligand blot assay of FIXaβ, FIXaγ and FIXaδ.
Plasmin, FIXaβ, FIXaγ and FIXaγ/FIXaδ mixture were electrophoresed using 12% non-reduced SDS-PAGE. The concentration of each protein (nanogram) loaded is indicated. The proteins were transferred to an Immobilon-P PVDF membrane and the membrane was blocked using 5% milk. A, The membrane was then incubated with 3 μg/mL Glu-plasminogen. To detect bound plasminogen, a goat polyclonal plasminogen antibody was used followed by a secondary anti-goat antibody. The blot was developed with the Amersham ECL kit. B, The membrane was incubated with 3 μg/mL DIP-plasmin (instead of Glu-plasminogen) and the bound DIP-plasmin was detected using plasminogen antibody as in panel ‘A’. C, The membrane was treated with 100 U/mL of CpB for 30 minutes at 37ºC prior to incubation with DIP-plasmin. Detection of bound DIP-plasmin was performed as in panel ′B′. D, The membrane was incubated with DIP-plasmin in the presence of EACA and detection of the bound DIP-plasmin was performed as in panel ′B′. Pm, plasmin; FIXa-L, light chain of FIXa; HγN, NH2-terminal of FIXaγ heavy chain; FIXa-HδC, C-terminal of FIXaδ heavy chain.
Effect of FIXaγ and FIXaδ on tPA-mediated plasminogen activation
Both plasminogen and tPA use their Kringle domains to bind to the C-terminal lysine residues in fibrin for colocalization at the clot site. Glu-plasminogen exists in closed T-state conformation and adopts the open R-state conformation upon binding to EACA or the C-terminal lysine residues on cell surface protein receptors [34–37]. Intact Glu-plasminogen (T-state) in the absence of ω-amino acids of the lysine class e.g., EACA, is severely attenuated in its activation by plasminogen activators, but the R-state that exists upon binding to EACA or the C-terminal lysine residues in the pertinent protein receptors is highly activatable [36]. The Glu-plasmin initially formed by proteolysis at Arg561-Val562 rapidly removes the NH2-terminal 77 residues in Glu-plasmin/Glu-plasminogen to yield Lys-plasmin/Lys-plasminogen, which exist in open R-conformation ± EACA [37]; the Lys-plasminogen is then activated to the final product Lys-plasmin.
Since Glu-plasminogen is the native circulating plasminogen, we studied enhancement of tPA-mediated activation of Glu-plasminogen by FIXaβ, FIXaγ and FIXaγ/FIXaδ (25/75) mixture. Detailed methods are given in the supplementary material. As shown in Fig 6, FIXaβ or FIXaγ did not potentiate the activation of Glu-plasminogen by tPA. In contrast, FIXaγ/FIXaδ potentiated tPA mediated plasminogen activation in a concentration dependent manner. In control experiments (Table S1), FIXaβ, FIXaγ or FIXaγ/FIXaδ mixture under similar conditions had no influence on the catalytic activity of tPA (synthetic substrate S-2288) or plasmin (synthetic substrate S-2251). Although we cannot exclude other mechanisms, it appears that the enhancement of tPA-mediated plasminogen activation by FIXaδ is likely mediated via the C-terminal lysine residues (Lys316 and Lys413) of FIXaδ which could allow both tPA and plasminogen/plasmin binding and colocalize them.
Fig 6. tPA-mediated activation of plasminogen by FIXaδ but not by FIXaβ or FIXaγ.
Each reaction mixture (100 μL) contained 5 mM CaCl2, 1 μM Glu-plasminogen, and 10 nM tPA in TBS pH 7.5. In one set of experiments, reaction mixtures contained 10 nM (diamonds), 50 nM (squares) or 100 nM (triangles) dEGR-IXaγ/dEGR-IXaδ (25/75) mixture. In other experiments, reaction mixtures contained 100 nM dEGR-IXaβ (circles), 100 nM dEGR-IXaγ (hexagon) or 100 nM BSA (inverted triangles). The reaction mixtures were incubated for 5–30 minutes at 37°C and 5 μL aliquots were removed at a given time, diluted 30-fold in TBS/BSA pH 7.5 and placed on ice. Plasmin activity was measured using synthetic substrate S-2251 as outlined in Materials and methods. The plasmin generated was calculated from a standard curve measuring S-2251 hydrolysis by purified plasmin. The values represent mean of three different experiments.
Enhancement effect of FIXaδ on plasma clot lysis
Initial experiments were performed to study the fibrinolytic effect of dEGR-IXaβ, dEGR-IXaγ and dEGR-IXaδ in the tPA (0.25 μg/mL)-induced clot lysis in FIX-depleted plasma. Addition of RVV-X to FIX-depleted plasma in the absence of tPA caused fibrin formation with minimal lysis as reflected by an increase in OD405 (Fig 7A). Simultaneous addition of tPA caused dissolution of the fibrin clot induced by tPA mediated conversion of plasminogen to plasmin. Further, addition of dEGR-IXaβ or dEGR-IXaγ had essentially no effect on promoting clot lysis. In contrast, addition of dEGR-IXaγ/dEGR-IXaδ mixture enhanced tPA-induced plasma clot lysis. Since dEGR-IXaγ had no effect, the enhanced clot lysis seen with dEGR-IXaγ/dEGR-IXaδ mixture can be attributed primarily to dEGR-IXaδ, which could bind plasminogen and tPA simultaneously. The midpoint of clot lysis at 7.5 nM and 15 nM dEGR-IXaδ in the dEGR-IXaγ/dEGR-IXaδ mixture was ~28min and ~32min, respectively as compared to ~36min without dEGR-IXaδ. Consistent with these observations, when NPP (Gray line, Fig 7A) was used instead of FIX-depleted plasma, the fibrinolysis was similar to the FIX-depleted plasma. Next, we examined whether increasing the concentration of tPA from 0.25 μg/mL to 0.5 μg/mL would result in enhanced fibrinolysis by FIX/FIXa in the RVV-X activation system. However, fibrinolysis was similar in both NPP (mid-point of clot lysis ~19 min) and FIX-depleted plasma (mid-point of clot lysis ~20 min). These data indicate that FIX/FIXa does not contribute to fibrinolysis when clotting is initiated with RVV-X. The reason(s) why FIX/FIXa does not contribute to fibrinolysis in this system is unknown.
Fig 7. Effect of FIX on plasma clot lysis assay.
A, Effect of FIXaδ on the FIX-depleted plasma clot lysis. Clot formation was initiated with dilute RVV-X, and fibrinolysis was induced by simultaneous addition of 0.25 μg/mL tPA. Fibrin formation and lysis were monitored by measuring the optical density at 405 nm as outlined under Materials and methods. The effects of addition of dEGR-IXaγ/dEGR-IXaδ (25/75) mixture (10 nM and 20 nM), dEGR-IXaγ (100 nM) and dEGR-IXaβ (100 nM) are presented. Control experiments consisted of no tPA ± dEGR-IXaβ, dGER-IXaγ or dEGR-IXaγ/dEGR-IXaδ mixture. The fibrinolysis observed with NPP is also presented. B, tPA mediated plasma clot lysis using FXIa as the clotting initiator. Concentration of FXIa used was 64 ng/mL and tPA was 0.5 μg/mL. NPP, 50% NPP:50% FIX-depleted plasma, and 20% NPP:80% FIX-depleted plasma were used. Control experiments consisted of no tPA.
Next, we examined if FIX/FIXa contributes to fibrinolysis when clotting is initiated by FXIa, a physiologic activator of FIX. Here, we used 64 ng/mL FXIa and 0.5 μg/mL tPA and 100% NPP, 50% NPP:50% FIX-depleted plasma, and 20% NPP:80% FIX-depleted plasma. These data are presented in Fig 7B. The mid-point of clot lysis was ~18 min for NPP, ~21 min for 50% NPP:50% FIX-depleted plasma, and ~25 min for 20% NPP:80% FIX-depleted plasma. These data clearly indicate that FIX/FIXa does contribute to fibrinolysis when a physiologic activator of FIX is used.
Discussion
The data presented here reveals that in the presence of Ca2+/PL, plasmin cleavage in FIXaβ at Lys316↓Gly317 (FIXaγ) leads to loss of catalytic activity. Notably, the cleaved fragment likely stays associated with FIXa as is the case in γ-thrombin [38]. This is due to the interaction of the cleaved C-terminal fragment with the NH2-terminal heavy chain of FIXa involving several salt-bridges (~10) and hydrogen bonds (~32) [39,40]. The loss of catalytic activity (~10-fold for synthetic substrate and ~30-fold for FX) could stem from the impairment of catalytic triad and FVIIIa binding (~80-fold) [41–43] as observed for γ-thrombin activation of fibrinogen and FXIII [44] as well as impairment of GpIbα binding to exosite-II in γ-thrombin [45,46].
The mass spec data revealed that plasmin cleaves FIXaγ at Lys413-Leu414 generating a second C-terminal lysine in FIXaδ. The ligand blots demonstrate that FIXaγ has one while FIXaδ has two binding sites for plasminogen/DIP-plasmin. This difference has important biologic implication in that FIXaδ by simultaneous binding to tPA and plasminogen enhances tPA-mediated plasminogen activation and plasma clot lysis. Since C-terminal Lys413 is missing in FIXaγ, it can only bind either plasminogen or tPA that is insufficient to bring them in close proximity for plasminogen activation and plasma clot lysis. The second cleavage in FIXaγ at Lys413↓Leu414 is consistent with plasmin cleavages in FXaα at Lys330↓Gly331 and Lys435↓Ser436 for converting it to a fibrinolytic enhancer. Notably, the second cleavage at Lys435↓Ser436 in FXaα is not obligatory, since FXaα already has C-terminal Lys448 [13,20]. However, plasmin cleavage at Lys413↓Leu414 in FIXaγ is essential for its conversion to a fibrinolytic enhancer [13].
The biochemical properties of FIXaγ/FIXaδ are in agreement with previous studies describing a pathway between coagulation and fibrinolysis involving plasmin modulation of FVa, FXa, and FVIIIa [9–13,15–18]. In these studies, plasmin was shown to obliterate procoagulant functions of these proteins, while converting them into tPA and plasminogen/plasmin receptors to facilitate fibrinolysis. In previous studies, plasmin cleaved FIX at multiple sites (introduction and Fig. 2B) in the absence of PL membrane surface [14]. Interestingly, in the absence of PL, plasmin also cleaved the light chain of FX/FXa at residue Lys43 [8,10,11]. In the presence of Ca2+/PL, we also did not observe cleavage at Arg318-Ser319 in FIXaβ. The reason(s) for this is unknown; however, cleavage at this site does not generate C-terminal lysine and cannot participate in tPA or plasminogen/plasmin binding. Because of the methodology used, Samis et al. [14] also did not detect proteolysis in FIX at Lys413-Leu414, which in all probability occurred. If so, although not tested, their product could enhance fibrinolytic activity as we have observed in Fig 7B. Although the data presented in Fig 2A are non-physiological in nature, the results demonstrate that cleavages at Lys316-Gly317 and Lys413-Leu414 are most likely needed for generation of fibrinolytic activity. While the mechanism for two binding sites (plasminogen and tPA) on FIXaδ is plausible, the data are equally consistent with FIXaδ in conjunction with appropriately situated fibrin, serving as an accelerating scaffold.
The present study highlights the intricate balance between coagulation and fibrinolysis. Although many coagulation factors are viewed as only procoagulants, they can also contribute to fibrinolysis. These findings are critical from a clinical perspective especially in the setting of trauma. Many patients presenting with major trauma have thromboelastography (TEG) performed upon presentation to the emergency department. Often, the TEG shows a shortened R time (reaction time) indicating activation of the clotting cascade, and increased LY30 (percentage decrease in 30 min post-Maximum Amplitude) indicating increased fibrinolytic activity. When patients present with increased LY30, tranexamic acid is administered based upon the CRASH-2 study [47,48]. Tranexamic acid, a lysine analog competes with plasmingeon/plasmin and t-PA which utilize C-terminal lysine in binding to the Kringle domains. Tranexamic acid administration decreases fibrinolysis and helps restore hemostasis. Thus, this study and others serve to further our understanding of the biochemical process during the period of clotting activation and increased fibrinolysis as occurs in trauma patients.
Addendum
Amy E. Schmidt, Kanagasabai Vadivel and Julian Whitelegge performed the experiments. Amy Schmidt, Kanagasabai Vadivel and S. Paul Bajaj conceived, designed, analyzed and interpreted the data, and wrote the manuscript.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grant HL141850. Amy E. Schmidt was supported by American fellowship from the American Association of University Women Educational Foundation and by predoctoral Fellowship 0315211Z from the American Heart Association Heartland Affiliate.
Footnotes
Disclosure of conflict of Interests
The authors state that they have no conflict of interest.
Essentials
Plasmin inactivates factor IX but does it also convert it to a fibrinolytic enhancer is not known
Perform NH2-terminal sequencing, mass spec and functional assays on plasmin cleaved factor (F) IXa
K316-G317 and K413-L414 cleavages in FIXa are required for its conversion to fibrinolytic enhancer
Plasmin processed FIXa enhanced clot lysis and plasminogen (Pg) activation by tissue Pg-activator
References
- 1.Yoshitake S, Schach BG, Foster DC, Davie EW, Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry 1985;24: 3736–3750. [DOI] [PubMed] [Google Scholar]
- 2.Di Scipio RG, Kurachi K, Davie EW. Activation of human factor IX (Christmas factor). J Clin Invest 1978;61: 1528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj SP, Rapaport SI, Russell WA. Redetermination of the rate-limiting step in the activation of factor IX by factor XIa and by factor VIIa/tissue factor. Explanation for different electrophoretic radioactivity profiles obtained on activation of 3H- and 125I-labeled factor IX. Biochemistry 1983;22: 4047–53. [DOI] [PubMed] [Google Scholar]
- 4.Lawson JH, Mann KG. Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. J Biol Chem. 1991;266: 11317–27. [PubMed] [Google Scholar]
- 5.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry 1991;30: 10363–70. [DOI] [PubMed] [Google Scholar]
- 6.Ponting CP, Marshall JM, Cederholm-Williams SA. Plasminogen: a structural review. Blood Coagul Fibrinolysis 1992;3: 605–14. [PubMed] [Google Scholar]
- 7.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemostasis 2005;93: 647–54. [DOI] [PubMed] [Google Scholar]
- 8.Pryzdial EL, Lavigne N, Dupuis N, Kessler GE. Plasmin converts factor X from coagulation zymogen to fibrinolysis cofactor. J Biol Chem 1999; 274: 8500–5. [DOI] [PubMed] [Google Scholar]
- 9.Grundy JE, Lavigne N, Hirama T, MacKenzie CR, Pryzdial EL. Binding of plasminogen and tissue plasminogen activator to plasmin-modulated factor X and factor Xa. Biochemistry 2001;40: 6293–302. [DOI] [PubMed] [Google Scholar]
- 10.Pryzdial EL, Kessler GE. Autoproteolysis or plasmin-mediated cleavage of factor Xaalpha exposes a plasminogen binding site and inhibits coagulation. J Biol Chem 1996;271: 16614–20. [DOI] [PubMed] [Google Scholar]
- 11.Grundy JE, Hancock MA, Meixner SC, MacKenzie RC, Koschinsky ML, Pryzdial EL. Plasminogen binds to plasmin-modulated factor Xa by Ca(2+) - and C-terminal lysine-dependent and -independent interactions. Thromb Haemost 2007;97: 38–44. [PubMed] [Google Scholar]
- 12.Pryzdial EL, Kessler GE. Kinetics of blood coagulation factor Xaalpha autoproteolytic conversion to factor Xabeta. Effect on inhibition by antithrombin, prothrombinase assembly, and enzyme activity. J Biol Chem 1996;271: 16621–26. [DOI] [PubMed] [Google Scholar]
- 13.Talbot K, Meixner SC, Pryzdial EL. Enhanced fibrinolysis by proteolysed coagulation factor Xa. Biochim Biophys Acta 2010;1804: 723–30. [DOI] [PubMed] [Google Scholar]
- 14.Samis JA, Ramsey GD, Walker JB, Nesheim ME, Giles AR. Proteolytic processing of human coagulation factor IX by plasmin. Blood 2000;95: 943–51. [PubMed] [Google Scholar]
- 15.Kalafatis M, Mann KG. The role of the membrane in the inactivation of factor va by plasmin. Amino acid region 307–348 of factor V plays a critical role in factor Va cofactor function. J Biol Chem 2001;276: 18614–23. [DOI] [PubMed] [Google Scholar]
- 16.Zeibdawi AR, Pryzdial EL. Mechanism of factor Va inactivation by plasmin. Loss of A2 and A3 domains from a Ca2+-dependent complex of fragments bound to phospholipid. J Biol Chem 2001;276: 19929–36. [DOI] [PubMed] [Google Scholar]
- 17.Nogami K, Nishiya K, Saenko EL, Takeyama M, Tanaka I, Yoshioka A, Shima M. Identification of a plasmin-interactive site within the A2 domain of the factor VIII heavy chain. Biochim Biophys Acta 2008;1784:753–63. [DOI] [PubMed] [Google Scholar]
- 18.Nogami K, Nishiya K, Saenko EL, Takeyama M, Ogiwara K, Yoshioka A, Shima M. Identification of plasmin-interactive sites in the light chain of factor VIII responsible for proteolytic cleavage at Lys36. J Biol Chem 2009;284:6934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pryzdial EL, Bajzár L, Nesheim ME. Prothrombinase components can accelerate tissue plasminogen activator-catalyzed plasminogen activation. J Biol Chem 1995;270: 17871–7. [DOI] [PubMed] [Google Scholar]
- 20.Leytus SP, Foster DC, Kurachi K, Davie EW. Gene for human factor X: a blood coagulation factor whose gene organization is essentially identical with that of factor IX and protein C. Biochemistry 1986;25: 5098–102. [DOI] [PubMed] [Google Scholar]
- 21.Husten EJ, Esmon CT, Johnson AE. The active site of blood coagulation factor Xa. Its distance from the phospholipid surface and its conformational sensitivity to components of the prothrombinase complex. J Biol Chem 1987;262: 12953–61. [PubMed] [Google Scholar]
- 22.Mathur A, Zhong D, Sabharwal AK, Smith KJ, Bajaj SP. Interaction of factor IXa with factor VIIIa. Effects of protease domain Ca2+ binding site, proteolysis in the autolysis loop, phospholipid, and factor X. J Biol Chem 1997;272: 23418–26. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt AE, Stewart JE, Mathur A, Krishnaswamy S, Bajaj SP. Na+ site in blood coagulation factor IXa: effect on catalysis and factor VIIIa binding. J Mol Biol 2005;350: 78–91. [DOI] [PubMed] [Google Scholar]
- 24.Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976. 73:1447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur A, Bajaj SP. Protease and EGF1 domains of factor IXa play distinct roles in binding to factor VIIIa. Importance of helix 330 (helix 162 in chymotrypsin) of protease domain of factor IXa in its interaction with factor VIIIa. J Biol Chem 1999;274: 18477–86. [DOI] [PubMed] [Google Scholar]
- 26.Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 1984;138:141–3. [DOI] [PubMed] [Google Scholar]
- 27.Whitelegge JP, Zhang H, Aguilera R, Taylor RM, Cramer WA. Full subunit coverage liquid chromatography electrospray ionization mass spectrometry (LCMS+) of an oligomeric membrane protein: cytochrome b(6)f complex from spinach and the cyanobacterium Mastigocladus laminosus. Mol Cell Proteomics 2002;1:816–27. [DOI] [PubMed] [Google Scholar]
- 28.Halfman CJ. Concentrations of binding protein and labeled analyte that are appropriate for measuring at any analyte concentration range in radioimmunoassays. Methods Enzymol 1981;74: 481–97. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973;22: 3099–108. [DOI] [PubMed] [Google Scholar]
- 30.Craig DA. The Cheng-Prusoff relationship: something lost in the translation. Trends Pharmacol Sci 1993;14: 89–91. [DOI] [PubMed] [Google Scholar]
- 31.Sperzel M and Huetter J. Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J Thromb Haemostasis 2007;5: 2113–8. [DOI] [PubMed] [Google Scholar]
- 32.Bajaj MS, Ogueli GI, Kumar Y, Vadivel K, Lawson G, Shanker S, Schmidt AE, Bajaj SP. Engineering kunitz domain 1 (KD1) of human tissue factor pathwayinhibitor-2 to selectively inhibit fibrinolysis: properties of KD1-L17R variant. J Biol Chem 2011; 286:4329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DE, Reisfeld RA. Disc electrophoresis in polyacrylamide gels: extension to new conditions of pH and buffer. Ann N Y Acad Sci. 1964;121: 373–81. [DOI] [PubMed] [Google Scholar]
- 34.Brockway WJ, Castellino FJ. Measurement of the binding of antifibrinolytic amino acids to various plasminogens. Arch Biochem Biophys 1972;151: 194–9. [DOI] [PubMed] [Google Scholar]
- 35.Violand BN, Byrne R, Castellino FJ. The effect of alpha-,omega-amino acids on human plasminogen structure and activation. J Biol Chem 1978;253: 5395–401. [PubMed] [Google Scholar]
- 36.Zhang L, Gong Y, Grella DK, Castellino FJ, Miles LA. Endogenous plasmin converts Glu-plasminogen to Lys-plasminogen on the monocytoid cell surface. J Thromb Haemost 2003;1: 1264–70. [DOI] [PubMed] [Google Scholar]
- 37.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost 2005;93:647–54. [DOI] [PubMed] [Google Scholar]
- 38.Rydel TJ, Yin M, Padmanabhan KP, Blankenship DT, Cardin AD, Correa PE, Fenton JW 2nd, Tulinsky A. Crystallographic structure of human gamma-thrombin. J Biol Chem 1994;269: 22000–6. [PubMed] [Google Scholar]
- 39.Zögg T, Brandstetter H. Structural basis of the cofactor- and substrate-assisted activation of human coagulation factor IXa. Structure 2009;17: 1669–78. [DOI] [PubMed] [Google Scholar]
- 40.Vadivel K, Schreuder HA, Liesum A, Schmidt AE, Goldsmith G, Bajaj SP. Sodium-site in serine protease domain of human coagulation factor IXa: evidence from the crystal structure and molecular dynamics simulations study. J Thromb Haemost 2019;17:574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mertens K, Celie PH, Kolkman JA, Lenting PJ. Factor VIII-factor IX interactions: molecular sites involved in enzyme-cofactor complex assembly. Thromb Haemost 1999; 82: 209–217. [PubMed] [Google Scholar]
- 42.Bajaj SP. Region of factor IXa protease domain that interacts with factor VIIIa: analysis of select hemophilia B mutants. Thromb Haemost 1999;82:218–25. [PubMed] [Google Scholar]
- 43.Schmidt AE, Bajaj SP. Structure-function relationships in factor IX and factor IXa. Trends Cardiovasc Med 2003;13: 39–45. [DOI] [PubMed] [Google Scholar]
- 44.Lewis SD, Lorand L, Fenton JW 2nd, Shafer JA. Catalytic competence of human alpha- and gamma-thrombin in the activation of fibrinogen and factor XIII. Biochemistry 1987;26: 7597–603. [DOI] [PubMed] [Google Scholar]
- 45.Jandrot-Perrus M, Didry D, Guillin MC, Nurden AT. Cross-linking of alpha and gamma-thrombin to distinct binding sites on human platelets. Eur J Biochem 1988;174: 359–67. [DOI] [PubMed] [Google Scholar]
- 46.Lechtenberg BC, Freund SM, Huntington JA. GpIbα interacts exclusively with exosite II of thrombin. J Mol Biol 2014;426: 881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejía-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 48.CRASH-2 collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011; 377:1096–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.