Summary
Variants in RORB have been reported in eight individuals with epilepsy, with phenotypes ranging from eyelid myoclonia with absence epilepsy to developmental and epileptic encephalopathies.
We identified novel RORB variants in 11 affected individuals from four families. One from whole genome sequencing and three from RORB screening of three epilepsy cohorts: developmental and epileptic encephalopathies (n=1021), overlap of generalized and occipital epilepsy (n=84) and photosensitivity (n=123). Following interviews and review of medical records, individual’s seizure and epilepsy syndromes were classified.
Three novel missense variants and one exon 3 deletion were predicted to be pathogenic by in silico tools, not found in population databases and located in key evolutionary conserved domains.
Median age of seizure onset was 3.5 years (0.5 to 10 years). Generalized, predominantly absence and myoclonic, and occipital seizures were seen in all families, often within the same individual (6/11). All individuals with epilepsy were photosensitive and 7/11 had cognitive abnormalities. EEGs showed generalized spike and wave and or polyspike and wave.
Here we show a striking RORB phenotype of overlap of photosensitive generalized and occipital epilepsy in both individuals and families. This is the first report of a gene associated with this overlap of epilepsy syndromes.
Keywords: Retinoid-Related Orphan Receptor β, GGE, IPOE, Photosensitivity, Intellectual disability
Introduction
Copy number variants have provided critical insight into identification of genes causing human disease. One example in the epilepsies is the identification of RORB, encoding the retinoid-related orphan receptor β, which lies within the 9q21.13 microdeletion1. There is only a single study reporting patients with pathogenic variants in RORB2. The epilepsy phenotypes in this study varied from developmental and epileptic encephalopathies (DEEs) to genetic generalized epilepsies, predominantly eyelid myoclonia with absence epilepsy associated with mild intellectual disability (ID)2.
RORB is present in immature neurons and is hypothesized to have a role in neuronal cell differentiation3. RORB has two differentially expressed isoforms, RORβ1 and RORβ2, which differ only in their short N-terminal domains and are identical in the DNA-binding domain. RORβ2 is expressed predominantly in the retina and pineal gland while RORβ1 is also expressed in cortex, spinal cord, and the pituitary3. Despite relatively little knowledge about human disorders associated with RORB mutations, there has been considerable interest in RORB in murine models. Targeted knock out mouse models of both isoforms produce a neurodevelopmental phenotype with visual and gait abnormalities, but seizures have not been observed4, 5.
In this study, we identified novel heterozygous RORB variants in eleven individuals from four families and describe the epilepsy phenotypes. Familial epilepsy with RORB variants is characterized by an overlap of photosensitive generalized and focal occipital epilepsy syndromes.
Methods
Cohort
Family A, with eight affected individuals, was referred for epilepsy genetic research and whole genome sequencing (WGS) was performed. Screening for RORB variants was performed in 1021 patients with DEE (family B and C identified), 84 individuals with an overlap of photosensitive GGE and occipital epilepsy either in the patient or their family (family D identified), and 123 individuals with photosensitive epilepsy (no variants identified).
Phenotyping
All available family members were interviewed using a standardized epilepsy questionnaire and seizure videos were reviewed6. Medical records, EEGs and neuroimaging were obtained. Seizures and epilepsy syndromes were diagnosed according to the 2017 International League Against Epilepsy classification7.
Molecular
Family A: WGS was performed on three individuals using an Illumina HiSeq X platform with a mean coverage of 30X. Reads were mapped against the human genome reference hg19. We selected coding variants with a frequency <0.0001 in population databases (1000 Genomes phase 3, and NHLBI GO Exome Sequencing Project [ESP], ExAC and gnomAD) and with an impact severity predicted by SnpEff of ‘HIGH’ or ‘MED’.
Family B: 34 genes including RORB were sequenced using molecular inversion probes targeting the coding exons and the intron-exon boundaries as previously described (>5bp)8.
Family C: An intragenic deletion in RORB was identified in family C by targeted oligonucleotide array comparative genomic hybridization (628 probes in RORB, average probe spacing 72 bp) to identify intragenic deletions and duplications.
Family D: Patients and families with overlap between photosensitive GGE and occipital lobe epilepsy were screened for RORB variants using Sanger sequencing with exon-specific primers (oligonucleotides available on request).
All variants were validated and segregation was performed with Sanger sequencing.
Ethics
Written informed consent was obtained from all patients and, in the case of minors or those with intellectual disability, their parents or legal guardians. The study was approved by the local ethics committees.
Results
Phenotypes
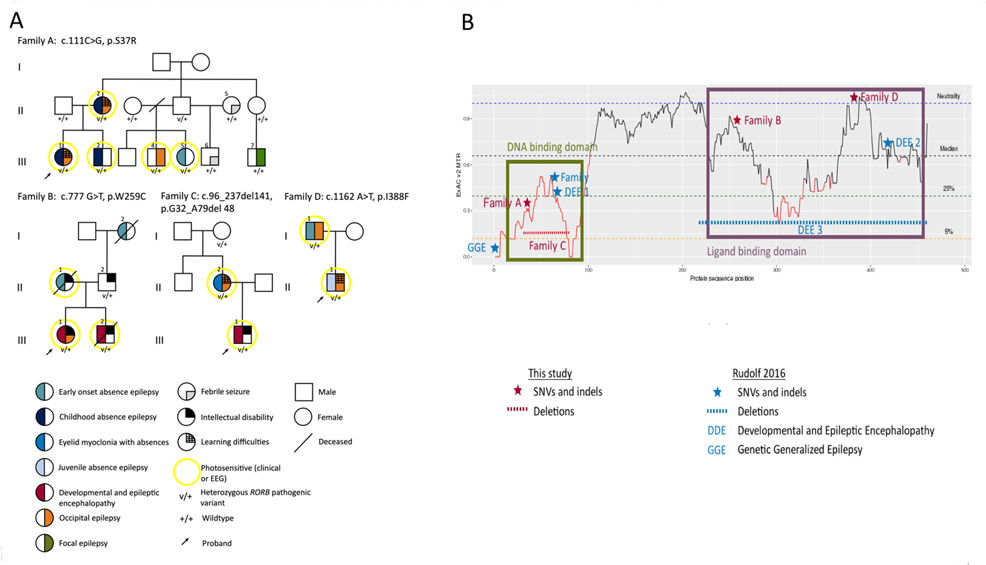
The four families included 14 individuals with RORB variants, of whom 11 had epilepsy, one had ID and two were unaffected (Figure 1A, Table 1). Clinical histories for all affected individuals are in the supplementary material.
Figure 1.
A, Pedigrees of the four families. B, Missense tolerance ratio (MTR) in the RORβ1 protein showing the variants found in the four families and previously reported individuals. DDE, developmental and epileptic encephalopathy; EEG, electroencephalographic; GGE, genetic generalized epilepsy; SNV, single nucleotide variant
TABLE 1.
Genetic variants and clinical features of study individuals
| A | B | C | D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case (gender/age) | II – 2 (F/46y) | III – 1* (F/17y) | III – 2* (M/15y) | III-4 (M/18y) | III – 5 (F/14y) | III – 1* (F/18y) | III-2* (M/#11y) | II-2 (F/40y) | III-1* (M/11y) | I-1 (M/48y) | II-1 (M/20y) | |
| RORB Variant | c.111C>G/p.Ser37Arg | c.777G>T/p.Trp259Cys | c.96_237del141/p.Gly32_Ala79del48 | c.1162A>T/p.Ile388Phe | ||||||||
| Pathogenic predictions | Sift – 0; Polyphen − 0.989; CADD – 25; GERP – 3.98 | Sift – 0; Polyphen − 1; CADD – 34; GERP – 5.5999 | Sift – 0.002; Polyphen − 0.943; CADD – 24.7; GERP – 5.73 | |||||||||
|
Sz onset age [offset] |
5y: A | 4y: A [7y] | 6y: A | 5y: Occipital Seizures [14y] | 2y: A [10 y] | 9m: MA | 9m: MA | 3y: Absence with eyelid myoclonia | 6m: M | 12m: A [13y] | 10y: Occipital Seizures | |
| Other Sz onset age | 10y: GTCS [31y]; 10y: Occipital Seizures |
5y: Occipital Seizures [7y] | 14y: GTCS | Nil | Nil | 12m: FS [3.5y]; 9 y: GTCS; 22m: M; 15y: Occipital Seizures |
16m: GTCS [20m]; 22m: M [11y] |
19y: GTCS; 35y: Occipital Seizures |
3y: A | 18m: FS [18m]; 18 y: GTCS [23y]; 44y: Occipital Seizures [44y] |
13y: EM [14y]; 13y: Absence status [13y]; 13y: GTCS [13y] |
|
| Syndrome | CAE + IPOE | CAE + IPOE | CAE | IPOE | EOAE | DEE – Epilepsy with myoclonic absences + Occipital Lobe Epilepsy | DEE − Epilepsy with myoclonic absences | EMAE + Occipital Lobe Epilepsy | DEE | EOAE + Occipital Lobe Epilepsy | JAE + IPOE | |
| Development (age DD noted) | LD | LD | N | N | N | DD (9m), Mild ID, 12y − G-tube | DD (4m) Severe ID | DD (18m), LD | DD (birth) Severe ID | N | Learning difficulties | |
| Medication trials | VPA, LTG, CBZ, TPM | VPA | VPA, ETX, LEV | VPA | Nil | CBZ, VPA, LTG, CLB, PB, ETX, LEV | CBZ, VPA, ETX, CLB, LTG, CZP | VPA, LTG | VPA, CLB, ETX, TPM, LEV, LTG | VPA, TPR, LTG | VPA, LEV | |
| Examination | N | N | N | N | N | All growth parameter < 3rd % | N | N | Mild hypertonia of limbs | N | N | |
| EEG | 15y: GSW, PSW, PPR; 40y: GSW | 4y: GSW, PSW, PPR (sleep) | 6y: GSW, PPR, OIRDA; 14y: GSW, PPR | 8y & 9y: PPR, GSW, PSW, CPS | 8y: PPR, CPS | 1y & 5y: GSW, PPR | 1y: GSW, PPR, 18m: GSW | 8y: GSW, PPR, 19y: PPR | 8m: GSW, PSW, PPR 15m: GSW | 44 y: BD | 13y: GSW, PPR 14y: Normal | |
| MRI | N | Not done | N | N | Not done | Not done | N | Not done | N | N | N | |
F: Female, M: Male, y: Years old, #:age at death
AED exposure in utero, A: Absence seizure, MA: Myoclonic absence seizures, M: Myoclonic seizures, GTCS: Generalised tonic clonic seizures, FS: Febrile seizure, CAE: childhood absence epilepsy, IPOE: Idiopathic photosensitive occipital lobe epilepsy, EOAE: Early onset absence epilepsy, DEE: Developmental and epileptic encephalopathy, EMAE: Eyelid-myoclonia with absence epilepsy, JAE: Juvenile absence epilepsy, LD: Learning difficulties, DD: Developmental delay, ID: Intellectual disability, G-tube: Gastrostomy tube, N: Normal, VPA: Sodium valproate, LTG: Lamotrigine, CBZ: Carbamazepine, TPM: Topiramate, ETX: Ethosuximide, LEV: Levetiracetam, PHT: Phenytoin, CLB: Clobazam, PB: Phenobarbital, CZP: Clonazepam, GSW: Generalised spike and wave, PSW: Polyspike and wave, PPR: Photo-paroxysmal response, OIRDA: Occipital intermittent rhythmic delta activity, CPS: Central parietal spikes, BD: Bitemporal delta
Mean age of seizure onset was 3.5 years (range 6 months to 10 years). Age of onset varied according to the initial seizure type: 3 had seizures with myoclonic semiology at 6–9 months (myoclonic absence in 2, myoclonic in 1), 6 had absence seizures at 1–6 years and 2 had occipital seizures at 5 and 10 years.
Each family had some members with focal seizures and other members with generalized seizures, while 6 individuals had both seizure types. Generalized seizure types included absence (6/11), absence with eyelid myoclonia (2/11), myoclonic absence (2/11), infrequent GTCS (7/11) and myoclonic seizures (3/11). 7/11 individuals with RORB variants had occipital seizures; no other types of focal seizures occurred. Their seizures comprised of a visual hallucination of colored phenomena, such as colored vision, blotches, or formed visual images, sometimes followed by fear, eye deviation, loss of vision, nausea and loss of awareness. Seizures evolved to bilateral tonic-clonic seizures in only one individual. All seven were photosensitive.
Cognitive difficulties occurred in 7/11. These ranged from individuals who struggled at school and left early due to learning difficulties (4/11) to individuals with developmental delay in infancy resulting in mild (1/11) or severe (2/11) ID. The three individuals with ID had seizure onset between 6 and 9 months and their mothers were on antiepileptic drugs (AED) during their pregnancy.
Nine patients showed generalized spike-wave (GSW) and/or polyspike-wave. Two individuals without GSW either did not have early EEGs or results of early EEGs were not available. Individual A-III-5 did not have an EEG until 8 years despite having well documented absence seizures from 2 years. A photoparoxysmal response on intermittent photic stimulation occurred in 10 individuals.
An overlap of generalized and occipital epilepsy syndromes occurred in all families. Absence epilepsy syndromes were the most common including childhood absence epilepsy (CAE) (3), juvenile absence epilepsy (JAE) (1), early onset absence epilepsy (EOAE) (2), epilepsy with myoclonic absence seizures (2) and eyelid myoclonia with absence epilepsy (EMAE). Idiopathic photosensitive occipital epilepsy (IPOE) was seen in four individuals. Occipital lobe epilepsy (OLE) occurred in three individuals without clear photosensitive induction of seizures, however, all had been photosensitive when they had absence seizures at a younger age (Table 1). Three individuals had a developmental and epileptic encephalopathy (DEE). Six individuals had both generalized and occipital epilepsies in a variety of combinations: CAE and IPOE (2), JAE and IPOE (1), EOAE and OLE (1), DEE and OLE (1) and EMAE & OLE (1).
In family B, the two children with DEE inherited their RORB variant from their father who had mild ID. He did not have a history of seizures but early history was not available. Information on his mother (B-I-2), who died prior to the study, was limited but she had learning difficulties and severe epilepsy with GTCS, absence seizures and episodes of nonconvulsive status epilepticus leaving her unable to care for her children. Her epilepsy worsened on carbamazepine and improved with sodium valproate.
There were two unaffected individuals that carried the familial RORB variant (A-II-4 and CI-2). There was one individual (A-III-7) with a different phenotype with childhood focal impaired awareness seizures who did not carry the familial RORB variant (supplementary material).
Molecular (Table 1, Figure 1B, Supplemental Table)
Novel missense RORB variants were identified in families A, B and D and a deletion was found in family C. All missense variants were predicted to be damaging by in-silico prediction methods, evolutionarily conserved, and not reported in ExAC, gnomAD, ESP or dbSNP. The p.Ser37Arg variant in family A affects the DNA-binding domain, an area with a low missense tolerance ratio (Figure 1B). It is predicted to be destabilizing as arginine is longer than serine with many possible rotamers that may interact with neighboring amino acids3. Variants in family B (p.Trp259Cys) and D (p.Ile388Phe) are likely involved in the regulation of RORβ activity and in recruitment of coactivators3. The deletion in family C included all of exon 3 encoding the DNA binding domain; deletion of the 141-bp exon would be predicted to cause a frameshift and premature truncation of the protein, though additional studies would be required to confirm the effect on transcription. No other plausible variants were found.
Discussion
Here, we present eleven individuals from four families with inherited RORB variants showing an overlap of photosensitive GGE and occipital epilepsy. These phenotypes occurred both as separate syndromes in different family members and also together within single individuals. We delineate the co-occurrence of occipital epilepsy in RORB epilepsies, with the known association of GGE and DEE published in the only study of RORB epilepsies to date. The previous study of a family and four sporadic individuals with RORB epilepsies reported a broad phenotypic spectrum encompassing GGE, predominantly eyelid myoclonia with absence epilepsy, and DEE. Individuals in the study had a cognitive profile ranging from learning disabilities to severe ID2. The phenotypes in these previously reported families are similar to what we have identified however they did not report any occipital epilepsies. Interestingly, eyelid myoclonia with absence epilepsy is a syndrome for which the occipital cortex is assumed to play a key role and may initiate the generalized epilepsy network.
Families with both generalized and occipital epilepsies occurring in different first- and second-degree members have been previously reported9–11. The finding of both generalized and focal epilepsy syndromes within a single individual is rare in common epilepsies, being reported in only 3.5% (39/1120) of individuals from 303 families9. It is considerably more frequent in families with photosensitive epilepsies; we found that 26% (21/82) of individuals with photosensitivity from 29 families had both generalized and focal epilepsy10.
RORB is expressed in layer IV cortical neurons and thalamic nuclei3. Both are integral to the thalamocortical network which underlies the generation of generalized spike-wave and generalized seizures. RORB is also expressed in the retina and necessary for the proliferation and differentiation of retinal cells3, 12. Although our patients did not show any visual abnormalities, all had clinical and/or electrical photosensitivity. A combination of thalamocortical network and visual network abnormalities may be critical to bring together this fascinating overlap of photosensitive GGE and occipital epilepsy.
Monogenic diseases often show a phenotypic spectrum within families. In RORB families, phenotypes ranged from occipital epilepsy (1), to GGE (4), a combination (6) or mild ID without seizures. Similar variability is seen in an epilepsy-movement disorder syndrome, Infantile Convulsions with Choreoathetosis Syndrome due to PRRT2 pathogenic variants, where family members can have paroxysmal kinesigenic dyskinesia, infantile epilepsy, both or can even be unaffected13.
The seizure type of absence with eyelid myoclonia is associated with photosensitivity and can occur as the key feature of the epilepsy syndrome EMAE or be a part of a DEE. Genetic causes of the DEEs associated with absence with eyelid myoclonia include CHD2, SYNGAP1 and NEXMIF (formally known as KIAA2022)14, 15. There has been little molecular success in identifying the genes causing an overlap between GGE and occipital epilepsy, with the GGE often considered to follow complex, or polygenic, inheritance9, 10. Conversely the overlap between generalized and occipital seizures in the DEE has been associated with a number of genes, such as CLN6 and the CAG trinucleotide repeat in Huntington’s disease 16–18. Here, we expand the phenotype associated with RORB variants making it the first gene to be associated with the overlap of photosensitive GGE and IPOE.
Supplementary Material
Acknowledgements
We thank the children and their families for participating in our research. We gratefully acknowledge support from the Health Research Council of New Zealand, Cure Kids New Zealand, the Ted and Mollie Carr Endowment Trust, the NIH (National Institute of Neurological Disorders and Stroke) and the National Health and Medical Research Council (NHMRC) of Australia. This work was supported by the Victorian Government’s Operational Infrastructure Support Program and the NHMRC Independent Research Institute Infrastructure Support Scheme (IRIISS) and the National Institutes of Health (NIH).
Footnotes
Disclosure of Conflicts of Interest
Prof Sadleir is funded by the Health Research Council of New Zealand and Cure Kids New Zealand. She is a consultant for the Epilepsy Consortium and has received travel grants from Seqirus and Nutricia. She has received research grants from Zynerba.
Prof Mefford is funded by the NIH (National Institute of Neurological Disorders and Stroke).
Prof Scheffer serves on the editorial boards of Neurology and Epileptic Disorders, may accrue future revenue on a pending patent re: Therapeutic compound; has received speaker honoraria from Athena Diagnostics, UCB, GlaxoSmithKline, Eisai, and Transgenomics; has received scientific advisory board honoraria from Nutricia, UCB, and GlaxoSmithKline, has received funding for travel from Athena Diagnostics, UCB, and GlaxoSmithKline; and receives/has received research support from the National Health and Medical Research Council, Australian Research Council, National Institutes of Health, Health Research Council of New Zealand, March of Dimes, the Weizmann Institute, Citizens United for Research in Epilepsy, US Department of Defense, and the Perpetual Charitable Trustees.
The remaining authors have no conflicts of interest.
Ethical Publication Statement:
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Boudry-Labis E, Demeer B, Le Caignec C, et al. A novel microdeletion syndrome at 9q21.13 characterised by mental retardation, speech delay, epilepsy and characteristic facial features. Eur J Med Genet. 2013; 56: 163–70. [DOI] [PubMed] [Google Scholar]
- 2.Rudolf G, Lesca G, Mehrjouy MM, et al. Loss of function of the retinoid-related nuclear receptor (RORB) gene and epilepsy. Eur J Hum Genet. 2016; 24: 1761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H, Aramaki M, Fu Y, et al. Retinoid-Related Orphan Receptor beta and Transcriptional Control of Neuronal Differentiation. Curr Top Dev Biol. 2017; 125: 227–55. [DOI] [PubMed] [Google Scholar]
- 4.André E, Conquet F, Steinmayr M, et al. Disruption of retinoid-related orphan receptor β changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998; 17: 3867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia L, Oh EC, Ng L, et al. Retinoid-related orphan nuclear receptor RORβ is an early-acting factor in rod photoreceptor development. Proc Natl Acad Sci USA. 2009; 106: 17534–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reutens TC, Howell RA, Gebert KE, et al. Validation of a questionnaire for clinical seizure diagnosis. Epilepsia. 1992; 33: 1065–71. [DOI] [PubMed] [Google Scholar]
- 7.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017; 58: 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiatt JB, Pritchard CC, Salipante SJ, et al. Single molecule molecular inversion probes for targeted, highaccuracy detection of low-frequency variation. Genome Res. 2013; 23: 843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epi4k consortium. Phenotypic analysis of 303 multiplex families with common epilepsies. Brain. 2017; 140: 2144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor I, Berkovic SF, Scheffer IE. Genetics of epilepsy syndromes in families with photosensitivity. Neurology. 2013; 80: 1322–9. [DOI] [PubMed] [Google Scholar]
- 11.Taylor I, Marini C, Johnson MR, et al. Juvenile myoclonic epilepsy and idiopathic photosensitive occipital lobe epilepsy: is there overlap? Brain. 2004; 127: 1878–86. [DOI] [PubMed] [Google Scholar]
- 12.Feng S, Xu S, Wen Z, et al. Retinoic acid-related orphan receptor RORbeta, circadian rhythm abnormalities and tumorigenesis (Review). Int J Mol Med. 2015. 35: 1493–500. [DOI] [PubMed] [Google Scholar]
- 13.Heron SE, Grinton BE, Kivity S, et al. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012; 90: 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvill GL, Heavin SB, Yendle SC, et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet, 2013. 45(7): p. 825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lange IM, Helbig KL, Weckhuysen S, et al. De novo mutations of KIAA2022 in females cause intellectual disability and intractable epilepsy. J Med Genet. 2016; 53: 850–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canafoglia L, Gilioli I, Invernizzi F, et al. Electroclinical spectrum of the neuronal ceroid lipofuscinoses associated with CLN6 mutations. Neurology. 2015; 85: 316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew SE, Goldberg YP, Kremer B, et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat Genet. 1993; 4: 398–403. [DOI] [PubMed] [Google Scholar]
- 18.Cloud LJ, Rosenblatt A, Margolis RL et al. Seizures in juvenile Huntington’s disease: frequency and characterization in a multicenter cohort. Mov Disord. 2012; 27: 1797–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.