Abstract
Background:
Necrotizing enterocolitis (NEC), a necrotic inflammation of the intestine, represents a major health problem in the very premature infant. Although prevention is difficult, the combination of ingestion of maternal-expressed breastmilk in conjunction with a probiotic provides the best protection. In this study, we establish a mechanism for breastmilk/probiotic protection.
Methods:
Ultra-high-performance liquid chromatography-tandem mass spectrometry of B. infantis secretions was used to identify an anti-inflammatory molecule. Indole-3-lactic acid (ILA) was then tested in an established human immature small intestinal cell line, necrotizing colitis enterocytes and other immature human enteroids for anti-inflammatory effects and to establish developmental function. ILA was also examined in immature and mature enterocytes.
Results:
We have identified indole-3-lactic acid, a metabolite of breastmilk tryptophan, as the anti-inflammatory molecule. This molecule is developmentally functional in immature but not mature intestinal enterocytes, ILA reduces the IL-8 response after IL-1β stimulus. It interacts with the transcription factor aryl hydrocarbon receptor (AHR) and prevents transcription of the inflammatory cytokine IL-8.
Conclusions:
This molecule produced by B. infantis (ATCC No. 15697) interaction with ingested breastmilk functions in a complementary manner and could become useful in the treatment of all at risk premature infants for necrotizing enterocolitis if safety and clinical studies are performed.
INTRODUCTION
This laboratory seeks to define the pathogenesis of necrotizing enterocolitis (NEC) (1). This condition occurs most commonly at the post-menstrual age of 28–31 weeks and results in an inflammatory necrosis of principally of the distal small intestine and the colon, leading to considerable morbidity, mortality and medical expense (2). We hypothesize that the condition is in part caused by an aberrant reaction of the immature intestine to colonizing bacteria (3–5). The immature intestine is not prepared to interact with the enormous numbers of colonizing bacteria encountered after birth when the newborn enters the extrauterine environment (6,7). We have reported that immature human intestinal cells favor inflammation over immune homeostasis with microbial-epithelial interaction (8). Inflammation can be evoked by commensal as well as pathogenic organisms (3) in part because of a developmentally regulated innate immune response and an increased surface expression of TLR-4 receptors (9) as well as overexpressed signaling molecules (NFKβ and IL-8) and under expressed regulatory molecules (SIGIRR, IRAK-M, A-20, etc.) (4).
However, paradoxically it has been shown that feeding mother’s-expressed breastmilk or probiotics to the premature can either prevent or reduce the severity of NEC (10, 11). Although expressed breastmilk contains many passively protective factors and microorganisms (12), we have reported that breastmilk ingestion can stimulate the proliferation of a so-called “pioneer” bacteria over formula-fed infants that are uniquely suited for activation of protective immune responses and anti-inflammation (13, 14). We have studied the role of these “pioneer” bacteria in an ex-vivo model of human premature intestine (15) and in vivo in premature mouse models (9). A common “pioneer” bacterium associated with ingested breastmilk is Bifidobacterium longum subsp infantis (B. infantis; ATCC No. 15697) (14). Feeding expressed breastmilk with probiotics is clinically the best way to prevent NEC (16). We have shown that this bacterium and its secretions can protect against an IL-1β (a common inflammatory cytokine in NEC) -induced inflammation (17–19). We have also shown that B. infantis secretory fractions separated from the organism are anti-inflammatory and require a TLR-4 receptor to be effective (15, 18).
In this study, we extend this observation to identify the anti-inflammatory molecule in secretions as indole-3-lactic acid (ILA), a breakdown molecule of breastmilk tryptophan metabolized by B. infantis. In addition, this anti-inflammatory molecule is uniquely functioning in the immature intestine. We provide evidence for the isolation and identification of this anti-inflammatory molecule and begin to determine its anti-inflammatory mechanism in the immature human and mouse small intestine.
METHODS
Cell cultures
H4 cells, a human immature non-transformed primary small intestinal epithelial cell line characterized by our laboratory were cultured as previously described (20). NEC-IEC were isolated and cultured from the viable margins of resected ileal NEC tissues from a NEC neonate at 25-wk gestation and were cultured as previously described (9). The use of two human cell lines had the permission of Partners IRB #2011P003833 at Massachusetts General Hospital. Caco-2 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured as previously described (21).
Organoid cultures
Human sample procedures were approved by institutional review board protocols IRB 1999P003833 (Brigham and Women’s Hospital, Boston, MA) and IRB 2016P000949 (Massachusetts General Hospital, Boston, MA) for the derivation of immature enterospheres (FEnS). The human immature intestinal organoids were derived as described in our previous publication (22). In this experiment the FEnS we used was from gestational age 15 and 22 weeks therapeutically aborted fetuses as described (22). When the culture reached confluence, it was apically treated with 5 mmol/L N-[(3,5-Difluorophenyl) acetyl]-L-alanyl-2-phenyl] glycine-1, 1-dimethylethyl ester (DAPT) in DMEM/F12 for 48 hours (h) as previously described to promote cell differentiation. The monolayers were treated with 20 μM of ILA for 24 hours before being stimulated with human IL-1β (1ng/ml for 24 h) basolaterally. The supernatants from the basolateral side were collected for an IL-8 protein assay by an enzyme-linked immune sorbent assay (ELISA).
Bacterial cultures and B. infantis secretory fragment separation
Bifidobacterium longum subsp infantis (B. infantis), obtained from ATCC (Manassas, VA) (ATCC No. 15697), were cultured anaerobically in a media modified from the combination of Mann-Rogosa-Sharpe (MRS) broth (DIFCO; BD Bio- science, Franklin Lakes, NJ) and H4 cell culture media (Supplemental TABLE S1) (8,18). B. infantis conditioned media at the stationary growth phase was prepared by centrifugation of probiotic cultures at 3700 rpm (equal 2936g) (Sorvall legend RT+ centrifuge, ThermoFisher Scientific, MA) for 10 min at 4°C and then by use of 0.22-μm filtration to eliminate residual bacteria. The tested filtrate was used for B. infantis secretory fractions (SFs) separation by high speed centrifugation (3220g, 30 min at 4°C) (Eppendorf centrifuge 5810R, Eppendorf North America, NY) with different sizes of the Amicon Ultra centrifugal filters (MilliporeSigma, MA). The secretory fractions (<3KD, 3–10KD and >10KD) were used to test anti-inflammatory effects in H4 cells before subjecting them to the characteristic identification.
Hydrocortisone treatment for H4 cells
H4 cells cultured in 12-well tissue culture plates to 40% confluence were treated with or without hydrocortisone (HC) (Sigma, St. Louis, MO) at 1μM for five days (23). Hydrocortisone, a trophic factor, has been used to convert immature cells to mature cells (28). The cells were collected for total RNA isolation and the aryl hydrocarbon receptor (AHR) mRNA was determined by real time quantitative reverse transcription PCR (qRT-PCR) as described later.
Identification of anti- inflammatory effective molecules in secretory size fractions.
Chemicals:
Liquid chromatography–mass spectrometry (LC-MS) grade water, acetonitrile and acetic acid were purchased from VWR (Milan, Italy).
Instrumentation and ultra-high-performance liquid chromatography-tandem mass spectrometry
Samples were filtered on a 0.45 μm nylon filter (Phenomenex®, Bologna, Italy) and directly injected into an ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Analyses were performed on a Nexera UHPLC system coupled to a hybrid Ion trap-Time of flight (IT-TOF) mass spectrometer (Shimadzu, Kyoto, Japan). For the separation of <3KD, 3–10KD and >10KD B. infantis fractions a Kinetex™ EVO C18 column with geometry 150 mm × 2.1 mm, 2.6 μm (Phenomenex®) was used, at a flow rate was 0.5 mL/min, with the following gradient: 0–18 min, 1–30%B, 18–19 min, 30–98%B, hold for 1.50 min, returning to 1%B in 0.1 min. Column oven was set to 45° C, photodiode array detection (PDA) was set to 254 and 280 nm. MS detection was performed in both positive and negative electrospray ionization (ESI) mode, interface temperature was set to 250°C, curve desolvation line: 250°C, nebulizer and drying gas 1.5 and 10L/min. MS1 range 100–1500, MS/MS: data dependent acquisition (DDA), precursors were searched in the range 100–1500 with execution trigger 105, collision energy 35%, dynamic exclusion 30s. The molecular formulas were obtained through Formula Predictor (Shimadzu). MS file was converted in mzXML format and analyzed by MZmine2. Data were deconvoluted, deisotoped and aligned. Spectral annotation was performed against HMDB and MassBank of North America (MoNA) database, with max mass accuracy tolerance of 10 ppm. Furthermore, Ultra high resolution MS analyses were performed on a Bruker SolariX XR FT-ICR 7T (Bruker Daltonics, Bremen, Germany). The sample was infused by a Hamilon syringe at 2 μL/min. The instrument was tuned with a standard solution of sodium trifluoroacetate. Mass Spectra were recorded in broadband mode in the range 100–1500 m/z, with an ion accumulation of 20 ms, with 32 scans using 4 million data points (4 M). Nebulizing (N2) and drying gases (air) were set at 1 and 4 mL/min, respectively, with a drying temperature of 200 °C. Both positive and negative ESI were employed. Identification of compounds based on accurate MS measurements was performed by Compound Crawler version 3.0 (Bruker Daltonics). For quantitative analysis, an external calibration method was employed, a calibration curve with five levels were built, triplicate analysis of each point was run. The UHPLC-MS/MS conditions were the same as described above. Extracted ion chromatogram (EIC) of ILA MS/MS transition 204–158 were employed.
Determination of the anti-inflammatory effects of B. infantis SFs and indole-3-lactic acid on H4, hydrocortisone-treated H4 (H4-HC), NEC-IEC and Caco2 cells.
H4 cells were pretreated with or without B. infantis SFs at the concentration of 10% or indole-3-lactic acid (ILA) (1 μM, 5 μM and 20 μM) for 24 h before IL-1β stimulation (1ng/ml) for 24 h or treated with B. infantis SFs or different doses of ILA alone. The IL8 secretion into the cell culture supernatants was determined by ELISA. The effects of ILA on IL-1β-induced IL8 secretion was also determined in H4-HC, NEC-IEC and Caco2 cells with the dose range (1μM and 5 μM of ILA).
Determination of the anti-inflammatory effects of ILA on immature and adult mouse intestinal organ culture
C57BL/6J and TLR-4 knockout mice (Jackson Laboratory) were bred and housed in a specific pathogen free facility. Animals were given water and standard laboratory chow ad libitum. Timed pregnant mice (embryonic day 18.5) were established as previous described (15). Immature mice from two mouse strains and C57BL/6J (8-week old male) adult mice small intestinal tissues were collected and cut into 3-mm pieces and maintained in organ culture media as described previously (15). After 1–2 h at 37°C, tissues were pretreated with and without ILA (1μM or 5 μM) for 24 h and then stimulated with 1ng/ml of recombinant mouse IL-1β (R&D Systems, Minneapolis, MN) for 24 h. Supernatants were collected and stored at −20°C for ELISA analysis. Animal procedures had been previously approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and Use committee (2018N000070).
Treatment with the aryl hydrocarbon receptor (AHR) inhibitor -CH223191 in H4 cells.
H4 cells were pretreated with or without various doses of AHR inhibitor- CH223191(Sigma, St. Louis, MO) for 30 minutes (24) and then treated as described with ILA (1μM) for 24 h before exposure to IL-1β (1ng/ml) for 24 h. The inhibitor is unique for ILA and is 80% effective. IL8 secretion into the cell culture supernatants was determined by ELISA.
Real-time quantitative reverse transcription PCR
The total RNA of the cells was isolated by Trizol combination with Neasy RNA isolation kit (ThermoFish Scientific, Grand Island, NY). RNA was reverse transcribed with random hexamers using an Advantage RT-for -PCR kit (Clontech, Mountain View, CA). The cDNA was amplified using iQ SYBR Green Supermix (Bio-Rad, Philadelphia, PA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers were amplified in all samples to represent the housekeeper gene. The change in the normalized transcript level was expressed relative to the control sample with a change of n in CT representing a 2(−n). Primer sequences used in this study were as follows:
Human GAPDH, forward, 5’- ATGGGGAAGGTGAAGGTCG-3’, and reverse, 5’- GGGGTCATTGATGGCAACAATA-3’
Human AHR, forward, 5’- CTTAGGCTCAGCGTCAGTTAC-3’, and reverse, 5’- CGTTTCTTTCAGTAGGGGAGGAT- 3’
LDH Cytotoxicity Assay
For each experiment with B. infantis size fragments (SF) or ILA, lactate dehydrogenase (LDH) cytotoxic assay was performed by using a LDH Assay Kit (Roche Applied Science, Branford, CT).
IL-8 ELISA
Secreted human IL-8 and mouse IL-8 homologue protein - Macrophage-inflammatory protein 2 (MIP-2) were measured by ELISA by using human IL-8 and mouse MIP2 detection kits (R&D Systems, Minneapolis, MN).
Statistical Analysis
All data are presented as the mean ±standard error of the mean (SEM). The unpaired Student’s t-test was used to compare the mean of two groups. One-way ANOVA and Turkey post-hoc test were used to compare the mean of multiple groups. Differences of p < 0.05 were considered significant (*p < 0.05, **p < 0.01, †p < 0.001) (GraphPad Prism 6).
RESULTS
Secretory fractions of Bifidobacterium longum subsp infantis are anti-inflammatory with H4 cells.
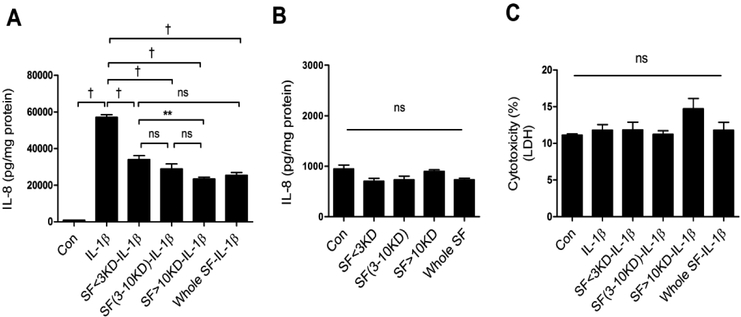
When bacterial secretions from Bifidobacterium longum subsp infantis (ATCC No. 15697) were specifically separated into different sized fractions (<3KD, 3–10KD, >10KD) and exposed to H4 cells before adding the inflammatory stimulus IL-1β (FIGURE 1), a highly significant reduction in IL-8 secretion was seen with all fractions (A). When the fractions were exposed to H4 cells in the absence of IL-1β they had no effect on inflammation(B) and the bacterial secretion fractions were not cytotoxic (C).
Fig 1. The effects of different secretory size fractions of B. infantis on anti-inflammation and cytotoxicity in H4 cells.
H4 cells were pretreated with or without B. infantis secretory size fractions (SFs) before IL-1β stimulation (A) or treated with SFs alone (B). The secretion of IL8 (A-B) and lactate dehydrogenase (LDH) (C) into the cell culture supernatant was determined by ELISA and cytotoxicity assay – LDH assay. Graphs represent means ± SEM (n=3) from three independent experiments. One -way ANOVA and Turkey post hoc tests were used for statistical analysis (**P<0.01, †P<0.001).
Identification of the B. infantis anti-inflammatory secretory molecule as indole-3-lactic acid.
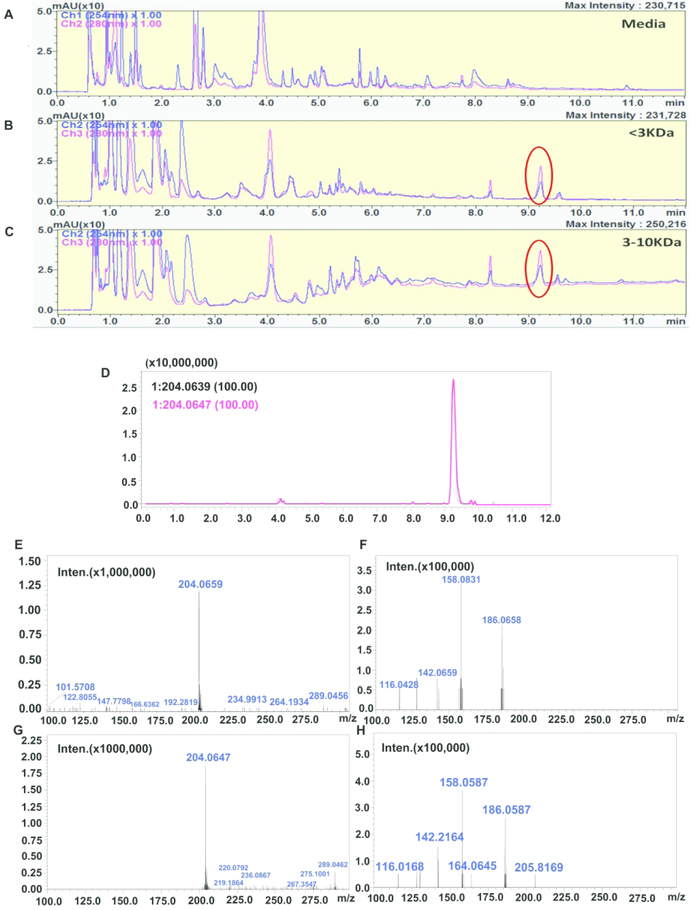
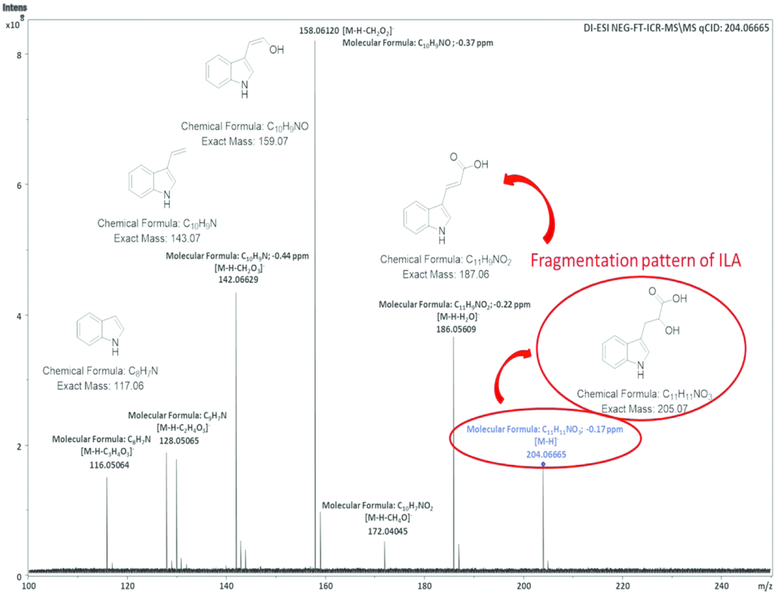
To identify metabolites in the different molecular weight fractions of B. infantis secretions, we employed Ultra high-performance liquid chromatography (UHPLC) coupled to high resolution Mass Spectrometry (HRMS) in a typical untargeted workflow (25, 26). Reversed phase (RP) chromatography ensured a good separation of both polar and non-polar analytes. The metabolite profile of secretions revealed the presence of multiple analytes such as amino acids, sugars, dipeptides, nucleosides, glycolipids and fatty acids. By comparison of UV-MS traces relative to the separation of cell media alone and <3, 3–10KD and >10KD (data not shown) B. infantis size fractions, an intense peak possessing absorbance at 280 nm was revealed, notably, this peak was absent in the media (FIGURE 2, panels A–C). The high-resolution MS spectrum showed an ion with m/z 204.0659 [M-H]− in negative ESI, with a molecular formula of C11H11NO3. This was identified as indole-3-lactic acid (ILA). The MS/MS fragmentation pattern and retention time (FIGURE 2, panels D–H) were consistent with those reported in on-line spectral libraries and with its standard compound. For further confirmation, the peak was collected in a series of consecutive liquid chromatography (LC) runs and analyzed by infusion through ultra-high-resolution Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR-MS), the detailed fragmentation pattern is shown in FIGURE 3. The amount of ILA in the different sized fractions was comprised between 22.17 to 33.12 μg/mL. The differences in ILA content among the fractions is probably due to different mechanical properties of the molecular cut-off centrifugal filters employed for the fractionation.
Fig 2. Comparison of ultra-high performance liquid chromatography ultra violet(UHPLCUV) profiles of the media, <3KD and 3–10KD B. infantis SF and identification of indole-3-lactic acid (ILA) as the structure of the specific peaks from B. infantis SF by an extracted ion chromatogram (EIC).
RP-UHPLC-UV chromatograms (280 and 254 nm) showing the comparison of B. infantis culture media with B. infantis secretion size fractions 3KD and 10–3KD. The circle shows the ILA peak which is absent in culture media and present in both of the B. infantis SF (A-C).
To confirm ILA, we compared the ILA standard compound retention time with the B. infantis 3K fraction. Figure (D) reports the EIC showing the ILA [M-H]− peak in B. infantis 3K fractions (black line) overlapped with the corresponding standard (pink line). MS and MS/MS (E-F) spectra of ILA peak in B. infantis 3K fraction compared to MS and MS/MS spectra of standard compound (G-H). The ILA identity is further confirmed by spectral matching with the on-line spectral libraries.
Fig3. B. infantis SF structure pattern was analyzed by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS).
Ultra-high-resolution mass spectrometry provides an accurate mass measurement and unambiguous molecular formula assignment. Thus, after isolation by RP-UHPLC of ILA peak this was infused in a FT-ICR mass spectrometer for structure elucidation. The MS/MS spectrum of isolated ILA shows its fragmentation pattern and molecular formula of each MS/MS ion with sub-ppm mass accuracy.
Indole-3-lactic acid (ILA) is anti-inflammatory in H4 cells.
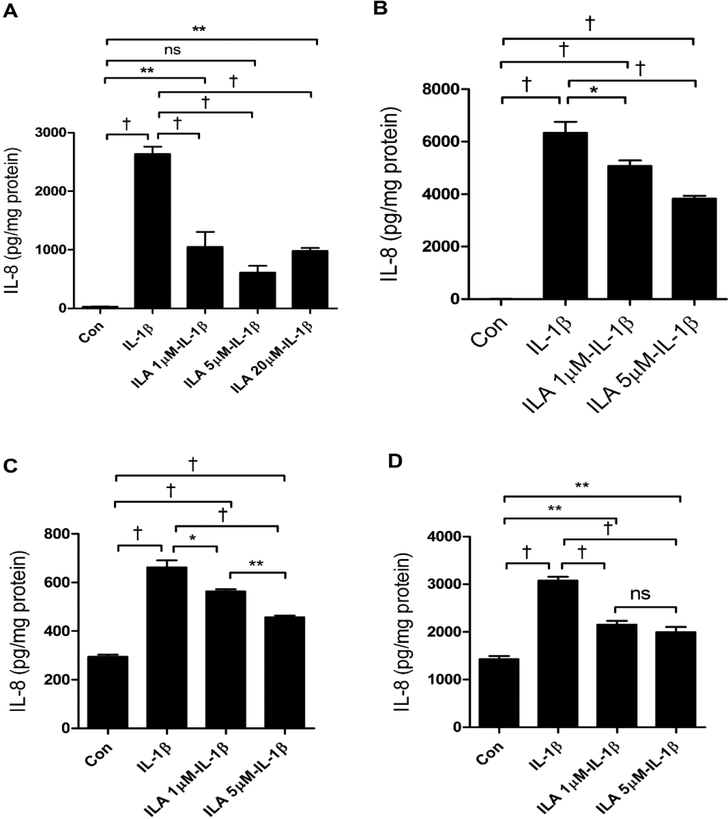
Having identified the peak in sized fractions of B. infantis secretions as ILA, we determined if ILA was anti-inflammatory in H4 cells. Accordingly, we incubated H4 cells with ILA before exposing them to IL-1β and then measuring IL-8 as a representative inflammatory cytokine. At levels of 1μM, 5μM and 20μM, ILA was significantly anti-inflammatory to the IL-1β stimulus (FIGURE 4A). However, at the same doses of ILA alone no inflammation was noted and the various doses were not cytotoxic (data not shown).
Fig4. The effect of indole-3-lactic acid (ILA) on anti-inflammation in H4 cells, NEC enterocytes and immature human intestinal organoids.
H4 cells (A), primary enterocytes isolated from resected intestine of a NEC patient (B), and immature human intestinal organoids isolated from therapeutically aborted fetuses at gestational age 15 -week (C) and 22- week (D) were pretreated with or without ILA before IL-1β stimulation. The secretion of IL8 into the cell culture supernatant was determined by ELISA (N = 3). Graphs represent the mean ± SEM from three independent experiments. One -way ANOVA and Turkey post hoc tests were used for statistical analysis (*P<0.05, **P<0.01, †P<0.001).
ILA is also anti-inflammatory in small intestine from a patient with NEC and other immature enterocytes.
To confirm that ILA was generally anti-inflammatory in immature intestine, we used a primary cell line isolated from resected small intestine from a NEC patient (FIGURE 4B) and organoids from the therapeutically aborted human fetuses at 15 (FIGURE 4C) and 22 (FIGURE 4D) weeks gestation. These enterocytes and organoids were exposed to ILA before adding IL-1β. The various concentrations of ILA resulted in a significant anti-inflammatory effect.
ILA causes anti-inflammation through an aryl hydrocarbon receptor (AHR) and responds to TLR-4 expression.
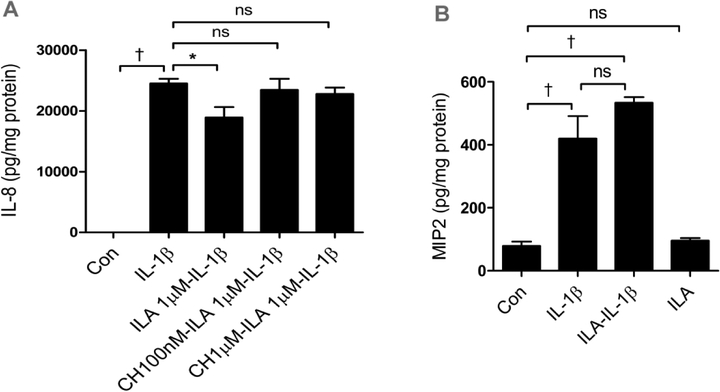
It has been reported that ILA interacts with an AHR in intestinal epithelial cells (27,28). To determine if this receptor/transcription factor is involved in the anti-inflammatory effect of H4 cells, we determined the mRNA expression of AHR and the effect of the AHR inhibitor CH223191(sigma, cat#08124, lot#0000042799) on ILA anti-inflammation after IL-1β stimulation. CH223191 is a potent and specific AHR antagonist which inhibited AHR activator induced AHR nuclear translocation and DNA binding with the half maximal inhibitory concentration (IC50) of 30nM (24). FIGURE 5A shows that inhibition of AHR abrogates the anti-inflammatory effects of ILA in H4 cells. We also tested ILA in TLR-4 knockout immature mice and found that anti-inflammation was lost without a TLR-4 receptor. (FIGURE 5B)
Fig5. ILA anti IL-1β-induced Il-8 induction is mediated by aryl hydrocarbon receptor (AHR) in H4 cells and TLR4 is required for ILA anti inflammation in immature intestine.
(A) H4 cells were pretreated with or without different doses of AHR inhibitor- CH223191 then treated with ILA before exposure to IL-1β. The secretion of IL8 into the supernatant was determined by ELISA. (B) TLR4 knockout immature mice (embryonic day 18.5) small intestinal organ cultures were pretreated with or without 5μM of ILA before IL-1β stimulation. The secretion of microphage inflammatory protein 2 (MP2) into the supernatant was determined by ELISA. Data are represented as the mean ±SEM, n=3 from three independent experiments for (A) and n=6, from the repeated experiments for (B). One -way ANOVA and Tukey post hoc tests were used for statistical analysis (*p<0.05 and †p<0.001).
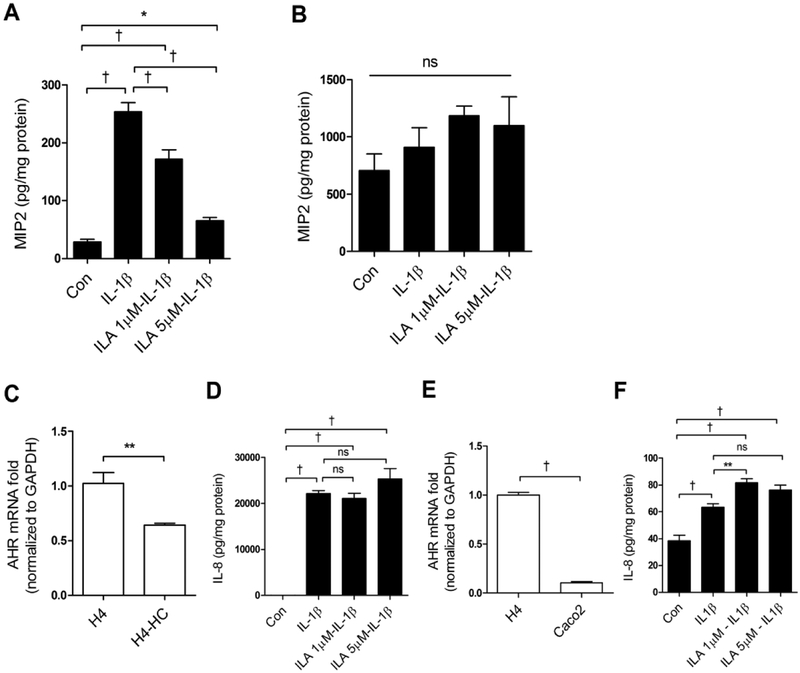
ILA effect is developmentally regulated.
To determine if ILA was anti-inflammatory in vivo in immature vs adult intestine, we prepared C57BL/6J immature and adult mouse small intestinal organ cultures and exposed them to ILA before IL-1β stimulation. Exposure to ILA resulted in a significant reduction in IL-1β inflammation in immature mouse (FIGURE 6A) but not adult (FIGURE 6B) small intestine. To determine if the effect of ILA was developmentally regulated in human intestines, we exposed ILA to H4 cells, H4 cells treated with hydrocortisone (a trophic agent shown to induce maturation of immature enterocytes) (23) and to Caco2 cells before an IL-1β stimulus (FIGURE 6C–F). AHR mRNA expression was reduced in H4 cells treated with hydrocortisone (C) and anti-inflammation of ILA was lost (D) AHR in Caco2 cells was reduced (E) and ILA had no effect on IL8 secretions (F).
Fig 6. AHR and ILA anti-inflammatory response to IL-1β are developmentally regulated.
C57BL6 (A) immature (embryonic day 18.5) and (B) adult (8 -week old male) mice small intestinal organ cultures were pretreated with or without ILA before IL-1β stimulation. The secretion of MP2 into the supernatant was determined by ELISA. (C) Expression of AHR mRNA in H4 cells and Hydrocortisone treated H4 (HC-H4) cells, (D) IL8 reaction in HC-H4 cells exposure to ILA before an IL-1β stimulus. (E) AHR mRNA in H4 and Caco2 cells, (F) IL8 secretions in Caco2 cells pretreated with or without ILA before IL-1β stimulus. Data are represented as the mean ± SEM, n=6 for mice and n=3 for cells (from three independent experiments). T-test was used for two-group and One -way ANOVA and Tukey post hoc tests were used for multiple-group statistical analysis (*p<0.05, ** p<0.01, †p<0.001).
Proposed mechanism for indole-3-lactic acid effect on immature enterocytes.
Taken together, we propose that indole-3-lactic acid, a breakdown product of the amino acid tryptophan, present in large amounts in breastmilk, is produced by B. infantis metabolism and mediates the mechanism of anti-inflammatory in immature human enterocytes (H4, NEC primary cell lines and immature enteroids). ILA requires an interaction with TLR-4 and with the AHR receptor to interfere with its transcription of the inflammatory cytokine IL-8 that causes excessive inflammation in the premature intestine resulting in NEC (Supplemental FIGURE S1).
DISCUSSION
We have previously reported that B. infantis (ATCC No. 15697) and its secretions were anti-inflammatory in ex-vivo experiments using a human immature primary small intestinal cell line (H4 cells) and in vivo in immature mouse small intestine (15, 18,19) suggesting that this “pioneer” bacterium known to exist as part of the initial colonizing microbiota of breastfed newborns and breastmilk itself potentially functions in concert to impart protection against the severe intestinal inflammatory necrosis leading to NEC (29, 30). We have reported that size fractions of B. infantis secretions (5–10KDa) when exposed to immature enterocytes protected against an LPS-stimulated inflammatory response in a primary human small intestinal cell line (18). We know from clinical studies that increased levels of IL-1β are found in the intestinal secretions of prematures developing NEC (31). We have also reported that anti-inflammatory secretions from B. infantis requires an increased enterocyte surface level expression of TLR-4 receptors (9,32) and affects the developmentally expressed transcription factor, AP-1, to interfere with IL-8 stimulated inflammation (17). More recently, using transcription profiles of RNA isolated from H4 cells exposed to B. infantis secretions followed by an IL-1β inflammatory stimulus, we have determined the transcription of tight junction genes (occludin and claudin) are increased and intercellular transport is reduced (21).
In this study, we extend these observations to determine the structure of the molecules in secretions which mediates enterocyte anti-inflammation. We compared the metabolite profiles of cell media with the 3K, 3–10K and >10K fractions after UHPLC-MS/MS analysis. (FIGURE 2). The accurate mass spectrometry measurement provided the molecular formula: C11H11NO3 and by tandem mass spectrometry fragmentation, assisted by spectral library matching, it was finally identified as indole-3-lactic acid (ILA) (FIGURE 3).
It is important to recognize that all strains of bifodobacteria studied are capable of producing indole-3-lactic acid (ILA) from tryptophan (33–34), but B. infantis represents a unique bacterium present in breastfed newborns. ILA is only anti-inflammatory in immature intestine and breastmilk contains tryptophan capable of producing ILA (35). This suggests that B. infantis, a known probiotic in infants, in combination with expressed breastmilk may be an important way to prevent NEC.
When ILA was incubated with H4 cells at the concentration found in secretions and at higher concentrations before a stimulus with IL-1β, the molecule was anti-inflammatory ex vivo in H4 cells (FIGURE 4A), in a primary cell line from resected small intestine in a patient with necrotizing enterocolitis (FIGURE 4B) and additionally in immature intestinal organoids (FIGURE 4C, 4D) suggesting that this was a generalized effect in immature enterocytes.
Since ILA is a potential ligand for the AHR in enterocytes, we determined the effect of ILA in immature enterocytes in which AHR was inhibited. We found that the inhibitor of AHR caused the anti-inflammatory effect to be lost suggesting a pathway for anti-inflammation in immature enterocytes (FIGURE 5A). When we used ILA and IL-1β in immature mice with the TLR-4 receptor knocked out, ILA also lost the anti-inflammatory effect suggesting that TLR-4 present in abundance on the surface of immature enterocytes was involved in the anti-inflammatory process (FIGURE 5B).
The evidence that ILA was only effective as an anti-inflammatory molecule in immature intestine existed in both mouse models and human enterocytes. We exposed wild type immature and adult organ cultures of mouse intestine to ILA before an inflammatory stimulus with IL-1β (FIGURE 6A–B). The anti-inflammatory effect was only present in the immature mouse. In addition, we measured the AHR gene in immature enterocytes treated with hydrocortisone to cause intestinal maturation and Caco2 cells. The receptor genetic level was high in immature enterocytes and lower in cortisone-treated immature enterocytes and Caco2 cells. In like manner, when ILA and IL-1β were used in the cortisone-treated and Caco2 cells, they lacked an anti-inflammatory response strongly suggesting that ILA is only anti-inflammatory in immature but not adult intestine.
Tryptophan is a substrate for intestinal bacteria leading to important metabolites. The health of the patient has become very important as investigators have addressed microbial-epithelial crosstalk (36). Of particular importance is the indole metabolites that use as ligands AHR as a translational receptor in enterocytes and immunocytes (37). This is particularly true in the early life when bifidobacteria are abundant in breastfed babies and in experimental animals have been shown to influence development of immunologic defense (38). It has been also been associated with inflammatory bowel disease. Low tryptophan levels and a decrease in AHR in active patients have been reported (39) and indole metabolites of tryptophan have been used in animal models to treat colitis (35). In like manner, the role of tryptophan metabolite deficiency has been associated with irritable bowel syndrome and metabolic syndrome. Furthermore, decreased levels of AHR receptors have been found in patients with multiple sclerosis (40).
It has previously been reported that a number of colonizing intestinal bacteria, particularly gram-negative organisms, can also metabolize the amino acid tryptophan to produce metabolic pathways whose production improves health and provides immunologic protection (33, 34). One such pathway utilizing indole metabolites (indole-3-lactic acid, indole-3-pyruvic acid, etc.) (33, 34) uses the aryl hydro-carbon receptor (AHR) to activate the immune system and other intestinal functions for purposes of homeostasis. For example, indole-3-pyruvic acid (IPA), an AHR agonist, can be used to inhibit inflammation associated with animal models of colitis (35). AHR is a ligand-activated transcription factor stimulated by dietary and microbial metabolites that plays an important role in maintaining homeostasis by intestinal mucosa. Indole-3-lactic acid is a metabolite of tryptophan (33).
Lactic acid bacteria are capable of converting amino acids to secondary metabolites. This process starts with a deamination step, which in the case of aromatic amino acids is operated by the enzyme aromatic aminotransferase that converts amino acids into alpha-ketoacids. These compounds are further converted by dehydrogenases to hydroxy acids. The presence of ILA has also been reported in Lactobacillus casei and L. helveticus (33, 36) and this molecule has shown anti-inflammatory activity in keratinocytes against ultraviolet-B induced damages (33). Increased amounts of these tryptophan metabolites were found in other bacterial species such as Megamonas hypermegale, Roseburia intestinalis, Ruminococcus obeum, E. rectale and F. prausnitzii, etc. (36).
We know that breastmilk, particularly colostrum, has high levels of the essential amino acid tryptophan (11, 12). We also know that ingestion of maternal-expressed breastmilk in prematures is protective against NEC (11, 12, 14,). It has also been reported that breastmilk-induced bacteria (B. infantis, Lactobacillus acidophilus and Bacteroides fragilis) known as “pioneer” bacteria, (14) are specifically stimulated with initial colonization with ingested breastmilk in premature infants compared to probiotics given to formula-fed infants (13). They can function together with probiotics to protect against the expression of NEC in prematures (13, 16).
Based on the results presented here, we hypothesize that Bifidobacterium longum subsp infantis, a known “pioneer” bacterium (13, 14), present in the intestine of premature infants fed mothers expressed breastmilk, metabolize breastmilk tryptophan to produce indole-3-lactic acid which acts to inhibit the transcription factor’s (AHR) stimulation of IL-8 only in the immature intestine that express TLR-4 on their surface and thus protect against the excessive intestinal inflammation seen in the premature and thought to be a contributing factor in necrotizing enterocolitis. Supplemental FIGURE S1 is a cartoon which depicts this proposed mechanism of anti-inflammation in immature enterocytes. A previous clinical study has shown that “pioneer” probiotics are most effective in preventing NEC when given with expressed breastmilk (16). Thus, this observation provides a mechanistic explanation to support the additive effect of breastmilk and probiotics in the prevention of NEC. Additional in vivo studies in animals are required and safety studies in humans before a large scale, single protocol clinical trial with ILA can be done to determine efficacy in immature intestine.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the following grants.
-NIDDK (P01-DK033506) “Barrier function of the GI tract in health and disease” PI W. Allan Walker
-Family Larsson-Rosenquist Foundation “The impact of breastmilk microbiome and protective factors on development of human intestinal disease” PI W. Allan Walker
-Beth Israel/Deaconess Medical Center (Award #01027741) “Impact of breastmilk in premature intestinal colonization” PI W. Allan Walker
Footnotes
DISCLOSURES
No conflict of interest, financial or otherwise, are declared by authors
REFERENCES
- 1).Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011. 364 (3):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Holman RC, et al. Necrotizing enterocolitis hospitalizations among neonates in the United States. Paediatr Perinat Epidemiol 2006; 20 (6):498–506. [DOI] [PubMed] [Google Scholar]
- 3).Claud EC, et al. Developmentally-regulated IκB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. PNAS 2004; 101(19): 7404–7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Nanthakumar N, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. Plos One 2011; 6: e17776–17785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Weng M, Walker WA. The role of gut microbiota in programming the immune phenotype. J Dev Orig Health Dis 2013; 4(3):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Groer MW, Gregory KE, Louis-Jacques A, Thibeau S, Walker WA. The very low birth weight infant microbiome and childhood health. Birth Defects Res (Part C) Embryo Today 2015; 105 (4):252–264. [DOI] [PubMed] [Google Scholar]
- 7).Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007;5: e177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Nanthakumar N, Fusunyan RD, Sanderson IR, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic basis for necrotizing enterocolitis. PNAS 2000; 97(11): 6043–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Meng D, Zhu W, Ganguli K, Shi H, Walker WA. Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretion on immature human enterocytes are mediated by TLR4 receptors. Am J Physiol Gastrointest Liver Physiol 2016; 311: G744–G753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nature Rev: Gastroenterol & Hepatol 2010; 7(9):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).O’Rourke L, et al. Tryptophan metabolic profile in term and preterm breast milk: implications for health. J Nutr Sci 2018; 7: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Donnet-Hughes A, Schriffin E, Walker WA. Protective properties of human milk and bacterial colonization of the neonatal gut In: Duggan C, Koletzko B, Watkins J, Walker WA. Nutrition In Pediatrics Basic Science and Clinical Aspects. 5thed Chinese Publications Inc., New Haven, CT: Eds. 2017; 250–260. [Google Scholar]
- 13).Gregory KE, et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 2016;4(1)68–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Jost T, Lacroix C, Braegger CP, Chassard C. New insights in gut microbiota establishment in healthy breast fed neonates. Plos One 2012;7: e44595–44803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Meng D, et al. The toll-like receptor −4 in human and mouse colonic epithelium is developmentally regulated: a possible role in necrotizing enterocolitis. Pediatr Res 2015; 77(3):416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Repa A, et al. Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula. Pediatr Res 2015; 77(2): 381–88. [DOI] [PubMed] [Google Scholar]
- 17).Cahill C, et al. Differential expression of the activator protein 1 transcription factor regulates interleukin-1 beta induction of interleukin 6 in the developing enterocyte. Plos One 2016; 11: e0145184–0145195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Ganguli K, Meng D, Rautava S, Lu L, Walker WA*, Nanthakumar N*. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol 2013; 304: G132–G141 (*shared senior authorship). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Guo S, Guo Y, Ergun A, Lu L, Walker WA*, Ganguli K*. Secreted metabolites of Bifidobacterium infantis and Lactobacillus acidophilus protect immature human enterocytes from IL-1β-induced inflammation: A transcription profiling analysis. Plos One 2015; 10: e0124549–0124557 (*shared senior authorship). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Sanderson IR, et al. Human immature enterocytes in vitro: modulation of the phenotype by extracellular matrix. PNAS 1996;93(15):7717–7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Guo S, et al. Secretions of Bifidobacteraium infantis and Lactobacillus acidophilus protect intestinal epithelial barrier function. J Pediatr Gastroenterol Nutr 2017; 64(3):404–412. [DOI] [PubMed] [Google Scholar]
- 22).Senger S, et al. Human immature-derived enterospheres provide insights on intestinal development and a novel model to study Necrotizing Enterocolitis (NEC). Cellular and Molecular Gastroenterology and Hepatology Journal 2018; 5(4):549–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Lu L, Li T, William G, Petit E, Walker WA. Hydrocortisone induces changes in gene expression and differentiation in immature human enterocytes. Am J Phys-Gastrointestinal and Liver Physiol 2011; 300: G425–G432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicological Sciences: An Official Journal Of The Society Of Toxicology 2010; 117(2):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Theodoridis GA, Gika HG, Want EJ, Wilson ID. Liquid chromatography–mass spectrometry based global metabolite profiling: A review. Anal Chim Acta 2012; 711:7–16. [DOI] [PubMed] [Google Scholar]
- 26).Cajka T, Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal Chem 2016;88(1): 524–545. [DOI] [PubMed] [Google Scholar]
- 27).Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos 2015;43(10):1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Metidji A, et al. Article: The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity 2018;49(2): 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Ganguli K, Walker WA. Treatment of necrotizing enterocolitis with probiotics. Gastroenterol ClinNorth Am 2012; 41(4): 733–746. [DOI] [PubMed] [Google Scholar]
- 30).Olsen R, Greisen G, Schrøder M, Brok J. Prophylactic probiotics for preterm infants: a systematic review and meta-analysis of observational studies. Neonatology 2016; 109(2): 105–112. [DOI] [PubMed] [Google Scholar]
- 31).Henry MCW, Moss RL. Necrotizing enterocolitis. Annu Rev Med 2009; 60: 111–124. [DOI] [PubMed] [Google Scholar]
- 32).Good M, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol 2015;8(5):1166–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun 2018; 9:3294–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Vogel CF, et al. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol 2007; 21(12): 2941–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Xu G, et al. Bifidobacterium grown on human milk oligosaccharides produce tryptophan metabolite Indole-3-lactic acid that significantly decreases inflammation in intestinal cells in vitro. The FASEB Journal 2018;32(1). [Google Scholar]
- 36).Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y. Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J Immunol 2018; 201(12): 3683–3693. [DOI] [PubMed] [Google Scholar]
- 37).Aragozzini F, Ferrari A, Pacini N, Gualandris R. Indole-3-lactic acid as a tryptophan metabolite produced by Bifidobacterium spp. Appl Environ Microbiol. 1979. September;38(3):544–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Laursen M, Bahl M, Michaelsen K, Licht T. First foods and gut microbes. Front Microbiol. 2017; 8: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Nikolaus S, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017. December;153(6):1504–1516. [DOI] [PubMed] [Google Scholar]
- 40).Rothhammer V. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 2016. June;22(6):586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.