Abstract
Introduction:
In patients with advanced cancer, prolongation of life with treatment often incurs substantial emotional and financial expense. Among hospitalized patients with cancer since acute kidney injury (AKI) is known to be associated with much higher odds for hospital mortality, we investigated whether renal replacement therapy (RRT) use in the intensive care unit (ICU) was a significant independent predictor of worse outcomes.
Methods:
We retrospectively reviewed patients admitted in 2005 to 2014 who were diagnosed with stage IV solid tumors, had AKI, and a nephrology consult. The main outcomes were survival times from the landmark time points, inpatient mortality, and longer term survival after hospital discharge. Logistic regression and Cox proportional regression were used to compare inpatient mortality and longer term survival between RRT and non-RRT groups. Propensity score-matched landmark survival analyses were performed with 2 landmark time points chosen at day 2 and at day 7 from ICU admission.
Results:
Of the 465 patients with stage IV cancer admitted to the ICU with AKI, 176 needed RRT. In the multivariate logistic regression model after adjusting for baseline serum albumin and baseline maximum Sequential Organ Failure Assessment (SOFA), the patients who received RRT were not significantly different from non-RRT patients in inpatient mortality (odds ratio: 1.004 [95% confidence interval: 0.598-1.684], P = .9892). In total, 189 patients were evaluated for the impact of RRT on long-term survival and concluded that RRT was not significantly associated with long-term survival after discharge for patients who discharged alive. Landmark analyses at day 2 and day 7 confirmed the same findings.
Conclusions:
Our study found that receiving RRT in the ICU was not significantly associated with inpatient mortality, survival times from the landmark time points, and long-term survival after discharge for patients with stage IV cancer with AKI.
Keywords: dialysis, stage IV cancer, hospice, ICU, SOFA score, palliative care
Introduction
An increasing body of data indicate the poor quality of life associated with renal replacement therapy (RRT),1 including those with acute kidney injury (AKI).2 In a general population of critically ill patients requiring RRT, AKI has been associated with mortality of 40% to 70%, and AKI is an independent risk factor for death.3,4 In patients with solid tumor in particular, the prevalence of AKI and chronic kidney disease has been studied extensively because of the negative effects of these conditions on cancer and overall mortality.5–7 The 1-year risk of AKI as defined by Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease in a large Danish study in patients with cancer was 17.5%.5 The Renal Insufficiency and Anticancer Medications 1 and 2 studies showed that the 2-year survival rate in patients with active malignancy was significantly lower for those with chronic kidney disease defined as glomerular filtration rate (GFR) less than 60 ml/min using Modification of Diet in Renal Disease equation.6 In cancer populations, AKI in hospitalized patients was associated with more than 4-fold higher odds of mortality (odds ratio [OR]: 4.47, 95% confidence interval [CI]: 3.16-6.32, P < .001).8 Use of RRT in the intensive care unit (ICU) was further evaluated and, in a multivariate analysis of patients who received at least 48 hours of continuous RRT, was a significant and independent predictor of higher mortality.9
With advances in cancer medicine, survival has improved. The goal is remission; however, as the complexities of cancer biology prevent full eradication of the disease in some cases, keeping the progression of cancer at bay is an acceptable and often celebrated option. To help patients through their difficult journey of treatments, our role as physicians is to provide the support necessitated by the individual situation using a patient-centered approach. The Institute of Medicine (IOM) defined the term “patient centered” as “providing care that is respectful of and responsive to individual patient preferences, needs, and values, and ensuring that patient values guide all clinical decisions.”10 Patients with advanced solid tumors whose disease has not responded to multiple lines of treatment admitted with AKI with indications for hemo-RRT is a typical situation that arises in a cancer center. Given the nephrotoxicity associated with chemotherapies and antibiotics, and the contribution of sepsis, infections, and urinary obstruction to AKI in patients with cancer, we are often led to question the benefit of RRT in these patients. There is evidence in the general population that the adjusted mortality rate of patients on maintenance RRT is nearly twice that of adults with cancer and more than twice that of adults with congestive heart failure or stroke.11 Therefore, exploring the outcomes of RRT for AKI in an advanced cancer population is of importance.
The objective of the study was to determine the impact of RRT on survival for ICU admitted patients with stage IV cancer and AKI, using propensity score matched landmark analyses at 2 separate landmark time points.
Methods
Study Design and Patient Population
We retrospectively reviewed all patients admitted to The University of Texas MD Anderson Cancer Center in 2005 to 2014 with stage IV solid tumors who were admitted with a diagnosis of AKI and for who a nephrology consult was obtained. This retrospective study was approved by the institutional review board at the University of Texas MD Anderson Cancer Center in accordance with the principles of the Declaration of Helsinki. The main end points of the study were long-term survival after hospital discharge, inpatient mortality, and survival times from the landmark time points. A data file of 1194 of unique patients’ records was created by extracting patients diagnosed with AKI on hospitalization using the International Classification of Diseases-9 diagnosis codes from the MD Anderson Enterprise Information Warehouse, which electronically stores patient data. Of the 900 that fit the inclusion criteria, 176 needed RRT during their stay in the ICU. We compared them with other ICU admitted patients in order to adjust for the intensity of illness using available Sequential Organ Failure Assessment (SOFA) scores.
Data extracted from the patients’ medical records included demographic, clinical, and laboratory data (creatinine and GFR). Demographic variables included age, sex, race/ethnicity, and vital status (alive vs dead). Clinical data included type of malignancy, diagnoses of peripheral vascular disease and/or dementia, and consults for RRT and palliative and hospice care. We specified the use of these variables based on a published 6-month prognostic model for mortality of patients who are maintained on RRT and concluded that older age, dementia, peripheral vascular disease, and decreased albumin were independent predictors of poor survival.12 Hospital admission data included length of hospital stay, length of ICU stay, SOFA score, and disposition. The first albumin level obtained during the first 3 days of ICU admission was extracted from laboratory data.
Landmark Survival Analysis
We performed landmark survival analyses at landmark time points of day 2 and day 7, including only those patients who survived until the landmark time points. Patients who started receiving RRT within the landmark time point were classified as RRT, and patients who did not receive RRT within the landmark time point were classified as no RRT regardless of receiving RRT later. At each landmark time point, RRT and no RRT groups were identified. Patients can receive RRT at any time during their ICU stay, and RRT patients are guaranteed to survive at least until the time of RRT; this is known as the guarantee-time bias or the immortal time bias.13 Landmark analysis is used extensively in medical research to correct for this type of bias inherent in an analysis of time-to-event outcome between groups (in this study, RRT and no RRT) that were determined during study follow-up.14
Propensity Score Matching
Propensity scores were calculated to reduce the selection bias in comparison between RRT and no RRT patients, based on logistic regression models, including age at admission, baseline serum albumin, baseline creatinine, baseline GFR, and maximum baseline SOFA. Using propensity scores in RRT (treated) and no-RRT (control) groups, we selected propensity score-matched no RRT patients, utilizing “greedy nearest neighbor matching,” which selects the control unit nearest to each treated unit sequentially without replacements. At each landmark time point, we identified propensity score-matched cohorts. Propensity score-matched cohorts were examined in standardized differences and variance ratios to evaluate the performance of matching.
Statistical Analysis
Patient characteristics were summarized for 4 RRT groups (RRT started within 2 days, RRT started during 3-7 days, RRT started after 7 days of ICU admission, and no RRT during hospitalization), RRT and no RRT groups at each landmark analysis, and propensity score matched cohorts at each landmark analysis.
We conducted 2-day and 7-day landmark analyses including all patients who survived until the landmark time point. Then, propensity score-matched cohorts (RRT vs no RRT) were selected utilizing propensity score matching method described in the previous section.
Survival times from the landmark time points, survival from start of RRT, longer term survival from hospital discharge, and inpatient mortality (discharged alive vs dead) were obtained. Survival times were censored at last follow-up for patients who were alive. Multivariate Cox proportional hazards regression was used to explore the association of RRT on survival times from the landmark time points using propensity score-matched cohorts. Inpatient mortality was explored using multivariate logistic regression model. Longer term survival from discharge to death was compared between RRT and no RRT during hospital stay, utilizing Cox regression model. P values less than .05 were considered significant. SAS 9.4 (SAS Institute INC, Cary, North Carolina) was used for data analysis.
Results
Among 900 hospitalized patients with stage IV solid tumors and a diagnosis of AKI, 465 had ICU visits during hospitalization, and 176 (20%) of them were treated with RRT. Baseline characteristics of the 465 ICU patients by RRT group are shown in Table 1. Patients who started RRT within 2 days from ICU had lower serum albumin level, higher creatinine, lower GFR, and higher SOFA score than the other RRT groups. The RRT started during 3- to 7-day group were comparable to RRT started within 2-day group in albumin level, GFR, and SOFA score. There were more males in RRT started later than 7 days of ICU admission group than other groups.
Table 1.
Summary Statistics and Comparison of Baseline Characteristics by RRT Group.
| RRT Started |
|||||
|---|---|---|---|---|---|
| Covariates | Within 2 Days of ICU Admission, N = 98 |
Within 3-7 Days of ICU Admission, n = 42 |
Later Than 7 Days of ICU Admission, N = 36 |
No RRT, N = 289 |
P Value |
| Categorical variables | n (%) | n (%) | n (%) | n (%) | |
| Gender | |||||
| Female | 49 (50%) | 19 (45.2%) | 8 (22.2%) | 141 (48.8%) | .0219 |
| Male | 49 (50%) | 23 (54.8%) | 28 (77.8%) | 148 (51.2%) | |
| Race/ethnicity | |||||
| Black | 19 (19.4%) | 8 (19%) | 6 (16.7%) | 49 (17%) | .6444 |
| Other | 22 (22.4%) | 13 (31%) | 12 (33.3%) | 64 (22.1%) | |
| White | 57 (58.2%) | 21 (50%) | 18 (50%) | 176 (60.9%) | |
| Patient’s cancer type | |||||
| Adrenal | 0 (0%) | 0 (0%) | 1 (2.8%) | 1 (0.3%) | .0161 |
| Breast | 12 (12.2%) | 5 (11.9%) | 0 (0%) | 25 (8.7%) | |
| GI | 29 (29.6%) | 9 (21.4%) | 9 (25%) | 55 (19%) | |
| Genitourinary | 9 (9.2%) | 6 (14.3%) | 11 (30.6%) | 44 (15.2%) | |
| Gynecology | 7 (7.1%) | 3 (7.1%) | 3 (8.3%) | 30 (10.4%) | |
| Lung and head/neck | 18 (18.4%) | 10 (23.8%) | 5 (l3.9%) | 73 (25.3%) | |
| Pancreatic and Bil | 4 (4.1%) | 4 (9.5%) | 1 (2.8%) | 25 (8.7%) | |
| Sarcoma/melanoma | 12 (12.2%) | 3 (7.1%) | 2 (5.6%) | 19 (6.6%) | |
| Thyroid | 0 (0%) | 0 (0%) | 3 (8.3%) | 9 (3.1%) | |
| Unknown Primary | 7 (7.1%) | 2 (4.8%) | 1 (2.8%) | 8 (2.8%) | |
| Patient died in the hospital | |||||
| No | 24 (24.5%) | 11 (26.2%) | 11 (30.6%) | 143 (49.5%) | <.0001 |
| Yes | 74 (75.5%) | 31 (73.8%) | 25 (69.4%) | 146 (50.5%) | |
| Patient received supportive care while in hospital | |||||
| No | 98 (100%) | 42 (100%) | 36 (100%) | 288 (99.7%) | .8941 |
| Yes | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) | |
| Patient diagnosed with peripheral vascular disease before hospital admission | |||||
| No | 98 (100%) | 42 (100%) | 34 (94.4%) | 280 (96.9%) | .1283 |
| Yes | 0 (0%) | 0 (0%) | 2 (5.6%) | 9 (3.1%) | |
| Continuous variables, mean ± SD | |||||
| Patient age at time of hospital admission | 59.21 ± 11.62 | 59.76 ± 13.11 | 59.28 ± l5.78 | 60.49 ± 13.57 | .8378 |
| Number of days patient was hospitalized | 14.46 ± 15.89 | 24.24 ± 21.34 | 45.50 ± 41.52 | 15.44 ± 17.39 | <.000l |
| Baseline serum albumin test | 2.66 ± 0.67 | 2.77 ± 0.57 | 2.92 ± 0.65 | 2.85 ± 0.68 | .0724 |
| Baseline creatinine | 3.24 ± 2.12 | 2.49 ± 2.05 | 1.70 ± 1.35 | 2.69 ± 2.47 | .0063 |
| GFR during first ICU admission | 30.37 ± 23.15 | 37.52 ± 27.10 | 72.53 ± 49.78 | 42.30 ± 35.79 | <.000l |
| ADM SOFA during first ICU admission | 11.43 ± 4.70 | 9.35 ± 3.91 | 8.00 ± 3.11 | 7.73 ± 3.86 | <.0001 |
| Max SOFA during first ICU admission | 16.29 ± 4.38 | 16.13 ± 4.05 | 13.34 ± 5.06 | 9.78 ± 4.36 | <.000l |
Abbreviations: GI, gastrointestinal; GFR, glomerular filtration rate; ICU, intensive care unit; RRT, renal replacement therapy; SD, standard error; SOFA, Sequential Organ Failure Assessment.
Landmark Survival Analysis
Among 465 ICU admitted patients, 39 were excluded from the 2-day landmark analysis, and 156 were excluded from the 7-day landmark analysis because of death before landmark time or censoring (Figure 1). In the 2-day landmark analysis, 426 patients were included, and 89 patients received RRT and 337 did not receive RRT within 2 days. In the 7-day landmark analysis, 309 patients were included and there were 82 received RRT and 227 did not receive within 7 days.
Figure 1.
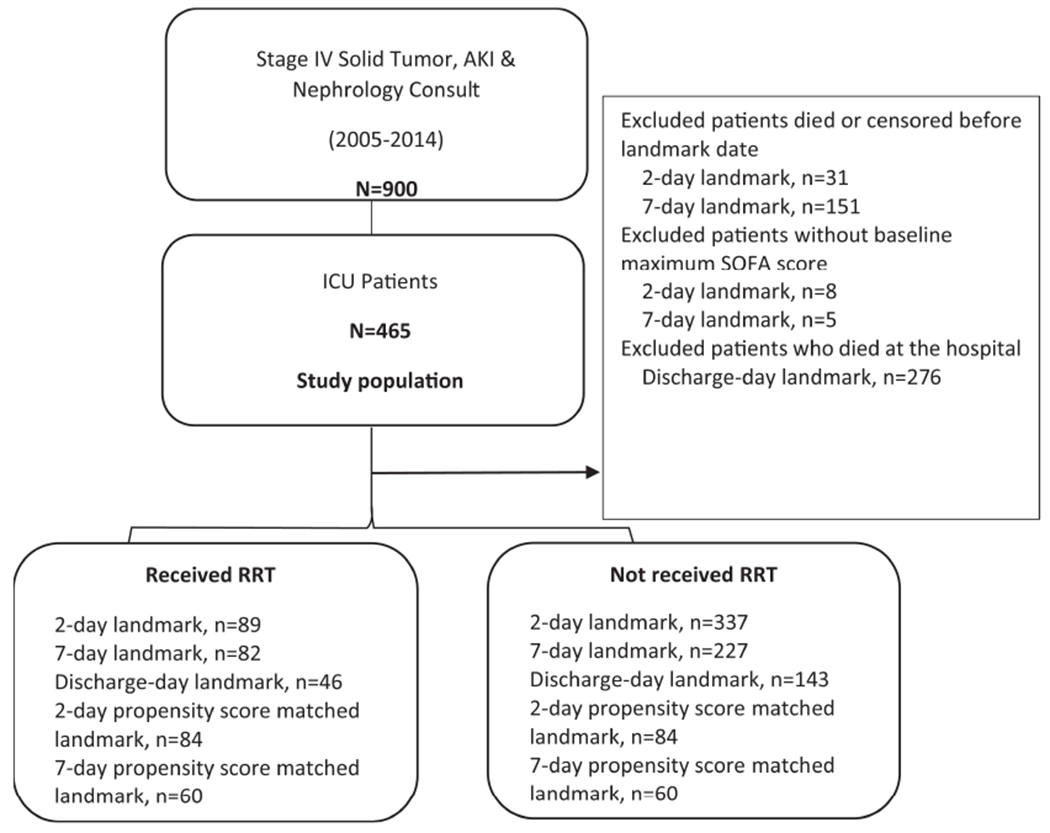
Flowchart of study population.
Patient characteristics of RRT and no RRT groups and propensity score-matched cohorts at 2-day landmark analysis are presented in Table 2, respectively, and patient characteristics of RRT and no RRT groups and propensity score-matched cohorts at 7-day landmark analysis are presented in Table 3, respectively. Significant differences were observed between RRT and no RRT groups in most variables, except age (Tables 2 and 3). After propensity score matching, there were no significant differences in variables used for propensity scores by standard mean difference and variance ratio, indicating the success of matching (Tables 2 and 3). Significant differences were observed between dialyzed and nondialyzed patients in most variables, except age.
Table 2.
Summary Statistics and Comparison of Baseline Characteristics for RRT and No RRT Groups for Landmark Analysis at Day 2.
| Patient’s Characteristics and Clinical Factors | RRT (n = 89) Mean ± SD |
No RRT (n = 337) Mean ± SD |
P Value | |
|---|---|---|---|---|
| Including all patients included in RRT and no RRT groups | ||||
| Patient age at time of hospital admission | 59.21 ± 11.17 | 60.36 ± 13.72 | .4125 | |
| Number of days patient was hospitalized | 15.39 ± 16.30 | 19.89 ± 23.12 | .0367 | |
| Baseline serum albumin test | 2.64 ± 0.68 | 2.87 ± 0.67 | .0049 | |
| Baseline creatinine | 3.25 ± 2.20 | 2.55 ± 2.41 | .0144 | |
| GFR during first ICU admission | 31.16 ± 23.84 | 45.70 ± 37.75 | <.0001 | |
| ADM SOFA during first ICU admission | 11.11 ± 4.62 | 7.85 ± 3.76 | <.0001 | |
| Max SOFA during first ICU admission | 16.21 ± 4.48 | 10.84 ± 4.88 | <.0001 | |
| Patient’s characteristics and clinical Factors | RRT (n = 84) Mean ± SD |
No RRT (n = 84) Mean ± SD |
Standardized mean difference | Variance ratio |
| Including patients in propensity score-matched cohorts | ||||
| Patient age at time of hospital admission | 59.11 ± 11.28 | 58.92 ± 14.71 | 0.01 | 0.59 |
| Baseline serum albumin test | 2.69 ± 0.67 | 2.69 ± 0.69 | 0.00 | 0.92 |
| Baseline creatinine | 3.21 ± 2.22 | 3.21 ± 3.22 | 0.00 | 0.47 |
| GFR during first ICU admission | 31.83 ± 24.23 | 34.96 ± 26.90 | −0.12 | 0.81 |
| Max SOFA during first ICU admission | 15.96 ± 4.45 | 15.73 ± 4.00 | 0.06 | 1.24 |
Abbreviations: GFR, glomerular filtration rate; ICU, intensive care unit; RRT, renal replacement therapy; SD, standard error; SOFA, Sequential Organ Failure Assessment.
Table 3.
Summary Statistics and Comparison of Baseline Characteristics for RRT and no RRT Groups for Landmark Analysis at Day 7.
| Patient’s Characteristics and Clinical Factors | RRT (n = 82) Mean ± SD |
No RRT (n = 227) Mean ± SD |
P Value | |
|---|---|---|---|---|
| Including all patients included in RRT and no RRT groups | ||||
| Patient age at time of hospital admission | 60.43 ± 12.84 | 60.20 ± 13.82 | .8946 | |
| Number of days patient was hospitalized | 24.39 ± 18.94 | 23.46 ± 25.54 | .7308 | |
| Baseline serum albumin test | 2.74 ± 0.63 | 2.93 ± 0.68 | .0281 | |
| Baseline creatinine | 3.23 ± 2.37 | 2.46 ± 2.58 | .0194 | |
| GFR during first ICU admission | 31.79 ± 25.38 | 52.02 ± 41.38 | <.0001 | |
| ADM SOFA during first ICU admission | 9.54 ± 4.27 | 6.99 ± 3.36 | <.0001 | |
| Max SOFA during first ICU admission | 15.30 ± 4.49 | 9.52 ± 4.38 | <.0001 | |
| Patient’s Characteristics and Clinical Factors | RRT (n = 60) Mean ± SD |
No RRT (n = 60) Mean ± SD |
Standardized mean difference | Variance ratio |
| Including patients in propensity score-matched cohorts | ||||
| Patient age at time of hospital admission | 59.59 ± 13.46 | 60.99 ± 13.43 | −0.10 | 1.00 |
| Baseline serum albumin test | 2.87 ± 0.61 | 2.81 ± 0.72 | −0.09 | 0.70 |
| Baseline creatinine | 3.26 ± 2.58 | 2.82 ± 1.89 | 0.20 | 1.86 |
| GFR during first ICU admission | 34.40 ± 28.23 | 33.87 ± 23.70 | 0.02 | 1.42 |
| Max SOFA during first ICU admission | 13.92 ± 4.33 | 13.32 ± 3.71 | 0.15 | 1.36 |
Abbreviations: ICU, intensive care unit; GFR, glomerular filtration rate; RRT, renal replacement therapy; SD, standard error; SOFA, Sequential Organ Failure Assessment.
Landmark survival analyses using all RRT and no RRT groups at the landmark time points (Table 4) and landmark survival analyses using propensity score-matched cohorts at the landmark time points are presented (Table 4). When age, baseline serum albumin, baseline creatinine level, baseline GFR, and maximum SOFA score were adjusted, RRT was not significantly associated with survival times from the landmark time point at each landmark (hazard ratio [HR] = 1.211 [95% CI: 0.879-1.669] at day 2; HR = 0.917 [95% CI: 0.620-1.356) at day 7]). Among 465 ICU patients, 276 (59.3%) died in the hospital; 66 (14.1%) were discharged on hospice, while 123 (26.5%) were discharged alive. Palliative care consultation was only obtained for 1 patient in the study population. Among 176 receiving RRT, 130 (73.8%) died in the hospital versus 146 (50.5%) of 289 without RRT died in the hospital. The median survival after ICU admission was 13 days in no RRT patients (Figure 2A). Among 176 patients who received RRT, the median time to RRT was 2 days (with the minimum of 0 and the maximum of 85 days), the median hospital survival after RRT was 5 days, and the median survival after RRT was 8 days (95% CI: 6-11 days). There was one patient who survived 3316 days after RRT. At day 14 from RRT, 38% were alive. At day 60 from RRT, 17% were alive (Figure 2B).
Table 4.
Landmark Survival Analysis on Survival Time From ICU Admission to Death.
| Hazard Ratio (95% CI) | P Value | |
|---|---|---|
| 2-day landmark analysis using RRT and no RRT groups | ||
| RRT vs No | 1.153 (0.882-1.506) | .2979 |
| Age at admit | 1.000 (0.992-1.007) | .9389 |
| Baseline serum albumin level | 0.781 (0.665-0.916) | .0024 |
| Baseline creatinine level | 1.014 (0.967-1.064) | .5585 |
| Baseline eGFR value | 0.998 (0.994-1.001) | .2493 |
| Baseline Max SOFA | 1.053 (1.030-1.076) | <.000l |
| 2-day landmark analysis using propensity score-matched cohorts | ||
| RRT vs No | 1.211 (0.879-1.669) | .24l3 |
| Age at admit | 0.998 (0.987-1.009) | .6925 |
| Baseline serum albumin level | 0.775 (0.598-1.004) | .0536 |
| Baseline creatinine level | 1.024 (0.955-1.099) | .5053 |
| Baseline eGFR value | 1.001 (0.993-1.009) | .8497 |
| Baseline Max SOFA | 1.058 (1.0l5-1.103) | .0082 |
| 7-day landmark analysis using RRT and no RRT groups | ||
| RRT vs No | 0.911 (0.660-1.256) | .5678 |
| Age at admit | 1.001 (0.993-1.010) | .8l43 |
| Baseline serum albumin level | 0.805 (0.666-0.974) | .0256 |
| Baseline creatinine level | 1.032 (0.982-1.085) | .2l28 |
| Baseline eGFR value | 1.001 (0.997-1.004) | .7290 |
| Baseline Max SOFA | 1.043 (1.0l3-1.073) | .0046 |
| 7-day landmark analysis using propensity score-matched cohorts | ||
| RRT vs No | 0.917 (0.620-1.356) | .6636 |
| Age at admit | 1.005 (0.99l-1.019) | .4620 |
| Baseline serum albumin level | 0.797 (0.594-1.068) | .1283 |
| Baseline creatinine level | 1.028 (0.928-1.139) | .5966 |
| Baseline eGFR value | 0.999 (0.990-1.008) | .8380 |
| Baseline Max SOFA | 1.054 (0.997-1.115) | .0650 |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment.
Figure 2.
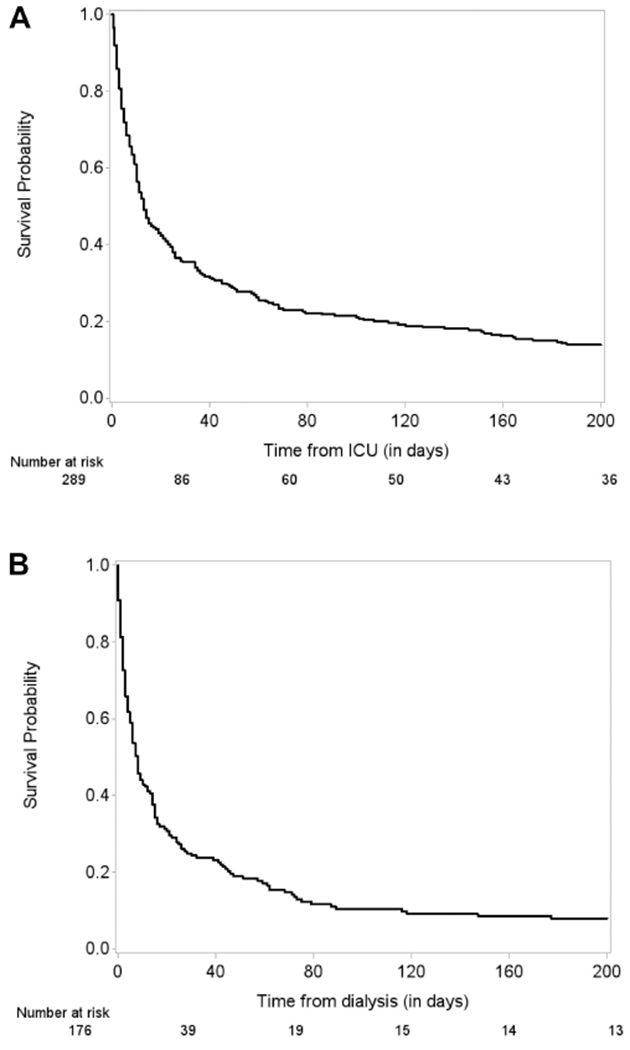
A, Overall survival (OS) for no renal replacement therapy (RRT) patients. Median survival for no RRT patients: 13 days. B, Kaplan-Meier curve of OS since initiation of RRT for RRT patients. Median survival from start of RRT for RRT patients: 8 days.
Inpatient Mortality and RRT
The association between inpatient mortality (died in the hospital as compared with discharged alive) and RRT (RRT vs no RRT) was explored using a multivariate logistic regression model. Of 465 patients, 176 had RRT versus 289 did not during the hospitalization. When baseline maximum SOFA score and baseline albumin level were adjusted, RRT was not significantly associated with inpatient mortality (OR: 1.004 [95%CI: 0.598-1.684], P = .9892; Table 5). When we excluded patients who started RRT later than 7 days from ICU admission and adjusted for baseline serum albumin and maximum SOFA score, RRT was not significantly associated with inpatient mortality (OR: 0.803 [95% CI: 0.447-1.441], P = .4618; Table 5).
Table 5.
Multivariate Logistic Regression Model on Hospital Death.
| Odds Ratio | 95% CI | P Value | ||
|---|---|---|---|---|
| All patients | ||||
| RRT vs No | 1.004 | 0.598 | 1.684 | .9892 |
| Baseline serum albumin level | 0.621 | 0.448 | 0.861 | .0042 |
| Baseline max SOFA | 1.225 | 1.161 | 1.293 | <.0001 |
| All patients excluding patients who started RRT later than 7 days from ICU admission | ||||
| RRT vs No | 0.803 | 0.447 | 1.441 | .4618 |
| Baseline serum albumin level | 0.618 | 0.439 | 0.869 | .0057 |
| Baseline max SOFA | 1.247 | 1.175 | 1.323 | <.0001 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment.
Impact of RRT on Long-Term Survival After Hospital Discharge
Among 189 patients who were discharged from the hospital alive, 46 received RRT and 143 did not during their hospital stay. Including these patients, the effect of RRT on longer term survival after discharge was evaluated. Renal replacement therapy was not associated with long-term survival after discharge (HR = 0.898 [95% CI: 0.622-1.297], P = .5652) with the median longer term survival after discharge being 41 days for RRT and 42 days for non-RRT patients (Figure 3).
Figure 3.
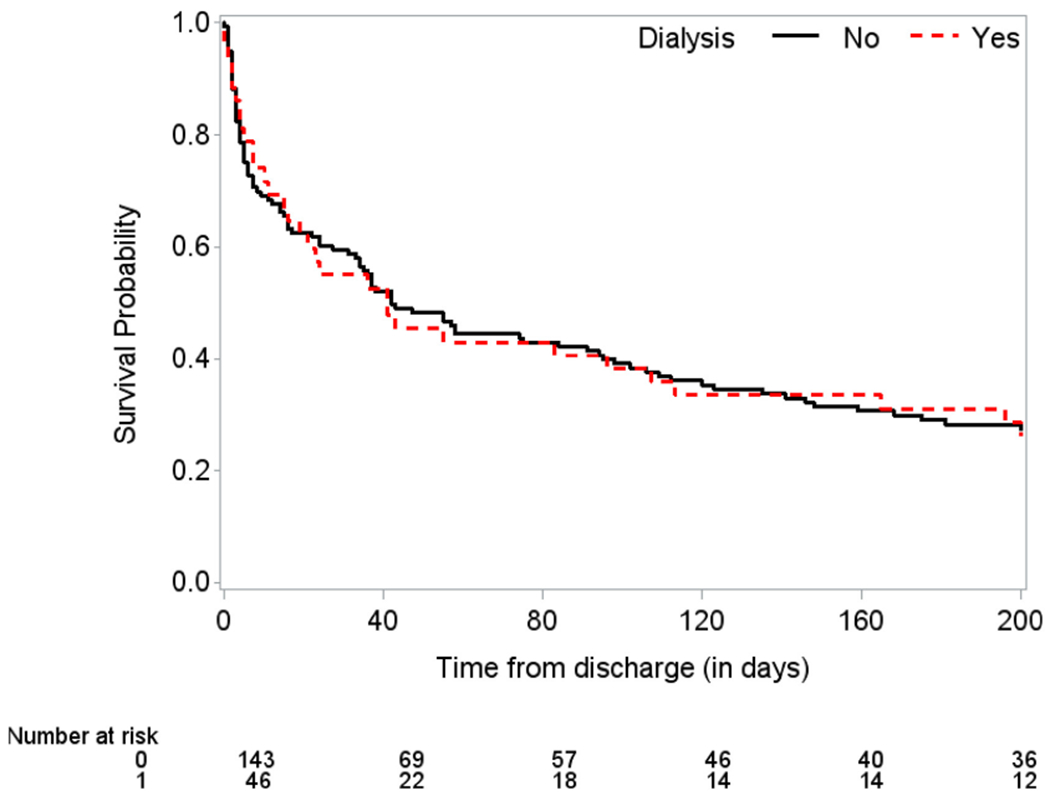
Kaplan-Meier curves of survival since discharge by renal replacement therapy (RRT) for patients discharged from the hospital. Median survival since discharge for RRT patients: 41 days. Median survival since discharge for no RRT patients: 42 days.
Discussion
After accounting for baseline maximum SOFA score and baseline albumin level, our results indicate that the use of RRT in stage IV solid tumor patients with AKI admitted to the ICU was not associated with better or worse survival. The inpatient mortality of ICU patients who received RRT was 51% and of those who did not 52% with a mean serum albumin of 2.8 and maximum SOFA score of 13. However, RRT was not significantly associated with survival after discharge from the hospital, where the difference of 1 day in median survival was noted between patients who received RRT and those who did not.
Stage IV solid tumor patients have a higher mortality than those with stage I to III disease, and mortality is further increased when kidney failure is a comorbidity. With advancements in cancer treatments, we have been able to prolong life, but the appropriateness of measures such as RRT is questioned when prolongation of dying incurs substantial emotional and financial expense.
Renal replacement therapy was also associated with a longer length of stay in the hospital with a median of 14 days when compared to the median of 11 days for patients who did not receive RRT. In a prospective study of 975 patients with all types of malignancies admitted to an ICU, patients who required RRT had worse survival than patients who did not.15 The study also demonstrated that kidney failure (HR = 1.77), performance status, the presence of uncontrolled cancer, and number of organs with failure were independent predictors of poor survival.15 The findings of our study of ICU patients are consistent with these findings in which our ICU patients’ median survival (after hospital discharge) only differed by maximum of 1 day in the RRT group versus no RRT group. Since almost half of our study cohort had been in the ICU, we sought to investigate the role of RRT in the ICU by controlling for intensity of illness using the SOFA score. We found that the patients with higher SOFA scores were more likely to receive RRT, which made sense because SOFA scores include renal failure. In a recent prospective cohort of 499 patients of patient older than 65 years of age admitted to an ICU, 361 patients who were offered RRT. The study conclusions were consistent with our findings in which the 90-day mortality was similar in patients who did and did not receive RRT.16
There has been increasing research and interest in creating tools to guide nephrologists in deciding on the appropriateness of offering RRT in patients with a poor prognosis. The Renal Physicians Association and the American Society of Nephrology published a clinical practice guideline, Shared Decision Making in the Appropriate Initiation of and Withdrawal from RRT, in 2000, which was later updated with a second edition in 2010.17 The guideline recommends a shared decision-making approach in which the physician and patient agree on a specific course of action based on a common understanding of the patient’s values, overall condition, prognosis, treatment goals and risks, and benefits of the chosen course compared to reasonable alternatives.18 With the increasing appreciation of the role of palliative care, a palliative approach to RRT has also been discussed, with the goals of promoting comfort, respecting patients’ wishes, improving quality of life, and reducing the discomfort associated with RRT.11 Using patients’ comorbidities, investigators have come up with prognostic tools to assist nephrologists and others in making decisions about initiating RRT.2,19,20 The use of the “surprise” question—“Would I be surprised if this patient died in the next year?”—has been used and validated in a study by Moss et al; the unadjusted odds of dying within 1 year for patients with end-stage renal disease in the “No, I would not be surprised” group were 3.5 times higher than for patients in the “Yes” group.21
Our study showed that RRT for AKI was not associated with increased survival in patients with advanced cancer. These findings support the usefulness of creating similar prognostic tools for patients with AKI and comorbidities including cancer to help guide patients, oncologists, and nephrologists in shared decision-making discussions prior to reaching agreement about the initiation of RRT. If RRT is started in such a patient, then it should be with a clear understanding by all parties involved of the likely high associated hospital mortality. For cases in which the patient’s outcome is uncertain, a time-limited trial of RRT can be implemented with agreed upon parameters in advance to establish whether the trial has been successful at its conclusion.22 The role of specialist palliative care physicians in such a situation could be very helpful to guide the decision-making.23 Palliative care consultation was underutilized in our study with less than 1% of our study population receiving a palliative care consult. This is even a lower percentage of palliative care consultations for AKI than that has been recently reported in the large 2012 National Inpatient Sample in which there were palliative care consultations for 8% of patients with AKI.24 Of the 41.6% patients who died during the hospitalization, 21% of the patients had hospice as their final discharge disposition.
Although there is evidence supporting the poor prognosis of patients with AKI undergoing RRT, the outcomes of RRT specifically in patients with advanced solid-tumor cancer have not been studied previously. Our study contributes this information to the literature and notes the particularly high hospital mortality of patients with advanced cancer having AKI in the ICU. With the increasing costs of medical care for patients with cancer and the longer survival of these patients, the cost of cancer care in the United States in 2010 was approximately $124.6 billion and is projected to increase by 27% to $157.77 billion in 20 20.25 In a recent study by Manzano et al, studying the rate of unplanned hospitalizations in Texas residents aged 66 years or older and initially diagnosed with gastrointestinal cancer between 2001 and 2007 (n = 2944), the most common noncancer reason for unplanned hospitalization was fluid and electrolyte disorders, affecting 8.3% of the patients.26 In another study by Obermeyer et al of Medicare fee-for-service beneficiaries with poor-prognosis cancer, those receiving hospice care compared to those not receiving hospice care had significantly lower rates of hospitalization, ICU admission (HR = 2.4, 95% CI: 2.3-2.5), and invasive procedures (RRT HR = 3.6, 95% CI: 3.1-4.2) at the end of life, along with significantly lower total costs during the last year of life.27 These studies indicate the significant cost attributed to the use of RRT at the end of life, and the potential cost savings if RRT that does not improve survival is not provided. We will be using data from this study to conduct a cost analysis in the near future.
A limitation of this study is its retrospective nature; however, we tried to overcome this shortcoming by performing propensity score-matched landmark analysis, matching for known significant predictors of poor mortality (including albumin, GFR, SOFA scores, and timing of RRT from the time of ICU admission).
Conclusions
Our study demonstrates that RRT for AKI in the ICU was not associated with worse hospital survival in patients with solid tumor stage IV cancer. Moreover, in the context of comparable longer term survival after hospital discharge in patients who did and did not receive RRT, there is the question of the impact on quality of life and suffering that these patients were subjected to at the end of life. Our findings will enable patients with stage IV cancer having AKI who are admitted to the ICU, their families, and their physicians to make a more informed decision about whether to proceed with RRT and other treatment options. Our study also demonstrates the low utilization of specialist palliative care physicians who can be most helpful by facilitating goals-of-care discussions when a patient with a serious illness such as stage IV cancer with AKI is admitted and decisions need to be made about RRT and other intensive procedures. Knowing the poor prognosis associated with RRT for AKI in the ICU, we advocate for a more comprehensive outpatient advance care planning process for patients with stage IV cancer that would identify the level of medical intervention that the patient would want to undergo in the event of future severe illness, including possible intensive treatments such as RRT.28,29
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The statistical analysis work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Evans RW, Manninen DL, Garrison LP, et al. The quality of life of patients with end-stage renal-disease. N Engl J Med. 1985; 312(9):553–559. [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM; VA/NIH Acute Renal Failure Trial Network. Predictors of health utility among 60-day survivors of acute kidney injury in the veterans affairs/national institutes of health acute renal failure trial network study. Clin J Am Soc Nephrol. 2010; 5(8):1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Investigators RRTS, Bellomo R, Cass A, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. [DOI] [PubMed] [Google Scholar]
- 4.Network VNARFT, Palevsky PM, Zhang JH, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christiansen CF, Johansen MB, Langeberg WJ, Fryzek JP, Sørensen HT. Incidence of acute kidney injury in cancer patients: a Danish population-based cohort study. Eur J Intern Med. 2011; 22(4):399–406. [DOI] [PubMed] [Google Scholar]
- 6.Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110(6):1376–1384. [DOI] [PubMed] [Google Scholar]
- 7.Janus N, Launay-Vacher V, Byloos E, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer. 2010; 103(12):1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salahudeen AK, Doshi SM, Pawar T, Nowshad G, Lahoti A, Shah P. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol. 2013;8(3):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salahudeen AK, Kumar V, Madan N, et al. Sustained low efficiency dialysis in the continuous mode (C-SLED): dialysis efficacy, clinical outcomes, and survival predictors in critically ill cancer patients. Clin J Am Soc Nephrol. 2009;4(8):1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leavitt M Medscape’s response to the institute of medicine report: crossing the quality chasm: a new health system for the 21st century. MedGenMed. 2001;3(2):2. [PubMed] [Google Scholar]
- 11.Grubbs V, Moss AH, Cohen LM, et al. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9(12):2203–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen LM, Ruthazer R, Moss AH, Germain MJ. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol. 2010;5(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan CJ. Landmark analysis: a primer. J Nucl Cardiol. 2019; 26(2):391–393. [DOI] [PubMed] [Google Scholar]
- 14.Dafni U Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. [DOI] [PubMed] [Google Scholar]
- 15.Soares M, Salluh JI, Carvalho MS, Darmon M, Rocco JR, Spector N. Prognosis of critically ill patients with cancer and acute renal dysfunction. J Clin Oncol. 2006;24(24):4003–4010. [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Adhikari NKJ, Burns KEA, et al. Selection and receipt of kidney replacement in critically Ill older patients with AKI. Clin JAm Soc Nephrol. 2019;14(4):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss AH. Ethical principles and processes guiding dialysis decision-making. Clin J Am Soc Nephrol. 2011;6(9):2313–2317. [DOI] [PubMed] [Google Scholar]
- 18.Moss AH. Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol. 2010; 5(12):2380–2383. [DOI] [PubMed] [Google Scholar]
- 19.Barrett BJ, Parfrey PS, Morgan J, et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis. 1997;29(2):214–222. [DOI] [PubMed] [Google Scholar]
- 20.Couchoud C, Labeeuw M, Moranne O, et al. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24(5): 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer JS, Holley JL. The role of time-limited trials in dialysis decision making in critically Ill patients. Clin J Am Soc Nephrol. 2016;11(2):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quill TE, Abernethy AP. Generalist plus specialist palliative care-creating a more sustainable model. N Engl J Med. 2013; 368(13):1173–1175. [DOI] [PubMed] [Google Scholar]
- 24.Chong K, Silver SA, Long J, et al. Infrequent provision of palliative care to patients with dialysis-requiring AKI. Clin J Am Soc Nephrol. 2017;12(11):1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levit L, Balogh E, Nass S, Ganz PA. eds. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 26.Manzano JG, Luo R, Elting LS, George M, Suarez-Almazor ME. Patterns and predictors of unplanned hospitalization in a population-based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32(31):3527–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Citko J, Moss AH, Carley M, Tolle S. The National POLST paradigm initiative, 2nd Edition #178. J Palliat Med. 2011; 14(2):241–242. [DOI] [PubMed] [Google Scholar]
- 29.Meghani SH, Hinds PS. Policy brief: the institute of medicine report dying in America: improving quality and honoring individual preferences near the end of life. Nurs Outlook. 2015;63(1):51–59. [DOI] [PubMed] [Google Scholar]


