Abstract
Inflammation is a hallmark of cancer and supports tumor growth, proliferation, and metastasis, but also inhibits T cell immunosurveillance and the efficacy of immunotherapy. The biology of cancer inflammation is defined by a cycle of distinct immunological steps that begins during disease conception with the release of inflammatory soluble factors. These factors communicate with host organs to trigger bone marrow mobilization of myeloid cells, trafficking of myeloid cells to the tumor, and differentiation of myeloid cells within the tumor bed. Tumor-infiltrating myeloid cells then orchestrate an immunosuppressive microenvironment and assist in sustaining a vicious cycle of inflammation that co-evolves with tumor cells. This Cancer-Inflammation Cycle acts as a rheostat or “inflammostat” that impinges upon T cell immunosurveillance and prevents the development of productive anti-tumor immunity. Here, we define the major nodes of the Cancer-Inflammation Cycle and describe their impact on T cell immunosurveillance in cancer. Additionally, we discuss emerging pre-clinical and clinical data suggesting that intervening upon the Cancer-Inflammation Cycle will be a necessary step for broadening the potential of immunotherapy in cancer.
Keywords: Cancer, Inflammation, Inflammostat, Macrophage, Myeloid Cells, T cells, Immunotherapy
1. Introduction
The immune system is hardwired with multiple checks and balances that combine to regulate the decision between elimination of non-self and the preservation of self. In cancer, immune surveillance is dependent on a balance of activating and inhibitory signals. For example, strategies to disrupt inhibitory signals (e.g. CTLA4 and PD1/PDL1) that negatively impact immunosurveillance in cancer have yielded promising treatments for patients. However, despite increasing indications for these therapies, only a minority of patients with cancer achieve significant clinical benefit. This observation reflects significant heterogeneity in the immune reaction to cancer that is detected across a wide-range of tumor histologies and even within the same tumor type. For instance, even in cancers where immunotherapy has demonstrated remarkable activity, such as non-small cell lung cancer (NSCLC) [1-4], kidney cancer [5-7], and melanoma [8, 9], many patients do not respond or ultimately relapse after initial disease control. Moreover, for several malignancies, including gastrointestinal cancers [10], brain cancers [11], and sarcoma [12], profound resistance to immunotherapy is the norm. Thus, in an effort to extend the benefits of immunotherapy, active investigations aim to identify determinants of response and resistance. One such determinant that has consistently emerged as a key mechanism of resistance is inflammation.
Inflammation is a hallmark of cancer and develops agnostically to tumor histology [13]. In this context, cancer-associated inflammation is defined by the recruitment of leukocytes to tumors and their release of soluble factors. Historically, inflammatory cells and the factors that they release within the tumor microenvironment have been perceived as the main mediators of cancer progression, metastasis and resistance to therapy. However, cancer-associated inflammation extends beyond the tumor microenvironment giving rise to chronic systemic inflammation which is characterized by increases in inflammatory cells and proteins detected in the peripheral blood of patients. Systemic chronic inflammation associates with poor outcomes to immunotherapy [14]. Moreover, accumulating evidence demonstrates that markers of systemic chronic inflammation are not only predictive of outcomes, but may directly mediate resistance to cytotoxic therapies (e.g. chemotherapy and radiotherapy) [15] and enforce immunosuppression by impinging upon the development of productive anti-tumor immunity [16, 17]. Taken together, inflammation has emerged as a major therapeutic target that will need to be reconciled to extend the benefit of cancer immunotherapy.
In this review, we discuss recent insights into the impact of inflammation on resistance to cancer immunotherapy. In our discussion, we highlight elegant studies in mice and humans to illustrate that factors secreted by tumor and host cells align to coordinate a systemic inflammatory reaction, acting as an inflammatory rheostat, which we call the “inflammostat”. As discussed in detail in subsequent sections, these molecules trigger mobilization of myeloid cells from the bone marrow, myeloid cell trafficking and infiltration into tumors, and the differentiation of infiltrating myeloid cells which then orchestrate immune suppression in the tumor microenvironment [18]. Within tumors, the myeloid cell reaction sustains the inflammostat creating a vicious cycle that fosters tumor cell survival, invasion and metastasis as well as evasion of the adaptive immune system [13, 19]. This biology emphasizes the complexity of the cancer-host interaction and identifies a series of well-defined events that may be targeted therapeutically to divert the inflammatory reaction to cancer from tumor-promoting to tumor-suppressive. A major challenge moving forward is in how to combine and sequence drugs to disrupt cancer inflammation and to prevent the emergence of compensatory mechanisms of inflammation that are equally supportive of cancer progression. It is important to be mindful that the inflammatory reaction in cancer may differ across tumor histologies and even within the same histology. As a result, our focus in this review is on fundamental mechanisms that define elements of the inflammatory process in cancer without an emphasis on any specific tumor histology.
2. The Cancer Inflammation Cycle
Cancer biology is defined by multiple hallmarks that coalesce to instruct its complexity [13]. These hallmarks include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Inflammation and genomic instability are key determinants and enablers of these hallmarks [13]. However, tumor cells must also evade immune elimination. Accordingly, cells arising from both the innate (e.g. myeloid cells) and adaptive (e.g. T and B lymphoid cells) arms of the immune system influence the evolution and biology of cancer. In this regard, myeloid cells, including monocytes, macrophages, dendritic cells, neutrophils, eosinophils and mast cells, are fundamental determinants of cancer biology [14, 20]. Myeloid cells are recruited almost universally to a developing tumor [18]. Within the tumor, myeloid cells secrete survival factors and remodel the surrounding microenvironment to promote tumor cell growth and to protect the tumor from immune attack. In addition, myeloid cells assist in tumor cell invasion of the surrounding extracellular matrix and in doing so, direct tumor cells to intravasate into the bloodstream and begin the metastatic journey to distant organs [21]. Overall, myeloid cells co-evolve with tumor cells and serve as major proponents for cancer development and progression.
Within tumors, macrophages represent the predominant recruited myeloid cell type [18]. Macrophages can be detected at the earliest stages of carcinogenesis where they persist through development of invasive disease and accompany tumor cells in the formation of metastatic lesions [22-24]. The life cycle of macrophages and their precursors is fundamental to cancer biology. This process, which we call the Cancer-Inflammation Cycle (Figure 1), is integral from cancer conception. Indeed, the initiation of the Cancer-Inflammation Cycle can precede the emergence of a tumor cell. For example, inflammation (e.g. gastritis, esophagitis, colitis, pancreatitis, hepatitis, etc) is a major risk factor for cancer development in humans [25]. Cancer inflammation is also a determinant of immunoediting (reviewed in detail elsewhere [26, 27]), a process during which tumor cells are sculpted, or edited, with decreased susceptibility to immune recognition and elimination. Immunoediting proceeds sequentially through distinct phases, termed “elimination”, “equilibrium”, and “escape”. This process is dependent on a dynamic interconnection between innate and adaptive immunity. During the elimination phase, innate immunity may contribute to productive anti-tumor immunity via cross-presentation of antigens by dendritic cells for priming of tumor-specific T cells [26, 27]. However, innate and adaptive immunity eventually vie for dominance in the equilibrium phase [26, 27]. During this phase, inflammation seeks to undermine the anti-tumor productivity of T cell immune surveillance. Ultimately, inflammation prevails, and tumor cells escape immune elimination leading to disease progression. In our description of the Cancer-Inflammation Cycle, our focus is on macrophages as well as macrophage precursors and their involvement in shaping T cell immunosurveillance in cancer. However, the Cancer-Inflammation Cycle is applicable to all innate immune cell subsets that accumulate in tumors and contribute to cancer biology.
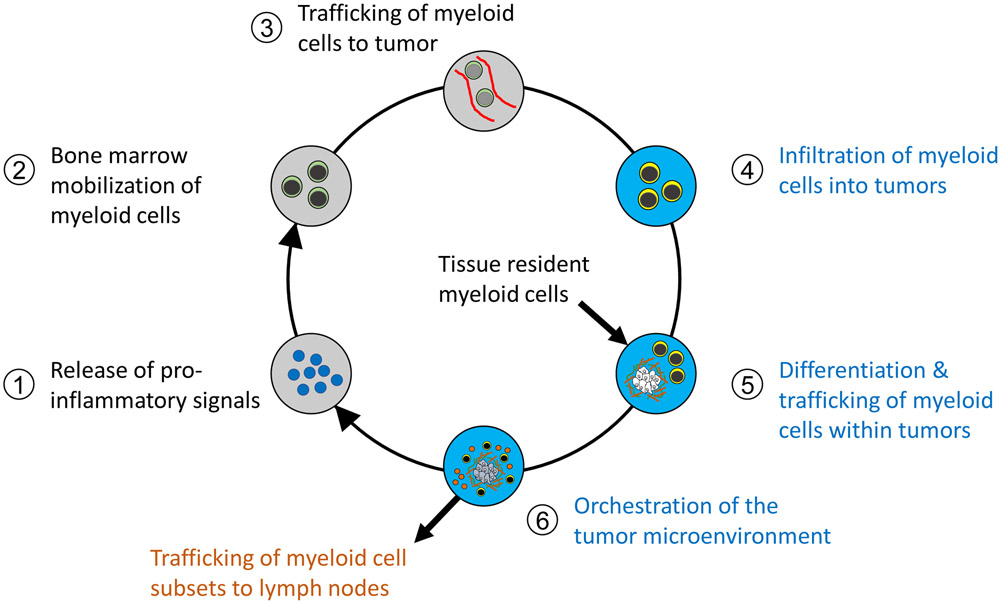
Figure 1. The Cancer-Inflammation Cycle.
Cancer inflammation is a cyclic process that is initiated at disease conception. The cycle is dynamic and evolves with cancer progression. This cycle can be divided into six major steps, starting with the release of pro-inflammatory signals that elaborate the mobilization (from bone marrow or adjacent tissues), recruitment, and differentiation of myeloid cells. Tumor-infiltrating myeloid cells then orchestrate a microenvironment that is supportive of cancer growth, metastasis, immune evasion, and therapeutic resistance. Each step is described above, with the anatomic location of each immunologic event given including processes occurring extra-tumoral (grey), intra-tumoral (blue), and within draining lymph nodes (orange).
The Cancer-Inflammation Cycle (Figure 1) is composed of multiple steps which we present below and describe in greater detail in subsequent sections of this Review. The first step of the Cancer-Inflammation Cycle involves the release of pro-inflammatory factors by tumor and stromal cells with the tumor microenvironment (step 1). These factors accumulate systemically and trigger bone marrow mobilization of monocytes (step 2). Monocytes then circulate through the bloodstream and traffic to (step 3) and infiltrate the tumor bed (step 4). Within the tumor, monocytes differentiate in response to cues from their surrounding microenvironment (step 5). This differentiation process is fundamental to defining the array of monocyte-derived cell phenotypes present within tumors which extend well beyond the classically defined macrophage subsets that have been described as M1 (i.e. tumor-suppressive) or M2 (i.e. tumor-supportive) macrophages [28-31]. For example, macrophage subsets can also be defined spatially within tumors as peri-vascular, stromal, and peri-tumoral. This infantry of monocyte-derived cells then orchestrates multiple tumor-dependent functions (step 6) including angiogenesis, matrix remodeling, immunosuppression, and metastasis which are hallmarks of cancer [13].
The Cancer-Inflammation Cycle coordinates the myeloid compartment in tumor and lymphoid tissues. For instance, bone marrow-derived myeloid cells are the predominant source of tumor-infiltrating myeloid cells and mediate resistance to therapies, including chemotherapy, radiotherapy, and immunotherapy [19, 32]. In mouse models of lung and pancreatic cancer, tissue-resident myeloid cells, such as embryonic-derived macrophages, have also been found to contribute to the myeloid reaction and serve distinct roles from bone marrow-derived cells in orchestrating the tumor microenvironment and supporting tumor development [33, 34]. Whereas some tumor-infiltrating myeloid cells may be retained indefinitely within tumors, others (e.g. dendritic cells) may ultimately exit tumors to enter draining lymph nodes for presentation of antigen [35]. In doing so, the Cancer-Inflammation Cycle converges with the Cancer-Immunity Cycle [36] which defines key processes fundamental to the productivity of T cell immunosurveillance in cancer. Specifically, the Cancer-Inflammation Cycle regulates the priming and effector phases of the Cancer-Immunity Cycle by directing the immunoregulatory functions of myeloid cells in lymph nodes and tumor, respectively. For instance, myeloid cells are fundamental in the priming of tumor-specific T cells and regulate the effector activity of T cells in tumors [37]. Thus, the Cancer-Inflammation Cycle details steps for maintaining the inflammatory reaction in cancer which is also a fundamental determinant of T cell immunosurveillance. In addition, it portrays a conceptual model that may be used to understand and potentially disrupt or even redirect inflammation in cancer.
Cancer inflammation is a major regulator of productive T cell immunity. Most commonly, inflammation is viewed as an obstacle to T cell immunosurveillance. However, the pliability of myeloid cells renders them vulnerable to re-education and as a result, to shifting their phenotype from immunosuppressive to immunostimulatory [14]. To this end, triggering a potent T cell immune response against cancer may lead to a reshaping of the infiltrating myeloid cell reaction which then becomes essential, rather than an obstacle, to the success of immunotherapy. Thus, the Cancer-Inflammation and Cancer-Immunity Cycles are interdependent and together may be viewed as containing the necessary elements for generating both pro- and anti-tumor inflammation. A major challenge is how to remove or redirect inhibitory elements of the inflammatory reaction that restrain the potential of productive T cell immunosurveillance without interfering with components necessary for a successful immune response.
3. Inflammation as a determinant of immune escape in cancer.
The capacity to evade immune recognition is characteristic of cancer [13] and is facilitated, at least in part, by chronic inflammation [19]. Tumor cells exploit four hallmarks of immune escape defined by decreased antigenicity, reduced immunogenicity, formation of an immunosuppressive microenvironment, and impairment of immune capacity, which refers to the ability of the immune system to produce anti-tumor activity [38]. Inflammation contributes to each of these hallmarks which we discuss below. For example, inflammation shapes tumor cell antigenicity, or the presence of antigens capable of eliciting an immune response. Tumors which lack sufficient antigenicity may appear invisible to the immune system. However, antigenicity can be toggled and in this regard, it can be enhanced by myeloid cell-derived reactive chemical entities (e.g. reactive oxygen species) which display mutagenic potential and trigger genome-wide DNA mutations in mice [39]. Similarly, in patients, chronic inflammation in the setting of Helicobacter Pylori infection has been shown to associate with distinct genetic alterations in the gastric epithelium [40]. This process appears mediated by upregulation of activation-induced cytidine deaminase (AID), a DNA-mutator enzyme [40]. Further, TNF-induced AID expression has been linked to genetic mutations in human colonic epithelial cells [41]. Thus, inflammation may be a determinant of mutational load in human cancer. In mouse models of lymphoma, sarcoma, and breast cancer, IFN-γ exposure has also been associated with DNA damage [42]. Overall, increased mutational burden in tumor cells supports the emergence of mutated neoantigens which may be recognized by the immune system as non-self [43-46]. However, inflammatory mediators (e.g. TNF-α) can also reduce the immunogenicity of tumor cells, such as by increasing PD-L1 expression [47]. In mouse models of melanoma and colorectal cancer, overexpression of PD-L1 on tumor cells is sufficient to promote immune evasion by immunogenic tumors [48]. In addition, mouse models show that IL-6 produced by inflammatory cells induces STAT3 signaling in tumor cells and their paracrine release of immunosuppressive molecules (e.g. VEGF, IL-10) which then impair cancer immunosurveillance [49]. Thus, inflammation is not only a determinate of tumor cell antigenicity but can coordinate the release of inflammatory mediators which reduce tumor cell immunogenicity, or the capacity to trigger a productive adaptive immune response.
Tumor cells must ultimately circumvent immune pressure arising from acquisition of antigens that provoke dissimilarity from self. Darwinian selection imposed by the immune system enforces the emergence of tumor cells with reduced antigenicity, reduced immunogenicity, or both [26, 38]. However, tumor cells also establish a microenvironment, of inflammatory cells and other stromal cells that restrain the productivity of T cell immunosurveillance. These inflammatory cells deplete vital nutrients (e.g. tryptophan and arginine) necessary for T cell effector activity [50], while also facilitating the production of immunosuppressive elements (e.g. adenosine) that impair anti-tumor T cell biology [51]. In doing so, the stromal microenvironment that surrounds tumor cells assumes a hostile phenotype that fosters immune dysfunction rather than activation. Chronic systemic inflammation in the setting of cancer may also impair the functional capacity, or fitness, of the immune system. To this end, a clinical study in patients with advanced adenoma of the colon showed that increased mobilization of myeloid cells from the bone marrow associates with decreased responsiveness to a MUC1 vaccine [52]. Indeed, myeloid cells can directly impinge upon adaptive immunity via multiple mechanisms, including depletion of key amino acids (e.g. tryptophan and arginine) that are critical for T cell function [53], and production of immunosuppressive molecules, such as reactive oxygen species and nitric oxide which impair T cell activity [54]. In addition, chronic inflammation impairs the generation of memory T cell responses [55], which might be important for preventing disease relapse. Thus, local and systemic inflammation in cancer may influence the quality of immune cells and in doing so, may impact the success of immunotherapy.
4. The Inflammostat
During cancer development, inflammation is detected not only locally within a developing tumor, but also systemically. Collectively, local and systemic inflammatory responses form an inflammostat that contributes to the capacity of tumor cells to evade immune recognition and elimination. Within the blood, systemic inflammation associates with increases in inflammatory markers, such as neutrophils, monocyte subsets, C-reactive protein (CRP), IL-6, and serum amyloid A (SAA). These markers correlate with poor prognosis but may also impinge on treatment outcomes. For example, multiple meta-analyses show that an elevated neutrophil-to-lymphocyte ratio (NLR) associates with poor survival in patients treated with chemotherapy, independent of tumor histology [56-58]. In addition, an elevated NLR in patients with melanoma and NSCLC is associated with poor outcomes to immune checkpoint blockade therapy and correlates with reduced overall survival for patients with renal cell carcinoma treated with high-dose IL-2 [59-63]. Similarly, accumulation within the blood of CD11b+ myeloid cells lacking HLA-DR correlates with decreased responsiveness to vaccines in patients [52]. CRP, which is primarily synthesized in the liver in response to IL-6, also associates with reduced survival in patients with melanoma treated with immune checkpoint blockade therapy [16, 64, 65]. However, these markers are not only prognostic but may also directly impair immune fitness. In support of this, CRP can inhibit T cell proliferation by disrupting calcium-dependent T cell signaling [65-67] and promote the expansion of myeloid cells with suppressive properties [68]. Similarly, SAA is an acute phase reactant produced primarily in the liver by hepatocytes and has been found to be a potent stimulator of myeloid cells leading to release of cytokines and chemokines, including IL-6, CXCL1, and G-CSF, which may then reinforce the systemic inflammatory reaction [69-71]. Taken together, the inflammostat, comprised of both systemic and local inflammation, is not only prognostic of poor outcomes in cancer but also a determinant of immunobiology.
The inflammostat is defined by the contexture of the inflammatory response to cancer and includes the type, polarity, and density of inflammatory immune cells (Figure 2). The parameters establishing this contexture consist of macrophages, immature myeloid cells, and dendritic cells as well as other innate immune cells (e.g. mast cells, eosinophils) that are detected locally within tumors and systemically in the blood. These cellular parameters associate with a polarity that defines their functional connotation and thus, their capacity to either support or inhibit productive T cell immunosurveillance in cancer. In the blood, systemic inflammation correlates with alterations in liver-specific acute phase reactants including increased CRP and SAA as well as a decrease in blood albumin levels [14]. Inflammatory mediators (e.g. G-CSF, CCL2, IL-8) released in response to cancer development trigger increased bone marrow mobilization of some myeloid cell subsets and interrupt the mobilization of others. For example, bone marrow mobilization of CD15+ neutrophils and CD14+ monocytes lacking HLA-DR accumulate in the blood of both mice and humans with systemic inflammation [52, 72, 73]. In contrast, bone marrow production of conventional dendritic cell subsets may be diminished, as seen in mice as well as patients with breast and pancreatic cancer [74]. Within tumors, myeloid cell accumulation is influenced by chemokine signatures established within the tumor microenvironment. To this end, chemokines lure circulating myeloid cells to gather within tumors where they then regulate T cell entry or exclusion. For example, CD103+ dendritic cells that infiltrate tumors can support T cell recruitment and activity via interferon-dependent release of CXCL9/10 [75]. In the MC38 colorectal cancer model, CXCL9 production by CD103+ dendritic cells was found to be critical for anti-tumor activity mediated by CXCR3+ T cells in the setting of PD1 blockade [76]. In contrast, increased macrophage accumulation in tumors most often portends a poor prognosis and commonly displays an inverse relationship with T cell infiltration in mouse and human cancers [77, 78]. To this end, macrophages expressing scavenger receptors (e.g. CD206 and CD163) are recognized for their capacity to orchestrate immunosuppression [79, 80]. However, the cellular phenotype of macrophages ultimately governs their behavior. For instance, myeloid cells can be induced by interferons to activate the STAT1 signaling pathway and in doing so, increase their expression of MHC and co-stimulatory molecules (e.g. CD86) that are critical for supporting T cell activation and effector activity [81, 82]. However, these signals also enhance the production of immunoregulatory molecules, such as PDL1 and IDO, which subsequently aim to dampen an ongoing immune response [83, 84]. Thus, the inflammostat is dynamic and not only defined by inflammatory cells and their mediators but also by their functional phenotype, or polarity.
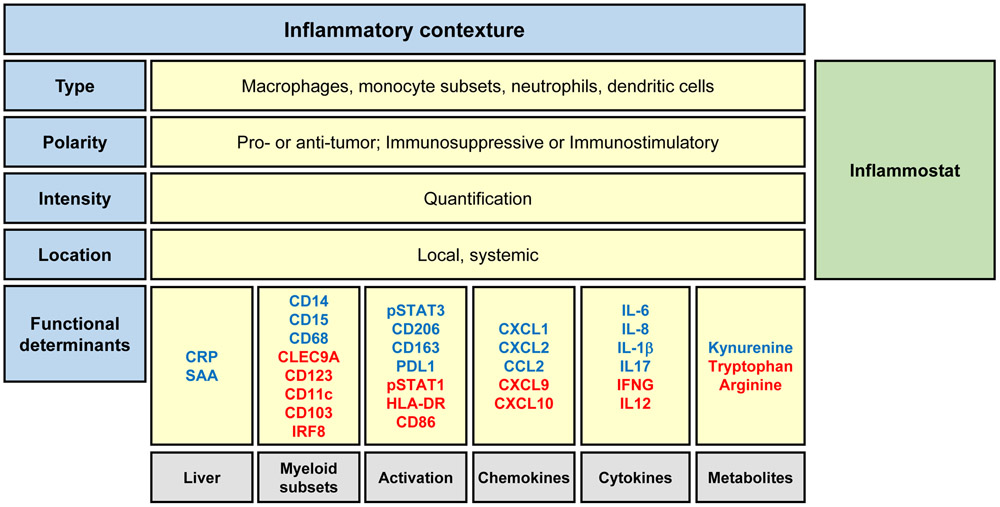
Figure 2. Graphical representation of the Inflammatory Contexture and Inflammostat.
The inflammatory contexture is defined as the type, polarity, intensity, and location of cancer inflammation. Parameters comprising the inflammatory contexture include myeloid cell subsets detected within the blood and tumor (type), the functional phenotype of myeloid cells (polarity), and the intensity of the inflammatory response (intensity) determined by cell density within tumors and the magnitude of inflammatory factors present within the bloodstream (location). The immunostat, a rheostat of the inflammatory reaction, is derived from the magnitude and polarity of the inflammatory contexture and serves as a fundamental regulator of immunosurveillance. A high inflammostat signifies an immunological state that is unlikely to respond to therapies that seek to solely engage T cell immunosurveillance by derailing immune checkpoints (e.g. CTLA4 and PD1/PDL1). The functional orientation of the inflammatory contexture is characterized by proteins and their activation status (e.g. phosphorylation) that predict the phenotype of inflammatory cells (e.g. immunostimulatory or immunosuppressive) and that associate with responses to immunotherapy. Shown are a select set of proposed determinants and their association with immune suppression (blue) or stimulation (red). Abbreviations: CRP, c-reactive protein; CXCL/CCL, chemokine motif ligands; IFNG, interferon gamma; IRF8, interferon regulatory factor 8; IL, interleukin; PDL1, programmed death-ligand 1; SAA, serum amyloid A; STAT, signal transducer and activator of transcription.
The importance of inflammation in cancer is increasingly appreciated. Preclinical models have established inflammation as a generalized mechanism of resistance to chemotherapy, radiotherapy, and immunotherapy. Despite this, strategies to merely disrupt or redirect elements of the inflammatory response have produced little clinical benefit for patients with advanced malignancies. In contrast, intervening on inflammation as an approach to prevent cancer development or its recurrence has produced promising outcomes. For example, exploratory results from a randomised, double-blind, placebo-controlled trial showed that blockade of IL-1β associated with decreased lung cancer incidence and mortality in patients with atherosclerosis and prior myocardial infarction [85]. Similarly, anti-inflammatory medications including aspirin, which irreversibly inactivates the cyclooxygenase (COX) enzyme, and celecoxib, a selective COX-2 inhibitor, have shown activity in reducing the risk of cancer for select patient populations in multiple prospective studies [14].Consistent with this, preclinical models of pancreatic cancer show that mere expression of oncogenic Kras is insufficient to promote cancer development in the absence of inflammation [86, 87]. Similarly, in mouse models of multiple myeloma, cancer progression is dependent on inflammatory mediators, specifically IL-18, that drive the generation of myeloid-derived suppressor cells to obstruct T cell immunosurveillance [88]. Together, these findings suggest that the inflammostat may assume distinct roles depending on the stage of cancer development. During the earliest stages of cancer conception, the inflammostat underpins tumor cell potential. However, in later stages of cancer, the inflammostat is a facilitator of cancer progression.
5. Blockade of the initiators of the cancer inflammation cycle
The Cancer-Inflammation Cycle defines multiple distinct steps that may be therapeutically targeted (Figure 3). For example, signaling pathways that coordinate the initiation of the cycle offer potential targets for therapy. One such target is focal adhesion kinase (FAK), a non-receptor tyrosine kinase family member that is activated in tumor cells [89-91]. FAK regulates secretion of multiple soluble factors involved in macrophage recruitment and polarization, granulocyte recruitment, and matrix remodeling [89, 90]. Tumor-derived chemokines, such as CCL2 and CXCL1, which lure macrophages and neutrophils, respectively, into the tumor microenvironment represent a second class of targets for intervening on cancer inflammation [72, 78, 92-98]. A third class of targets includes genomic drivers of cancer, such as oncogenic Kras, which trigger tumor cells to release soluble factors, including ICAM-1 and GM-CSF, which then attract macrophages early during tumor development to facilitate tumor growth, metastasis, and T cell exclusion [22, 99, 100]. In a mouse model of lung cancer, Myc was also shown to cooperate with oncogenic Kras activation to support inflammation and simultaneously, to drive immunosuppression by facilitating tumor cell release of CCL9 and IL23. Whereas IL23 coordinated exclusion of adaptive immunity, CCL9 attracted macrophages to tumors in a manner that required sustained Myc activation [101]. To this end, Myc deactivation was found to reverse inflammation as well as immunosuppression and correlated with tumor regression. Thus, strategies to intervene on oncogene-driven signaling pathways or on downstream molecules may hold promise for reprogramming the immunological state of tumors.
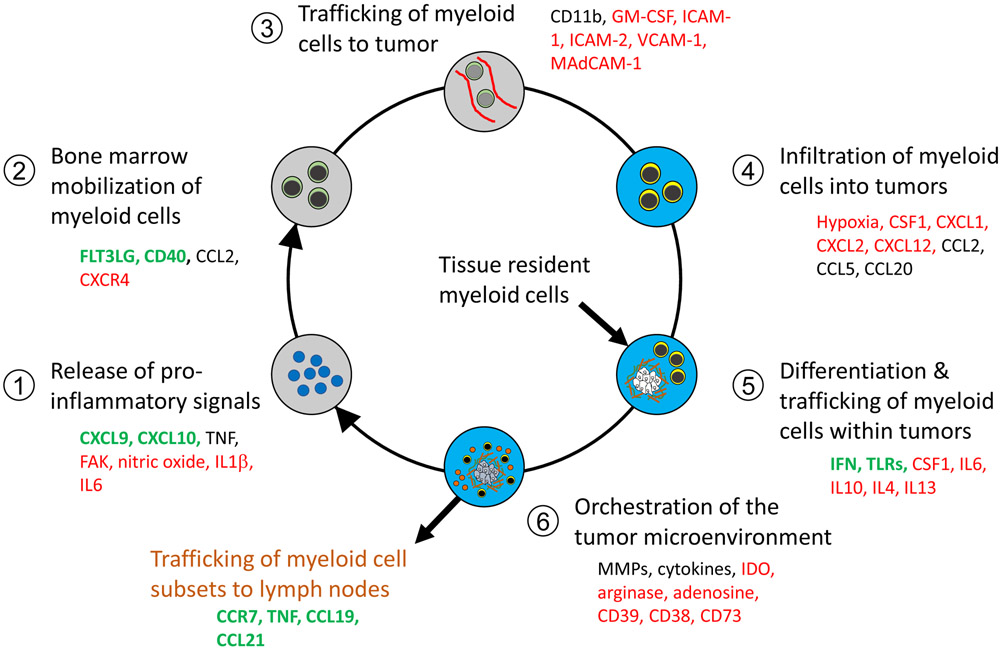
Figure 3. Regulatory factors that shape the Cancer-Inflammation Cycle.
Each step of the Cancer-Inflammation Cycle is coordinated by an array of factors. Factors shown in green may stimulate anti-tumor immunity, whereas factors shown in red engage an inflammatory response that more commonly suppresses anti-tumor immunity. Factors that are in black may support either the stimulation or suppression of anti-tumor immunity. Together, these factors contribute to the inflammatory contexture of cancer and establish an inflammatory rheostat (“inflammostat”). In addition, these factors identify potential therapeutic targets that may be derailed to disrupt the Cancer-Inflammation Cycle as a strategy to disengage the pro-tumorigenic potential of inflammation and redirect the inflammatory response with immunostimulatory properties. Abbreviations: CSF1, colony stimulating factor 1; IL, interleukin; CXCL/CCL, chemokine motif ligands; FAK, focal adhesion kinase; FLT3Lg, Fms related tyrosine kinase 3 ligand; ICAM, intracellular adhesion molecule; IDO, indoleamine 2,3-dioxgenase; IFN, interferon; MAdCAM-1, mucosal addressin cell adhesion molecule 1; MMP, matrix metalloproteinase; TLR, toll like receptor; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule.
Tumors can adapt swiftly to changes in their surrounding microenvironment. For example, tumor cells respond to cytotoxic stress (e.g. radiation and chemotherapy) by increasing their release of myeloid chemoattractants (e.g. CCL2 and CSF1) which then support neovascularization and elaboration of immunosuppression within tumors [94, 102]. However, disruption of a single chemokine signaling pathway can also invoke compensatory mechanisms that sustain pro-tumorigenic inflammation, immunosuppression and foster therapeutic resistance. For example, blocking the recruitment of either CXCR2+ neutrophils or CCR2+ monocytes is sufficient in a mouse model of pancreatic cancer to trigger a reciprocal increase in the alternative cell population [95]. This compensatory response to disruption of the inflammatory reaction thwarts the efficacy of chemotherapy but can be circumvented by dual targeting of both the CCR2 and CXCR2 signaling pathways. Similarly, mouse models have revealed that inhibition of CSF1R as a strategy to deplete tumor-associated macrophages arouses cancer-associated fibroblasts which release chemoattractants to facilitate the recruitment of CXCR2+ neutrophils [103]. Together, these findings illustrate the significance of redundancy mechanisms that coordinate the myeloid reaction in cancer. However, while blockade of inflammatory elements trigger compensatory mechanisms, withdrawal of chemokine blockade has been found in a mouse breast cancer model to accelerate cancer progression as a result of a rebound in myeloid cell recruitment [104]. This finding raises important concerns with the translation of strategies aimed at long-term disruption of myeloid cell trafficking in cancer. One approach to circumventing this issue may be to incorporate chemokine blockade administered temporally with cytotoxic- and immune-based therapies to transiently disrupt the recruitment of myeloid cell subsets that act to inhibit treatment efficacy. Nonetheless, these preclinical studies suggest that the cancer inflammostat is hardwired with resilience and as a result, it may not be sufficient to disrupt a single mechanism underpinning the initiation and recruitment of inflammation but rather require a coordinated attack on multiple mechanisms and steps within the Cancer-Inflammation Cycle.
6. Myeloid-dependent orchestration of immune suppression in cancer
Tumor-infiltrating myeloid cells undergo differentiation to establish extraordinary diversity that is detected transcriptionally as well as spatially within tumors. However, the precise mechanisms by which this infantry of myeloid cells cooperates to direct biology within tumors is still not completely understood. Despite this, it is clear that myeloid cells are influential in defining the immunological state of the tumor microenvironment. To this end, tumor-infiltrating macrophages deplete essential amino acids necessary for T cell effector function. For instance, indoleamine 2,3 dioxygenase (IDO), produced by macrophages, catabolizes tryptophan (Trp) to kynurenine (Kyn). In mouse models of cancer, IDO regulates immune tolerance by depleting tryptophan levels needed for T cell activity and by producing tryptophan metabolites, such as kynurenine, that support immunosuppression [105-107]. Increased IDO activity in patients with cancer is marked by a decrease in the serum Trp/Kyn ratio and associates with a poor prognosis [105, 108]. Similarly, neutrophils express and release arginase into their surrounding microenvironment to deplete arginine levels [50]. Arginine is an important amino acid involved in cellular proliferation, protein synthesis, metabolite production (e.g. polyamines and nitric oxide), and T cell biology. Increased arginase expression is a classic mechanism of T cell suppression mediated by myeloid cells [54, 109-111]. In cancer patients, plasma levels of arginine are commonly decreased [112]. Notably, decreased arginine concentrations directly affect T cell metabolism and in doing so, impair T cell survival and their anti-tumor activity [113]. Taken together, myeloid cells shape the metabolic state of the host as well as the tumor microenvironment with implications for the efficacy of T cell immunosurveillance in cancer.
Although myeloid cells residing within tumors are fundamental to defining the immunological state of the tumor microenvironment, macrophages residing outside of tumors also regulate immunosurveillance in cancer [114]. To this end, depletion of extratumoral macrophages in secondary lymphoid organs may be sufficient to facilitate the infiltration of T cells into spontaneously-arising tumors. Moreover, T cell-dependent anti-tumor efficacy achieved with chemotherapy and a CD40 agonist was found to be dependent on elimination of extratumoral macrophages [114]. It is noteworthy, though, that this same immunotherapy approach, in which chemotherapy is combined with a CD40 agonist, was effective at facilitating T cell infiltration into implanted tumors without a requirement for elimination of extratumoral macrophages [114]. This observation implies that extratumoral macrophages are co-opted during spontaneous cancer development and that mechanisms of immune resistance may differ depending on the tumor model investigated. Nonetheless, elements of inflammation occurring outside of tumors may be critical in defining T cell immunosurveillance in cancer.
Tumor-infiltrating macrophages can regulate immunosurveillance in cancer by intermingling with T cells in the stromal microenvironment that surrounds tumor cells. In this regard, macrophages form long-lived interactions with T cells and in doing so, can sequester T cells away from tumor cells [77]. Within tumors, macrophages upregulate immunoregulatory molecules, such as PD-L1, which protects them during antigen presentation from T cell-mediated killing [115], but also imparts them with the capacity to restrain both T cell proliferation and release of cytokines [116, 117]. Although IFN-γ is a major mechanism for upregulation of PD-L1 on myeloid cells, PD-L1 expression can be triggered by multiple mechanisms. For example, hypoxia can upregulate PD-L1 on myeloid cells in multiple mouse models of cancer [117]. Tumor cells can also instruct myeloid cells to upregulate PD-L1 in a COX2/PGE2 dependent manner [116]. However, the capacity of myeloid cells to inhibit T cells in a cell-contact dependent manner extends beyond PD-L1. Macrophages and other antigen presenting cells can express other checkpoint molecules, such as B7S1, which affect T cell differentiation in tumors and limit their expansion and cytolytic activity [118]. Signaling proteins, such as PI3Kγ and BTK, also engender tumor-infiltrating macrophages in mouse models of breast and pancreatic cancer with a transcriptional program that supports T cell suppression [119, 120].
Within tumors, myeloid cells actively remodel the extracellular matrix as if attempting to repair a wound. In this process, they respond to signals, such as ATP, which can be released by dying tumor cells [121]. Myeloid cells use P2 adrenergic receptors (e.g. P2X7) to sense extracellular ATP (eATP), which acts as a Danger-Associated Molecular Pattern (DAMP) to trigger inflammation [122, 123]. For example, eATP acts as a chemoattractant for circulating neutrophils [124-128]. During inflammation, eATP also undergoes phosphohydrolysis by ectonucleotidases. Specifically, eATP is degraded to AMP by CD39 and then hydrolyzed by CD73 to adenosine and inorganic phosphate [51]. CD39 is expressed by an array of tumor-infiltrating leukocytes, including regulatory T cells, effector T cells, and myeloid cells. Notably, CD39 is upregulated on activated T cells; is suggested to mark tumor-reactive effector T cells within tumors; but is also associated with reduced T cell polyfunctionality [45, 129-131]. In contrast, CD39 expression on myeloid cells correlates with their capacity to suppress T cell functions [132]. Similarly, while CD73 is expressed on macrophages, multiple cell subsets can express CD73 including tumor cells, regulatory T cells, lymphocytes, and dendritic cells [133]. Together, CD39 and CD73 are important factors regulating adenosine production.
Whereas adenosine supports wound healing in non-malignant settings [134, 135], production of adenosine in cancer is associated with immunosuppression [51]. The suppressive activity of adenosine is ascribed to activation of adenosine A2A receptors (A2AR) which elicit the expansion of regulatory T cells but also impair the cytotoxic, proliferative and cytokine release potential of effector T cells [51]. Myeloid cells also express A2ARs and respond to adenosine by restraining anti-tumor T cell activity [136]. In a mouse model of colon adenocarcinoma, CD39 antibodies were used to inhibit eATP conversion to adenosine and in doing so, were found to augment eATP-P2-mediated proinflammatory responses [137]. In this study, blockade of CD39 enzymatic activity triggered intra-tumoral macrophage depletion and NALP3 inflammasome activity leading to generation of IL-1β and IL-18. Altering the inflammatory reaction in tumors with CD39 blockade also enhanced T cell proliferation and effector function as well as reversed anti-PD1 resistance. Combining antibodies that block both CD39 and CD73 to disrupt their sequential activity for producing adenosine has also been explored using peripheral blood mononuclear cells isolated from breast cancer patients [138]. In this model system, addition of ATP suppressed human T cell proliferation in response to CD3-CD28 stimulation. This suppression was more effectively reversed with combined antibody blockade of both CD39 and CD73 compared to either alone. These data support a role for CD39 as a pivotal regulator in balancing the pro-inflammatory effects of eATP with CD73-dependent production of adenosine which favors immunosuppression. Overall, it is evident that tumor-infiltrating myeloid cells convey a variety of regulatory strategies capable of supporting tumor cell evasion of T cell immunosurveillance.
7. Toggling the phenotype of the inflammatory reaction in tumors
Although cancer inflammation is most commonly skewed toward promoting tumor growth and inhibiting immune activation, the inherent pliability of myeloid cells raises the possibility that inflammation may be redirected to have anti-tumor and immunostimulatory properties. As such, the Cancer-Inflammation Cycle may also be viewed as a model for devising strategies capable of harnessing the therapeutic potential of the inflammatory response to cancer.
The phenotype of myeloid cells is defined by an array of signals received from the surrounding microenvironment. For example, CSF-1, IL-4, IL-13, and IL-10 are cytokines that contribute to the development of myeloid cells displaying immunosuppressive features. In contrast, IFN-γ and acute Toll-Like Receptor (TLR) stimulation (e.g. LPS) have long been associated with instructing myeloid cells with anti-tumor properties [28, 139]. In cancer, inflammatory monocytes are recruited to tumors and are most commonly viewed as myeloid-derived suppressor cells [73]. However, in mouse models of pancreatic cancer, this same myeloid cell subset can be engendered with anti-tumor and anti-stromal properties in response to systemic activation of the CD40 signaling pathway [140-142]. In this regard, CD40 stimulation triggers the release of IFN-γ which induces inflammatory monocytes with anti-tumor properties and instructs them to facilitate the release of matrix metalloproteinases within tumors that then transiently resolve elements of the stromal matrix. This anti-stromal response sensitizes tumor cells to cytotoxic chemotherapy. Similarly, partial activation of CD11b, an integrin involved in the recruitment of myeloid cells to sites of inflammation, can redirect myeloid cells with immunostimulatory properties capable of fostering productive T cell immunosurveillance in a mouse model of pancreatic cancer [143]. Inhibition of class IIa histone deacetylase (HDAC) also stimulates the recruitment and differentiation of macrophages that are highly phagocytic and in doing so, sensitizes mouse breast tumors to chemotherapy and immune checkpoint blockade [144]. Thus, the myeloid response to cancer may be harnessed for therapeutic benefit, both by re-education of existing tumor-associated myeloid cells towards an anti-tumor phenotype and by de novo recruitment of myeloid cells with anti-tumor properties.
The fate of tumor-infiltrating myeloid cells is ultimately defined by the tumor microenvironment. As such, maintaining an anti-tumor shift in the phenotype of cancer inflammation is challenged by the inevitable tendency to revert to a pro-tumor state. For example, lactic acid produced by tumor cells as a by-product of glycolysis supports a pro-tumor phenotype in macrophages [145]. Similarly, macrophages are lured into hypoxic regions in tumors in response to hypoxia-induced Semaphorin 3A (Sema3A) and as a result, acquire a pro-tumor phenotype. Disrupting the Sema3A/Neuropilin-1 signaling axis in mouse models of cancer prevents macrophage recruitment to hypoxic regions and supports T cell-dependent anti-tumor immunity [146]. The importance of instructing and maintaining cancer inflammation with an anti-tumor phenotype is accentuated by its requisite role in T cell-dependent tumor immunity.
For example, macrophages responding to cytokines (e.g. IFN-γ and GM-CSF) released by tumor-infiltrating T cells trigger endogenous immunity and eliminate tumor cells in models of ovarian cancer [147]. This finding reveals an interdependent relationship between cancer inflammation and adaptive immunity. Whereas cancer inflammation can suppress T cell immunosurveillance, T cells can engender cancer inflammation with anti-tumor functions and in doing so, inflammation may then nurture a productive Cancer-Immunity Cycle. Thus, establishing productive T cell immunosurveillance may be a tipping point for successfully toggling and maintaining the inflammostat in a position supportive of immune-mediated elimination of cancer.
The anti-tumor potential of myeloid cells is determined by a balance of inhibitory and stimulatory signals. For macrophages, this balance is critical to defining their capacity to phagocytose tumor cells and mediate anti-tumor activity. As such, tumor cells evade clearance by macrophages by overexpressing cell surface proteins, including CD47, CD24, and β2M [148-150], which act as ‘don’t eat me’ signals and inhibit the phagocytic machinery of macrophages. Disrupting these signals with monoclonal antibodies has shown therapeutic promise in models of human solid cancers as well as in patients with Non-Hodgkin’s Lymphoma [151, 152]. However, merely blocking inhibitory signals without provision of activation stimuli may not be sufficient for persuading macrophages to attack tumor cells [153]. Moreover, the metabolic state of macrophages may be fundamental to engaging their appetite for eliminating tumor cells [153]. To this end, TLR9 agonists can rewire macrophages with a metabolic state that shunts tricarboxylic acid cycle intermediates for de novo lipid biosynthesis that is necessary for their phagocytosis of tumor cells [153]. This process also imparts macrophages with antitumor potential capable of overcoming inhibitory signals mediated by CD47 on tumor cells. Thus, toggling the phenotype of macrophages is a promising approach for converting the inflammatory reaction from a therapeutic obstacle to a clinical opportunity.
8. Combined targeting of the Cancer-Immunity and Cancer-Inflammation Cycles
Inflammation is a major determinant of anti-tumor immunity and acts as a rheostat to finetune T cell immunosurveillance. However, mere targeting of the Cancer-Inflammation Cycle has yet to reproducibly demonstrate clinical benefit in patients with advanced cancer. Given the interconnection between cancer inflammation and T cell immunosurveillance, preclinical and clinical studies are investigating the benefit of combining strategies that intervene on inflammation and concurrently, potentiate effector T cell activity while overcoming mechanisms of T cell exhaustion. To do this, three major approaches are being studied in which immune checkpoint inhibitors are combined with therapies that (i) disrupt cytokine and chemokine signals (e.g. FAK, CSF1R, CXCR2), (ii) activate myeloid cells to bridge innate and adaptive immunity (e.g. CD40, TLR9), and (iii) inhibit inflammatory elements involved in metabolic dysregulation (e.g. IDO, arginase, adenosine).
It has become increasingly clear that multiple tumor intrinsic mechanisms of cancer inflammation converge to regulate T cell immunosurveillance. Derailing these signaling pathways may shift the immunological state of tumors from immune-resistant to -sensitive. For example, one approach is to inhibit signals that promote the recruitment of immunosuppressive myeloid cells to tumors. FAK activity in tumor cells facilitates the release of multiple chemoattractants involved in establishing myeloid-dependent immunosuppression in tumors [89-91]. Blockade of FAK signaling in models of pancreatic cancer impairs myeloid cell recruitment and in doing so, unveils the efficacy of immune checkpoint blockade [91]. In patients, defactinib, an oral ATP-competitive FAK inhibitor is being tested in combination with anti-PD1 therapy for the treatment of advanced cancer. Preliminary results show safety of this combination and suggest potential clinical activity [154].
Whereas FAK inhibition aims to impair the release of a range of chemoattractants that orchestrate immunosuppression in cancer, an alternative approach is to intervene selectively on distinct soluble factors that foster cancer inflammation. For example, tumor and host cells produce CSF1 which signals through CSF1R to regulate macrophage phenotype and to mediate macrophage homing and survival. In patients with advanced cancer, CSF1R inhibition is tolerable and demonstrates biological activity as seen by elimination of tumor-associated macrophages. However, with the exception of tenosynovial giant cell tumors [155-157]. CSF1R-directed therapies have not produced clinical activity as monotherapy in patients. In mice, anti-CSF1R therapy triggers a compensatory accumulation of neutrophils within tumors, which support tumor growth. The chemokine C-X-C motif receptor 2 (CXCR2) mediates neutrophil homing via interaction with multiple chemokines, including CXCL1, CXCL5, and CXCL8. In mouse models of sarcoma and pancreatic cancer, inhibition of CXCR2 in combination with anti-PD1 therapy reduces metastases and improves the efficacy of anti-PD1 immunotherapy [96, 158]. Further, anti-CSF1R therapy combines with CXCR2 inhibition to unleash the capacity of anti-PD1 checkpoint inhibition to trigger T cell-dependent immunity in mouse models of cancer [103]. Ongoing clinical studies are evaluating CSF1R blockade as well as CXCR1/2 inhibition in combination with checkpoint inhibitor therapy for the treatment of solid and hematologic malignancies ( NCT02777710, NCT03927105, NCT03697564, NCT02880371, NCT03502330, NCT03238027, NCT02526017, NCT02829723, NCT03161431).
Myeloid cells are central to the development of productive T cell immunosurveillance in cancer. Acting as a bridge to alert the adaptive immune system of non-self, myeloid cells process and present antigens to prime and activate antigen-specific T cells. Classically, this process is mediated by dendritic cells which much be “licensed” with T cell stimulatory capacity [159-162]. In this regard, stimulation of CD40 expressed on dendritic cells triggers their cross-presentation of antigens and subsequent priming of T cells. Agonistic CD40 monoclonal antibodies can substitute for CD40L, the natural ligand of CD40 which is expressed on activated T cells [163-165]. In patients, CD40 antibodies have produced modest activity as monotherapy with some responses reported in patients with melanoma [166, 167]. However, mere stimulation of the CD40 pathway produces no clinical benefit for most patients and similarly, shows limited activity in many mouse models of cancer [167]. However, CD40 is fundamental to the efficacy of T cell immunotherapy, where anti-tumor activity mediated by tumor-specific T cells is reliant on CD40 and CD40L [168]. For these reasons, CD40 agonists are now being studied for their capacity to sensitize tumors to immune checkpoint blockade. For example, CD40 agonism can induce APCs to secrete IL-12 which decreases PD1 expression on tumor-infiltrating T cells and in doing so, conditions murine tumors for enhanced responsiveness to anti-CTLA4 and anti-PD1 therapy [169]. In addition, preclinical models show that CD40 agonists can enhance the therapeutic potential of tumors responsive to dual immune checkpoint blockade with anti-PD1 and anti-CTLA4 [170]. Together, these preclinical data suggest a role for CD40 agonists in settings where checkpoint blockade produces anti-tumor activity but does not achieve complete remission. However, for pancreatic tumors where neither CD40 agonism or dual checkpoint inhibitor blockade produces significant activity, the combination in preclinical models is also ineffective. This treatment resistance, though, may be overcome by depleting extra-tumoral macrophages [114] or by genetic ablation of CXCL1 release by tumor cells [92]. In patients with advanced pancreatic cancer, CD40 agonists have been combined with anti-PD1 therapy and found to be safe [171]. However, in this study, chemotherapy was also included making it unclear whether tumor responses seen in patients were produced by chemotherapy or the combination.
Whereas CD40 agonists have mainly been used as a strategy to trigger systemic immune activation, TLR agonists have been administered intratumorally to invoke chemokine and antigen release and to enhance T cell infiltration locally [172, 173]. TLRs recognize conserved microbial molecules, so-called pathogen-associated molecular patterns (PAMPs). In mice, intratumoral injection of a TLR7 agonist shifts the intratumoral macrophage population from immunosuppressive to immunostimulatory [174]. Similarly, TLR9, which is activated by microbial DNA, has garnered interest for its potential to enhance the activity of anti-PD1 treatment in patients with advanced melanoma [175]. A CpG-oligodeoxynucleotide (CMP-001) designed to activate TLR9 via intratumoral injection has also been studied in combination with anti-PD1 therapy for the treatment of patients with PD-1 refractory advanced melanoma. Of 68 patients treated in the phase I setting, the objective response rate was 22% and produced increases in CXCL10, PDL1 and tumor-infiltrating CD8 T cells [176]. Thus, strategies to redirect the local inflammatory response in cancer may sensitize tumors to T cell-directed immunotherapy.
Cancer inflammation is sustained by multiple signals which may be disrupted therapeutically. One such example is interleukin-6, a prototypical inflammatory cytokine that plays a central role in the acute phase response. IL-6 activates STAT3 locally in tumor and stromal cells as well as systemically, such as in hepatocytes which respond to IL-6 by elaborating the production of CRP and SAA [177], [178]. Broadly, IL-6 has pleiotropic capacity and impacts multiple biological processes including inflammation, bone homeostasis, metabolism and T cell differentiation [179, 180]. In the context of cancer, IL-6 can be produced by both tumor and host cells. Additionally, IL-6 can be detected in the peripheral blood of patients and is associated with poor outcomes when elevated [181-185]. In pre-clinical models, IL-6 blockade in combination with anti-PD-L1 therapy elicits productive anti-tumor immunity and effectively controls tumor growth [186, 187]. An early phase clinical trial investigating the combination of anti-IL-6 therapy and checkpoint inhibition for the treatment of melanoma is ongoing ( NCT03999749).
Cancer inflammation produces derangements in metabolism which can impair the functional fitness of the immune system. In this regard, IDO is a key enzyme involved in tryptophan metabolism. Reduced tryptophan levels and increased tryptophan metabolites combine to alter T cell and dendritic cell function, and in doing so, inhibit productive anti-tumor immunosurveillance [107]. In tumors inflamed with T cell infiltrates, IDO is often increased due to IFN-γ produced by tumor-infiltrating lymphocytes [107]. In preclinical models of cancer, IDO inhibition combines to enhance the efficacy of immune checkpoint blockade [188]. In patients with melanoma, IDO inhibition has been combined with anti-PD1 therapy but unexpectedly was found not to significantly improve outcomes compared to anti-PD1 therapy alone [189, 190]. However, recent biomarker analyses suggest that IDO inhibition may need to be considered for select patient populations. For instance, a decreasing Trp/Kyn ratio after treatment with anti-PD1 therapy has been found to associate with worse overall survival in patients with melanoma and renal cell carcinoma [108]. This finding highlights the potential relevance of metabolic aberrations in governing the efficacy of cancer immunotherapy. To this end, extracellular metabolites represent a distinct class of immunosuppressive molecules. For example, adenosine produced from eATP released within tumors in the setting of hypoxia and cell death can inhibit T cell immunosurveillance. Adenosine engages with A2AR, including A2a and A2b adenosine receptors, on immune cells producing diverse immunoregulatory effects [51, 191]. In mouse models of cancer, acquired resistance to anti-PD-L1 therapy is associated with upregulation of CD38. CD38 contributes to accumulation of adenosine in tumors and in doing so, inhibits CD8 T cell function and the efficacy of anti-PDL1 immunotherapy [192]. Blockade of CD38 reversed this biology and sensitized tumors to anti-PDL1 therapy. Alternative strategies to disrupt the adenosine pathway include targeting CD73 and A2AR. In mice, inhibition of A2AR synergizes with anti-PD1 therapy to delay tumor outgrowth, reduce metastasis, and improve survival [193-195]. Similarly, treatment with anti-CD73 to block adenosine production combines with anti-PD1 therapy in multiple mouse models of cancer to produce T cell dependent anti-tumor activity [196]. Together, these studies form the basis for ongoing clinical trials investigating the CD39-CD73-adenosine signaling pathway as monotherapy and in combination with immune checkpoint blockade [197].
9. Concluding Remarks
The immune system is characterized by remarkable diversity and specificity that combine to create an exceptional therapeutic opportunity for distinguishing cancer from normal tissue. Harnessing the immune system for therapy is now standard of care for many cancers. However, while immunotherapy can produce exceptional responses in some patients including durable complete remissions, the majority of patients still do not respond. Multiple mechanisms may shape immune-resistance in cancer, but inflammation has emerged as a fundamental determinant. Inflammation acts as a rheostat, which we have termed the inflammostat, to finetune T cell-dependent immunosurveillance. This inflammostat is defined by the degree and phenotype of local and systemic inflammatory responses associated with cancer. Once triggered, inflammation is self-propagating and reinforced in a vicious cycle. By understanding the biology that drives inflammation in cancer, inflammatory-related biomarkers (Figure 2) may allow for mapping out the Cancer-Inflammation Cycle for individual patients. Functional determinants associated with liver inflammation, bone marrow mobilization, immune cell activation, myeloid cell chemotaxis, inflammatory molecules, and metabolism have been identified that associate with treatment and survival outcomes. Many of these determinants can be detected readily with blood sampling and may provide an opportunity for non-invasive and efficient evaluation of the inflammatory status of a patient. Inclusion of peripheral markers of inflammation in future clinical trials is needed to robustly define the utility of these markers and associations with tumor biology. Ultimately, profiling the inflammostat may enable tailoring specific therapeutic approaches for intervening on cancer inflammation with the goal to unleash productive T cell immunosurveillance and produce durable complete remissions for cancer patients.
Acknowledgements
This works was supported by grants from the National Institutes of Health grants T32 HL007439-41 (M.M.W.), R01 CA197916 (G.L.B.); U01 CA224193-01 (G.L.B.), the Stand Up to Cancer (SU2C) Innovative Research Grant SU2C-AACR-IRG 13-17 (G.L.B.), and grant support from the Robert L. Fine Cancer Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Robert L. Fine Cancer Research Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
G.L.B. is a consultant/advisory board member for Seattle Genetics, Aduro Biotech, AstraZeneca, Bristol-Myers Squibb, Genmab, Merck, Shattuck Labs, Boehringer Ingelheim, and BiolineRx; reports receiving commercial research grants from Incyte, Bristol-Myers Squibb, Verastem, Halozyme, Biothera, Newlink, Novartis, and Janssen. G.L.B. is an inventor of intellectual property and recipient of royalties related to CAR T cells that are licensed by the University of Pennsylvania to Novartis. No additional potential conflicts of interest were disclosed by M.M.W.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, Group IMS, Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC, N Engl J Med 378(24) (2018) 2288–2301. [DOI] [PubMed] [Google Scholar]
- [2].Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Ozguroglu M, Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer, N Engl J Med 377(20) (2017) 1919–1929. [DOI] [PubMed] [Google Scholar]
- [3].Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, K.-. Investigators, Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer, N Engl J Med 375(19) (2016)1823–1833. [DOI] [PubMed] [Google Scholar]
- [4].Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr., Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR, Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer, N Engl J Med 373(17) (2015) 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B, CheckMate I, Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma, N Engl J Med 378(14) (2018) 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T, K.-. Investigators, Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N Engl J Med 380(12) (2019) 1116–1127. [DOI] [PubMed] [Google Scholar]
- [7].Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr., Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK, Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N Engl J Med 380(12) (2019) 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J, Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma, N Engl J Med 377(14) (2017) 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, Pembrolizumab versus Ipilimumab in Advanced Melanoma, N Engl J Med 372(26) (2015) 2521–32. [DOI] [PubMed] [Google Scholar]
- [10].Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr., PD-1 Blockade in Tumors with Mismatch-Repair Deficiency, N Engl J Med 372(26) (2015) 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, Voloschin A, Ramkissoon SH, Ligon KL, Latek R, Zwirtes R, Strauss L, Paliwal P, Harbison CT, Reardon DA, Sampson JH, Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143, Neuro Oncol 20(5) (2018) 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA, Movva S, Davis LE, Okuno SH, Reed DR, Crowley J, Butterfield LH, Salazar R, Rodriguez-Canales J, Lazar AJ, Wistuba LH II Baker RG Maki D Reinke S Patel, Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial, Lancet Oncol 18(11) (2017) 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144(5) (2011) 646–74. [DOI] [PubMed] [Google Scholar]
- [14].Liu M, Kalbasi A, Beatty GL, Functio Laesa: Cancer Inflammation and Therapeutic Resistance, J Oncol Pract 13(3) (2017) 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grivennikov SI, Greten FR, Karin M, Immunity, inflammation, and cancer, Cell 140(6) (2010) 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Damuzzo V, Solito S, Pinton L, Carrozzo E, Valpione S, Pigozzo J, Arboretti Giancristofaro R, Chiarion-Sileni V, Mandruzzato S, Clinical implication of tumor-associated and immunological parameters in melanoma patients treated with ipilimumab, Oncoimmunology 5(12) (2016) e1249559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Samak R, Edelstein R, Israel L, Immunosuppressive effect of acute-phase reactant proteins in vitro and its relevance to cancer, Cancer Immunol Immunother 13(1) (1982) 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qian BZ, Pollard JW, Macrophage diversity enhances tumor progression and metastasis, Cell 141(1) (2010) 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hanahan D, Coussens LM, Accessories to the crime: functions of cells recruited to the tumor microenvironment, Cancer Cell 21(3) (2012) 309–22. [DOI] [PubMed] [Google Scholar]
- [20].Stone ML, Beatty GL, Cellular determinants and therapeutic implications of inflammation in pancreatic cancer, Pharmacol Ther 201 (2019) 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Condeelis J, Pollard JW, Macrophages: obligate partners for tumor cell migration, invasion, and metastasis, Cell 124(2) (2006) 263–6. [DOI] [PubMed] [Google Scholar]
- [22].Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, Storz P, Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions, Cancer Discov 5(1) (2015) 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH, Dynamics of the immune reaction to pancreatic cancer from inception to invasion, Cancer Res 67(19) (2007) 9518–27. [DOI] [PubMed] [Google Scholar]
- [24].Clark CE, Beatty GL, Vonderheide RH, Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer, Cancer Lett 279(1) (2009) 1–7. [DOI] [PubMed] [Google Scholar]
- [25].Ferrone C, Dranoff G, Dual roles for immunity in gastrointestinal cancers, J Clin Oncol 28(26) (2010) 4045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schreiber RD, Old LJ, Smyth MJ, Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion, Science 331(6024) (2011) 1565–70. [DOI] [PubMed] [Google Scholar]
- [27].Dunn GP, Old LJ, Schreiber RD, The three Es of cancer immunoediting, Annu Rev Immunol 22 (2004) 329–60. [DOI] [PubMed] [Google Scholar]
- [28].Mantovani A, Sica A, Locati M, Macrophage polarization comes of age, Immunity 23(4) (2005) 344–6. [DOI] [PubMed] [Google Scholar]
- [29].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA, Macrophage activation and polarization: nomenclature and experimental guidelines, Immunity 41(1) (2014) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carmona-Fontaine C, Deforet M, Akkari L, Thompson CB, Joyce JA, Xavier JB, Metabolic origins of spatial organization in the tumor microenvironment, Proc Natl Acad Sci U S A 114(11) (2017) 2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang YK, Wang M, Sun Y, Di Costanzo N, Mitchell C, Achuthan A, Hamilton JA, Busuttil RA, Boussioutas A, Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry, Nat Commun 10(1) (2019) 3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Coussens LM, Zitvogel L, Palucka AK, Neutralizing tumor-promoting chronic inflammation: a magic bullet?, Science 339(6117) (2013) 286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, Yokoyama WM, Hawkins WG, Fields RC, Randolph GJ, DeNardo DG, Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression, Immunity 47(2) (2017) 323–338 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici N, Baudesson de Chanville C, Combadiere B, Geissmann F, Savina A, Combadiere C, Boissonnas A, Macrophages of distinct origins contribute to tumor development in the lung, J Exp Med 215(10) (2018) 2536–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alvarez D, Vollmann EH, von Andrian UH, Mechanisms and consequences of dendritic cell migration, Immunity 29(3) (2008) 325–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, Immunity 39(1) (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [37].Gardner A, Ruffell B, Dendritic Cells and Cancer Immunity, Trends Immunol 37(12) (2016)855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Beatty GL, Gladney WL, Immune escape mechanisms as a guide for cancer immunotherapy, Clin Cancer Res 21(4) (2015) 687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Canli O, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, Neumann T, Horst D, Lower M, Sahin U, Greten FR, Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis, Cancer Cell 32(6) (2017) 869–883 e5. [DOI] [PubMed] [Google Scholar]
- [40].Shimizu T, Marusawa H, Matsumoto Y, Inuzuka T, Ikeda A, Fujii Y, Minamiguchi S, Miyamoto S, Kou T, Sakai Y, Crabtree JE, Chiba T, Accumulation of somatic mutations in TP53 in gastric epithelium with Helicobacter pylori infection, Gastroenterology 147(2) (2014) 407–17 e3. [DOI] [PubMed] [Google Scholar]
- [41].Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T, Kinoshita K, Honjo T, Chiba T, Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers, Gastroenterology 135(3) (2008) 889–98, 898 e1-3. [DOI] [PubMed] [Google Scholar]
- [42].Takeda K, Nakayama M, Hayakawa Y, Kojima Y, Ikeda H, Imai N, Ogasawara K, Okumura K, Thomas DM, Smyth MJ, IFN-gamma is required for cytotoxic T cell-dependent cancer genome immunoediting, Nat Commun 8 (2017) 14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, Rizvi NA, Merghoub T, Levine AJ, Chan TA, Wolchok JD, Greenbaum BD, A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy, Nature 551(7681) (2017) 517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy, Science, 2015, pp. 69–74. [DOI] [PubMed] [Google Scholar]
- [45].Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, Duan K, Ang N, Poidinger M, Lee YY, Larbi A, Khng AJ, Tan E, Fu C, Mathew R, Teo M, Lim WT, Toh CK, Ong BH, Koh T, Hillmer AM, Takano A, Lim TKH, Tan EH, Zhai W, Tan DSW, Tan IB, Newell EW, Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates, Nature 557(7706) (2018) 575–579. [DOI] [PubMed] [Google Scholar]
- [46].Stevanovic S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, Hinrichs CS, Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer, Science 356(6334) (2017) 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu Y, Chang SS, Lin WC, Hsu JM, Hsu YH, Kim T, Chang WC, Hsu JL, Yamaguchi H, Ding Q, Wang Y, Yang Y, Chen CH, Sahin AA, Yu D, Hortobagyi GN, Hung MC, Deubiquitination and Stabilization of PD-L1 by CSN5, Cancer Cell 30(6) (2016) 925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH, PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity, J Exp Med 214(4) (2017) 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H, Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells, Nat Med 10(1) (2004) 48–54. [DOI] [PubMed] [Google Scholar]
- [50].Grohmann U, Bronte V, Control of immune response by amino acid metabolism, Immunol Rev 236 (2010) 243–64. [DOI] [PubMed] [Google Scholar]
- [51].Vijayan D, Young A, Teng MWL, Smyth MJ, Targeting immunosuppressive adenosine in cancer, Nat Rev Cancer 17(12) (2017) 709–724. [DOI] [PubMed] [Google Scholar]
- [52].Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ, MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study, Cancer Prev Res (Phila) 6(1) (2013) 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bronte V, Zanovello P, Regulation of immune responses by L-arginine metabolism, Nat Rev Immunol 5(8) (2005) 641–54. [DOI] [PubMed] [Google Scholar]
- [54].Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system, Nat Rev Immunol 9(3) (2009) 162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stelekati E, Shin H, Doering TA, Dolfi DV, Ziegler CG, Beiting DP, Dawson L, Liboon J, Wolski D, Ali MA, Katsikis PD, Shen H, Roos DS, Haining WN, Lauer GM, Wherry EJ, Bystander chronic infection negatively impacts development of CD8(+) T cell memory, Immunity 40(5) (2014) 801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E, Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis, J Natl Cancer Inst 106(6) (2014) dju124. [DOI] [PubMed] [Google Scholar]
- [57].Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, Wang Q, Yang W, Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies, Cancer Treat Rev 58 (2017) 1–13. [DOI] [PubMed] [Google Scholar]
- [58].Li X, Dai D, Chen B, Tang H, Xie X, Wei W, The value of neutrophil-to-lymphocyte ratio for response and prognostic effect of neoadjuvant chemotherapy in solid tumors: A systematic review and meta-analysis, J Cancer 9(5) (2018) 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, Stonehouse-Lee S, Sherry VE, Gilbert E, Eaby-Sandy B, Mutale F, DiLullo G, Cohen RB, Vachani A, Langer CJ, Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer, Lung Cancer 106 (2017) 1–7. [DOI] [PubMed] [Google Scholar]
- [60].Cassidy MR, Wolchok RE, Zheng J, Panageas KS, Wolchok JD, Coit D, Postow MA, Ariyan C, Neutrophil to Lymphocyte Ratio is Associated With Outcome During Ipilimumab Treatment, EBioMedicine 18 (2017) 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Fruh M, Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab, Lung Cancer 111 (2017) 176–181. [DOI] [PubMed] [Google Scholar]
- [62].Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P, Savoia P, Mandala M, Simeone E, Valpione S, Altomonte M, Spagnolo F, Cocorocchio E, Gandini S, Giannarelli D, Martinoli C, Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab, Ann Oncol 27(4) (2016) 732–8. [DOI] [PubMed] [Google Scholar]
- [63].Kuzman JA, Stenehjem DD, Merriman J, Agarwal AM, Patel SB, Hahn AW, Alex A, Albertson D, Gill DM, Agarwal N, Neutrophil-lymphocyte ratio as a predictive biomarker for response to high dose interleukin-2 in patients with renal cell carcinoma, BMC Urol 17(1) (2017) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nyakas M, Aamdal E, Jacobsen KD, Guren TK, Aamdal S, Hagene KT, Brunsvig P, Yndestad A, Halvorsen B, Tasken KA, Aukrust P, Maelandsmo GM, Ueland T, Prognostic biomarkers for immunotherapy with ipilimumab in metastatic melanoma, Clin Exp Immunol 197(1) (2019) 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Weber JS, Tang H, Hippeli L, Qian M, Wind-Rotolo M, Larkin JMG, Wolchok JD, Sznol M, Robert C, Woods DM, Laino AS, Hodi FS, Serum IL-6 and CRP as prognostic factors in melanoma patients receiving single agent and combination checkpoint inhibition, ASCO Annual Meeting 2019, Journal of Clinical Oncology, Chicago, IL, 2019, pp. 100–100. [Google Scholar]
- [66].Mortensen RF, Braun D, Gewurz H, Effects of C-reactive protein on lymphocyte functions. III. Inhibition of antigen-induced lymphocyte stimulation and lymphokine production, Cell Immunol 28(1) (1977) 59–68. [DOI] [PubMed] [Google Scholar]
- [67].Zhang L, Liu SH, Wright TT, Shen ZY, Li HY, Zhu W, Potempa LA, Ji SR, Szalai AJ, Wu Y, C-reactive protein directly suppresses Th1 cell differentiation and alleviates experimental autoimmune encephalomyelitis, J Immunol 194(11) (2015) 5243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jimenez RV, Kuznetsova V, Connelly AN, Hel Z, Szalai AJ, C-Reactive Protein Promotes the Expansion of Myeloid Derived Cells With Suppressor Functions, Front Immunol 10 (2019) 2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ye RD, Sun L, Emerging functions of serum amyloid A in inflammation, J Leukoc Biol 98(6) (2015) 923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD, Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2, Blood 113(2) (2009) 429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].He R, Sang H, Ye RD, Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R, Blood 101(4) (2003) 1572–81. [DOI] [PubMed] [Google Scholar]
- [72].Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC, Inflammatory Monocyte Mobilization Decreases Patient Survival in Pancreatic Cancer: A Role for Targeting the CCL2/CCR2 Axis, Clin Cancer Res 19(13) (2013) 3404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Veglia F, Perego M, Gabrilovich D, Myeloid-derived suppressor cells coming of age, Nat Immunol 19(2) (2018) 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Meyer MA, Baer JM, Knolhoff BL, Nywening TM, Panni RZ, Su X, Weilbaecher KN, Hawkins WG, Ma C, Fields RC, Linehan DC, Challen GA, Faccio R, Aft RL, DeNardo DG, Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance, Nat Commun 9(1) (2018) 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Spranger S, Dai D, Horton B, Gajewski TF, Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy, Cancer Cell 31(5) (2017) 711–723 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, Freeman GJ, Boland GM, Luster AD, Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy, Immunity 50(6) (2019) 1498–1512 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J, Ouakrim H, Regnier F, Lupo A, Alifano M, Damotte D, Donnadieu E, Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment, Proc Natl Acad Sci U S A 115(17) (2018) E4041–E4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM, Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy, Cancer Discov 1(1) (2011) 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Ostling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, Sund M, Brage SE, Fuxe J, Rolny C, Li F, Ravetch JV, Karlsson MC, Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis, Cell Rep 15(9) (2016) 2000–11. [DOI] [PubMed] [Google Scholar]
- [80].Ruffell B, Coussens LM, Macrophages and therapeutic resistance in cancer, Cancer Cell 27(4) (2015) 462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Piccolo V, Curina A, Genua M, Ghisletti S, Simonatto M, Sabo A, Amati B, Ostuni R, Natoli G, Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk, Nat Immunol 18(5) (2017) 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Beatty GL, Paterson Y, Regulation of tumor growth by IFN-gamma in cancer immunotherapy, Immunol Res 24(2) (2001) 201–10. [DOI] [PubMed] [Google Scholar]
- [83].Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ, Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade, Cell 167(6) (2016) 1540–1554 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Benci JL, Johnson LR, Choa R, Xu Y, Qiu J, Zhou Z, Xu B, Ye D, Nathanson KL, June CH, Wherry EJ, Zhang NR, Ishwaran H, Hellmann MD, Wolchok JD, Kambayashi T, Minn AJ, Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade, Cell 178(4) (2019) 933–948 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial, Lancet 390(10105) (2017) 1833–1842. [DOI] [PubMed] [Google Scholar]
- [86].Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M, Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence, Cancer Cell 19(6) (2011) 728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M, Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice, Cancer Cell 11(3) (2007) 291–302. [DOI] [PubMed] [Google Scholar]
- [88].Nakamura K, Kassem S, Cleynen A, Chretien ML, Guillerey C, Putz EM, Bald T, Forster I, Vuckovic S, Hill GR, Masters SL, Chesi M, Bergsagel PL, Avet-Loiseau H, Martinet L, Smyth MJ, Dysregulated IL-18 Is a Key Driver of Immunosuppression and a Possible Therapeutic Target in the Multiple Myeloma Microenvironment, Cancer Cell 33(4) (2018) 634–648 e5. [DOI] [PubMed] [Google Scholar]
- [89].Sulzmaier FJ, Jean C, Schlaepfer DD, FAK in cancer: mechanistic findings and clinical applications, Nat Rev Cancer 14(9) (2014) 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Serrels A, Lund T, Serrels B, Byron A, McPherson RC, von Kriegsheim A, Gomez-Cuadrado L, Canel M, Muir M, Ring JE, Maniati E, Sims AH, Pachter JA, Brunton VG, Gilbert N, Anderton SM, Nibbs RJ, Frame MC, Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity, Cell 163(1) (2015) 160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG, Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy, Nat Med 22(8) (2016) 851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, Richman LP, Lin JH, Sun YH, Rech AJ, Balli D, Hay CA, Sela Y, Merrell AJ, Liudahl SM, Gordon N, Norgard RJ, Yuan S, Yu S, Chao T, Ye S, Eisinger-Mathason TSK, Faryabi RB, Tobias JW, Lowe SW, Coussens LM, Wherry EJ, Vonderheide RH, Stanger BZ, Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy, Immunity 49(1) (2018) 178–193 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG, Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses, Cancer Res 73(3) (2013) 1128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, Ben-Josef E, Beatty GL, Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma, Clin Cancer Res 23(1) (2017) 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE, Fields RC, DeNardo DG, Hawkins WG, Goedegebuure P, Linehan DC, Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma, Gut 67(6) (2018) 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Steele CW, Karim SA, Leach JD, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TR, Strathdee D, Biankin AV, Nibbs RJ, Barry ST, Sansom OJ, Morton JP, CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma, Cancer Cell 29(6) (2016) 832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, D'Antuono R, Montani E, Garcia-Escudero R, Guccini I, Da Silva-Alvarez S, Collado M, Eisenberger M, Zhang Z, Catapano C, Grassi F, Alimonti A, Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer, Nature 515(7525) (2014) 134–7. [DOI] [PubMed] [Google Scholar]
- [98].Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA, Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression, Cancer Discov 6(1) (2016) 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D, Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia, Cancer Cell 21(6) (2012) 836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH, Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer, Cancer Cell 21(6) (2012) 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, Littlewood TD, Evan GI, Myc Cooperates with Ras by Programming Inflammation and Immune Suppression, Cell 171(6) (2017) 1301–1315 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, Torres-Hernandez A, Pergamo M, Ochi A, Zambirinis CP, Pansari M, Rendon M, Tippens D, Hundeyin M, Mani VR, Hajdu C, Engle D, Miller G, The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression, Nature 532(7598) (2016) 245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, Mulligan C, Nam B, Hockstein N, Denstman F, Shakamuri S, Speicher DW, Weeraratna AT, Chao T, Vonderheide RH, Languino LR, Ordentlich P, Liu Q, Xu X, Lo A, Pure E, Zhang C, Loboda A, Sepulveda MA, Snyder LA, Gabrilovich DI, Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors, Cancer Cell 32(5) (2017) 654–668 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW, CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis, Nature 475(7355) (2011) 222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA, Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity, Blood 115(17) (2010) 3520–30. [DOI] [PubMed] [Google Scholar]
- [106].Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL Jr., Mellor AL, Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase, Science 297(5588) (2002) 1867–70. [DOI] [PubMed] [Google Scholar]
- [107].Munn DH, Mellor AL, IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance, Trends Immunol 37(3) (2016) 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Li H, Bullock K, Gurjao C, Braun D, Shukla SA, Bosse D, Lalani AA, Gopal S, Jin C, Horak C, Wind-Rotolo M, Signoretti S, McDermott DF, Freeman GJ, Van Allen EM, Schreiber SL, Stephen Hodi F, Sellers WR, Garraway LA, Clish CB, Choueiri TK, Giannakis M, Metabolomic adaptations and correlates of survival to immune checkpoint blockade, Nat Commun 10(1) (2019) 4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Murray PJ, Amino acid auxotrophy as a system of immunological control nodes, Nat Immunol 17(2) (2016) 132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sica A, Mantovani A, Macrophage plasticity and polarization: in vivo veritas, J Clin Invest 122(3) (2012) 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V, Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells, Immunol Rev 222 (2008) 162–79. [DOI] [PubMed] [Google Scholar]
- [112].Vissers YL, Dejong CH, Luiking YC, Fearon KC, von Meyenfeldt MF, Deutz NE, Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency?, Am J Clin Nutr 81(5) (2005) 1142–6. [DOI] [PubMed] [Google Scholar]
- [113].Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, Zamboni N, Sallusto F, Lanzavecchia A, L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity, Cell 167(3) (2016) 829–842 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH, Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages, Gastroenterology 149(1) (2015) 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O'Brien S, Moon EK, Cantu E, Danet-Desnoyers G, Ra HJ, Litzky L, Akimova T, Beier UH, Hancock WW, Albelda SM, Eruslanov EB, Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer, Sci Transl Med 11(479) (2019) eaat1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S, COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells, Proc Natl Acad Sci U S A 114(5) (2017) 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S, PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation, J Exp Med 211(5) (2014) 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Li J, Lee Y, Li Y, Jiang Y, Lu H, Zang W, Zhao X, Liu L, Chen Y, Tan H, Yang Z, Zhang MQ, Mak TW, Ni L, Dong C, Co-inhibitory Molecule B7 Superfamily Member 1 Expressed by Tumor-Infiltrating Myeloid Cells Induces Dysfunction of Anti-tumor CD8(+) T Cells, Immunity 48(4) (2018) 773–786 e5. [DOI] [PubMed] [Google Scholar]
- [119].Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EE, Varner JA, PI3Kgamma is a molecular switch that controls immune suppression, Nature 539(7629) (2016) 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM, Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer, Cancer Discov 6(3) (2016) 270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, Seror C, Metivier D, Perfettini JL, Zitvogel L, Kroemer G, Chemotherapy induces ATP release from tumor cells, Cell Cycle 8(22) (2009) 3723–8. [DOI] [PubMed] [Google Scholar]
- [122].Cekic C, Linden J, Purinergic regulation of the immune system, Nat Rev Immunol 16(3) (2016) 177–92. [DOI] [PubMed] [Google Scholar]
- [123].Kroemer G, Galluzzi L, Kepp O, Zitvogel L, Immunogenic cell death in cancer therapy, Annu Rev Immunol 31 (2013) 51–72. [DOI] [PubMed] [Google Scholar]
- [124].Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S, The P2X7 Receptor in Infection and Inflammation, Immunity 47(1) (2017) 15–31. [DOI] [PubMed] [Google Scholar]
- [125].Idzko M, Ferrari D, Eltzschig HK, Nucleotide signalling during inflammation, Nature 509(7500) (2014) 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG, Purinergic signaling: a fundamental mechanism in neutrophil activation, Sci Signal 3(125) (2010) ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG, ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors, Science 314(5806) (2006) 1792–5. [DOI] [PubMed] [Google Scholar]
- [128].Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P, Extracellular ATP drives systemic inflammation, tissue damage and mortality, Cell Death Dis 5 (2014) e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Canale FP, Ramello MC, Nunez N, Araujo Furlan CL, Bossio SN, Gorosito Serran M, Tosello Boari J, Del Castillo A, Ledesma M, Sedlik C, Piaggio E, Gruppi A, Acosta Rodriguez EA, Montes CL, CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells, Cancer Res 78(1) (2018) 115–128. [DOI] [PubMed] [Google Scholar]
- [130].Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, Chang SC, Grunkemeier G, Leidner R, Bell RB, Weinberg AD, Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors, Nat Commun 9(1) (2018) 2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Raczkowski F, Rissiek A, Ricklefs I, Heiss K, Schumacher V, Wundenberg K, Haag F, Koch-Nolte F, Tolosa E, Mittrucker HW, CD39 is upregulated during activation of mouse and human T cells and attenuates the immune response to Listeria monocytogenes, PLoS One 13(5) (2018) e0197151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, Dietl J, Lutz MB, Mittelbronn M, Wischhusen J, Hausler SFM, Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape, J Immunother Cancer 4 (2016) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Young A, Mittal D, Stagg J, Smyth MJ, Targeting cancer-derived adenosine: new therapeutic approaches, Cancer Discov 4(8) (2014) 879–88. [DOI] [PubMed] [Google Scholar]
- [134].Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN, Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors, Am J Pathol 160(6) (2002) 2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN, Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors, J Exp Med 186(9) (1997) 1615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Cekic C, Day YJ, Sag D, Linden J, Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment, Cancer Res 74(24) (2014) 7250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, Madore J, Lepletier A, Aguilera AR, Sundarrajan A, Jacoberger-Foissac C, Wong C, Dela Cruz T, Welch M, Lerner AG, Spatola BN, Soros VB, Corbin J, Anderson AC, Effern M, Holzel M, Robson SC, Johnston RL, Waddell N, Smith C, Bald T, Geetha N, Beers C, Teng MWL, Smyth MJ, Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity, Cancer Discov 9(12) (2019) 1754–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, Courtois R, Dejou C, Jecko D, Becquart O, Rispaud-Blanc H, Gauthier L, Rossi B, Chanteux S, Gourdin N, Amigues B, Roussel A, Bensussan A, Eliaou JF, Bastid J, Romagne F, Morel Y, Narni-Mancinelli E, Vivier E, Paturel C, Bonnefoy N, Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies, Cell Rep 27(8) (2019) 2411–2425 e9. [DOI] [PubMed] [Google Scholar]
- [139].Long KB, Beatty GL, Harnessing the antitumor potential of macrophages for cancer immunotherapy, Oncoimmunology 2(12) (2013) e26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL, IFNgamma and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma, Cancer Discov 6(4) (2016) 400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH, CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans, Science 331(6024) (2011) 1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Beatty GL, O'Dwyer PJ, Clark J, Shi JG, Newton RC, Schaub R, Maleski J, Leopold L, Gajewski TF, Phase I study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of the oral inhibitor of indoleamine 2,3-dioxygenase (IDO1) INCB024360 in patients (pts) with advanced malignancies, J Clin Oncol 31 (2013) suppl; abstr 3025. [Google Scholar]
- [143].Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, Breden MA, Li X, Krisnawan VE, Khan SQ, Schwarz JK, Rogers BE, Fields RC, Hawkins WG, Gupta V, DeNardo DG, Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies, Sci Transl Med 11(499) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, Johnson SF, Carrasco RD, Lazo S, Bronson RT, Davis SP, Lobera M, Nolan MA, Letai A, Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages, Nature 543(7645) (2017) 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R, Functional polarization of tumour-associated macrophages by tumour-derived lactic acid, Nature 513(7519) (2014) 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M, Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity, Cancer Cell 24(6) (2013) 695–709. [DOI] [PubMed] [Google Scholar]
- [147].Spear P, Barber A, Rynda-Apple A, Sentman CL, Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN-gamma and GM-CSF, J Immunol 188(12) (2012) 6389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ, Weissman IL, CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy, Nature 572(7769) (2019) 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, McKenna KM, Ho PY, Cheng RZ, Chen JY, Barkal LJ, Ring AM, Weissman IL, Maute RL, Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy, Nat Immunol 19(1) (2018) 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL, CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis, Cell 138(2) (2009) 271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, Tran T, Lynn J, Chen JY, Volkmer JP, Agoram B, Huang J, Majeti R, Weissman IL, Takimoto CH, Chao MP, Smith SM, CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma, N Engl J Med 379(18) (2018) 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL, The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors, Proc Natl Acad Sci U S A 109(17) (2012) 6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Liu M, O'Connor RS, Trefely S, Graham K, Snyder NW, Beatty GL, Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated 'don't-eat-me' signal, Nat Immunol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wang-Gillam A, Lockhart AC, Tan BR, Suresh R, Lim KH, Ratner L, Morton A, Huffman J, Marquez S, Boice N, DeNardo DG, Phase I study of defactinib combined with pembrolizumab and gemictabine in patients with adanced cancer, ASCO Annual Meeting, Chicago, IL, 2018, pp. 380–380. [Google Scholar]
- [155].Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, Ries C, Brillouet A, Muller C, Jegg AM, Broske AM, Dembowski M, Bray-French K, Freilinger C, Meneses-Lorente G, Baehner M, Harding R, Ratnayake J, Abiraj K, Gass N, Noh K, Christen RD, Ukarma L, Bompas E, Delord JP, Blay JY, Ruttinger D, CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study, Lancet Oncol 16(8) (2015) 949–56. [DOI] [PubMed] [Google Scholar]
- [156].Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, Staddon AP, Cohn AL, Shapiro GI, Keedy VL, Singh AS, Puzanov I, Kwak EL, Wagner AJ, Von Hoff DD, Weiss GJ, Ramanathan RK, Zhang J, Habets G, Zhang Y, Burton EA, Visor G, Sanftner L, Severson P, Nguyen H, Kim MJ, Marimuthu A, Tsang G, Shellooe R, Gee C, West BL, Hirth P, Nolop K, van de Rijn M, Hsu HH, Peterfy C, Lin PS, Tong-Starksen S, Bollag G, Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor, N Engl J Med 373(5) (2015) 428–37. [DOI] [PubMed] [Google Scholar]
- [157].Papadopoulos KP, Gluck L, Martin LP, Olszanski AJ, Tolcher AW, Ngarmchamnanrith G, Rasmussen E, Amore BM, Nagorsen D, Hill JS, Stephenson J Jr., First-in-Human Study of AMG 820, a Monoclonal Anti-Colony-Stimulating Factor 1 Receptor Antibody, in Patients with Advanced Solid Tumors, Clin Cancer Res 23(19) (2017) 5703–5710. [DOI] [PubMed] [Google Scholar]
- [158].Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL, Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy, Sci Transl Med 6(237) (2014) 237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ, T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions, Nature 393(6684) (1998) 480–3. [DOI] [PubMed] [Google Scholar]
- [160].Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR, Help for cytotoxic-T-cell responses is mediated by CD40 signalling, Nature 393(6684) (1998) 478–80. [DOI] [PubMed] [Google Scholar]
- [161].Lanzavecchia A, Immunology. Licence to kill, Nature 393(6684) (1998) 413–4. [DOI] [PubMed] [Google Scholar]
- [162].Ridge JP, Di Rosa F, Matzinger P, A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell, Nature 393(6684) (1998) 474–8. [DOI] [PubMed] [Google Scholar]
- [163].French RR, Chan HT, Tutt AL, Glennie MJ, CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help, Nat Med 5(5) (1999) 548–53. [DOI] [PubMed] [Google Scholar]
- [164].Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI, Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40, Nat Med 5(7) (1999) 780–7. [DOI] [PubMed] [Google Scholar]
- [165].Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE, CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy, Nat Med 5(7) (1999) 774–9. [DOI] [PubMed] [Google Scholar]
- [166].Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O'Dwyer PJ, Running KL, Huhn RD, Antonia SJ, Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody, J Clin Oncol 25(7) (2007) 876–83. [DOI] [PubMed] [Google Scholar]
- [167].Beatty GL, Li Y, Long KB, Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists, Expert Rev Anticancer Ther 17(2) (2017) 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AHR, Ugel S, Sasso MS, Qualls JE, Kratochvill F, Zanovello P, Molon B, Ries CH, Runza V, Hoves S, Bilocq AM, Bindea G, Mazza EMC, Bicciato S, Galon J, Murray PJ, Bronte V, T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells, Cancer Cell 30(3) (2016) 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Ngiow SF, Young A, Blake SJ, Hill GR, Yagita H, Teng MW, Korman AJ, Smyth MJ, Agonistic CD40 mAb-Driven IL12 Reverses Resistance to Anti-PD1 in a T-cell-Rich Tumor, Cancer Res 76(21) (2016) 6266–6277. [DOI] [PubMed] [Google Scholar]
- [170].Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH, Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma, Cancer Immunol Res 3(4) (2015) 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].O'Hara MH, O'Reilly EM, Rosemarie M, Varadhachary G, Wainberg ZA, Ko A, Fisher GA, Rahma O, Lyman JP, Cabanski CR, Carpenter EL, Hollmann T, Gherardini PF, Kitch L, Selinsky C, LaVallee T, Trifan OC, Dugam U, Hubbard-Lucey VM, Vonderheide RH, Abstract CT004: A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients, American Association for Cancer Research Annual Meeting, Cancer Res, Atlanta, GA: , 2019, p. Abstact nr CT004. [Google Scholar]
- [172].Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK, Long SR, Hoppe RT, Janssen R, Candia AF, Coffman RL, Levy R, In Situ Vaccination with a TLR9 Agonist and Local Low-Dose Radiation Induces Systemic Responses in Untreated Indolent Lymphoma, Cancer Discov 8(10) (2018) 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R, In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study, J Clin Oncol 28(28) (2010) 4324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Vamer JA, Pu M, Messer KS, Guiducci C, Coffman RL, Kitaura K, Matsutani T, Suzuki R, Carson DA, Hayashi T, Cohen EE, Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer, JCI Insight 2(18) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ, Barve M, Daniels GA, Wong DJ, Schmidt EV, Candia AF, Coffman RL, Leung ACF, Janssen RS, SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase lb, Multicenter Study, Cancer Discov 8(10) (2018) 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Milhem M, Gonzales R, Medina T, Kirkwood JM, Buchbinder E, Mehmi I, Niu J, Shaheen M, Weight R, Margolin K, Luke J, Morris A, Mauro D, Krieg AM, Ribas A, Abstract CT144: Intratumoral toll-like receptor 9 (TLR9) agonist, CMP-001, in combination with pembrolizumab can reverse resistance to PD-1 inhibition in a phase lb trial in subjects with advanced melanoma, American Association for Cancer Research Annual Meeting, Cancer Res, Chicago, IL, 2018, p. Abstract nr CT144. [Google Scholar]
- [177].Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, Black TA, Chien AL, Majmundar KS, Thompson JC, Yee SS, O'Hara M, Aggarwal C, Xin D, Shaked A, Gao M, Liu D, Borad MJ, Ramanathan RK, Carpenter EL, Ji A, de Beer MC, de Beer FC, Webb NR, Beatty GL, Hepatocytes direct the formation of a pro-metastatic niche in the liver, Nature (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Gabay C, Kushner I, Acute-phase proteins and other systemic responses to inflammation, N Engl J Med 340(6) (1999) 448–54. [DOI] [PubMed] [Google Scholar]
- [179].Sansone P, Bromberg J, Targeting the interleukin-6/Jak/stat pathway in human malignancies, J Clin Oncol 30(9) (2012) 1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Kang S, Tanaka T, Narazaki M, Kishimoto T, Targeting Interleukin-6 Signaling in Clinic, Immunity 50(4) (2019) 1007–1023. [DOI] [PubMed] [Google Scholar]
- [181].Thiounn N, Pages F, Flam T, Tartour E, Mosseri V, Zerbib M, Beuzeboc P, Deneux L, Fridman WH, Debre B, IL-6 is a survival prognostic factor in renal cell carcinoma, Immunol Lett 58(2) (1997) 121–4. [DOI] [PubMed] [Google Scholar]
- [182].Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R, Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma, J Clin Oncol 13(3) (1995) 575–82. [DOI] [PubMed] [Google Scholar]
- [183].Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecchi A, Pirisi M, Emanuelli G, Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival, Cancer Immunol Immunother 55(6) (2006) 684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Martin F, Santolaria F, Batista N, Milena A, Gonzalez-Reimers E, Brito MJ, Oramas J, Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer, Cytokine 11(1) (1999) 80–6. [DOI] [PubMed] [Google Scholar]
- [185].Zhang GJ, Adachi I, Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma, Anticancer Res 19(2B) (1999) 1427–32. [PubMed] [Google Scholar]
- [186].Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, Zhong X, Zimmers TA, Ostrowski MC, Ludwig T, Bloomston M, Bekaii-Saab T, Lesinski GB, IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer, Gut 67(2) (2016) 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H, Combined Blockade of IL6 and PD-1/PD-L1 Signaling Abrogates Mutual Regulation of Their Immunosuppressive Effects in the Tumor Microenvironment, Cancer Res 78(17) (2018) 5011–5022. [DOI] [PubMed] [Google Scholar]
- [188].Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF, Upregulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells, Sci Transl Med 5(200) (2013) 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, Luke JJ, Balmanoukian AS, Schmidt EV, Zhao Y, Gong X, Maleski J, Leopold L, Gajewski TF, Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037), J Clin Oncol 36 (2018) 3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, Demidov L, Robert C, Larkin J, Anderson JR, Maleski J, Jones M, Diede SJ, Mitchell TC, Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO- 301/KEYNOTE-252): a phase 3, randomised, double-blind study, Lancet Oncol 20(8) (2019) 1083–1097. [DOI] [PubMed] [Google Scholar]
- [191].Allard B, Beavis PA, Darcy PK, Stagg J, Immunosuppressive activities of adenosine in cancer, Curr Opin Pharmacol 29 (2016) 7–16. [DOI] [PubMed] [Google Scholar]
- [192].Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, Villalobos PA, Cascone T, Liu X, Tan L, Lorenzi PL, Huang A, Zhao Q, Peng D, Fradette JJ, Peng DH, Ungewiss C, Roybal J, Tong P, Oba J, Skoulidis F, Peng W, Carter BW, Gay CM, Fan Y, Class CA, Zhu J, Rodriguez-Canales J, Kawakami M, Byers LA, Woodman SE, Papadimitrakopoulou VA, Dmitrovsky E, Wang J, Ullrich SE, Wistuba II, Heymach JV, Qin FX, Gibbons DL, CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade, Cancer Discov 8(9) (2018) 1156–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK, Adenosine Receptor 2A Blockade Increases the Efficacy of Anti-PD-1 through Enhanced Antitumor T-cell Responses, Cancer Immunol Res 3(5) (2015) 506–17. [DOI] [PubMed] [Google Scholar]
- [194].Leone RD, Sun IM, Oh MH, Sun IH, Wen J, Englert J, Powell JD, Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models, Cancer Immunol Immunother 67(8) (2018) 1271–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ, Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor, Cancer Res 74(14) (2014) 3652–8. [DOI] [PubMed] [Google Scholar]
- [196].Allard B, Pommey S, Smyth MJ, Stagg J, Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs, Clin Cancer Res 19(20) (2013) 5626–35. [DOI] [PubMed] [Google Scholar]
- [197].Vigano S, Alatzoglou D, Irving M, Menetrier-Caux C, Caux C, Romero P, Coukos G, Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function, Front Immunol 10 (2019) 925. [DOI] [PMC free article] [PubMed] [Google Scholar]